Characterization of the Key Odorants of Mastic Gum (Pistacia lentiscus var. Chia) from Two Different Countries
Abstract
1. Introduction
2. Materials and Methods
2.1. Mastic Gum Samples
2.2. Chemicals
2.3. Color Measurement
2.4. Aroma Compound Analysis
2.4.1. Aroma Extraction
2.4.2. Identification of the Aroma Compounds
2.4.3. Determination of the Aroma-Active Compounds (AACs)
2.5. Sensory Analysis
2.6. Statistical Data Analysis
3. Results and Discussions
3.1. Color Properties of the Mastic Gum Samples
3.2. Volatile Profile of the Mastic Gum Samples
3.2.1. Monoterpenes
3.2.2. Monoterpenoids
3.2.3. Sesquiterpenes
3.2.4. Alcohols
3.2.5. Other Compounds
3.3. Aroma-Active Compounds (AACs) of the Mastic Gum Samples
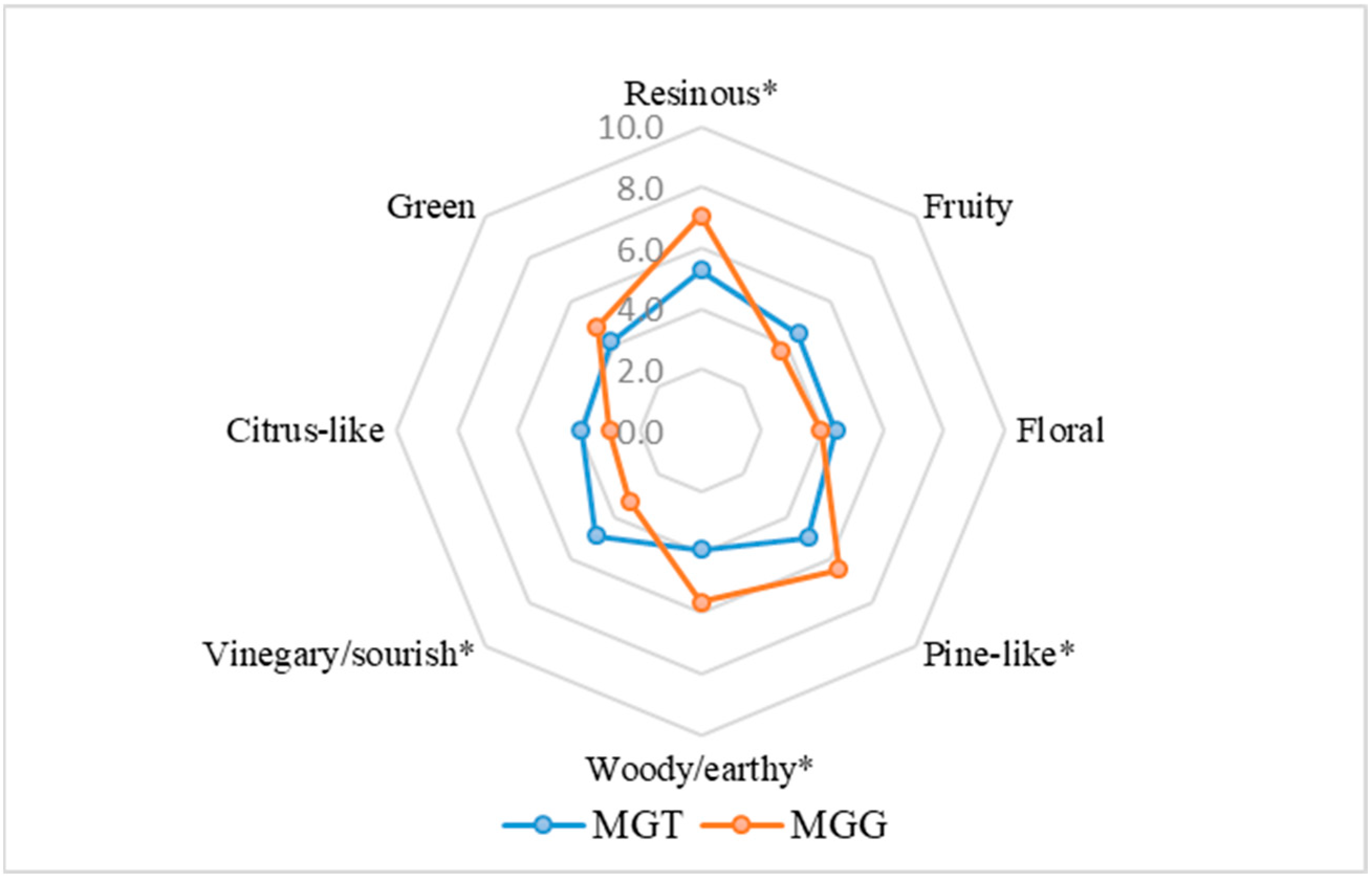
3.4. Sensory Analysis of the Mastic Gum Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MG | Mastic gum |
| MGT | Mastic gum from Türkiye |
| MGG | Mastic gum from Greece |
| AEDA | Aroma Extract Dilution Analysis |
| GC | Gas Chromatography |
| MS | Mass Spectrometer |
| FID | Flame Ionization Detector |
| GC-MS-O | Gas Chromatography-Mass Spectrometry-Olfactometry |
| GC-O | Gas Chromatography-Olfactometry |
| AACs | Aroma-active compounds |
| FD | Flavor Dilution |
References
- Nefzi, K.; Ben Jemaa, M.; Baraket, M.; Dakhlaoui, S.; Msaada, K.; Nasr, Z. In vitro antioxidant, antibacterial and mechanisms of action of ethanolic extracts of five Tunisian plants against bacteria. Appl. Sci. 2022, 12, 5038. [Google Scholar] [CrossRef]
- Daferera, D.; Pappas, C.; Tarantilis, P.A.; Polissiou, M. Quantitative analysis of α-pinene and β-myrcene in mastic gum oil using FT-Raman spectroscopy. Food Chem. 2002, 77, 511–515. [Google Scholar] [CrossRef]
- Boke Sarikahya, N.; Okkali, G.S.; Coven, F.O.; Isen, F.; Goren, A.C.; Nalbantsoy, A. Chemical characteristics and biological activity screening of Pistacia lentiscus mastic gum and leaves from Türkiye. J. Sci. Food Agric. 2024, 104, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Yosr, Z.; Yahya Imen, B.H.; Rym, J.; Chokri, M.; Mohamed, B. Sex-related differences in essential oil composition, phenol contents and antioxidant activity of aerial parts in Pistacia lentiscus L. during seasons. Ind. Crops Prod. 2018, 121, 151–159. [Google Scholar] [CrossRef]
- Pachi, V.K.; Mikropoulou, E.V.; Gkiouvetidis, P.; Siafakas, K.; Argyropoulou, A.; Angelis, A.; Mitakou, S.; Halabalaki, M. Traditional uses, phytochemistry and pharmacology of Chios mastic gum (Pistacia lentiscus var. Chia, Anacardiaceae): A review. J. Ethnopharmacol. 2020, 254, 112485. [Google Scholar] [CrossRef]
