Abstract
(1) Background: Digital health technologies (DHTs) are increasingly being utilized to facilitate receiving rehabilitation services remotely, offering innovative solutions to enhance recovery outcomes. This scoping review examines the role of DHT in home-based stroke rehabilitation, focusing on its applications, effectiveness, and limitations. It identifies key advancements and future directions for improving stroke recovery through technological innovations. (2) Methods: Using Arksey and O’Malley’s framework, a systematic search was conducted across multiple databases to identify studies involving DHT for home-based stroke rehabilitation. Eligible studies incorporated technologies for monitoring and evaluation. Data extraction followed PRISMA-ScR guidelines, synthesizing findings across various research designs. (3) Results: Ten studies were reviewed, categorizing technologies into wearable devices, smartphones, and sensor-based solutions. These tools primarily assessed mobility, upper extremity function, cognitive function, daily living activities, and continuous physiological monitoring. High feasibility and usability were reported, though challenges included small sample sizes and user-centered design limitations. (4) Conclusions: Most DHTs used for evaluating and monitoring home-based stroke rehabilitation are wearable and sensor-based, mainly focusing on mobility and upper extremity function. Their application is effective, but limitations remain. Future research should address these gaps to enhance usability and coverage.
1. Introduction
Stroke remains one of the leading causes of death and disability worldwide, imposing a significant burden on individuals, families, and healthcare systems [1,2]. The economic impact of stroke rehabilitation is also substantial, as long-term care costs are often required, placing a strain on healthcare systems worldwide [3]. According to recent estimates, approximately 6.5 to 6.6 million stroke-related deaths occur annually, a number that reflects the growing impact of this condition globally [4]. Stroke incidence continues to rise, largely due to the increasing aging population and the rising prevalence of risk factors such as hypertension, diabetes, and a sedentary lifestyle [5,6]. Following a stroke, disability is highly prevalent, and patients often face a range of physical and cognitive challenges, including upper extremity (UE) dysfunction, balance disorders, aphasia, dysphagia, and cognitive impairments [7]. The aftermath of a stroke can significantly impact a person’s ability to return to work and perform daily activities, ultimately affecting their quality of life [8]. Approximately 25–74% of stroke survivors require assistance with daily activities, placing a substantial burden on caregivers and healthcare facilities [9]. Early and effective rehabilitation is crucial in the first two months following a stroke, as this period is essential for minimizing the risk of permanent disability and improving recovery outcomes [10].
Also, stroke rehabilitation is no longer confined to hospital settings; a significant component of recovery can take place in the home environment. Home-based rehabilitation allows for continuous monitoring, evaluation, and intervention, which is particularly important for stroke patients who live alone or suffer from cognitive disorders [11]. Home-based digital health tools can support self-management, track progress, and provide ongoing feedback to both patients and healthcare providers [12,13]. This approach offers the potential to reduce the burden on hospitals and outpatient services while ensuring that patients receive the necessary care in the comfort of their homes [14].
As technological advancements continue to reshape healthcare, DHTs are emerging as powerful tools in stroke rehabilitation [15]. DHTs not only improve the efficiency and outcomes of rehabilitation, but they also provide more accessible options for patients [16]. For example, DHT can facilitate remote rehabilitation services, making it possible to reach individuals in rural or underserved areas who may not have access to specialized rehabilitation facilities [17,18]. Moreover, the use of DHT has proven beneficial in uncommon circumstances, such as during the COVID-19 pandemic, when in-person rehabilitation sessions were disrupted by lockdowns and social distancing measures [19]. The field of DHT requires further clarification, not only in terms of the technology itself but also in evaluating its efficacy, effectiveness, and implementation ability. Recent literature reviews have shown that home-based technologies are widely used in stroke rehabilitation, with each technology offering specific benefits and limitations. Additionally, designing for motivation and considering the home environment are crucial factors in developing effective home-based stroke rehabilitation technologies [11]. The most influential research on this topic occurred between 1994 and 2012, with a strong emphasis on randomized clinical trials. Virtual reality technology, in particular, has become a prominent area of research within DHT for stroke rehabilitation [20]. Furthermore, self-monitoring interventions using DHT, which provide feedback and enable self-observation, have proven to be particularly effective in supporting behavior change among stroke patients [21].
However, there is a gap in the literature regarding the specific types of technologies used for home-based rehabilitation monitoring and evaluating patient performance. This is critical for promoting greater independence and safety for patients in their home environments. Therefore, in this scoping review, we aim to explore the digital health technologies currently used to monitor and evaluate home-based stroke rehabilitation. By reviewing the available evidence, we seek to synthesize how these technologies are applied in practice and to provide guidance for future technological development and research initiatives.
2. Materials and Methods
2.1. Design
The research question guiding this review was:
“What digital health technologies are currently used to monitor and evaluate home-based stroke rehabilitation? What is the clinical focus, technological characteristics, reliability, clinical utility, and related recommendations of these technologies for technologists and researchers?”
This scoping review adhered to the methodology proposed by Arksey and O’Malley, which involves defining research questions, selecting appropriate studies, organizing data, and synthesizing and presenting the findings [22].
The protocol for this review was prospectively registered on the Open Science Framework (OSF) and is available at https://doi.org/10.17605/OSF.IO/QWJXV.
