The Combination of Nitrogen (N2) Pyrolysis and Carbon Dioxide (CO2) Activation for Regenerating Spent Activated Carbon
Abstract
1. Introduction
2. Materials and Methods
2.1. Spent GAC
2.2. Thermogravimetric and Elemental Analyses of Spent GAC
2.3. Thermal Regeneration Experiments
2.4. Determinations of Textural and Chemical Characteristics of Regenerated AC Products
3. Results
3.1. Thermochemical Characteristics of Spent GAC
3.2. Pore Properties of Spent GAC and Its Regenerated Products
- The regenerated samples exhibited significantly improved pore properties compared to the spent GAC (e.g., BET surface area of 425.17 m2/g and total pore volume of 0.184 cm3/g). The variations in pore property trends were not consistent with pyrolysis temperature and holding time. Interestingly, the maximum pore properties (BET surface area of 605.38 m2/g and total pore volume of 0.272 cm3/g) were obtained under the minimal process conditions (800 °C and 0 min holding time).
- The CO2-activated products generally displayed even higher pore properties than both the spent GAC and the pyrolysis-regenerated products except in the cases of KN-800-00 and KNC-800-00. It can be attributed to further activation by the interaction of the CO2 gas with carbon (AC or char) due to the following chemical reaction [1]:C(solid) + CO2(gas) → 2CO(gas)Above 800 °C, CO2 gas reacted with the carbon matrix, thus opening the pores, which resulted in higher pore properties. The maximal pore properties (BET surface area 723.23 m2/g, and total pore volume 0.327 cm3/g) of the CO2-activated GAC products