Evaluating the Impact of Artificial Saliva Formulations on Stainless Steel Integrity
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Morphological and Chemical Composition
3.2. Structural Analysis
3.3. Hardness Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.T.; Gbureck, U.; Rackwitz, L.; Nöth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How Smart Do Biomaterials Need to Be? A Translational Science and Clinical Point of View. Adv. Drug Deliv. Rev. 2013, 65, 581–603. [Google Scholar] [CrossRef] [PubMed]
- Masaeli, R.; Zandsalimi, K.; Tayebi, L. Biomaterials Evaluation: Conceptual Refinements and Practical Reforms. Ther. Innov. Regul. Sci. 2019, 53, 120–127. [Google Scholar] [CrossRef]
- Harun, W.S.W.; Kamariah, M.S.I.N.; Muhamad, N.; Ghani, S.A.C.; Ahmad, F.; Mohamed, Z. A Review of Powder Additive Manufacturing Processes for Metallic Biomaterials. Powder Technol. 2018, 327, 128–151. [Google Scholar] [CrossRef]
- Shahmir, H.; Al-Asadi, N.K.F.; Bani-Asad, Z.J.A.A. Comparison of Microstructure, Mechanical Properties and Biocompatibility of CoCrFeNiMn High-Entropy Alloy with 316L Stainless Steel. Intermetallics 2024, 167, 108215. [Google Scholar] [CrossRef]
