Corrosion of Buried Pipelines by Stray Current in Electrified Railways: Mechanism, Influencing Factors, and Protection
Abstract
1. Introduction
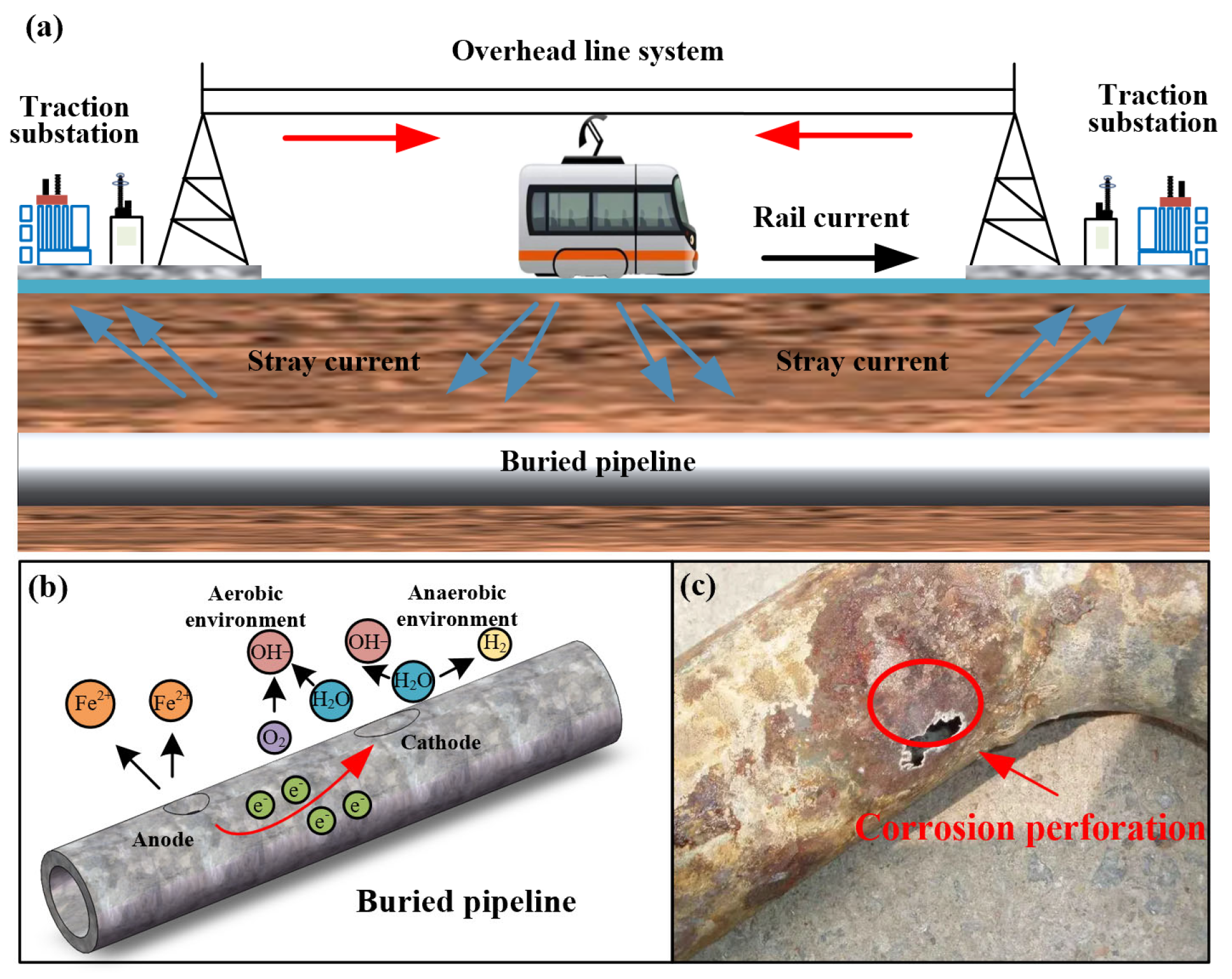
2. Stray Current Corrosion Mechanism and Interference Characteristics
2.1. Corrosion Mechanism and Interference Characteristics of DC Stray Current
