Abstract
Leaching from cement can lead to a loss in performance and durability and can also have an environmental impact. Therefore, it is an important aspect to consider when new cements are being developed and where concrete is to be placed that could lead to the contamination of groundwater. Calibrated thermodynamic models can provide very useful predictions in a matter of seconds for any cement-based material. However, such models need to include accurate representations of the solid-solution nature of the C-S-H gels that are included for the incongruent dissolution of calcium and silica. This study presents the calibration of a thermodynamic model employing the pH-REdox-Equilibrium geochemical software 3.8.7, written in C (PHREEQC 3.8.7), to model the change in the pH and the leaching of calcium (Ca) and silica (Si) from cement against the Ca/Si ratio and over time. The predicted concentrations of Ca and Si and the pH in the leachate were calculated using three solid-solution C-S-H gel models that were taken from the cemdata18 database, namely, CSHQ, CSH3T, and tobermorite–jennite, which have not been analysed before and show good agreement. The calibrated model was used to predict leaching from a CEM II/A-L cement and a blended CEM I + fly-ash with a cement replacement level of 35%. The effect of a sulphate environment (Na2SO4) was also analysed.
1. Introduction
Leaching from cement-based materials is the gradual release of various chemical components, such as calcium (Ca) and silica (Si), into the surrounding environment and the breaking down of solid phases, such as calcium–silica–hydrate (C-S-H) and portlandite. It can affect performance and durability, as well as have a negative environmental impact, if these components enter groundwater or sensitive soils. An increase in calcium in groundwater can affect its alkalinity, with the resulting change in the pH affecting the drinking water quality and aquatic life. Any new cement or concrete should, therefore, consider leaching, especially if they are in environmentally sensitive areas. Not understanding leaching not only affects the durability and longevity of concrete structures but also poses potential environmental risks. Understanding cement leaching is a necessary consideration as new sustainable cement and concrete are developed, so their behaviour in this environment is crucial for determining their viability.
Leaching is affected by the environment and temperature and can take several weeks, months, or even years to observe. Therefore, calibrated prediction models that account for changing temperature and accurately represent the incongruent dissolution of calcium and silica within appropriate solid-solution models of C-S-H gels are vital to understand and mitigate the effects of cement leaching.
Haga et al. [1] and Hidalgo et al. [2] reported on the dissolution of cement into pore water and the measurement of the changing pH and ion concentrations. Cement paste dissolution models were developed by Atkinson et al. [3] and Berner [4] to model the long-term change in the composition of the leachate. Faucon et al. [5] concluded in their work that hardened cement paste was altered by ongoing leaching and dissolution, and changes in the solid hydrate phases occurred as a result. Others [6,7,8] summarised in their findings on the leaching of cement in deionised water that diffusion was the main transport mechanism of ions into the leachate. Haga et al. [1] found that research into the structural changes of hardened cement pastes due to dissolution had hardly been reported, and Liu et al. [9,10] reported on the dissolution kinetics of aluminosilicate precursors to provide a comparative framework for Si behaviour in leaching.
Hidalgo et al. [2] provided a summary of the influence of C-S-H and portlandite on the chemical properties of the liquid phase in a hydrating cement. The high initial pH (>13) created from the dissolution of sodium and potassium hydroxides from the cement was lowered to approximately 12.5 because of the dissolution of other constituent minerals, including portlandite and C-S-H. They reported that previous research [11,12] measured the ongoing decalcification of C-S-H pastes due to leaching until a constant value of the Ca/Si ratio was reached.
C-S-H is the main hydrate phase of Portland cement. The hyphens are used to symbolise the variable composition and poorly defined crystalline structure [13]. The C-S-H Ca/Si ratio and calcium concentration ultimately control the type of gel formed [2,14], which can change due to due to the addition of supplementary cementitious materials (SCMs) or leaching and chemical attack by external sulphates and/or carbonation [15]. There are many descriptions of C-S-H. Taylor [13] provided C-S-H (I) and C-S-H (II), which are similar to tobermorite and jennite, respectively [2]. C-S-H’s solubility can be modelled using solid-solution models [16,17,18] or, to a limited extent, using a surface complexation approach [15,19,20].