- Bekmurzayeva, A.; Duncanson, W.J.; Azevedo, H.S.; Kanayeva, D. Surface Modification of Stainless Steel for Biomedical Applications: Revisiting a Century-Old Material. Mater. Sci. Eng. C 2018, 93, 1073–1089. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Chen, T.; Dong, C.; Zhang, K.; Bao, X. A Short Review of Medical-Grade Stainless Steel: Corrosion Resistance and Novel Techniques. J. Mater. Res. Technol. 2024, 29, 2788–2798. [Google Scholar] [CrossRef]
- Mocnik, P.; Kosec, T.; Kovac, J.; Bizjak, M. The Effect of pH, Fluoride and Tribocorrosion on the Surface Properties of Dental Archwires. Mater. Sci. Eng. C 2017, 78, 682–689. [Google Scholar] [CrossRef]
- Dawes, C.; Wong, S.F. The Effects of Consumption of Acidic Beverages on Salivary pH and Dental Erosion. J. Dent. 2019, 87, 103158. [Google Scholar] [CrossRef]
- Lenander-Lumikari, M.; Loimaranta, V. Saliva and Oral Microflora in Health and Disease. Adv. Dent. Res. 2000, 14, 40–47. [Google Scholar] [CrossRef]
- Arab-Nozari, M.; Shokrzadeh, M.; Zamehran, N.; Charati, J.Y.; Nahvi, A. Trimming and pH Effects on Nickel Ion Release from Stainless Steel Crowns of Primary Teeth. J. Dent. Indones. 2020, 27, 125–130. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, Y.; Yin, L.; Yuan, Z.; Lan, Y.; Xu, D.; Yang, C.; Yang, K. Improved Corrosion Resistance and Biofilm Inhibition Ability of Copper-Bearing 304 Stainless Steel Against Oral Microaerobic Streptococcus mutans. J. Mater. Sci. Technol. 2021, 66, 112–120. [Google Scholar] [CrossRef]
- Simionescu, N.; Ravoiu, A.; Benea, L. Electrochemical In Vitro Properties of 316L Stainless Steel for Orthodontic Applications. Rev. Chim. 2019, 70, 1144–1148. [Google Scholar] [CrossRef]
- Elagli, K.; Traisnel, M.; Hildebrand, H.F. Electrochemical behaviour of titanium and dental alloys in artificial saliva. Electrochim. Acta 1993, 38, 1769–1774. [Google Scholar] [CrossRef]
- Fusayama, T.; Katayori, T.; Nomoto, S. Corrosion of Gold and Gold Alloys. J. Dent. Res. 1963, 42, 1183–1197. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.M. Saliva Artificial Preparation for Corrosion Studies. Corros. Sci. 1977, 17, 971–982. [Google Scholar] [CrossRef]