In accordance with the JBI methodology for scoping reviews, a critical appraisal of individual sources of evidence was not undertaken, as the objective was to map the existing literature rather than assess study quality [23]. The review process was also aligned with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews checklist (PRISMA-ScR) to ensure comprehensive reporting [24]. All stages of this review were carried out with careful attention to detail, ensuring both rigor and reproducibility in the process. To facilitate the study selection and data extraction, we utilized Covidence software (Veritas Health Innovation, Melbourne, Vic, Australia, available at www.covidence.org), which streamlined the review process and ensured consistency in the handling of included studies [25].
2.2. Search Strategy
The search strategy was developed by the lead author [M.-A.C.] and a health sciences librarian [C.M.] and peer-reviewed by an academic librarian. To locate records, we created searches on the concepts of digital technology, stroke, rehabilitation, and home self-management using a combination of controlled vocabulary (i.e., MeSH) and keywords. The digital technology search included MeSH terms that cover a wide selection of health technologies such as physiologic monitoring, artificial intelligence, smartphones, virtual reality, wearable devices, and more. Other MeSH terms included were stroke, self-management, and physical therapy modalities. The search was limited to English articles. The original search was created in Medline (Ovid) [Appendix A] and translated to PsycINFO (Ovid), Scopus, and CINAHL with Full Text (EBSCOhost). To locate grey literature, we searched for conference proceedings in IEEE Xplore: IEEE Electronic Library (IEL); EMBASE (Ovid); and Google. All records were exported to Endnote (Clarivate, PA, USA) and deduplicated. Following deduplication, records were exported to Covidence (Veritas Health Innovation, Melbourne, Vic, Australia). All other search strategies are available in Appendix A.
2.3. Inclusion and Exclusion Criteria
Inclusion criteria for this scoping review are as follows: studies that involve patients who have experienced a stroke, are undergoing rehabilitation in a home environment, and use technology as assistive technology or for evaluative and monitoring purposes. Exclusion criteria include conceptual and theoretical studies, such as those focused on frameworks and policies.
2.4. Study Selection Process
The study selection process (Figure 1) was carried out using Covidence by three researchers based on predefined eligibility criteria. Initially, 1409 studies were identified from databases and registers. After removing six duplicate records (one manually and five via Covidence), 1403 studies proceeded to the screening stage. Following title and abstract screening, 1243 studies were excluded, leaving 57 studies for full-text assessment. All 57 studies were successfully retrieved, but 47 were excluded due to various reasons, including inappropriate setting (n = 4), intervention (n = 2), study design (n = 8), theoretical focus (n = 3), interventional study design (n = 2), wrong patient population (n = 4), incorrect technological specifications (n = 19), and absence of specific technology mention (n = 5). Consequently, 10 studies met the inclusion criteria and were included in this scoping review, with no ongoing or unclassified studies.
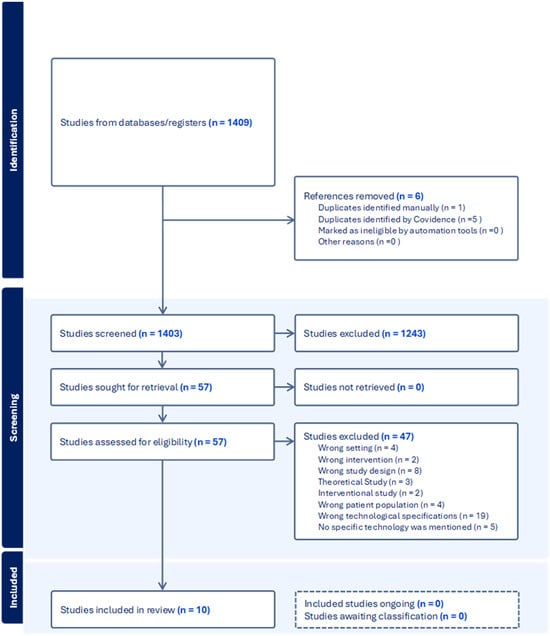
Figure 1.
Study selection process.
2.5. Data Extraction and Analysis
Data extraction from the selected studies was performed using customized charting forms created by the research team. Two of the authors, S.A. and J.B.H., conducted the data extraction, while P.S. reviewed and made any necessary revisions to the charted information, ensuring completeness and accuracy. M.K. performed data synthesis, analyses, and material preparation.
3. Results
Below are the summarized results from the included studies:
3.1. Characteristics of the Included Studies
The included studies were published between 2002 and 2021 and were conducted in the United States (n = 2) [26,27], Scotland (n = 1) [28], Italy (n = 1) [29], Canada (n = 1) [30], Sweden (n = 1) [31], Switzerland (n = 1) [32], Thailand (n = 1) [33], France (n = 1) [34], and Korea (n = 1) [35]. Included studies comprised longitudinal observational studies (n = 3) [26,30,32], feasibility studies (n = 3) [29,33,35], a construct validation study (n = 1) [28], a qualitative observational study (n = 1) [31], a prospective experimental pre/post-design study (n = 1) [27], and a design and development study (n = 1) [34].