- Trabelsi, H.; Cherif, O.A.; Sakouhıi, F.; Villeneuve, P.; Renaud, J.; Barouh, N.; Boukhchina, S.; Mayer, P. Total lipid content, fatty acids and 4-desmethylsterols accumulation in developing fruit of Pistacia lentiscus L. growing wild in Tunisia. Food Chem. 2012, 131, 434–440. [Google Scholar] [CrossRef]
- Dogan, Y.; Baslar, S.; Aydin, S.; Mert, H.H. A study of the soil-plant interactions of Pistacia lentiscus L. distributed in the western Anatolian part of Turkey. Acta Bot. Croat. 2003, 62, 73–88. [Google Scholar]
- Isfendiyaroglu, M. Effects of some physical and biochemical factors on the rooting of mastic tree (Pistacia lentiscus var. chia Duham) cuttings. J. Agric. Fac. Ege Univ. 2003, 40, 25–32. [Google Scholar]
- Salman, E.; Guclu, G.; Pehlivan, Z.Y.; Kelebek, H.; Selli, S. Changes in volatile, key odorants and bioactive properties of pomegranate juice during processing into concentrate. Food Chem. 2025, 472, 142856. [Google Scholar] [CrossRef]
- Xynos, N.; Termentzi, A.; Fokialakis, N.; Skaltsounis, L.A.; Aligiannis, N. Supercritical CO2 extraction of mastic gum and chemical characterization of bioactive fractions using LC-HRMS/MS and GC–MS. J. Supercrit. Fluids 2018, 133, 349–356. [Google Scholar] [CrossRef]
- Rigling, M.; Fraatz, M.A.; Trogel, S.; Sun, J.; Zorn, H.; Zhang, Y. Aroma investigation of Chios mastic gum (Pistacia lentiscus Variety Chia) using Headspace Gas Chromatography combined with Olfactory detection and chiral analysis. J. Agric. Food Chem. 2019, 67, 13420–13429. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, M.; Racioppi, R. Characterization of the volatile fraction of mastic oil and mastic gum. Nat. Prod. Res. 2022, 36, 3460–3463. [Google Scholar] [CrossRef]
- Negro, C.; De Bellis, L.; Miceli, A. Chemical composition and antioxidant activity of Pistacia lentiscus essential oil from Southern Italy (Apulia). J. Essent. Oil Res. 2015, 27, 23–29. [Google Scholar] [CrossRef]
- Chetschik, I.; Kneubühl, M.; Chatelain, K.; Schlüter, A.; Bernath, K.; Hühn, T. Investigations on the aroma of cocoa pulp (Theobroma cacao L.) and its influence on the odor of fermented cocoa beans. J. Agric. Food Chem. 2017, 66, 2467–2472. [Google Scholar] [CrossRef] [PubMed]
- Satora, P.; Pater, A. The influence of different non-conventional yeasts on the odour-active compounds of produced beers. Appl. Sci. 2023, 13, 2872. [Google Scholar] [CrossRef]
- Papageorgiou, V.P.; Mellidis, A.S.; Argyriadou, N. The chemical composition of the essential oil of mastic gum. J. Essent. Oil Res. 1991, 3, 107–110. [Google Scholar] [CrossRef]