- Amin, H.F.; Shehab, G.; Rahman, S. The Effect of Fluoride Ion Concentration and pH on the Corrosion and Cytotoxic Behavior of Ni–Cr Alloy. Corr. Prev. Control 2002, 49, 105–116. [Google Scholar]
- Marques, I.D.S.; Pereira, F.A.; Conti, P.C.R.; Oltramari-Navarro, P.V.P.; Navarro, R.L. Artificial Saliva Formulations: A Review of Their Characteristics and Clinical Indications. Gerodontology 2022, 39, 265–274. [Google Scholar] [CrossRef]
- Khairnar, M.R.; Wadkar, P.; Aher, G.; Sonawane, K.; Das, S. In Vitro Evaluation of Corrosion Behavior of Orthodontic Stainless Steel Wires in Artificial Saliva. J. Orthod. Sci. 2021, 10, 24. [Google Scholar]
- Wang, R.; Chen, W.; Wang, J.; Zhang, Y.; Liu, H. Influence of Fluoride and pH on the Corrosion Behavior of Dental Alloys in Artificial Saliva. J. Mater. Sci. Mater. Med. 2020, 31, 41. [Google Scholar]
- Chiba, A.; Sakakura, S.; Kobayashi, K.; Kusayanagi, K. Dissolution Amounts of Nickel, Chromium and Iron from SUS 304, 316 and 444 Stainless Steels in Sodium Chloride Solutions. J. Mater. Sci. 1997, 32, 1995–2000. [Google Scholar] [CrossRef]