Three articles involved small sample sizes, with 1–5 participants [29,33,35], while six articles included medium sample sizes, ranging from 6 to 16 participants [26,27,28,31,32,34]. One article reported a large sample size of 25 participants [30]. Feasibility testing was addressed in all the included studies as part of the research stage. Seven studies focused on patients in the chronic phase of stroke [26,27,28,29,30,31,34], one included both subacute and chronic phases [32], and one involved healthy participants [35]. Additionally, one study focused on frozen shoulder, which applies to stroke, but did not specify the stroke phase [33].
3.2. DHT Characteristics and Goals
The findings from the included studies emphasize the effectiveness of various technologies in supporting home-based rehabilitation and remote monitoring and evaluation. The included studies’ characteristics, objectives, and outcomes are detailed in Table 1.

Table 1.
Summary of included articles.
3.2.1. Clinical Focus Addressed in the Included Studies
Seven articles investigated mobility measurement, with four addressing the UE [28,32,33,34], two focusing on the lower extremities (LE) [26,29], and one examining the sit-to-stand transition [30]. One article focused on the actual and perceived use of the paretic UE [27], one explored cognitive support for activities of daily living (ADL) [31], and another addressed physiological monitoring [35].
3.2.2. Types of Technologies Used
Five articles explored wearable devices, including SECOSP [29], IMUs [32], SULAM [28], accelerometers [27], and wireless physiological monitoring devices (ECG and EMG) [35], while three articles examined sensor-based monitoring systems such as SAM [26], Pressure-Sensitive Bed Mats [30], and the SyMPATHy cup [34]. One article discusses the Tentaculus System (TS) as a cognitive assistive technology [31], and one article focuses on the iJoint web application and 3D simulation [33].
3.2.3. Accuracy and Reliability of Wearable Devices for Stroke Rehabilitation
Four studies examined the accuracy and reliability of wearable devices for stroke rehabilitation. One study revealed that SECOSP demonstrated superior accuracy compared to an IMU, with a mean error lower than 0.6%. It outperformed a widely used step-monitoring system, indicating its potential for precise step counting in mobility assessment [29].
Another study showed that the SULAM system effectively monitored UE activity throughout daily routines with minimal interference. The study provided preliminary evidence of its capability to objectively evaluate rehabilitation interventions aimed at improving UE function [28].
Another study found that participants who received high-dose accelerometer-based feedback reported significant perceived gains in both the amount and quality of paretic UE use. The feedback increased awareness of paretic limb utilization [27].
One study demonstrated that a wireless physiological signal monitoring system effectively filtered interference and captured continuous ECG and EMG data during daily activities. The system’s wireless connectivity enabled post-stroke and motor rehabilitation in home environments where standard monitoring devices were not accessible [35].
3.2.4. Accuracy and Reliability of Smartphone- and Sensor-Based Solutions for Stroke Patient Rehabilitation
One study found that SAM, unlike conventional pedometers, provides accurate and reliable measurements of cadence and total stride counts in hemiparetic stroke patients. This suggests that portable microprocessor-based gait monitoring can effectively quantify home- and community-based ambulatory activity levels in this population [26].
One study found that using Pressure-Sensitive Bed Mats, sit-to-stand (SiSt) durations were measured and showed variations among different populations, with post-stroke adults exhibiting longer durations compared to young and elderly healthy adults. These findings provide valuable data for monitoring patients in extended-care facilities or smart home environments [30].
A separate study showed that the SyMPATHy cup was well accepted by eight out of nine patients in rehabilitation settings or at home. These patients had minimal concerns about the design and usability of the cup, indicating its potential as an effective tool for monitoring arm and hand activity. This suggests that the cup could be a valuable tool for monitoring UE activity in rehabilitation settings [34].
One study found that The Tentaculus System (TS), an assistive technology solution, has the potential to support individuals with cognitive impairments in their everyday activities. These technologies could become more frequently used in the near future, and the study’s development process may serve as a foundation for guiding future AT implementations [31].
One study indicated that the iJoint web application could assist medical practitioners by enabling them to monitor patient exercises, detect errors, and support the rehabilitation process through telerehabilitation tracking. Preliminary findings suggest its usefulness in treatment planning [33].
4. Discussion
This scoping review explored the role of DHTs in monitoring and evaluating home-based stroke rehabilitation. Our findings suggest that DHTs are increasingly feasible and effective for evaluating and monitoring diverse aspects of stroke recovery in home settings. The review categorized technologies based on their clinical focus and technological features, identifying both strengths and limitations in current applications.
4.1. Clinical Focus of Digital Monitoring
Among the studies reviewed, mobility was the most frequently targeted domain. This is consistent with the critical importance of mobility for independence after stroke [36,37]. Technologies such as the SAM and the SECOSP demonstrated high accuracy and reliability in mobility assessment. SAM effectively tracked stride counts in chronic hemiparetic stroke patients [26], while SECOSP outperformed conventional IMU-based systems [29]. Similarly, pressure-sensitive mats and accelerometers reliably measured transitional movements such as SiSt, offering valuable feedback for tracking recovery progress [30].
Upper extremity function, another vital area of stroke rehabilitation, was addressed in several studies using technologies like SULAM, vibrotactile wristbands, and the SyMPATHy smart cup [28,32,33,34]. These tools supported both monitoring and sensory–motor enhancement, with preliminary evidence supporting their effectiveness in promoting UE recovery [28,33]. Accelerometer-based feedback increased patients’ awareness of paretic limb use, although it did not always translate to objective functional improvement [27]. A recent scoping review confirmed that most wearable technology applications in stroke rehabilitation have focused on UE recovery, particularly hemiparetic arms, while LE monitoring remains understudied. Our findings align with this observation but expand on it by highlighting the monitoring and evaluation angle, not just intervention [38].