were produced at 900 °C and a 30 min holding time. These results show that, under optimized pyrolysis-activation conditions, approximately 70% of the pore properties can be achieved, making these regenerated GACs highly promising for water treatment applications.
- As shown in Figure 2, the nitrogen adsorption–desorption isotherms of spent GAC (KN) and the regenerated products (KN-900-30 and KNC-900-30) displayed both Type I (major) and Type VI (minor) patterns, typically indicating the presence of both micropores and mesopores [29]. Type I curves exhibited steep uptake at low relative pressures, corresponding to high micropore adsorption potential. On the other hand, the Type VI isotherms featured slight hysteresis loops, starting around a relative pressure of approximately 0.30. Figure 3 further confirms the presence of mesopores with the recorded peak at approximately 4.0 nm, while an upward trend below 2.0 nm on the left of the curve indicates a microporous structure. For more precise micropore distribution analysis, models such as Dubinin–Astakhov (DA) and Horvath–Kawazoe (HK) are recommended [29,30].
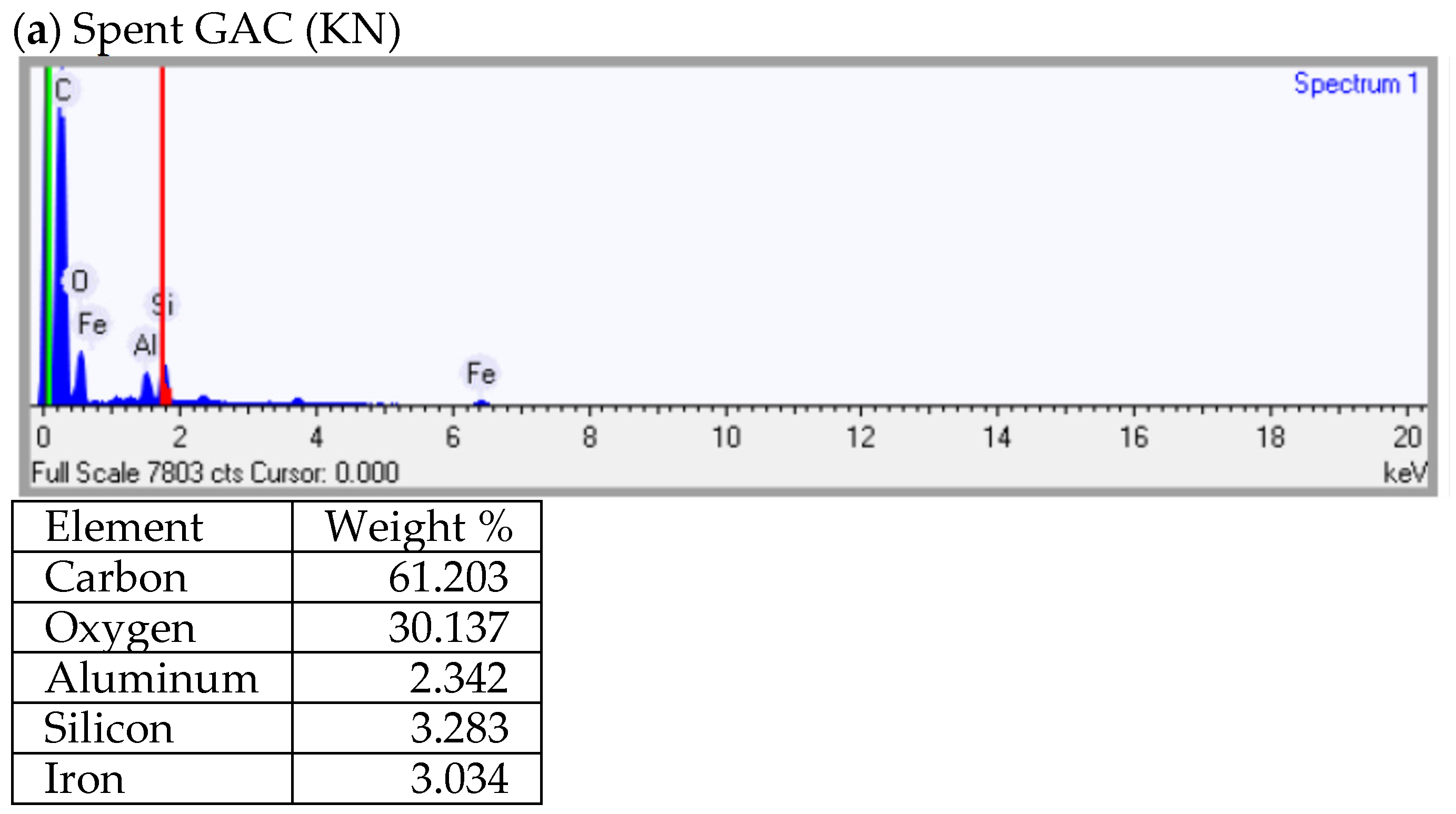
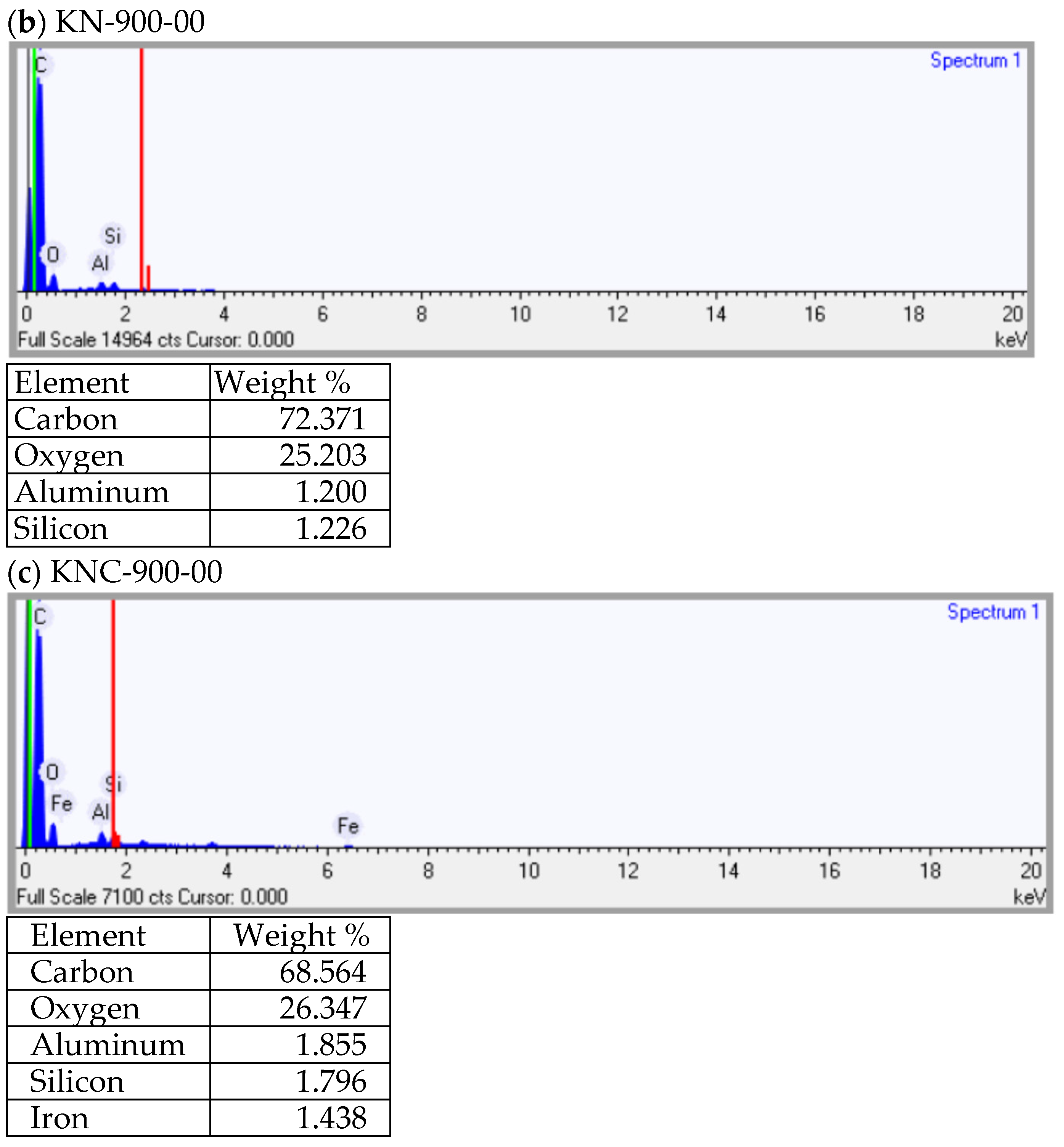
3.3. Textural and Chemical Characteristics of Spent GAC and Its Regenerated Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marsh, H.; Rodriguez-Reinoso, F. Activated Carbon; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; CRC Press: Bocca Raton, FL, USA, 2005. [Google Scholar]