- Paraschos, S.; Magiatis, P.; Gikas, E.; Smyrnioudis, I.; Skaltsounis, A.L. Quality profile determination of Chios mastic gum essential oil and detection of adulteration in mastic oil products with the application of chiral and non-chiral GC–MS analysis. Fitoterapia 2016, 114, 12–17. [Google Scholar] [CrossRef]
- Tabanca, N.; Nalbantsoy, A.; Kendra, P.E.; Demirci, F.; Demirci, B. Chemical characterization and biological activity of the mastic gum essential oils of Pistacia lentiscus var. Chia from Turkey. Molecules 2020, 25, 2136. [Google Scholar] [CrossRef]
- Pachi, V.K.; Mikropoulou, E.V.; Dimou, S.; Dionysopoulou, M.; Argyropoulou, A.; Diallinas, G.; Halabalaki, M. Chemical profiling of Pistacia lentiscus var. Chia resin and essential oil: Ageing markers and antimicrobial activity. Processes 2021, 9, 418. [Google Scholar] [CrossRef]
- Kelebek, H.; Sonmezdag, A.S.; Guclu, G.; Cengiz, N.; Uzlasir, T.; Kadiroglu, P.; Selli, S. Comparison of phenolic profile and some physicochemical properties of Uzun pistachios as influenced by different harvest period. J. Food Process. Preserv. 2020, 44, e14605. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, Y. Color distribution of a shade guide in the value, chroma, and hue scale. J. Prosthet. Dent. 2008, 100, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Kilic-Buyukkurt, O.; Guclu, G.; Sevindik, O.; Kelebek, H.; Kadiroglu Kelebek, P.; Selli, S. Characterization of aroma and aroma-active compounds of black carrot (Daucus carota L. ssp. sativus var. atrorubens Alef.) pomace by aroma extract dilution analysis. Heliyon 2024, 10, e35013. [Google Scholar] [CrossRef] [PubMed]
- Chetschik, I.; Granvogl, M.; Schieberle, P. comparison of the key aroma compounds in organically grown, raw west-african peanuts (Arachis hypogaea) and in ground, pan-roasted meal produced thereof. J. Agric. Food Chem. 2008, 56, 10237–10243. [Google Scholar] [CrossRef]
- Sonmezdag, A.S.; Kelebek, H.; Selli, S. Elucidation of hulling induced changes in the aroma and aroma-active compounds of cv. Uzun pistachio (Pistacia vera). J. Sci. Food and Agric. 2019, 99, 4702–4711. [Google Scholar] [CrossRef] [PubMed]
- Dhouibi, I.; Jridi, M.; Flamini, G.; Jabeur, H.; Masmoudi, M.; Bouaziz, M. Volatile and phenolic contents and antioxidant and antibacterial properties of Tunisian milk thistle and mastic oils. Euro Mediterr. J. Environ. Integr. 2020, 5, 59. [Google Scholar] [CrossRef]