- Narmada, I.B.; Alida; Farha, N.J.; Virgianti, I.D.; Larasati, P.P.; Nugraha, A.P.; Noor, T.N.E.B.T.A. Release of Nickel and Chromium Ions from Stainless Steel Orthodontics Bracket: A Review. Res. J. Pharm. Technol. 2023, 16, 4935–4942. [Google Scholar] [CrossRef]
- Lv, R.; Tang, X.; Ying, Z.; Ai, H.; Sun, H.; Zhang, W.; Wang, Y.; Cheng, J.; Yan, L. Corrosion Mechanism and Properties of 316L Stainless Steel in NaCl–KCl Molten Salt at High Temperatures. Crystals 2025, 15, 280. [Google Scholar] [CrossRef]

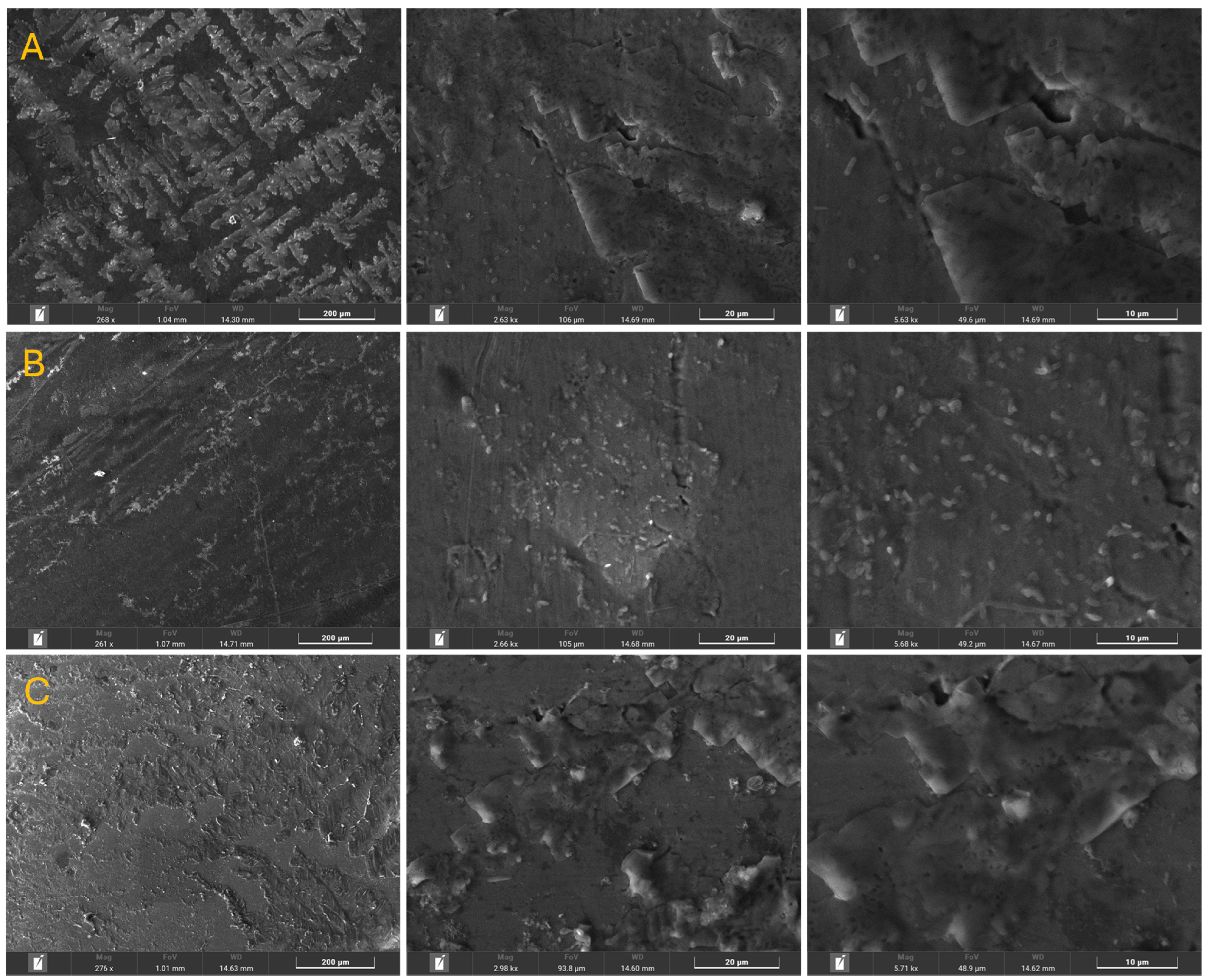
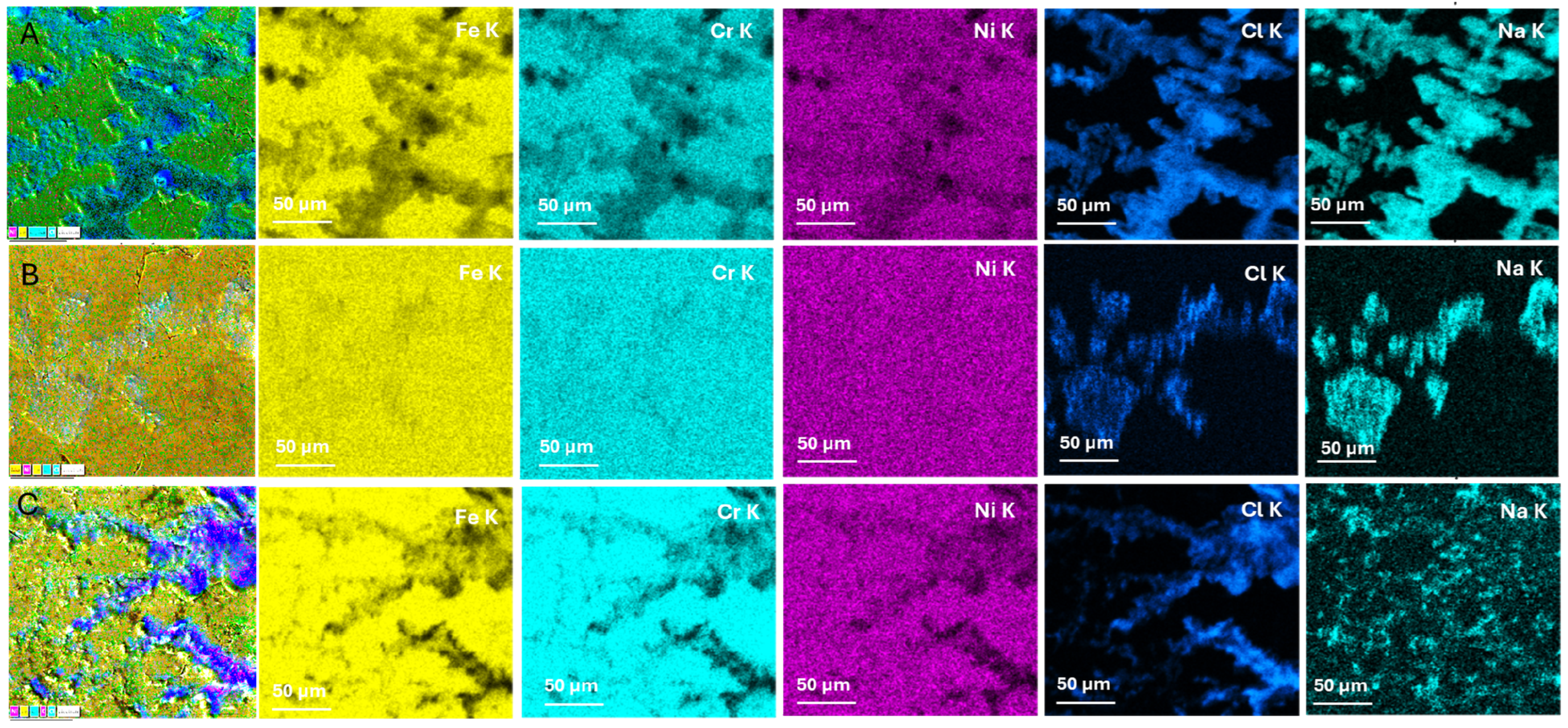
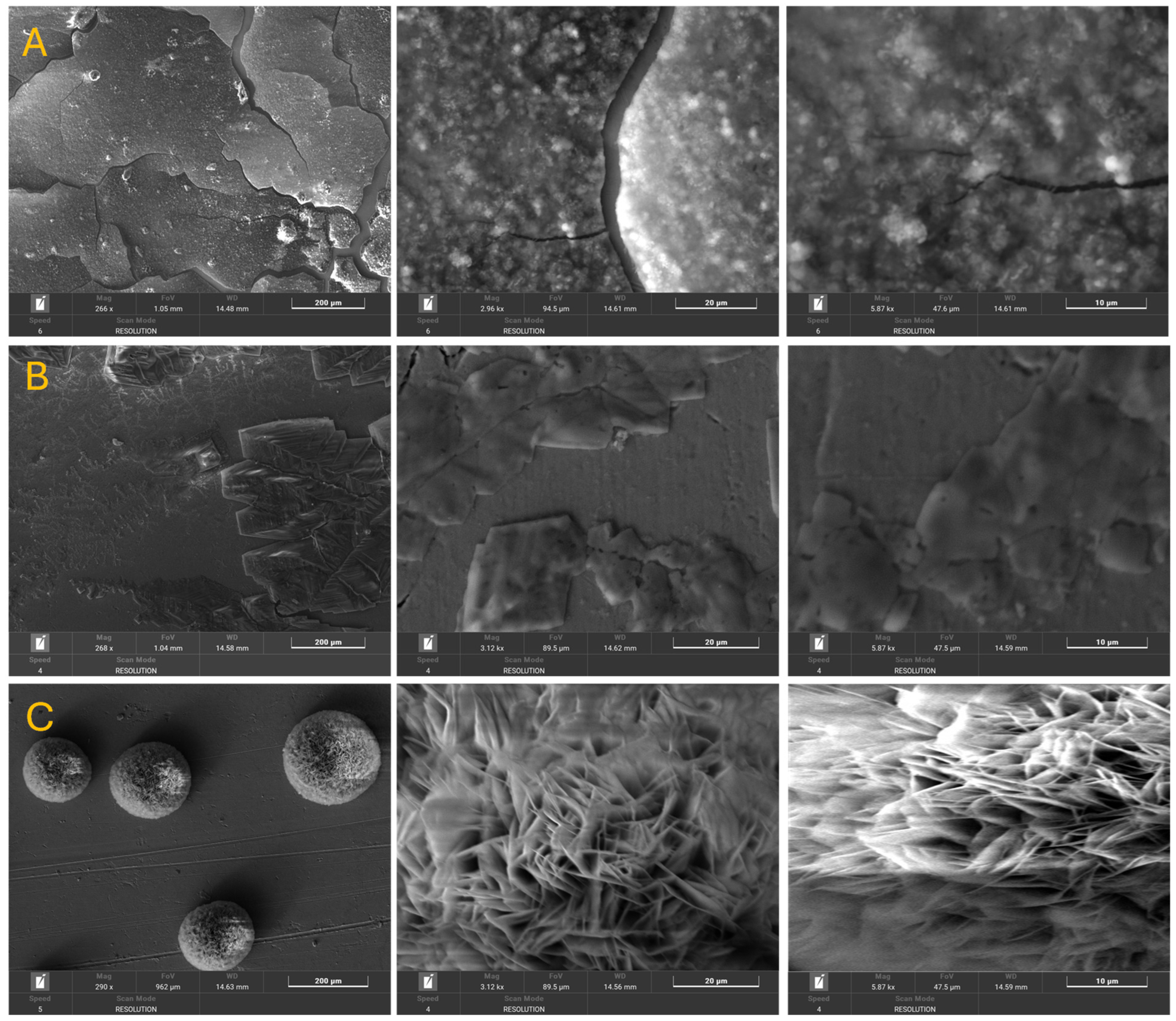
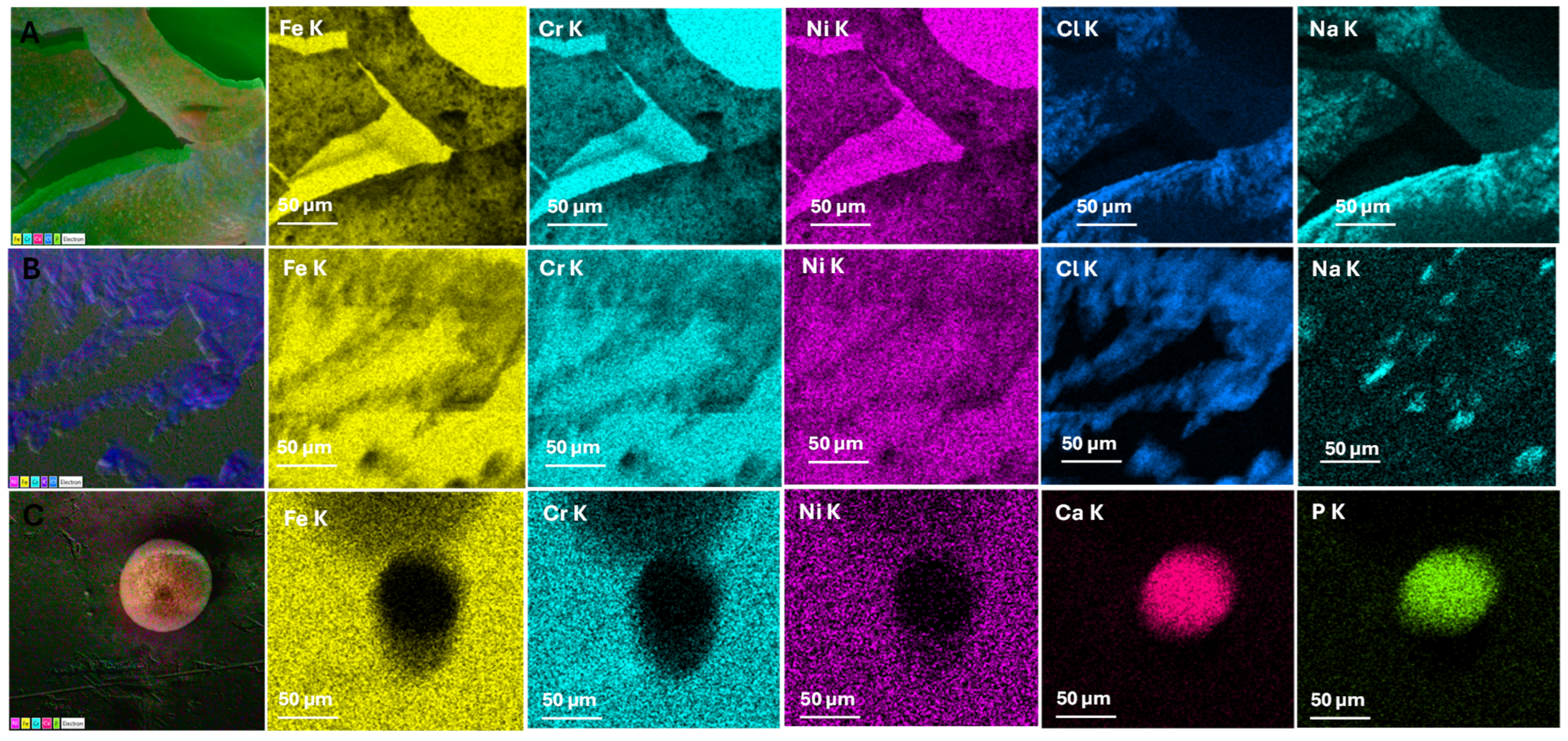
| Element | Initial Sample (%) | Afnor Saliva (%) | Fusayama/Meyer Saliva (%) | Fletcher Saliva (%) | |||
|---|---|---|---|---|---|---|---|
| 7 Days | 28 Days | 7 Days | 28 Days | 7 Days | 28 Days | ||
| Fe | 67.920 | 67.770 | 67.840 | 67.860 | 67.850 | 67.640 | 67.450 |
| Cr | 17.167 | 17.105 | 17.118 | 17.152 | 17.061 | 16.955 | 16.813 |
| Mn | 1.269 | 1.273 | 1.299 | 1.266 | 1.254 | 1.271 | 1.299 |
| Ni | 10.238 | 10.260 | 10.180 | 10.121 | 10.12 | 10.270 | 10.310 |
| Cu | 0.368 | 0.387 | 0.388 | 0.380 | 0.385 | 0.398 | 0.390 |