Others have employed discrete solid phases (DSPs) to model C-S-H, which provide reasonable comparisons with measured data of changing pH values and Ca and Si concentrations for molar Ca/Si ratios ranging from 2.7 to 0. Deriving DSPs for any ideal or non-ideal solid solution has been proposed by Walker et al. [18] and Kulik et al. [21]. This was further developed by Holmes et al. [22,23], who derived DSPs for the three end-member CSH3T and four end-member CSHQ gel solubility models within the Cemdata18 database [15]. These have been successfully used within PHREEQC 3.8.7 [24] to model various plain and blended cements as well as the effect of harsh environments, including carbonation, sulphate, and sea water. The geochemical software HYDCEM [25,26,27,28,29], developed for users to set up and model their own cement blends, also uses DSPs to model various cement systems.
PHREEQC has been used by many authors over the years to model leaching from cementitious as well as waste materials, which is summarised in Table 1.

Table 1.
Previous studies using PHREEQC for modelling cementitious material leaching.
This paper compared measured data from 777 C-S-H gel equilibrated solutions for pH, Ca (mmol/L), and Si (mmol/L) to develop a calibrated model using a CEM I cement and the PHREEQC geochemical software to simulate leaching from the cement using solid-solution gel models from the cemdata18 database, namely, CSHQ, CSH3T, and tobermorite–jennite, which have not been reported previously in the literature for this application. Analysis was undertaken using the previously developed DSPs for these gel models as well as the SOLID_SOLUTION data block in PHREEQC. The calibrated model was then further developed to model the dissolution of a CEM II/A-L and a blended CEM I + fly-ash with cement replacement levels of 20 and 35%. The effect of a sulphate environment (Na2SO4) on leaching was also predicted for all three cement types.
2. Materials and Methods
Walker et al. [18] reported that there are over 45 published C-S-H gel solubility models with Ca/Si ratios (mol/mol) of greater than 0.8. These, however, do not include those C-S-H solid solutions developed for the cemdata18 [15] database, namely, tobermorite–jennite, CSH3T, and CSHQ. These C-S-H solid solutions were employed here to predict the expected changes in pH, Ca, and Si against changing Ca/Si ratios using the PHREEQC geochemical code. The measured data from 777 C-S-H gel equilibrated solutions for pH, Ca (mmol/L), and Si (mmol/L), taken from Walker et al. [18], are shown in Figure 1. Using the selection criteria used by Walker et al. [18], namely, a curing temperature range of 17–30 °C and a curing time of ≥1 week, the final Ca/Si ratio of the C-S-H was measured, calculated, or calculable, the liquid phase was fully characterised, and the data were real. The solubility data in Figure 2 were used to compare the predicted pH, Ca, and Si against the C-S-H Ca/Si ratio. These data were deemed to be representative of C-S-H gel near equilibrium near room temperature (17–30 °C), with a curing time of one week or more.
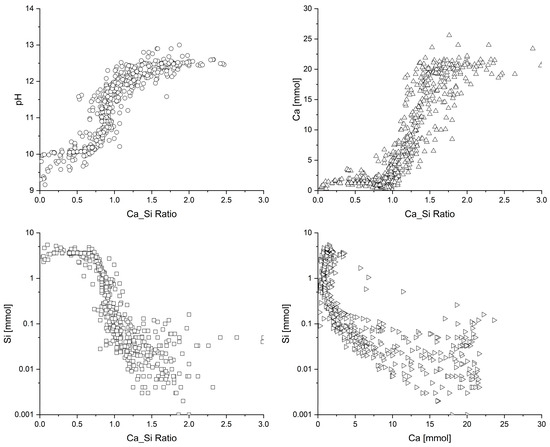
Figure 1.
C-S-H gel solubility data from 777 C-S-H gel equilibrated solutions for pH, Ca (mmol/L), and Si (mmol/L) taken from Walker et al. [16].
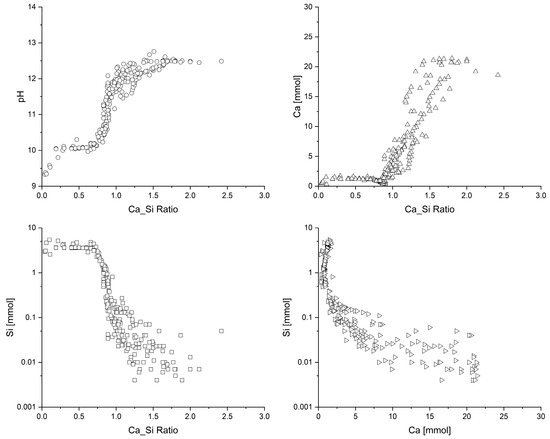
Figure 2.
Selected C-S-H gel solubility data for comparison with PHREEQC predictions.