- Koutsoudaki, C.; Krsek, M.; Rodger, A. Chemical composition and antibacterial activity of the essential oil and the gum of Pistacia lentiscus var. Chia. J. Agric. Food Chem. 2005, 53, 7681–7685. [Google Scholar] [CrossRef]
- Zachariadis, G.A.; Langioli, A.V. Headspace solid phase microextraction for terpenes and volatile compounds determination in mastic gum extracts, mastic oil and human urine by GC–MS. Anal. Lett. 2012, 45, 993–1003. [Google Scholar] [CrossRef]
- Miyamoto, T.; Okimoto, T.; Kuwano, M. Chemical composition of the essential oil of mastic gum and their antibacterial activity against drug-resistant Helicobacter pylori. Nat. Prod. Bioprospecting 2014, 4, 227–231. [Google Scholar] [CrossRef]
- Boelens, M.H.; Jimenez, R. Chemical composition of the essential oils from the gum and from various parts of Pistacia lentiscus L. (Mastic Gum Tree). Flavour. Fragr. J. 1991, 6, 271–275. [Google Scholar] [CrossRef]
- Magiatis, P.; Melliou, E.; Skaltsounis, A.L.; Chinou, I.B.; Mitaku, S. Chemical composition and antimicrobial activity of the essential oils of Pistacia lentiscus var. Chia. In Biogeochemistry of Marine Dissolved Organic Matter; Hansell, D.A., Carlson, C.A., Eds.; Academic Press: Cambridge, MA, USA, 1999; Volume 65, pp. 749–752. [Google Scholar] [CrossRef]
- Duru, M.E.; Cakir, A.; Kordali, S.; Zengin, H.; Harmandar, M.; Izumi, S.; Hirata, T. Chemical composition and antifungal properties of essential oils of three Pistacia species. Fitoterapia 2003, 74, 170–176. [Google Scholar] [CrossRef]
- Gkogka, E.; Hazeleger, W.C.; Posthumus, M.A.; Beumer, R.R. The antimicrobial activity of the essential oil of Pistacia lentiscus var. Chia. J. Essent. Oil Bear. Plants 2013, 16, 714–729. [Google Scholar] [CrossRef]
- Xanthis, V.; Fitsiou, E.; Voulgaridou, G.P.; Bogadakis, A.; Chlichlia, K.; Galanis, A.; Pappa, A. Antioxidant and cytoprotective potential of the essential oil Pistacia lentiscus var. Chia and its major components myrcene and α-pinene. Antioxidants 2021, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Kıvçak, B.; Akay, S.; Demirci, B.; Başer, K. Chemical composition of essential oils from leaves and twigs of Pistacia lentiscus, Pistacia lentiscus var. Chia, and Pistacia terebinthus from Turkey. Pharm. Biol. 2004, 42, 360–366. [Google Scholar] [CrossRef]
- Sehaki, C.; Molinie, R.; Mathiron, D.; Fontaine, J.X.; Jullian, N.; Ayati, F.; Fernane, F.; Gontier, E. Metabolomics-based profiling via a chemometric approach to investigate the antidiabetic property of different parts and origins of Pistacia lentiscus L. Metabolites 2023, 13, 275. [Google Scholar] [CrossRef]