- Suzuki, M. Adsorption Engineering; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Hwang, S.Y.; Lee, G.B.; Kim, J.H.; Hong, B.U.; Park, J.E. Pre-treatment methods for regeneration of spent activated carbon. Molecules 2020, 25, 4561. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, X.; Wen, J. Study on the harm of waste activated carbon and novel regeneration technology of it. IOP Conf. Ser. Earth Environ. Sci. 2021, 769, 022047. [Google Scholar] [CrossRef]
- Srivastava, A.; Gupta, B.; Majumder, A.; Gupta, A.K.; Nimbhorkar, S.K. A comprehensive review on the synthesis, performance, modifications, and regeneration of activated carbon for the adsorptive removal of various water pollutants. J. Environ. Chem. Eng. 2021, 9, 106177. [Google Scholar] [CrossRef]
- Santos, D.H.D.S.; Xiao, Y.; Chaukura, N.; Hill, J.M.; Selvasembian, R.; Zanta, C.L.P.S.; Meili, L. Regeneration of dye-saturated activated carbon through advanced oxidative processes: A review. Heliyon 2022, 8, e10205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, G.; Zhang, Y.; Su, Y.; Zhang, H. The deactivation mechanisms, regeneration methods and devices of activated carbon in applications. J. Clean. Prod. 2024, 476, 143751. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, Q.; Shi, Z.; Zhang, Z.; Ma, Y. Microwave regeneration of spent activated carbon for the treatment of ester-containing wastewater. RSC Adv. 2016, 6, 60815–60825. [Google Scholar] [CrossRef]
- Ma, A.; Zheng, X.; Liu, C.; Peng, J.; Li, S.; Zhang, L.; Liu, C. Study on regeneration of spent activated carbon by using a clean technology. Green Process. Synth. 2017, 6, 499–510. [Google Scholar] [CrossRef]