The PHREEQC [24] analysis was undertaken using the chemical and physical properties of a commercially available CEM I cement, as described in Table 1. The chemical compositions of the materials were determined by X-ray fluorescence (XRF). The phase composition was calculated using normalisation.
Thermodynamic Modelling
Thermodynamic calculations were carried out using the PHREEQC geochemical software along with the cemdata18 thermodynamic database to simulate leaching and provide predictions of the Ca, Si, and pH in the leachate at 25 °C. The CSHQ, CSH3T, and tobermorite–jennite C-S-H solution models were analysed using their derived DSPs [22,23] and the SOLID_SOLUTIONS data block in PHREEQC.
The thermodynamic model was based on the image shown in Figure 3, where 100 g of cement was placed in 1000 mL of pure water (Solution 1) with a water/solid (w/s) ratio of 10. Every fourteen days, Solution 1 was replaced with 1000 mL of pure water (Solution 0) until all the C-S-H and portlandite was dissolved. Separate PHREEQC models, depending on the C-S-H solid solution employed, provided simulations of the changing pH, calcium (mmol/L), and silica (mmol/L) in the replaced leachate (Solution 1) as well as the Ca/Si ratio of the C-S-H solid phase. The analysis included 266 changes, which was equivalent to 10 years.
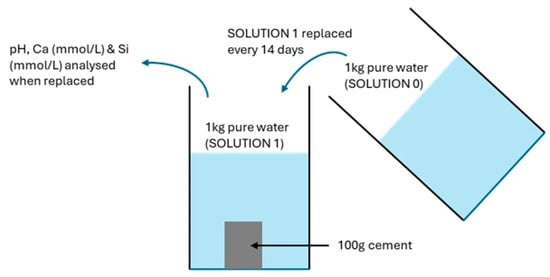
Figure 3.
PHREEQC leaching conceptualisation model.
The PHREEQC inputs for Solution 0 and Solution 1 are shown and described in Table 2. The CEM I data input for the model within the EQUILIBRIUM_PHASES data block is shown in Table 3.

Table 2.
Composition of CEM I cement.

Table 3.
PHREEQC input for solution data.
The thermodynamic end-members of the three C-S-H gel solid solutions, taken from cemdata18, are summarised in Table 4. The PHREEQC input data for the SOLID_SOLUTIONS data block are shown in Table 5. The TRANSPORT data block in PHREEQC (Table 6) was used to replace Solution 1 with Solution 0 (Figure 3) at regular, defined intervals.

Table 4.
PHREEQC input for the cement data and phases in EQUILIBRIUM_PHASES.

Table 5.
Solid-solution end-members (taken from [15]).

Table 6.
PHREEQC input for the solid-solution calculations.
The modelling approach assumed that equilibrium simulations between the evolving solid-phase assemblage and its pore solution accurately represented the near-equilibrium conditions presumed to dominate the system during leaching. This was predicated on the knowledge that dissolution and precipitation reactions occurring during leaching are generally rapid in relation to the underlying transport. A second assumption was that the discretisation of the solid-solution reactions, as described in our previous work [22,23], accurately represented the dissolution of these solids whilst increasing the speed of computation significantly.
3. Results
Using the input data above to analyse the cement described in Table 1, Figure 4 shows a comparison of the changes in Ca, Si, and pH during leaching against the C-S-H Ca/Si ratio using (1) the CSHQ gel model employing the DSP [22,23] and (2) the solid-solution option in PHREEQC. As may be seen, both approaches gave similar predictions, with the DSP showing kinks across the compositional range. This was a result of the non-ideal nature of the CSHQ DSP gel model, where imperfect mixing and incomplete solubility led to the ‘jagged’ output shown. While both provided clear predictions, the SOLID_SOLUTIONS data block was employed here throughout.