- Papanicolaou, D.; Melanitou, M.; Katsaboxakis, K. Changes in chemical composition of the essential oil of Chios “mastic resin” from Pistacia lentiscus var. Chia tree during solidification and storage. Dev. Food Sci. 1995, 37, 303–310. [Google Scholar] [CrossRef]
- Niebler, J.; Buettner, A. Identification of odorants in frankincense (Boswellia sacra Flueck.) by aroma extract dilution analysis and two-dimensional gas chromatography–mass spectrometry/olfactometry. Phytochemistry 2015, 109, 66–75. [Google Scholar] [CrossRef]
- Amanpour, A.; Guclu, G.; Kelebek, H.; Selli, S. Characterization of key aroma compounds in fresh and roasted terebinth fruits using aroma extract dilution analysis and GC–MS-Olfactometry. Microchem. J. 2019, 145, 96–104. [Google Scholar] [CrossRef]
- Ma, L.; Sun, Y.; Wang, X.; Zhang, H.; Zhang, L.; Yin, Y.; Wu, Y.; Du, L.; Du, Z. The characteristic of the key aroma-active components in white tea using GC-TOF-MS and GC-olfactometry combined with sensory-directed flavor analysis. J. Sci. Food Agric. 2023, 103, 7136–7152. [Google Scholar] [CrossRef]
- Yetisen, M.; Guclu, G.; Kelebek, H.; Selli, S. Elucidation of key aroma enhancement in cloudy lemon juices by the addition of peel oil using GC–MS-Olfactometry. Int. J. Food Sci. Technol. 2022, 57, 5280–5288. [Google Scholar] [CrossRef]
- Sawamura, M.; Onishi, Y.; Ikemoto, J.; Minh Tu, Y.T.; Lan Phi, N.T. Characteristic odour components of bergamot (Citrus bergamia Risso) essential oil. Flavour. Fragr. J. 2006, 21, 609–615. [Google Scholar] [CrossRef]
- Buettner, A.; Schieberle, P. Evaluation of aroma differences between hand-squeezed juices from Valencia late and Navel oranges by quantitation of key odorants and flavor reconstitution experiments. J. Agric. Food Chem. 2001, 49, 2387–2394. [Google Scholar] [CrossRef] [PubMed]
| Color Properties | MGT | MGG | p 1 |
|---|---|---|---|
| L* | 61.92 ± 0.06 | 47.36 ± 0.01 | ** |
| a* | −0.93 ± 0.01 | −1.79 ± 0.02 | ** |
| b* | 6.74 ± 0.11 | 27.23 ± 0.01 | ** |
| C* | 6.80 ± 0.11 | 27.29 ± 0.01 | ** |
| H° | −82.14 ± 0.21 | −86.23 ± 0.03 | ** |
| Concentration (µg/kg) 2 | ||||||
|---|---|---|---|---|---|---|
| No | LRI 1 | Aroma Compounds | MGT | MGG | p 3 | Identification 4 |
| Monoterpenes | ||||||
| 1 | 1011 | α-Pinene | 2179.05 ± 126.24 | 4417.56 ± 422.71 | ** | LRI, MS, std |
| 2 | 1057 | Camphene | 3.43 ± 0.08 | 30.28 ± 2.68 | ** | LRI, MS, std |
| 3 | 1096 | β-Pinene | 82.51 ± 7.85 | 134.98 ± 11.77 | ** | LRI, MS, std |
| 4 | 1113 | Sabinene | 13.23 ± 0.38 | 20.74 ± 0.51 | ** | LRI, MS, std |