- Durán-Jiménez, G.; Stevens, L.A.; Hodgins, G.R.; Uguna, J.; Ryan, J.; Binner, E.R.; Robinson, J.P. Fast regeneration of activated carbons saturated with textile dyes: Textural, thermal and dielectric characterization. Chem. Eng. J. 2019, 378, 121774. [Google Scholar] [CrossRef]
- Oladejo, J.; Shi, K.; Chen, Y.; Luo, X.; Gang, Y.; Wu, T. Closing the active carbon cycle: Regeneration of spent activated carbon from a wastewater treatment facility for resource optimization. Chem. Eng. Process. Process Intensif. 2020, 150, 107878. [Google Scholar] [CrossRef]
- Huang, F.; Liu, C.; Cheng, S.; Li, T. Microwave thermal regeneration characteristics of spent activated carbon based on a coupled electromagnetic, heat and mass transfer multiphase porous media model. Energy 2024, 292, 130437. [Google Scholar] [CrossRef]
- Li, Y.; Jin, H.; Liu, W.; Lu, Y.; Li, J. Study on regeneration of waste powder activated carbon through pyrolysis and its adsorption capacity of phosphorus. Sci. Rep. 2018, 8, 778. [Google Scholar] [CrossRef]
- Cazetta, A.L.; Junior, O.P.; Vargas, A.M.M.; da Silva, A.P.; Zou, X.; Asefa, T.; Almeida, V.C. Thermal regeneration study of high surface area activated carbon obtained from coconut shell: Characterization and application of response surface methodology. J. Anal. Appl. Pyrolysis 2013, 101, 53–60. [Google Scholar] [CrossRef]
- Yang, H.-C.; Lee, M.-W.; Eun, H.-C.; Kim, H.-J.; Lee, K.; Seo, B.-K. Thermal decontamination of spent activated carbon contaminated with radiocarbon and tritium. Processes 2020, 8, 1359. [Google Scholar] [CrossRef]