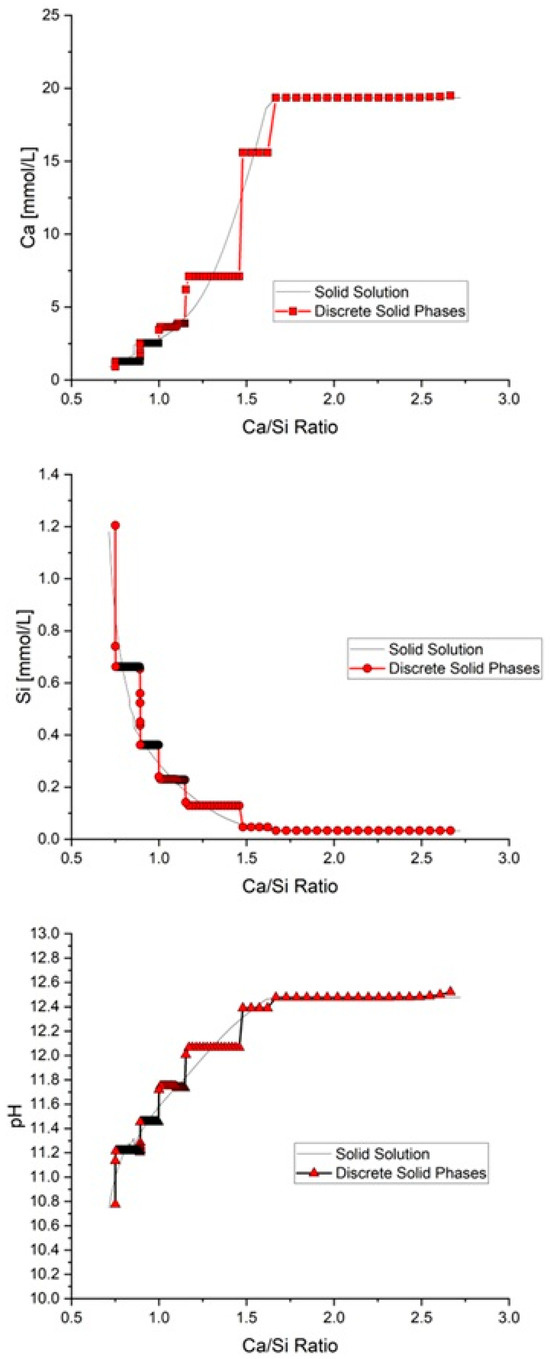
Figure 4.
Comparison between DSP and solid-solution solver in PHREEQC.
The results from the predicted leaching of the cement described in Table 1, undergoing the water changes shown in Figure 3, are shown in Figure 5 for the three C-S-H solid-solution gel models in Table 4. As may be seen, all three models provided reasonable predictions of the selected C-S-H gel solubility data for the Ca/Si ratios from 2.7 to 0.5 shown. While all three C-S-H gel models provided good comparisons with the measured data from the literature, CSH3T performed slightly better. In terms of pH, for example, the tobermorite–jennite model underestimated at Ca/Si ratios greater than 1.0. It also appeared to overestimate the Ca and Si concentrations at higher Ca/Si ratios.
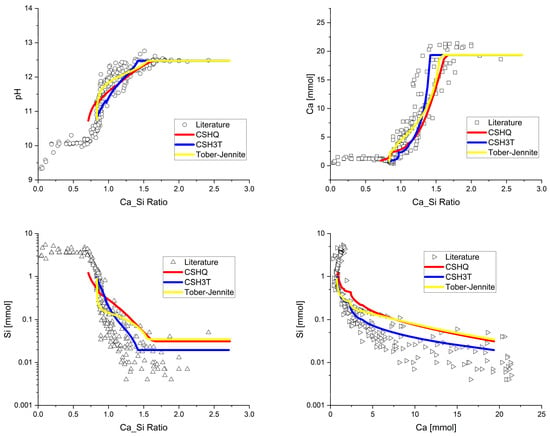
Figure 5.
Changes in pH, Ca, and Si at 25 °C.
4. Discussion
The leaching of cement is a long-term process by which material is removed according to its relative solubility. At a practical level, this can be simulated in several ways, such as the ‘bucket brigade’ calculations used here, in which a quantity of the pore solution is removed at each reaction (or time) step and replaced with fresh water, and re-equilibration of the system is carried out to perform the next discrete leaching step. There are other approaches, including coupled chemical-transport methods, in which a hypothetical column (or a single cell) has its contents moved into an adjacent cell at each reaction step, and the system is re-equilibrated. This approach has the advantage of spatial as well as temporal simulations and is, therefore, capable of representing dispersion effects and more complex phenomena.
The method described here has the advantage of simplicity and rapid run-times and has proved robust and reliable. Irrespective of the conceptual model underlying a leaching calculation, the predictions are very similar, showing the cement pore solution is buffered by one dominant hydrate—the most soluble salt in the assemblage—until exhausted.
Subsequently, the hydrate assemblage re-equilibrates with the pore solution, establishing a new solution chemistry, governed by the hydrates in the solid. This state continues (theoretically, at least) until all the solid is removed from the system.