Conversely, only a few studies targeted cognition and activities of daily living (ADLs) [31]. This is a notable gap, considering the high prevalence of post-stroke cognitive impairments and their impact on independence and quality of life [39]. TS, a sensor-based cognitive assistive technology, showed potential to enhance ADL performance by guiding users through daily tasks [31]. Given the relevance of cognition in functional recovery, more research is warranted in this domain [40].
Additionally, physiological status monitoring received limited attention, though it holds promise for comprehensive at-home stroke care [41]. One study demonstrated the feasibility of using a wireless wearable device for continuous ECG and EMG signal tracking, even in environments where traditional monitoring tools are inaccessible [35]. This technology enables clinicians to remotely assess cardiac and muscular health, potentially reducing the need for frequent hospital visits [42].
4.2. Types and Characteristics of Technologies
The majority of DHTs fell into two categories: wearable devices and smartphone and sensor-based solutions. Wearable technologies, such as SECOSP, SULAM, and GM-IMU, effectively measured physical activity, particularly UE and LE movement [28,29,32]. Accelerometer-based devices and wireless physiological monitoring systems further enabled remote feedback and data collection [27,35].
Smartphone and sensor-based tools, including SAM [26], SyMPATHy [34], pressure-sensitive mats [30], and the TS [31], also demonstrated strong performance in tracking specific rehabilitation metrics. These technologies were generally found to be reliable and usable, although some limitations such as discomfort, rigid design, and small sample sizes may hinder broader adoption [28,32,35].
Importantly, while wearables dominate the monitoring domain, the field of DHT interventions is increasingly trending toward virtual reality [43] and AI-based approaches in today’s healthcare landscape [44]. This divergence highlights the need for convergence between monitoring and intervention tools. More interdisciplinary collaboration between healthcare professionals, engineers, and data scientists is essential to develop holistic, human-centered solutions for patient adherence and daily use [45].
4.3. Reliability and Clinical Utility
Most included studies demonstrated that wearable and sensor-based technologies provide high levels of accuracy and reliability, particularly in monitoring mobility and motor function. For instance, SECOSP showed superior performance compared to IMUs [29], SAM reliably tracked stride counts in stroke patients [26], and SULAM enabled low-interference monitoring of daily arm activity [28]. Similarly, pressure mats effectively measured SiSt transitions across different populations [30], while the SyMPATHy cup and Tentaculus System received positive user feedback regarding usability and acceptability [31,34].
However, several challenges persist. In some cases, accelerometer feedback enhanced patients’ perceived limb function without corresponding objective improvements in motor outcomes, indicating a disconnect between subjective experiences and clinical progress [27]. Moreover, the design of physiological monitoring devices still requires refinement to improve user comfort and facilitate engagement during long-term use [35].
A recent meta-analysis further supports the potential of home-based digital rehabilitation, demonstrating that it can achieve outcomes comparable to traditional in-clinic services in domains such as upper extremity motor ability, static balance, stroke-related quality of life, and self-reported function [46]. While previous reviews have broadly explored home-based stroke care, our review focuses specifically on the monitoring and evaluation functions of digital health technologies, offering a more detailed analysis of these aspects.
In addition to performance considerations, several studies also highlighted technical and usability challenges that could impact real-world adoption. Issues such as device discomfort, difficulties in handling feedback mechanisms [34], and risks associated with sensor misplacement [32] were frequently reported. Addressing these user-centered concerns is crucial in rehabilitation settings, where comfort, usability, and patient adherence are critical to success.
4.4. Recommendations for Technologists and Researchers
Technology-wise, improvements can be made at the hardware and software levels to enhance stroke rehabilitation. For example, the design and usability of devices can be improved by developing flexible, skin-friendly sensors or smart garments, which would not only increase patient comfort but also provide richer data on body movements [34,35]. Also, the development of comprehensive monitoring tools that incorporate features like pressure mapping, limb configuration, and detailed activity data would make systems more robust and clinically relevant, ultimately improving rehabilitation outcomes [30,34]. From a software perspective, integrating advanced analytics through machine learning algorithms could significantly improve system functionality, enabling the classification of movements, detection of abnormalities, and real-time alerts for healthcare providers [35].
Research-wise, expanding the technologies to monitor other body parts and testing them on larger, more diverse patient populations would improve system versatility and validity, enhancing their broader applications [28,33]. For instance, combining accelerometer-based feedback with behavioral interventions, including exercise programs, could help address learned non-use and better motivate patients toward recovery [27]. Moreover, integrating multiple modalities, such as pairing systems like SECOSP with IMU sensors, would offer a more comprehensive view of patient activity by capturing specific motion percentages and movement intensity throughout the day [29]. Yet, significant gaps remain. Technologies that address cognitive function, ADLs, or whole-body rehabilitation are underrepresented [47]. Future studies should emphasize more comprehensive approaches that integrate cognitive and motor rehabilitation, interdisciplinary development, AI integration, and larger, more diverse samples to ensure generalizability and impact [48,49,50].