| Nb | 0.013 | 0.015 | 0.014 | 0.015 | 0.014 | 0.015 | 0.015 |
| Mo | 2.067 | 2.061 | 2.065 | 2.057 | 2.053 | 2.140 | 2.175 |
| Si | 0.580 | 0.520 | 0.480 | 0.530 | 0.520 | 0.610 | 0.590 |
| V | 0.074 | 0.070 | 0.070 | 0.073 | 0.074 | 0.061 | 0.061 |
| Co | 0.263 | 0.286 | 0.295 | 0.325 | 0.295 | 0.315 | 0.386 |
| W | 0.034 | 0.039 | 0.044 | 0.043 | 0.044 | 0.042 | 0.045 |
| P | - | 0.175 | 0.200 | 0.182 | 0.327 | 0.281 | 0.465 |
| S | - | 0.039 | - | - | - | - | - |
| Element | Initial (%) | Afnor 28 Days (%) | Fusayama/Meyer 28 Days (%) | Fletcher 28 Days (%) | Trend (28 Days vs. Initial) |
|---|---|---|---|---|---|
| Fe | ~67.8 | ~67.6 | ~67.3 | ~67.7 | Slight decrease in acidity; ~stable in neutral/basic |
| Cr | ~17.1 | ~17.2 | ~17.5 | ~17.1 | Slight increase in acidity (enriched); ~stable elsewhere |
| Ni | ~12.7 | ~12.6 | ~12.0 | ~12.6 | Notable decrease in acidity; minimal change in others |
| Mo | ~2.1 | ~2.2 | ~2.3 | ~2.1 | Slight increase in acidity; ~stable in neutral/basic |
| Cu | ~0.2 | ~0.2 | ~0.2 | ~0.2 | Essentially no change (trace element) |
| Co | ~0.06 | ~0.06 | ~0.05 | ~0.06 | Essentially no change (trace element) |