- Baghirzade, B.S.; Zhang, Y.; Reuther, J.F.; Saleh, N.B.; Venkatesan, A.K.; Apul, O.G. Thermal regeneration of spent granular activated carbon presents an opportunity to break the forever PFAS cycle. Environ. Sci. Technol. 2021, 55, 5608–5619. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Du, E. The effects of thermal regeneration conditions and inorganic compounds on the characteristics of activated carbon used in power plant. Energy Procedia 2012, 17 Pt A, 444–449. [Google Scholar] [CrossRef]
- Álvarez, P.M.; Beltrán, F.J.; Gómez-Serrano, V.; Jaramillo, J.; Rodríguez, E.M. Comparison between thermal and ozone regenerations of spent activated carbon exhausted with phenol. Water Res. 2004, 38, 2155–2165. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, Y.S.; Jeon, S.B.; Seo, J.B.; Jung, J.H.; Oh, K.J. Improvement of thermal regeneration of spent granular activated carbon using air agent: Application of sintering and deoxygenation. Korean J. Chem. Eng. 2014, 31, 1641–1650. [Google Scholar] [CrossRef]
- Toledo, R.B.C.; Aragón-Tobar, C.F.; Gámez, S.; de la Torre, E. Reactivation process of activated carbons: Effect on the mechanical and adsorptive properties. Molecules 2020, 25, 1681. [Google Scholar] [CrossRef]
- Nasir, M.Z.M.; Indiran, G.; Zaini, M.A.A. Assessment of thermal regeneration of spent commercial activated carbon for methylene blue dye removal. Part. Sci. Technol. 2021, 39, 504–510. [Google Scholar] [CrossRef]