4.5. Methodological Considerations
The methodological robustness across the included studies reveals several recurring challenges that influence the strength and generalizability of their findings.
Firstly, small sample sizes were a pervasive limitation across almost all studies [26,27,28,29,30,31,32,33,34,35]. Many investigations involved fewer than ten participants [26,28,29,31,32,33,34], which restricts statistical power and undermines the generalizability of results to broader clinical populations. For instance, some studies evaluated devices only in individuals without impairments [28] or solely at a specific functional level (e.g., Tinetti Level 2 stroke patients [29]), thus narrowing applicability.
Secondly, restricted participant diversity further affected external validity. Several studies included only participants with mild impairments [31] or excluded those with particular sensory challenges (e.g., pain or touch sensitivity) [28]. This selective sampling limits the ability to apply findings across the full spectrum of stroke or rehabilitation populations [26,28,32].
Third, short-term and highly controlled study designs were common [27,29,33]. Many systems were tested only under supervised, short-duration sessions in laboratory or rehabilitation center settings, rather than through prolonged independent home use. This lack of real-world evaluation introduces uncertainty about device performance in naturalistic environments where user behavior and environmental factors vary greatly [30,34].
Additionally, the assessment focus was often narrow. Several studies validated only single technical aspects of devices (e.g., concurrent validity or step counting accuracy) [28,29], while omitting broader evaluations like discriminant validity, sensitivity to change, or usability over time. Moreover, few studies examined how these technologies influence clinical outcomes or rehabilitation progress, leaving important translational questions unanswered [30,32].
Overall, while the included studies provided valuable insights into emerging technologies for stroke rehabilitation and home-based monitoring, the evidence base remains preliminary. Larger, longer-term, and more ecologically valid studies are needed to strengthen confidence in the clinical applicability and real-world effectiveness of these digital health interventions.
4.6. Strengths and Limitations of This Review
A key strength of this review is its clarification of the current state of the art in the use of DHTs for monitoring and evaluating stroke rehabilitation. This provides a foundation for researchers to address gaps in existing evidence or expand on the current literature. Moreover, the specific focus on evaluation and monitoring—rather than intervention—offers novel insights that may contribute to best practices for at-home support and independent living of stroke patients.
However, this research area is still emerging, and this scoping review included only 10 papers, which limits our ability to formulate strong recommendations. Additionally, the variety of applications and the diversity of study protocols prevent us from making highly specific recommendations or suggesting a follow-up systematic review focused on a particular topic. Furthermore, aspects related to medical device certification, data privacy regulations, and equity of access to digital health technologies were not explicitly addressed in the included studies, representing important areas for future research and consideration.
5. Conclusions
DHTs for monitoring and evaluating home-based stroke rehabilitation. The findings indicate that wearable, sensor-based, and smartphone-linked technologies are increasingly feasible for assessing mobility and upper extremity function in real-world settings. However, critical aspects such as cognitive monitoring, ADLs, and whole-body rehabilitation remain insufficiently addressed in the existing literature. While the reviewed technologies show promising accuracy and usability, important challenges around patient comfort, system adaptability, and integration of advanced analytics were identified.
Given the small number of studies and notable methodological heterogeneity, these findings should be interpreted with caution. Future research should prioritize the development of comprehensive, patient-centered monitoring solutions validated across larger and more diverse populations. Moreover, greater attention to regulatory certification, data privacy protections, and equitable access will be essential to ensure that emerging digital health technologies can be safely, effectively, and ethically integrated into home-based stroke rehabilitation programs.
Author Contributions
M.-A.C. conceptualized the study and developed the search strategy in collaboration with C.M. Screening, data extraction, and materials preparation were conducted by M.-A.C., S.A., J.B.H., P.S. and M.K. The initial draft of the manuscript was prepared by M.K. and M.-A.C., while all authors contributed to the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Gerry McDole Professorship in Improved Healthcare Delivery to Rural, Remote, and Underserved Populations of Manitoba 2020–2023 Grant.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
This work was supported by the University of Manitoba College of Rehabilitation Sciences.