For the cement example shown in this paper, the portlandite was completely depleted after 280 days or 20 cycles. C-S-H was still present but will be depleted by approximately 5000 days (~360 cycles). The depletion of ettringite after c. 2000 days (~142 cycles) will cause a further reduction in Ca in the solution. It should be noted, however, that secondary phases, such as zeolites or amorphous silica, may precipitate under certain conditions that can modify silica availability in ways that may not be fully accounted for in equilibrium-based models. These uncertainties suggest that while thermodynamic models provide valuable insights, experimental measurement and validation remain crucial for refining predictions.
The model was used to provide predictions for the dissolution of a CEM II/A-L and a blended CEM I + fly-ash cement, as described in Table 7, Table 8, and Table 9, taken from [40,41], respectively. The fly ash was blended with the CEM I with a cement replacement level of 35%.

Table 7.
PHREEQC input for the replacement of Solution 1 with Solution 0 every 14 days.

Table 8.
Composition of CEM II/A-L cement.

Table 9.
Composition of fly ash (pulverised fuel ash).
The SOLID_SOLUTIONS data block for the CEM II/A-L cement was identical to that shown in Table 5, whereas for the CEM I + 35% fly-ash model, the input shown in Table 10 was employed to account for the C-A-S-H gel model. The predicted changes in the Ca, Si, and Al concentrations and pH over time are shown in Figure 6. While there were minimal differences between the predictions for the CEM I and CEM II/A-L cements, the blended cement was somewhat different. This was due to the sequential leaching and eventual depletion of the hydrate minerals from the blended cements.

Table 10.
PHREEQC input for the C-A-S-H solid-solution calculations.
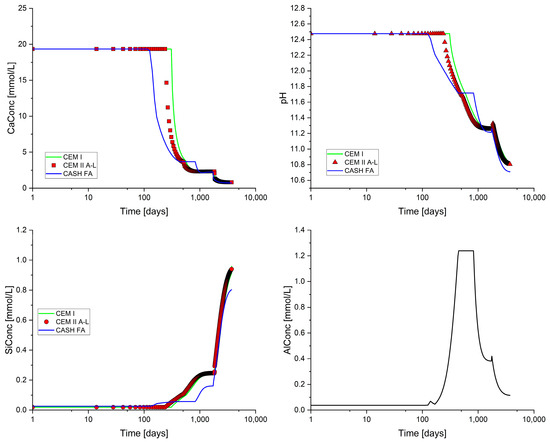
Figure 6.
Changes in pH, Ca, Si, and Al for the CEM I, CEM II/A-L, and CEM I + 35% fly-ash cements at 25 °C.
The change in the hydrate phases over time with ongoing exposure to 25 g/L of Na2SO4 is shown in Figure 7. The depletion of C-S-H and portlandite was much faster with the precipitation of thaumasite and higher volumes of ettringite than would be expected in pure water. These predictions are similar to the work of [42,43] and in terms of thaumasite precipitation, the lowering of the pH, and the expansion due to sulphate exposure, respectively. Previous work [44,45] has shown that the maximum rate of thaumasite formation is between 5 and 10 °C, but is stable above this range.
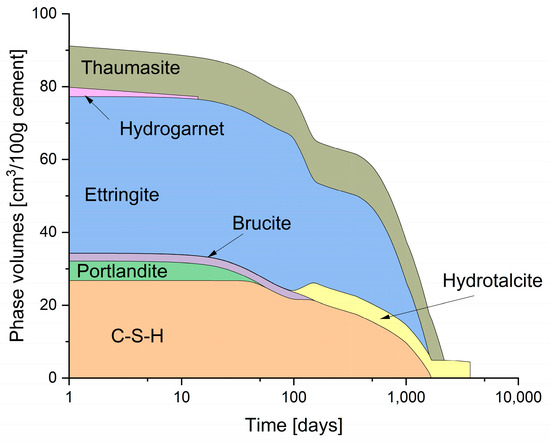
Figure 7.
Phase assemblage of CEM-I over time, subject to reaction and leaching with a sodium sulphate solution.
5. Conclusions
Cement leaching is an important process to consider, as it can adversely affect performance and durability and the environment within which it is placed. The ongoing development of new sustainable cementitious materials means leaching will need to be considered to minimise the environmental impact and ensure the longevity of the concrete. While the monitoring of natural leaching is very time-consuming (taking several years, typically), this work showed that thermodynamic predictive models employing three C-S-H gel solid-solution models (CSHQ, CSH3T, and tobermorite–jennite), which have not been used before, can simulate the incongruent dissolution of calcium and silica from this phase within seconds. These models can provide rapid, robust, and reliable predictions (within 10% of measured data overall) of the C-S-H internal Ca/Si ratio and the leachate’s pH and calcium and silica concentrations. They can also provide predictions of how the hydrate phases will break down over time.