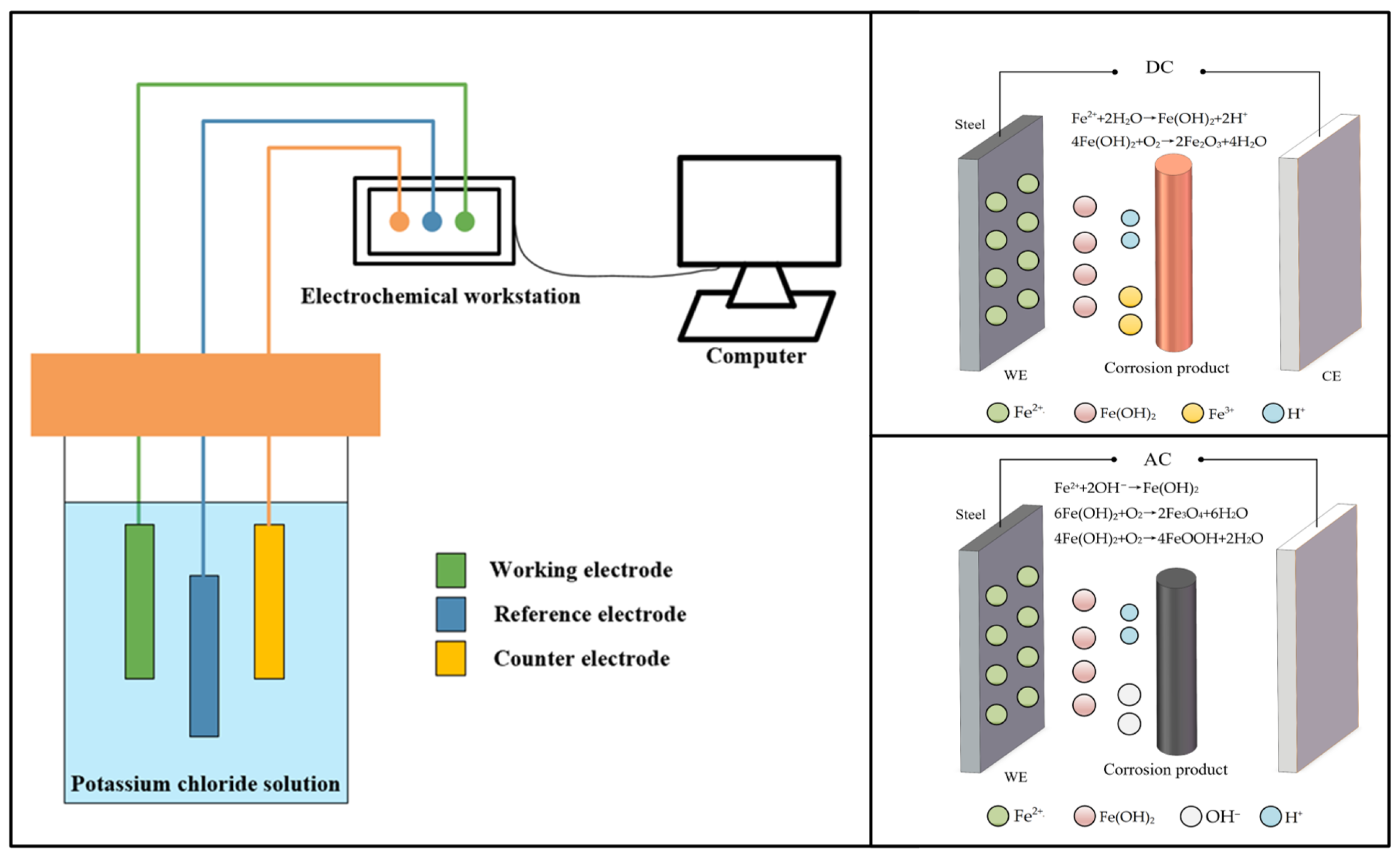
2.1.1. Corrosion Mechanism of DC Stray Current in Urban Rail Transit
2.1.2. Interference Characteristics of DC Stray Current
2.2. Corrosion Mechanism and Interference Characteristics of AC Stray Current
2.2.1. Rectification Model
2.2.2. Alkalization Mechanism and Autocatalytic Mechanism
2.2.3. AC Depolarization Theory