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Abbreviations
The following abbreviations are used in this manuscript:
| DHT | digital health technology |
| UE | upper extremity |
| LE | lower extremity |
| SiSt | sit-to-stand |
| ECG | electrocardiogram |
| EMG | electromyogram |
| SAM | Step Activity Monitor |
| SECOSP | Sensorized Codivilla Spring |
| SULAM | Strathclyde Upper-Limb Activity Monitor |
| IMU | inertial measurement unit |
| VR | virtual reality |
| TS | Tentaculus System |
Appendix A
- Medline (Ovid) Search; 1946–26 January 20231 exp stroke/2 exp Cerebral Hemorrhage/ s3 (stroke or strokes or cva* or poststroke* or apoplexy).tw,kw.4 ((cerebro* or brain or brainstem or cerebral*) adj3 (infarct* or accident*)).tw,kf.5 brain attack*.tw,kw.6 exp artificial intelligence/7 exp Monitoring, Physiologic/8 exp Monitoring, Ambulatory/9 Biofeedback, psychology/10 Self-Help Devices/11 exp Man-Machine Systems/12 automation/13 exp Computer Simulation/14 exp Video Games/15 exp wearable electronic devices/16 exp Cell Phone/ or Mobile Applications/ or Computers, Handheld/17 Electronic Mail/18 exp Touch Perception/19 wireless technology/20 (artificial intelligen* or AI or neural network* or (automat* adj2 recogni*) or machine learning).tw,kf.21 robot*.tw,kw.22 (video gam* or videogam* or exergam* or exer gam*).tw,kw.23 ambient assisted living.tw,kw.24 ambient intelligen*.tw,kw.25 (assistive adj3 (device* or technolog* or self-help)).tw,kf.26 ((ambient or smart or intelligent) adj2 (environment* or home* or house*)).tw,kf.27 (intelligent adj2 system*).tw,kf.28 ((technolog* or comput*) adj5 (ambient or non-wearable* or nonwearable* or unobtrusiv* or non-intrusive or nonintrusive or pervasive or ubiquitous or non-contact or noncontact or smart or intelligen* or passive)).tw,kf.29 (home adj2 (automation or device or module)).tw,kw.30 (digital technolog* or smart technolog*).tw,kw.31 ((monitor* or track*) adj2 (biomedical or medical or personal or home* or patient* or health or activit* or ambulat* or physiolog*)).tw,kf.32 (robot* or automat* or computer aided or computer assisted or power assist*).tw,kw.33 (virtual realit* or VR or simulat*).tw,kw.34 ((interactiv* or virtual) adj2 (environment or technolog*)).tw,kf.35 augmented realit*.tw,kw.36 (smartphone or smart-phone*).tw,kw.37 ((mobile or cell or smart or handheld) adj2 (device or phone*)).tw,kf.38 (iphone* or android* or ipad*).tw,kw.39 (personal digital assistant* or handheld computer* or handheld device*).tw,kw.40 mobile app*.tw,kw.41 haptic*.tw,kw.42 biofeedback.tw,kw.43 ((force or tactile or touch or tactual or electr*) adj2 (feedback or perception)).tw,kf.44 sensory substitution.tw,kw.45 piezoelectric*.tw,kw.46 (vibrotactile or vibration).tw,kw.47 wearable*.tw,kw.48 sensory aids/49 ((intelligent or smart) adj1 (home* or technolog* or sensor? or environment)).tw,kw.50 (at-home or home* or house* or residence or abode or residential or apartment or condo or domicile or dwelling or take-home).tw,kw.51 exp Self Care/52 Self-Management/53 (self-care or self-manage*).tw,kw.54 (rehabilitat* or rehab or “occupational therap*” or physiotherap* or “physical therap*”).tw,kw.55 rehabilitation/ or “activities of daily living”/ or neurological rehabilitation/ or stroke rehabilitation/ or telerehabilitation/56 exp Physical Therapy Modalities/57 Occupational Therapy/58 or/1-5 [Stroke Search]59 or/6-49 [Digital Technology Search]60 or/50-53 [At-Home Search]61 or/54-57 [Rehab Search]62 58 and 59 and 60 and 61 (829)63 limit 62 to english (815)
References
- Feigin, V.L.; Krishnamurthi, R.V.; Parmar, P.; Norrving, B.; Mensah, G.A.; Bennett, D.A.; Barker-Collo, S.L.; Moran, A.E.; Sacco, R.L.; Truelsen, T.C.; et al. Update on the Global Burden of Ischemic and Hemorrhagic Stroke in 1990–2013: The GBD 2013 Study. Neuroepidemiology 2015, 45, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Gund, B.M.; Jagtap, P.N.; Ingale, V.B.; Patil, R.Y. Stroke: A Brain Attack. IOSR J. Pharm. 2013, 3, 1–23. [Google Scholar]
- Majersik, J.J.; Woo, D. The enormous financial impact of stroke disability. Neurology 2020, 94, 377–378. [Google Scholar] [CrossRef] [PubMed]
- eClinicalMedicine. The rising global burden of stroke. eClinicalMedicine 2023, 59, 102028. [Google Scholar] [CrossRef]
- Katan, M.; Luft, A.R. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef]
- Otite, F.O.; Liaw, N.; Khandelwal, P.; Malik, A.M.; Romano, J.G.; Rundek, T.; Sacco, R.L.; Chaturvedi, S. Increasing prevalence of vascular risk factors in patients with stroke. Neurology 2017, 89, 1985–1994. [Google Scholar] [CrossRef]
- Noé-Sebastián, E.; Balasch-Bernat, M.; Colomer-Font, C.; Moliner-Muñoz, B.; Sanchez-Leiva, C.R.; Ugart, P.; Llorens, R.; Ferri-Campos, J. Disability after stroke: A longitudinal study in moderate and severe stroke patients included in a multidisciplinary rehabilitation program. Rev. Neurol. 2017, 64, 385–392. [Google Scholar]