| 5 | 1167 | β-Myrcene | 1448.49 ± 76.38 | 1056.05 ± 91.49 | ** | LRI, MS, std |
| 6 | 1196 | Limonene | 72.6 ± 6.98 | 73.13 ± 5.96 | ns | LRI, MS, std |
| 7 | 1212 | β-Phellandrene | 4.25 ± 0.42 | 3.06 ± 0.22 | ** | LRI, MS, std |
| 8 | 1265 | p-Cymene | 1.93 ± 0.18 | 6.1 ± 0.43 | ** | LRI, MS, std |
| 9 | 1431 | Perillene | 44.51 ± 2.43 | 14.62 ± 0.6 | ** | LRI, MS, std |
| 10 | 1666 | Citral | 9.34 ± 0.25 | 59.23 ± 3.83 | ** | LRI, MS, std |
| 11 | 1679 | (Z)-verbenone | 2.75 ± 0.14 | nd | ** | LRI, MS, std |
| Total | 3862.1 | 5815.8 | ||||
| Monoterpenoids | ||||||
| 12 | 1497 | Linalool | 4.65 ± 0.3 | 44.71 ± 3.44 | ** | LRI, MS, std |
| 13 | 1648 | Myrtenal | 0.62 ± 0.03 | 11.69 ± 0.55 | ** | LRI, MS, tent. |
| 14 | 1651 | (E)-Pinocarveol | 3.56 ± 0.31 | 33.76 ± 2.9 | ** | LRI, MS, std |
| 15 | 1669 | (E)-Verbenol | 4.89 ± 0.39 | 85.06 ± 1.02 | ** | LRI, MS, std |
| 16 | 1763 | Myrtenol | 0.47 ± 0.02 | 16.66 ± 0.27 | ** | LRI, MS, tent. |
| 17 | 1830 | (E)-Anethole | 5.66 ± 0.09 | 7.51 ± 0.04 | ** | LRI, MS, std |
| 18 | 1836 | (E)-Carveol | nd | 13.01 ± 1.3 | ** | LRI, MS, std |
| 19 | 1985 | Perilla alcohol | 2.49 ± 0.04 | 3.84 ± 0.18 | ** | LRI, MS, std |
| Total | 22.34 | 216.24 | ||||
| Sesquiterpenes | ||||||
| 20 | 1585 | β-Caryophyllene | 66.42 ± 3.66 | 25.07 ± 0.47 | ** | LRI, MS, std |
| 21 | 1667 | α-Humulene | 4.59 ± 0.45 | nd | ** | LRI, MS, std |
| Total | 71.01 | 25.07 | ||||
| Sesquiterpene oxide | ||||||
| 22 | 1953 | Caryophyllene oxide | 8.74 ± 0.41 | 12.08 ± 1.2 | * | LRI, MS, std |
| Total | 8.74 | 12.08 | ||||
| Diterpenoid | ||||||
| 23 | 2551 | Geranyl linalool | 2.95 ± 0.16 | 12.57 ± 0.18 | ** | LRI, MS, std |
| Total | 2.95 | 12.57 | ||||
| Alcohols | ||||||
| 24 | 1036 | 2-Methyl-3-buten-2-ol | 10.74 ± 0.63 | 36.06 ± 0.95 | ** | LRI, MS, std |
| 25 | 1152 | 3-Penten-2-ol | 2.48 ± 0.23 | 69.34 ± 4.42 | ** | LRI, MS, std |
| 26 | 1226 | 2-Hexanol | 1.42 ± 0.01 | 21.33 ± 1.96 | ** | LRI, MS, std |
| 27 | 1322 | 2-Methyl-2-buten-1-ol | nd | 5.2 ± 0.12 | ** | LRI, MS, std |
| 28 | 1480 | 2-Ethylhexanol | nd | 6.46 ± 0.34 | ** | LRI, MS, std |
| Total | 14.64 | 138.39 | ||||
| Volatile phenols | ||||||
| 29 | 1390 | 2-Methylanisole | 25.69 ± 1.48 | 54.98 ± 4.04 | ** | LRI, MS, std |
| 30 | 2126 | Methyl isoeugenol | nd | 2.33 ± 0.09 | ** | LRI, MS, tent. |
| Total | 25.69 | 57.31 | ||||
| Volatile acid | ||||||
| 31 | 1452 | Acetic acid | 4.53 ± 0.26 | 60.3 ± 0.67 | ** | LRI, MS, std |
| Total | 4.53 | 60.3 | ||||
| Aldehydes | ||||||
| 32 | 1447 | α-Campolenal | 1.63 ± 0.15 | 69.95 ± 1.23 | ** | LRI, MS, tent. |