Having access to these C-S-H gel models provides users with the opportunity to simulate leaching and predict the changes in the chemical composition under a range of conditions. However, predicting the changing silica concentration remains a challenge due to uncertainties around the possibility of amorphous silica-rich gels forming. Experimental validation will remain essential to refine model parameters and improve the predictions shown here, as C-S-H structures have complex and non-ideal dissolution behaviours.
Author Contributions
Conceptualization, N.H.; Methodology, N.H.; Software, N.H.; Validation, M.T.; Investigation, M.T.; Writing—original draft, N.H.; Writing—review & editing, M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The input files will be made available by contacting the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations were used in this manuscript:
| Ca | Calcium |
| Si | Silica |
| Ca/Si | Calcium-to-silica ratio |
| C-S-H | Calcium–silica–hydrate |
| SCMs | Supplementary cementitious materials |
| DSPs | Discrete solid phases |
References
- Haga, K.; Sutou, S.; Hironaga, M.; Tanaka, S.; Nagasaki, S. Effects of porosity on leaching of Ca from hardened ordinary Portland cement paste. Cem. Concr. Res. 2005, 35, 1764–1775. [Google Scholar] [CrossRef]
- Hidalgo, A.; Petit, S.; Domingo, C.; Alonso, C.; Andrade, C. Microstructural characterization of leaching effects in cement pastes due to neutralisation of their alkaline nature: Part I: Portland cement pastes. Cem. Concr. Res. 2007, 37, 63–70. [Google Scholar] [CrossRef]
- Atkinson, A.; Everitt, N.M.; Guppy, R.M.; UKAEA Harwell Lab. (UK). Materials Development Division. Evolution of pH in a Radwaste Repository: Internal Reactions Between Concrete Constituents; UKAEA Harwell Lab. (UK). Materials Development Division: Harwell, UK, 1988.
- Berner, U.R. Modelling the incongruent dissolution of hydrated cement minerals. Radiochim. Acta 1988, 44–45, 387–394. [Google Scholar] [CrossRef]
- Faucon, P. Contribution of Nuclear Magnetic Resonance Techniques to the Study of Cement Paste Water Degradation. In Proceedings of the 10th International Congress on the Chemistry of Cement, 3, Gothenburg, Sweden, 2–6 June 1997; Available online: https://cir.nii.ac.jp/crid/1574231876455520768.bib?lang=en (accessed on 9 March 2025).
- Adenot, F.; Buil, M. Modelling of the corrosion of the cement paste by deionized water. Cem. Concr. Res. 1992, 22, 489–496. [Google Scholar] [CrossRef]
- Carde, C.; François, R.; Torrenti, J.-M. Leaching of both calcium hydroxide and C-S-H from cement paste: Modeling the mechanical behavior. Cem. Concr. Res. 1996, 26, 1257–1268. [Google Scholar] [CrossRef]
- Adenot, F.; Richet, C. Modelling of the chemical degradation of a cement paste. In Mechanisms of Chemical Degradation of Cement-Based Systems; CRC Press: Boca Raton, FL, USA, 1997; p. 341. [Google Scholar]
- Liu, J.; Doh, J.-H.; Ong, D.E.L.; Kiely, F.L. Effect of thermal pretreatment on the reactivity of red mud valorized as aluminosilicate precursor for geopolymer production. Constr. Build. Mater. 2024, 445, 137943. [Google Scholar] [CrossRef]
- Liu, J.; Doh, J.-H.; Ong, D.E.; Wang, S.; Yang, Y.; Dinh, H.L.; Zi, G. Correlation between dissolubilities of Si, Al, and Fe from aluminosilicate precursor and strength of fly ash-based geopolymer. Constr. Build. Mater. 2023, 393, 132107. [Google Scholar] [CrossRef]
- Thomas, J.J.; Chen, J.J.; Allen, A.J.; Jennings, H.M. Effects of decalcification on the microstructure and surface area of cement and tricalcium silicate pastes. Cem. Concr. Res. 2004, 34, 2297–2307. [Google Scholar] [CrossRef]