- Treger, I.; Shames, J.; Giaquinto, S.; Ring, H. Return to work in stroke patients. Disabil. Rehabil. 2007, 29, 1397–1403. [Google Scholar] [CrossRef]
- Miller, E.L.; Murray, L.L.; Richards, L.G.; Zorowitz, R.D.; Bakas, T.; Clark, P.C.; Billinger, S.A. Comprehensive Overview of Nursing and Interdisciplinary Rehabilitation Care of the Stroke Patient: A Scientific Statement From the American Heart Association. Stroke 2010, 41, 2402–2448. [Google Scholar] [CrossRef]
- Miller, E. Assessment of the effectiveness of early post-stroke rehabilitation. Wiad. Lek. 2008, 61, 252–257. [Google Scholar]
- Chen, Y.; Abel, K.T.; Janecek, J.T.; Chen, Y.; Zheng, K.; Cramer, S.C. Home-based technologies for stroke rehabilitation: A systematic review. Int. J. Med. Inform. 2019, 123, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Shafeeq, D.M.; Khan, D.Z. Digital health interventions for diabetes self-management: Harnessing technology for improved outcomes. Int. J. Diabetes Res. 2023, 5, 5–11. [Google Scholar] [CrossRef]
- Morton, K.; Dennison, L.; May, C.R.; Murray, E.; Little, P.; McManus, R.J.; Yardley, L. Using digital interventions for self-management of chronic physical health conditions: A meta-ethnography review of published studies. Patient Educ. Couns. 2017, 100, 616–635. [Google Scholar] [CrossRef] [PubMed]
- Denecke, K.; May, R.; Borycki, E.; Kushniruk, A. Digital health as an enabler for hospital@home: A rising trend or just a vision? Front. Public Health 2023, 11, 1137798. [Google Scholar] [CrossRef]
- Malik, A.N.; Tariq, H.; Afridi, A.; Rathore, F.A. Technological advancements in stroke rehabilitation. JPMA J. Pak. Med. Assoc. 2022, 72, 1672–1674. [Google Scholar]
- Laut, J.; Porfiri, M.; Raghavan, P. The Present and Future of Robotic Technology in Rehabilitation. Curr. Phys. Med. Rehabil. Rep. 2016, 4, 312–319. [Google Scholar] [CrossRef]
- Schmeler, M.R.; Schein, R.M.; McCue, M.; Betz, K.L. Telerehabilitation Clinical and Vocational Applications for Assistive Technology: Research, Opportunities, and Challenges. Int. J. Telerehabilitat. 2009, 1, 59–72. [Google Scholar] [CrossRef]
- Cary, M.P.; Spencer, M.; Carroll, A.; Hand, D.H.; Amis, K.; Karan, E.; Cannon, R.F.; Morgan, M.S.; Hoenig, H.M. Benefits and Challenges of Delivering Tele-rehabilitation Services to Rural Veterans. Home Healthc. Now 2016, 34, 440–446. [Google Scholar] [CrossRef]
- Zou, C.; Harvard, A.; Qian, J.; Fox, B.I. A systematic review of digital health technologies for the care of older adults during COVID-19 pandemic. Digit. Health 2023, 9, 20552076231191050. [Google Scholar] [CrossRef]
- Fatehi, V.; Salahzadeh, Z.; Mohammadzadeh, Z. Mapping and analyzing the application of digital health for stroke rehabilitation: Scientometric analysis. Disabil. Rehabil. Assist. Technol. 2024, 20, 321–330. [Google Scholar] [CrossRef]
- Wang, S.; Kassavou, A. Digital Health Behavioural Interventions to Support Physical Activity and Sedentary Behaviour in Adults after Stroke: A Systematic Literature Review with Meta-Analysis of Controlled Trials. Behav. Sci. 2023, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Peters, M.D.; Godfrey, C.; McInerney, P.; Munn, Z.; Tricco, A.C.; Khalil, H. Scoping reviews. JBI Man. Evid. Synth. 2020, 10, 10.46658. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Babineau, J. Product review: Covidence (systematic review software). J. Can. Health Libr. Assoc. J. L’association Bibliothèques Santé Can. 2014, 35, 68–71. [Google Scholar] [CrossRef]
- Macko, R.F.; Haeuber, E.; Shaughnessy, M.; Coleman, K.L.; Boone, D.A.; Smith, G.V.; Silver, K.H. Microprocessor-based ambulatory activity monitoring in stroke patients. Med. Sci. Sports Exerc. 2002, 34, 394–399. [Google Scholar] [CrossRef]
- Whitford, M.; Schearer, E.; Rowlett, M. Effects of in home high dose accelerometer-based feedback on perceived and actual use in participants chronic post-stroke. Physiother. Theory Pract. 2020, 36, 799–809. [Google Scholar] [CrossRef]
- Vega-González, A.; Granat, M.H. Continuous monitoring of upper-limb activity in a free-living environment. Arch. Phys. Med. Rehabil. 2005, 86, 541–548. [Google Scholar] [CrossRef]
- Giansanti, D.; Tiberi, Y.; Silvestri, G.; Maccioni, G. New wearable system for step-counting telemonitoring and telerehabilitation based on the Codivilla spring. Telemed. J. E Health 2008, 14, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Arcelus, A.; Herry, C.L.; Goubran, R.A.; Knoefel, F.; Sveistrup, H.; Bilodeau, M. Determination of sit-to-stand transfer duration using bed and floor pressure sequences. IEEE Trans. Biomed. Eng. 2009, 56, 2485–2492. [Google Scholar] [CrossRef]