2.2.4. Oscillation Model
2.2.5. Passivation Damage and Pitting AC Corrosion Theory
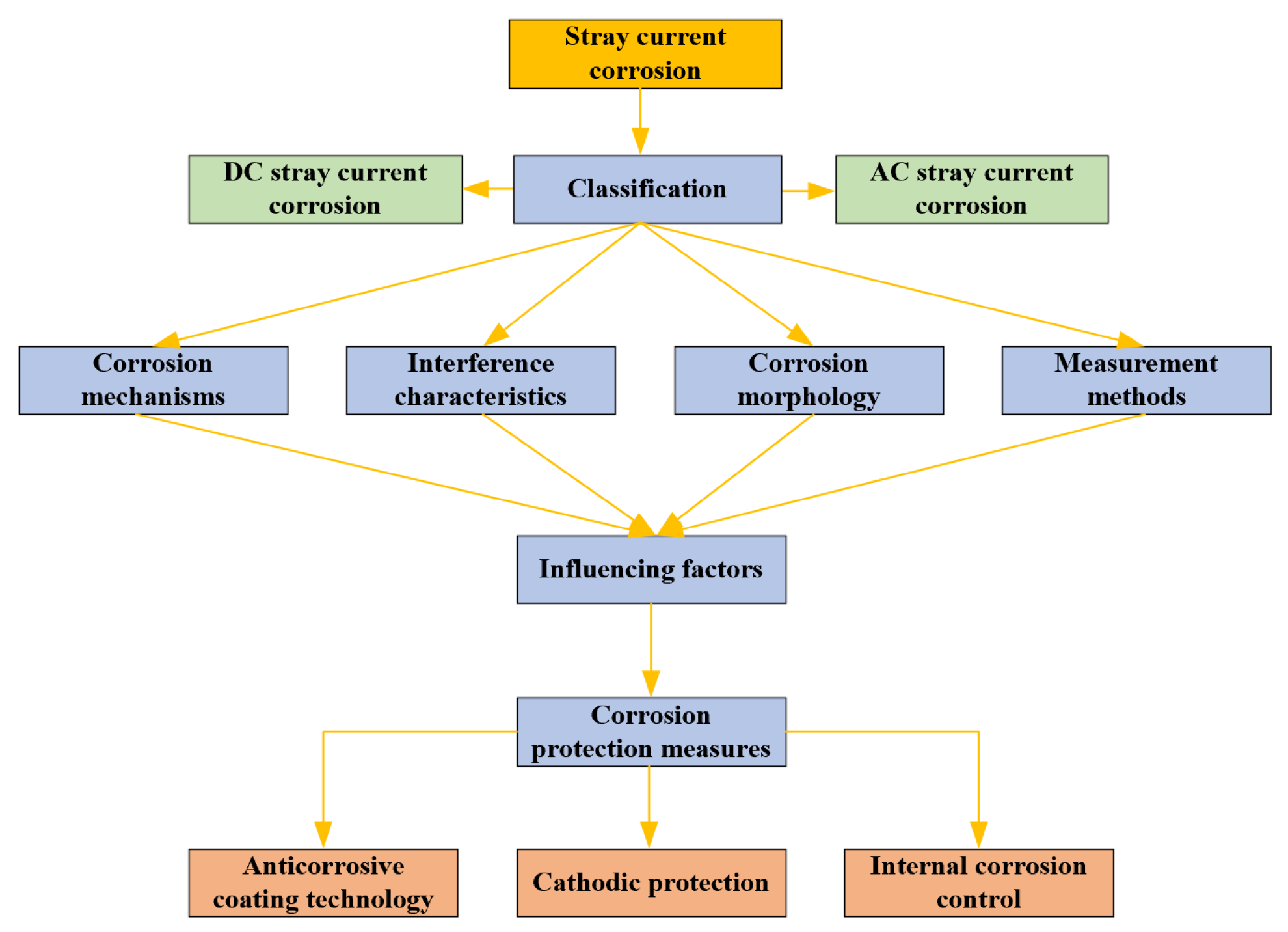
3. Stray Current Corrosion Morphology, Measurement Method, and Influencing Factors
3.1. Corrosion Morphology of DC Stray Current
3.2. Corrosion Morphology of AC Stray Current
3.3. Methods for Measuring DC and AC Stray Currents