- Harris, A.W.; Manning, M.C.; Tearle, W.M.; Tweed, C.J. Testing of models of the dissolution of cements—Leaching of synthetic C-S-H gels. Cem. Concr. Res. 2002, 32, 731–746. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry; Thomas Telford Publishing: London, UK, 1997. [Google Scholar]
- Flint, E.P.; Wells, L.S. Study of the system CaO-SiO2-H2O at 30 °C and of the reaction of water on anhydrous calcium silicates. J. Res. Natl. Bur. Stand. 1934, 12, 751–783. [Google Scholar] [CrossRef]
- Lothenbach, B.; Kulik, D.A.; Matschei, T.; Balonis, M.; Baquerizo, L.; Dilnesa, B.; Miron, G.D.; Myers, R.J. Cemdata18: A chemical thermodynamic database for hydrated Portland cements and alkali-activated materials. Cem. Concr. Res. 2019, 115, 472–506. [Google Scholar] [CrossRef]
- Kulik, D.A. Improving the structural consistency of C-S-H solid solution thermodynamic models. Cem. Concr. Res. 2011, 41, 477–495. [Google Scholar] [CrossRef]
- Kulik, D.A.; Kersten, M. Aqueous Solubility Diagrams for Cementitious Waste Stabilization Systems: II, End-Member Stoichiometries of Ideal Calcium Silicate Hydrate Solid Solutions. J. Am. Ceram. Soc. 2001, 84, 3017–3026. [Google Scholar] [CrossRef]
- Walker, C.; Sutou, S.; Oda, C.; Mihara, M.; Honda, A. Calcium silicate hydrate (C-S-H) gel solubility data and a discrete solid phase model at 25 °C based on two binary non-ideal solid solutions. Cem. Concr. Res. 2016, 79, 1–30. [Google Scholar] [CrossRef]
- Haas, J.; Nonat, A. From C-S-H to C-A-S-H: Experimental study and thermodynamic modelling. Cem. Concr. Res. 2015, 68, 124–138. [Google Scholar] [CrossRef]
- Churakov, S.V.; Labbez, C. Thermodynamics and Molecular Mechanism of Al Incorporation in Calcium Silicate Hydrates. J. Phys. Chem. C 2017, 121, 4412–4419. [Google Scholar] [CrossRef]
- Kulik, D.A.; Miron, G.D.; Lothenbach, B. A structurally-consistent CASH+ sublattice solid solution model for fully hydrated C-S-H phases: Thermodynamic basis, methods, and Ca-Si-H2O core sub-model. Cem. Concr. Res. 2022, 151, 106585. [Google Scholar] [CrossRef]
- Holmes, N.; Tyrer, M. Employing Discrete Solid Phases to represent C-S-H solid solutions in the cemdata07 thermodynamic database to model cement hydration using PHREEQC. Appl. Sci. 2022, 12, 10039. [Google Scholar] [CrossRef]
- Holmes, N.; Walker, C.; Tyrer, M.; Kelliher, D. Deriving discrete solid phases from CSH-3T and CSHQ end-members to model cement hydration in PHREEQC. In Proceedings of the Civil Engineering Research in Ireland (CERI) Conference, Dublin, Ireland, 25–26 August 2022; pp. 28–33. [Google Scholar]
- Parkhurst, D.J.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport and Inverse Geochemical Calculations; CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 2013. [Google Scholar]
- Ogoro, E.; Holmes, N.; Kelliher, D.; Tyrer, M. Predicting mortar compressive strength using HYDCEM. In Proceedings of the Civil Engineering Research in Ireland (CERI) Conference, Cork, Ireland, 27–28 August 2020. [Google Scholar]
- Holmes, N.; Kelliher, D.; Tyrer, M. HYDCEM: A new cement hydration model. In Proceedings of the 2nd International Conference on Sustainable Building Materials, Eindhoven, The Netherlands, 12–15 August 2019; Caprai, V., Brouwers, H.J.H., Eds.; pp. 66–74. [Google Scholar]
- Holmes, N.; Kelliher, D.; Tyrer, M. Modelling the addition of limestone in cement using HYDCEM. In Proceedings of the 39th Cement and Concrete Science Conference, Bath, UK, 9–10 September 2019; Ball, R., Dams, B., Ferrandiz-Mas, V., Ke, X., Paine, K., Tyrer, M., Walker, P., Eds.; pp. 23–27. [Google Scholar]
- Holmes, N.; Kelliher, D.; Tyrer, M. Thermodynamic cement hydration modelling using HYDCEM. In Proceedings of the Civil Engineering Research in Ireland (CERI) 2020, Cork, Ireland, 27–28 August 2020. [Google Scholar]