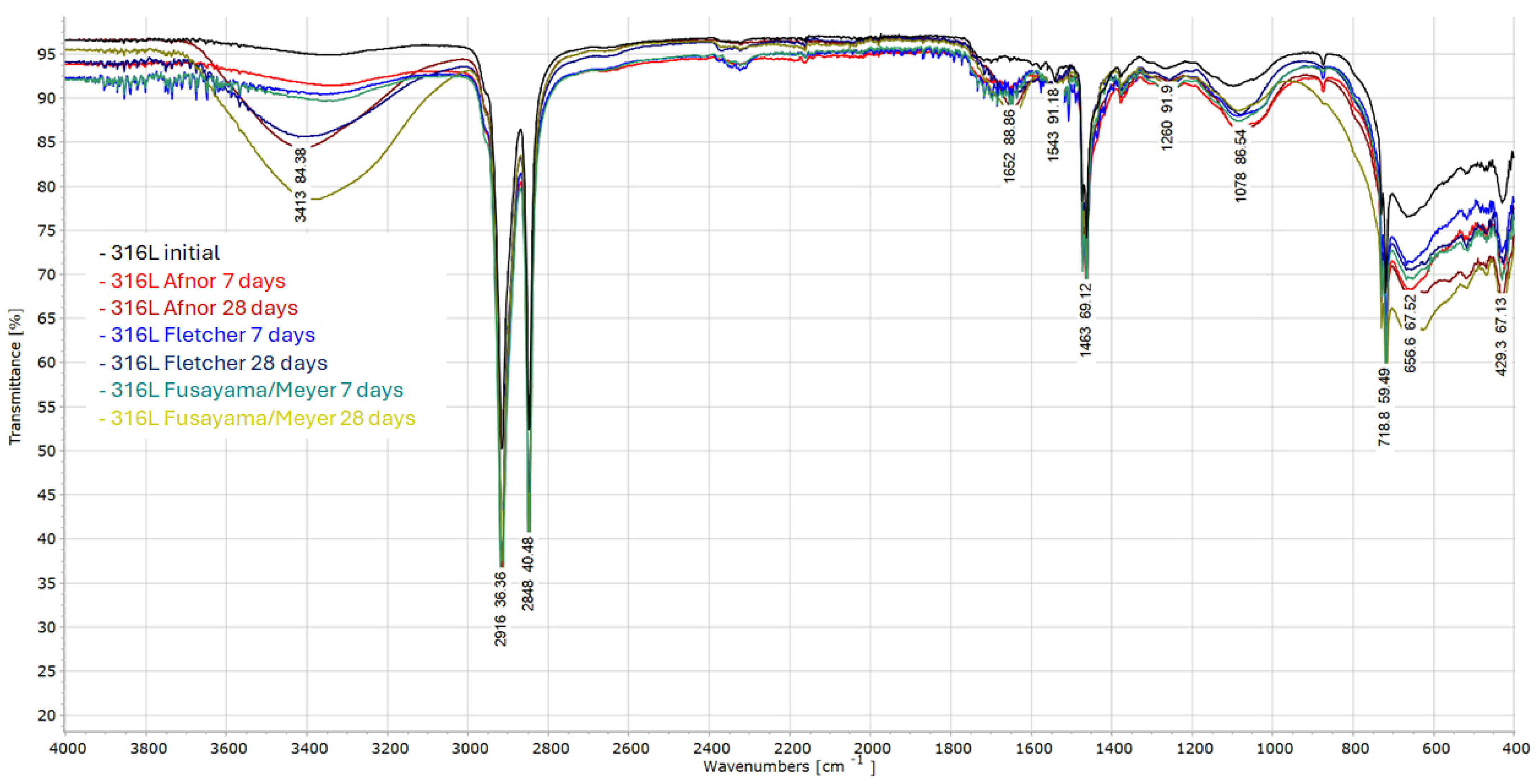
| Wavenumber (cm−1) | Possible Assignment | Interpretation |
|---|---|---|
| ~3414, 3438 | O–H stretching (H2O, hydroxyls) | Surface hydration or hydroxylation, strongest in Fusayama/Meyer for 28 days |
| 2916, 2848 | C–H stretching | Possible organic residue adsorption from artificial saliva |
| 1652, 1543 | Amide I/II, H–O–H bending | Associated with water or organic layer accumulation |
| 1463, 1386 | C–H bending, COO− | Presence of degraded organics or adsorbed molecules |
| 1230, 1078 | P=O, P–O–C, S=O | Phosphate/sulfate species, intense in Fusayama/Meyer |
| 718, 669 | Metal–O, Cl− | Cr–O, Fe–O, or chloride species, evident in Fletcher |
| 429, 671 | Metal oxide lattice | Broadening indicates surface oxide changes over time |
| Saliva Type | D1 (7 Days) [µm] | D2 (7 Days) [µm] | HV (7 Days) | HRA (7 Days) | D1 (28 Days) [µm] | D2 (28 Days) [µm] | HV (28 Days) | HRA (28 Days) |
|---|---|---|---|---|---|---|---|---|
| Afnor | 98.25 | 96.19 | 196.1 | 56.5 | 75.75 | 101.92 | 234.9 | 60.3 |
| Fusayama/Meyer | 96.82 | 100.18 | 191.1 | 55.9 | 93.22 | 97.09 | 204.7 | 57.5 |