- Lindqvist, E.; Borell, L. The match between experienced difficulties in everyday activities after stroke and assistive technology for cognitive support. Technol. Disabil. 2010, 22, 89–98. [Google Scholar] [CrossRef]
- Leuenberger, K.; Gonzenbach, R.; Wachter, S.; Luft, A.; Gassert, R. A method to qualitatively assess arm use in stroke survivors in the home environment. Med. Biol. Eng. Comput. 2017, 55, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Chiensriwimol, N.; Chan, J.H.; Mongkolnam, P.; Mekhora, K. Monitoring frozen shoulder exercises to support clinical decision on treatment process using smartphone. Procedia Comput. Sci. 2017, 111, 129–136. [Google Scholar] [CrossRef]
- Bobin, M.; Anastassova, M.; Boukallel, M.; Ammi, M. Design and study of a smart cup for monitoring the arm and hand activity of stroke patients. IEEE J. Transl. Eng. Health Med. 2018, 6, 2100812. [Google Scholar] [CrossRef]
- Jeong, J.-W.; Lee, W.; Kim, Y.-J. A real-time wearable physiological monitoring system for home-based healthcare applications. Sensors 2021, 22, 104. [Google Scholar] [CrossRef]
- Parmar, M.J.; Gandhi, N.V. The Relationship between Balance and Mobility in Poststroke Survivors: A Narrative Review. J. Soc. Indian Physiother. 2025. [Google Scholar] [CrossRef]
- Tse, T.; Douglas, J.; Lentin, P.; Carey, L. Measuring participation after stroke: A review of frequently used tools. Arch. Phys. Med. Rehabil. 2013, 94, 177–192. [Google Scholar] [CrossRef]
- Toh, S.F.M.; Fong, K.N.K.; Gonzalez, P.C.; Tang, Y.-M. Application of Home-Based Wearable Technologies in Physical Rehabilitation for Stroke: A Scoping Review. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 1614–1623. [Google Scholar] [CrossRef]
- Rohde, D.; Gaynor, E.; Large, M.; Mellon, L.; Hall, P.; Brewer, L.; Bennett, K.; Williams, D.; Dolan, E.; Callaly, E. The impact of cognitive impairment on poststroke outcomes: A 5-year follow-up. J. Geriatr. Psychiatry Neurol. 2019, 32, 275–281. [Google Scholar] [CrossRef]
- McDonald, M.W.; E Black, S.; A Copland, D.; Corbett, D.; Dijkhuizen, R.M.; Farr, T.D.; Jeffers, M.S.; Kalaria, R.N.; Karayanidis, F.; Leff, A.P.; et al. Cognition in stroke rehabilitation and recovery research: Consensus-based core recommendations from the second Stroke Recovery and Rehabilitation Roundtable. Int. J. Stroke 2019, 14, 774–782. [Google Scholar] [CrossRef]
- Clarke, M. The need for an integrated approach to remote monitoring of physiological data and activity data. J. Telemed. Telecare 2014, 20, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Banchs, J.E.; Scher, D.L. Emerging role of digital technology and remote monitoring in the care of cardiac patients. Med. Clin. North Am. 2015, 99, 877–896. [Google Scholar] [CrossRef] [PubMed]
- Rajashekar, D.; Boyer, A.T.; Larkin-Kaiser, K.A.; Dukelow, S.P. Technological Advances in Stroke Rehabilitation: Robotics and Virtual Reality. Phys. Med. Rehabil. Clin. N. Am. 2023, 35, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Baysal, Z. AI-Driven Rehabilitation Robots: Enhancing Physical Therapy for Stroke and Injury Recovery. Next Front. Life Sci. AI 2024, 8, 155. [Google Scholar] [CrossRef]
- Gregorio, S.I.F.; Montané-Jiménez, L.G. Towards the improvement of computer-assisted medical activities for stroke rehabilitation. In Proceedings of the 2022 10th International Conference in Software Engineering Research and Innovation (CONISOFT), San José Chiapa, Mexico, 24–28 October 2022; pp. 102–111. [Google Scholar]
- Hestetun-Mandrup, A.M.; Toh, Z.A.; Oh, H.X.; He, H.-G.; Martinsen, A.C.T.; Pikkarainen, M. Effectiveness of digital home rehabilitation and supervision for stroke survivors: A systematic review and meta-analysis. Digit. Health 2024, 10, 20552076241256861. [Google Scholar] [CrossRef]
- Cisek, K.; Kelleher, J.D. Current Topics in Technology-Enabled Stroke Rehabilitation and Reintegration: A Scoping Review and Content Analysis. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 3341–3352. [Google Scholar] [CrossRef]
- Stockbridge, M.D.; Bunker, L.D.; Hillis, A.E. Reversing the Ruin: Rehabilitation, Recovery, and Restoration After Stroke. Curr. Neurol. Neurosci. Rep. 2022, 22, 745–755. [Google Scholar] [CrossRef]
- Senadheera, I.; Hettiarachchi, P.; Haslam, B.S.; Nawaratne, R.; Sheehan, J.; Lockwood, K.J.; Alahakoon, D.; Carey, L.M. AI Applications in Adult Stroke Recovery and Rehabilitation: A Scoping Review Using AI. Sensors 2024, 24, 6585. [Google Scholar] [CrossRef]
- Sun, X.; Xu, K.; Shi, Y.; Li, H.; Li, R.; Yang, S.; Jin, H.; Feng, C.; Li, B.; Xing, C. Discussion on the rehabilitation of stroke hemiplegia based on interdisciplinary combination of medicine and engineering. Evid. Based Complement. Altern. Med. 2021, 2021, 6631835. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).