- Fagbohun, E.O.; Wang, Q.; Spessato, L.; Zheng, Y.; Li, W.; Olatoye, A.G.; Cui, Y. Physicochemical regeneration of industrial spent activated carbons using a green activating agent and their adsorption for methyl orange. Surf. Interfaces 2022, 29, 101696. [Google Scholar] [CrossRef]
- Márquez, P.; Benítez, A.; Chica, A.F.; Martín, M.A.; Caballero, A. Evaluating the thermal regeneration process of massively generated granular activated carbons for their reuse in wastewater treatments plants. J. Clean. Prod. 2022, 366, 132685. [Google Scholar] [CrossRef]
- Yuan, H.; Ye, J.; Yang, J.; Wang, H.; Ni, X.; Li, D.; Chen, Y. Green regeneration of spent activated carbon from antibiotics purification as remarkable absorbent for aqueous Cd2+ removal. J. Hazard. Mater. Adv. 2023, 12, 100361. [Google Scholar] [CrossRef]
- Tsai, W.T.; Chen, H.P.; Hsieh, M.F.; Sun, H.F.; Chien, S.F. Regeneration of spent bleaching earth by pyrolysis in a rotary furnace. J. Anal. Appl. Pyrolysis 2002, 63, 157–170. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lai, C.W. Adsorption of herbicide paraquat by clay mineral regenerated from spent bleaching earth. J. Hazard. Mater. 2006, 134, 144–148. [Google Scholar] [CrossRef]
- Pollard, S.J.T.; Sollars, C.J.; Perry, R. A clay-carbon adsorbent derived from spent bleaching earth: Surface characterisation and adsorption of chlorophenols from aqueous solution. Carbon 1992, 30, 639–645. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Condon, J.B. Surface Area and Porosity Determinations by Physisorption: Measurements and Theory; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- So, J.Y.; Cho, H.R. Thermal characteristics of spent activated carbon generated from air cleaning units in Korean nuclear power plants. Nucl. Eng. Technol. 