- Holmes, N.; Kelliher, D.; Tyrer, M. Simulating cement hydration using HYDCEM. Constr. Build. Mater. 2020, 239, 117811. [Google Scholar] [CrossRef]
- Halim, C.E.; Short, S.A.; Scott, J.A.; Amal, R.; Low, G. Modelling the leaching of Pb, Cd, As, and Cr from cementitious waste using PHREEQC. J. Hazard. Mater. 2005, 125, 45–61. [Google Scholar] [CrossRef]
- Walker, C.S.; Savage, D.; Tyrer, M.; Ragnarsdottir, K.V. Modeling the degradation of Ordinary Portland Cement using PHREEQC. In Proceedings of the 27th Cement and Concrete Science Conference, London, UK, 17–18 September 2007. [Google Scholar]
- Tiruta-Barna, L. Using PHREEQC for modelling and simulation of dynamic leaching tests and scenarios. J. Hazard. Mater. 2008, 157, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Martens, E.; Jacques, D.; Van Gerven, T.; Wang, L.; Mallants, D. Geochemical modeling of leaching of Ca, Mg, Al, and Pb from cementitious waste forms. Cem. Concr. Res. 2010, 40, 1298–1305. [Google Scholar] [CrossRef]
- Hareeparsad, S.; Tiruta-Barna, L.; Brouckaert, C.J.; Buckley, C.A. Quantitative geochemical modelling using leaching tests: Application for coal ashes produced by two South African thermal processes. J. Hazard. Mater. 2011, 186, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, G.; Etschmann, B.; Van Gerven, T.; Vandecasteele, C. Mechanisms and modelling of antimonate leaching in hydrated cement paste suspensions. Cem. Concr. Res. 2012, 42, 1307–1316. [Google Scholar] [CrossRef]
- Solpuker, U.; Sheets, J.; Kim, Y.; Schwartz, F.W. Leaching potential of pervious concrete and immobilization of Cu, Pb and Zn using pervious concrete. J. Contam. Hydrol. 2014, 161, 35–48. [Google Scholar] [CrossRef]
- Han, S.-C.; Jo, Y.; Yun, J.-I. Chemical degradation of fly ash blended concrete with the seasonal variation of rainwater in a radioactive waste repository: A thermodynamic modeling approach. Cem. Concr. Res. 2021, 141, 106326. [Google Scholar] [CrossRef]
- Vega-Garcia, P.; Schwerd, R.; Schwitalla, C.; Johann, S.; Scherer, C.; Helmreich, B. Leaching prediction for vertical test panels coated with plaster and mortars exposed under real conditions by a PHREEQC leaching model. Chemosphere 2021, 280, 130657. [Google Scholar] [CrossRef]
- Bandow, N.; Gartiser, S.; Ilvonen, O.; Schoknecht, U. Evaluation of the impact of construction products on the environment by leaching of possibly hazardous substances. Environ. Sci. Eur. 2018, 30, 14. [Google Scholar] [CrossRef]
- Holmes, N.; Tyrer, M.; West, R.P.; Lowe, A.; Kelliher, D. Using PHREEQC to model cement hydration. Constr. Build. Mater. 2022, 319, 126–129. [Google Scholar] [CrossRef]
- Shaji, N.; Holmes, N.; Tyrer, M. Early Age Assessment of a New Course of Irish Fly Ash as a Cement Replacement. Appl. Sci. 2024, 14, 4128. [Google Scholar] [CrossRef]
- Schmidt, T.; Lothenbach, B.; Romer, M.; Neuenschwander, J.; Scrivener, K. Physical and microstructural aspects of sulfate attack on ordinary and limestone blended Portland cements. Cem. Concr. Res. 2009, 39, 1111–1121. [Google Scholar] [CrossRef]
- Lothenbach, B.; Bary, B.; Le Bescop, P.; Schmidt, T.; Leterrier, N. Sulfate ingress in Portland cement. Cem. Concr. Res. 2010, 40, 1211–1225. [Google Scholar] [CrossRef]
- Schmidt, T.; Lothenbach, B.; Scrivener, K.L.; Romer, M.; Rentsch, D. Conditions for Thaumasite formation. In Proceedings of the 12th International Congress on the Chemistry of Cement, Montreal, QC, Canada, 8–13 July 2007; p. 12. [Google Scholar]
- Nixon, P.; Longworth, I.H. Thaumasite Expert Group Report: Reviews After Three Years’ Experience. 2003. Available online: https://api.semanticscholar.org/CorpusID:106878720 (accessed on 7 May 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).