| 33 | 1514 | Isoneral | 3.63 ± 0.36 | 10.64 ± 0.65 | ** | LRI, MS, std |
| Total | 5.26 | 80.59 | ||||
| Ketones | ||||||
| 34 | 1341 | Sulcatone | 1.98 ± 0.18 | 3.66 ± 0.19 | ** | LRI, MS, tent. |
| 35 | 1610 | 2-Undecanone | nd | 5.07 ± 0.1 | ** | LRI, MS, tent. |
| Total | 1.98 | 8.73 | ||||
| Ester | ||||||
| 36 | 1499 | Linalyl acetate | 1.55 ± 0.15 | nd | ** | LRI, MS, std |
| Total | 1.55 | 0.00 | ||||
| General total | 4021 | 6427 | ** | |||
| No | LRI 1 | Compounds | Odor Descriptions | Flavor Dilution, FD 2 | |
|---|---|---|---|---|---|
| MGT | MGG | ||||
| 1 | 1011 | α-Pinene | Forest like, resinous | 256 | 512 |
| 2 | 1057 | Camphene | Pungent, spicy, pine-like | nd | 2 |
| 3 | 1096 | β-Pinene | Resinous, terpene-like | 128 | 128 |
| 4 | 1167 | β-Myrcene | Pine-like, greenish | 256 | 128 |
| 5 | 1196 | Limonene | Citrusy, fruity | 32 | 16 |
| 6 | 1265 | p-Cymene | Citrusy | nd | 2 |
| 7 | 1226 | 2-Hexanol | Wine like | nd | 4 |
| 8 | 1390 | 2-Methylanisole | Woody, fresh | 2 | 4 |
| 9 | 1431 | Perillene | Greenish, pea like | 16 | 64 |
| 10 | 1452 | Acetic acid | Vinegar like | 4 | 4 |
| 11 | 1447 | α-Campolenal | Grassy | nd | 4 |
| 12 | 1497 | Linalool | Floral | 256 | 128 |
| 13 | 1480 | Unknown | Earthy | 2 | nd |
| 14 | 1648 | Myrtenal | Spicy, pungent | nd | 16 |
| 15 | 1669 | (E)-Verbenol | Pine-like | 2 | 4 |
| 16 | 1679 | (E)-Verbenone | Camphorous | nd | 4 |
| 17 | 1953 | Caryophyllene oxide | Woody | nd | 4 |
| 18 | 1985 | Perilla alcohol | Greenish | nd | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kilic-Buyukkurt, O.; Guclu, G.; Kelebek, H.; Selli, S. Characterization of the Key Odorants of Mastic Gum (Pistacia lentiscus var. Chia) from Two Different Countries. Appl. Sci. 2025, 15, 5329. https://doi.org/10.3390/app15105329
Kilic-Buyukkurt O, Guclu G, Kelebek H, Selli S. Characterization of the Key Odorants of Mastic Gum (Pistacia lentiscus var. Chia) from Two Different Countries. Applied Sciences. 2025; 15(10):5329. https://doi.org/10.3390/app15105329
Chicago/Turabian StyleKilic-Buyukkurt, Ozlem, Gamze Guclu, Hasim Kelebek, and Serkan Selli. 2025. "Characterization of the Key Odorants of Mastic Gum (Pistacia lentiscus var. Chia) from Two Different Countries" Applied Sciences 15, no. 10: 5329. https://doi.org/10.3390/app15105329
APA StyleKilic-Buyukkurt, O., Guclu, G., Kelebek, H., & Selli, S. (2025). Characterization of the Key Odorants of Mastic Gum (Pistacia lentiscus var. Chia) from Two Different Countries. Applied Sciences, 15(10), 5329. https://doi.org/10.3390/app15105329