2017, 49, 873–880. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Zhuang, X.; Gan, Z.; Zhou, J.; Zhang, Y.; Zhang, H. Insight into biomass pyrolysis mechanism based on cellulose, hemicellulose, and lignin: Evolution of volatiles and kinetics, elucidation of reaction pathways, and characterization of gas, biochar and bio-oil. Combust. Flame 2022, 242, 112142. [Google Scholar] [CrossRef]
- Steiner, C. Considerations in biochar characterization. In Agricultural and Environmental Applications of Biochar: Advances and Barriers; Guo, M., He, Z., Uchimiya, S.M., Eds.; Soil Science Society of America: Madison, WI, USA, 2016; pp. 87–100. [Google Scholar]
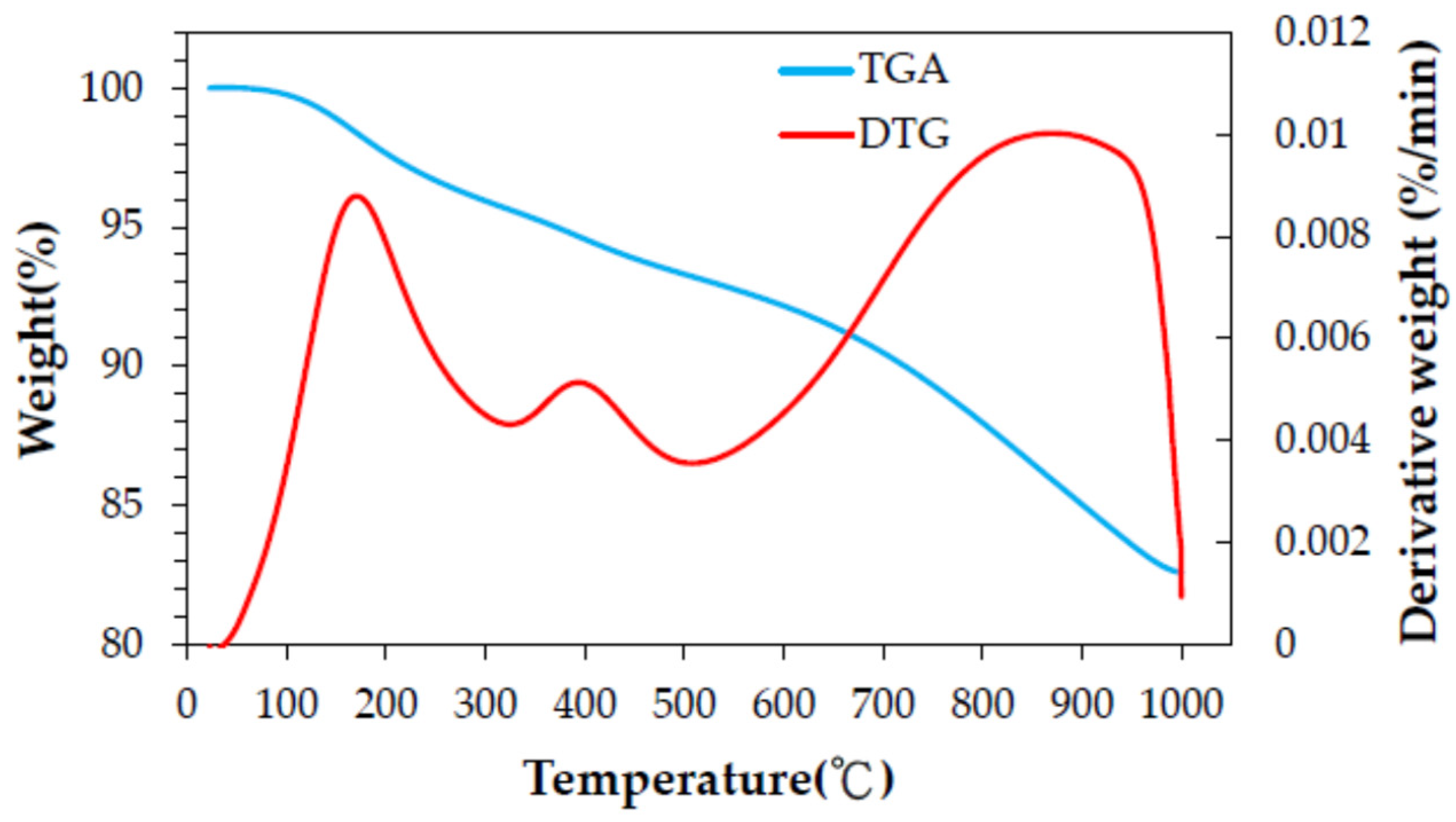
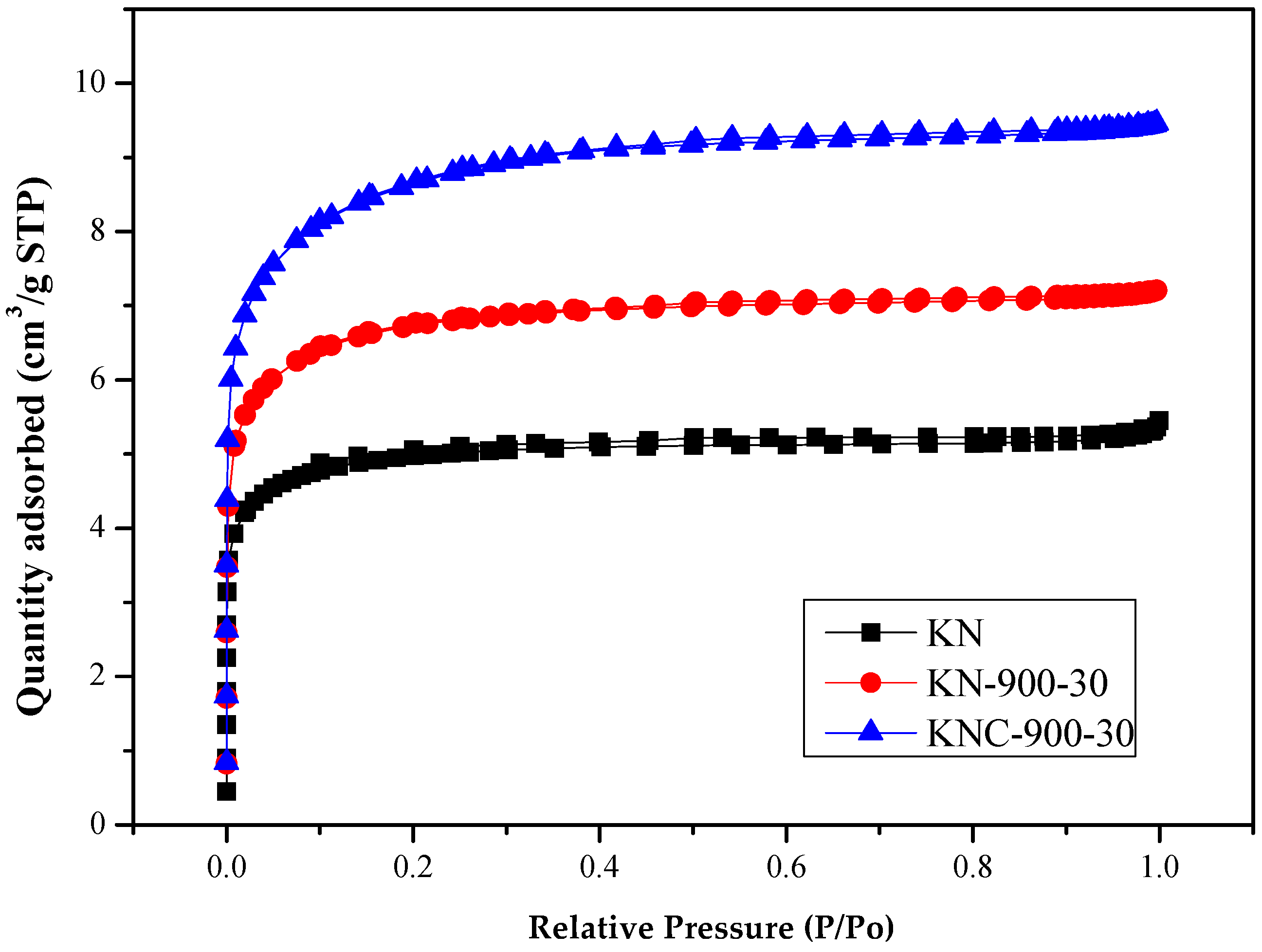

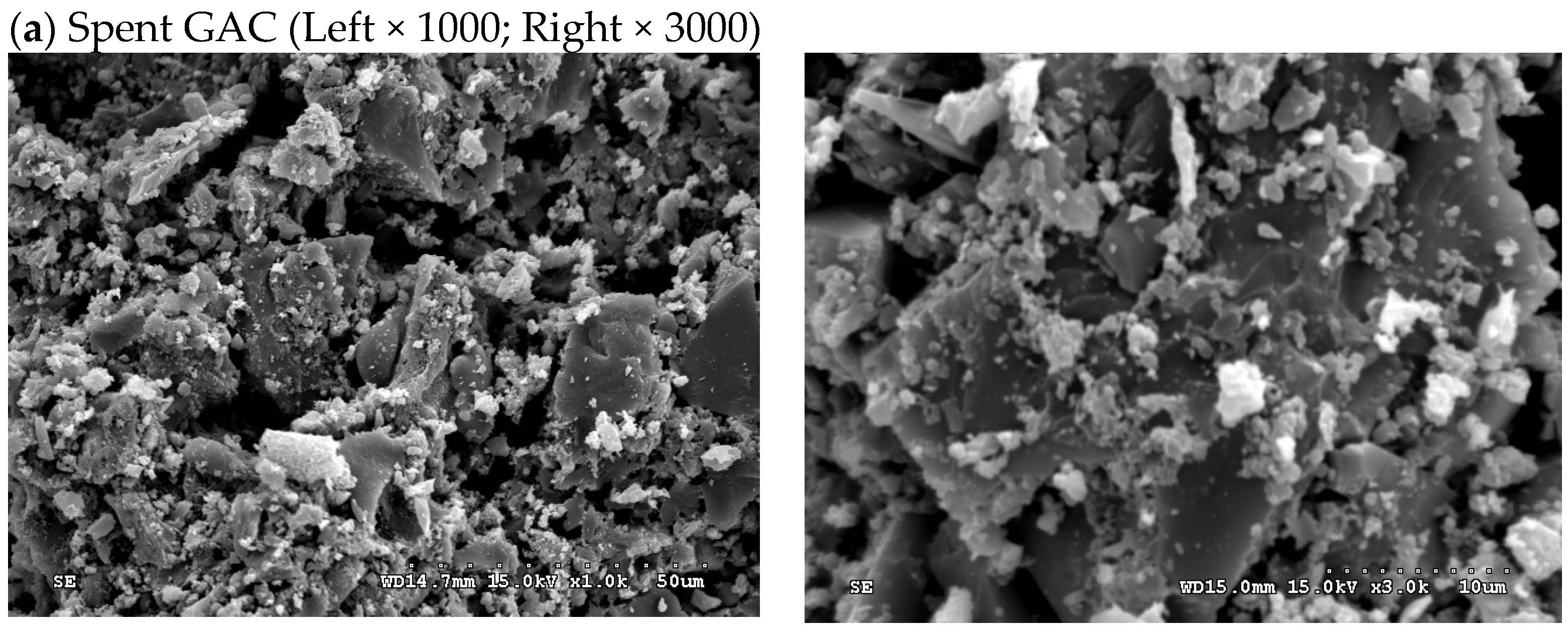
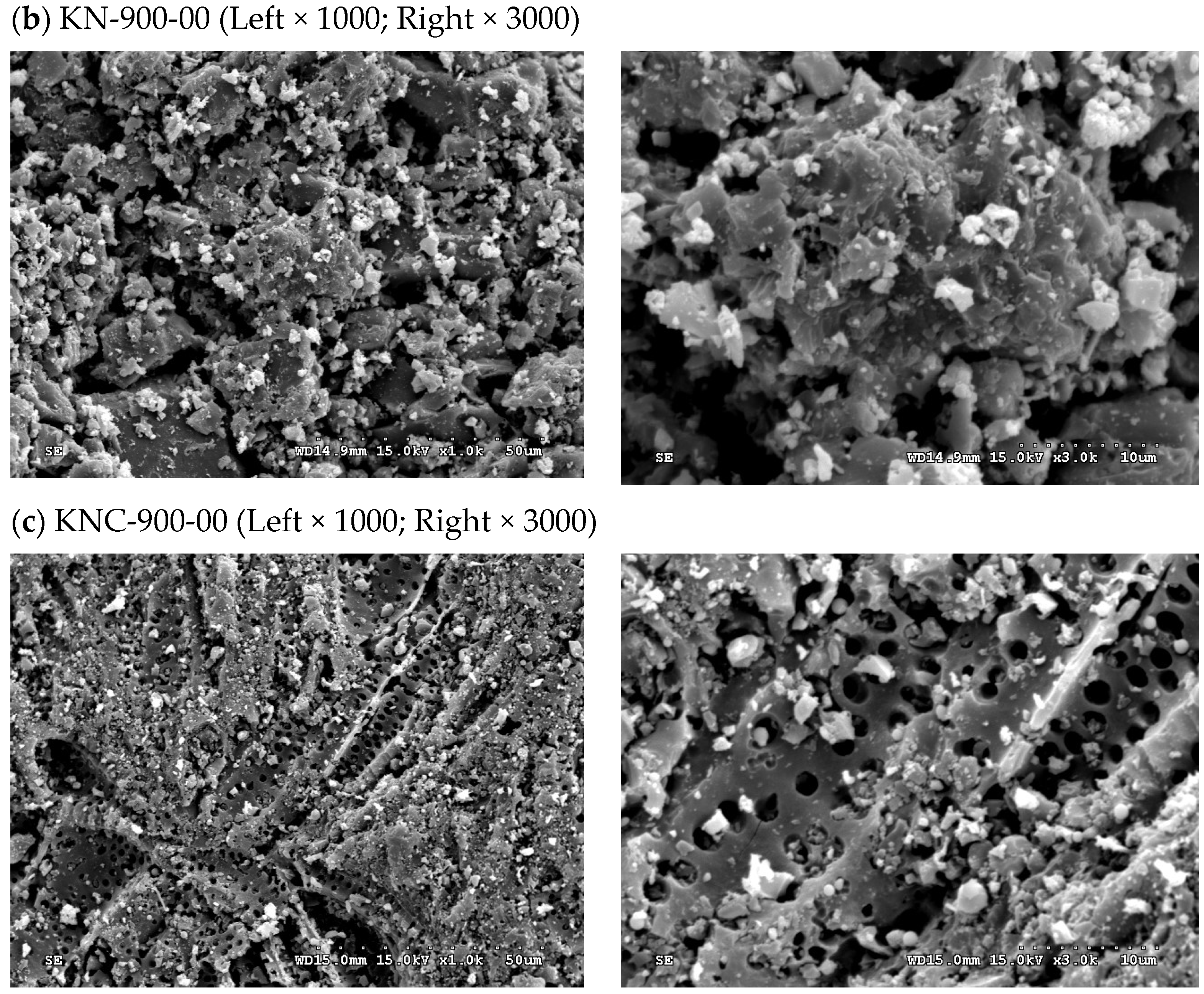
| Property a | Value |
|---|---|
| Moisture (wt%) | 9.99 ± 0.50 |
| Ash (wt%) | 13.58 ± 0.66 |
| Volatile matter (wt%) | 13.34 ± 0.95 |
| Fixed carbon b (wt%) | 73.08 |
| Spent GAC (KN) and Regenerated GAC Product a | SBET b (m2/g) | Smicro c (m2/g) | Vt d (cm3/g) | Vmicro c (cm3/g) |
|---|---|---|---|---|
| KN | 425.17 | 324.04 | 0.184 | 0.129 |
| KN-800-00 | 605.38 | 385.99 | 0.272 | 0.156 |
| KNC-800-00 | 538.00 | 426.68 | 0.231 | 0.169 |
| KN-800-30 | 516.43 | 422.74 | 0.220 | 0.170 |
| KNC-800-30 | 578.22 | 405.79 | 0.254 | 0.163 |
| KN-800-60 | 523.99 | 413.95 | 0.224 | 0.165 |
| KNC-800-60 | 647.15 | 470.20 | 0.283 | 0.188 |
| KN-850-00 | 531.67 | 444.32 | 0.223 | 0.175 |