| Fletcher | 97.97 | 98.90 | 191.3 | 55.9 | 90.62 | 79.27 | 256.9 | 62.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buruiana, D.L.; Bogatu, N.L.; Muresan, A.C.; Herbei, E.E.; Trus, C.; Ghisman, V. Evaluating the Impact of Artificial Saliva Formulations on Stainless Steel Integrity. Appl. Sci. 2025, 15, 5345. https://doi.org/10.3390/app15105345
Buruiana DL, Bogatu NL, Muresan AC, Herbei EE, Trus C, Ghisman V. Evaluating the Impact of Artificial Saliva Formulations on Stainless Steel Integrity. Applied Sciences. 2025; 15(10):5345. https://doi.org/10.3390/app15105345
Chicago/Turabian StyleBuruiana, Daniela Laura, Nicoleta Lucica Bogatu, Alina Crina Muresan, Elena Emanuela Herbei, Constantin Trus, and Viorica Ghisman. 2025. "Evaluating the Impact of Artificial Saliva Formulations on Stainless Steel Integrity" Applied Sciences 15, no. 10: 5345. https://doi.org/10.3390/app15105345
APA StyleBuruiana, D. L., Bogatu, N. L., Muresan, A. C., Herbei, E. E., Trus, C., & Ghisman, V. (2025). Evaluating the Impact of Artificial Saliva Formulations on Stainless Steel Integrity. Applied Sciences, 15(10), 5345. https://doi.org/10.3390/app15105345