| KNC-850-00 | 555.93 | 400.13 | 0.249 | 0.161 |
| KN-850-30 | 519.14 | 397.93 | 0.222 | 0.158 |
| KNC-850-30 | 541.13 | 399.76 | 0.239 | 0.160 |
| KN-850-60 | 521.42 | 420.76 | 0.222 | 0.168 |
| KNC-850-60 | 634.97 | 433.99 | 0.278 | 0.174 |
| KN-900-00 | 558.38 | 451.83 | 0.237 | 0.179 |
| KNC-900-00 | 569.21 | 409.04 | 0.260 | 0.164 |
| KN-900-30 | 569.32 | 411.53 | 0.249 | 0.165 |
| KNC-900-30 | 723.23 | 457.25 | 0.327 | 0.185 |
| KN-900-60 | 496.08 | 387.65 | 0.212 | 0.154 |
| KNC-900-60 | 699.88 | 385.89 | 0.316 | 0.155 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Y.-C.; Chen, W.-S.; Tsai, C.-H.; Tsai, W.-T. The Combination of Nitrogen (N2) Pyrolysis and Carbon Dioxide (CO2) Activation for Regenerating Spent Activated Carbon. Appl. Sci. 2025, 15, 5336. https://doi.org/10.3390/app15105336
Ye Y-C, Chen W-S, Tsai C-H, Tsai W-T. The Combination of Nitrogen (N2) Pyrolysis and Carbon Dioxide (CO2) Activation for Regenerating Spent Activated Carbon. Applied Sciences. 2025; 15(10):5336. https://doi.org/10.3390/app15105336
Chicago/Turabian StyleYe, Ya-Chen, Wen-Shing Chen, Chi-Hung Tsai, and Wen-Tien Tsai. 2025. "The Combination of Nitrogen (N2) Pyrolysis and Carbon Dioxide (CO2) Activation for Regenerating Spent Activated Carbon" Applied Sciences 15, no. 10: 5336. https://doi.org/10.3390/app15105336
APA StyleYe, Y.-C., Chen, W.-S., Tsai, C.-H., & Tsai, W.-T. (2025). The Combination of Nitrogen (N2) Pyrolysis and Carbon Dioxide (CO2) Activation for Regenerating Spent Activated Carbon. Applied Sciences, 15(10), 5336. https://doi.org/10.3390/app15105336