3.4. Factors Affecting Stray Current Corrosion
3.4.1. Environmental Factors
3.4.2. Electrical Factors
3.4.3. Other Factors
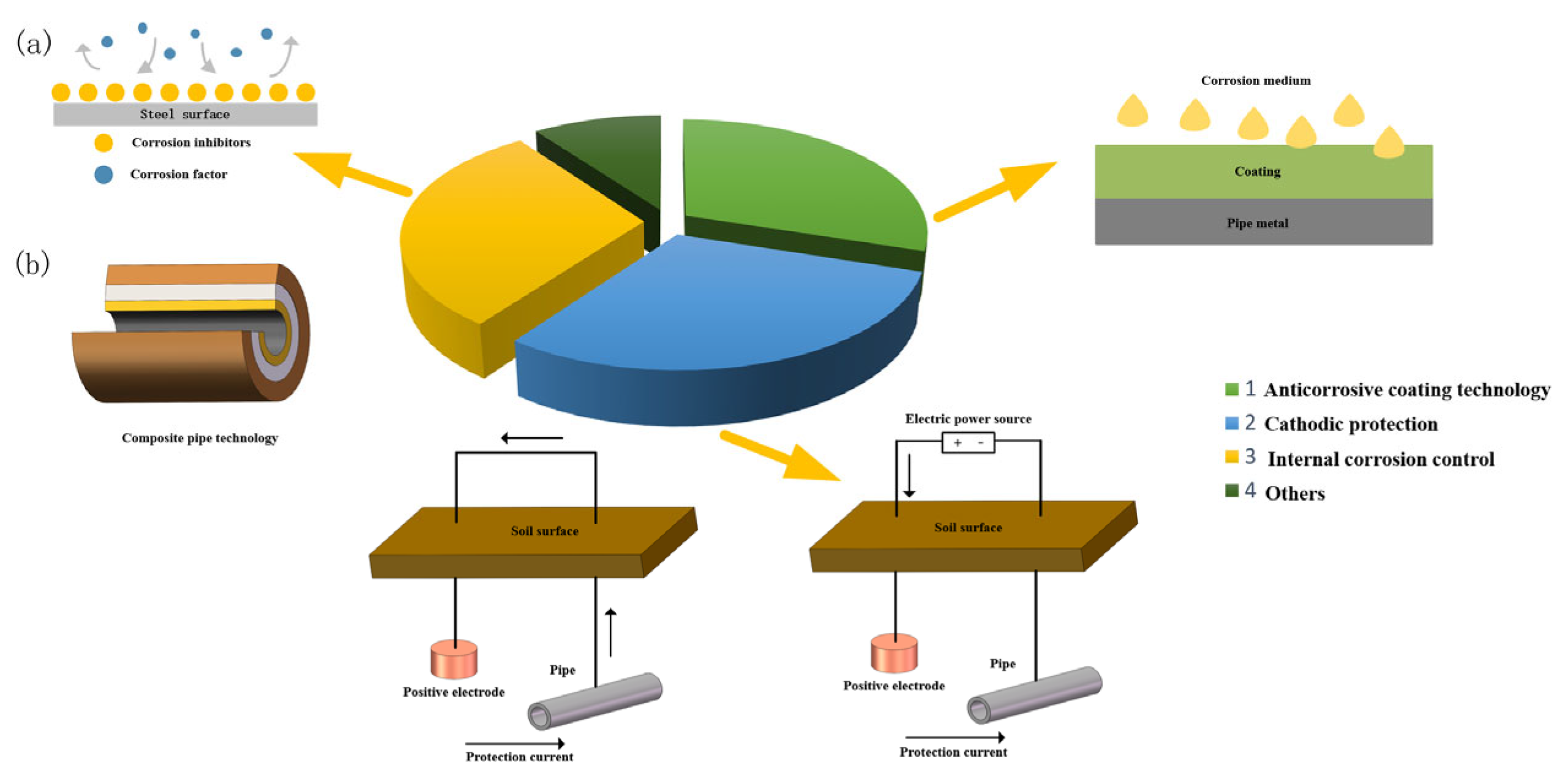
4. Corrosion Protection Measures
4.1. Anticorrosive Coating
4.1.1. Organic Anti-Corrosion Coating
4.1.2. Inorganic Nonmetal Anticorrosive Coating
4.1.3. New Coatings
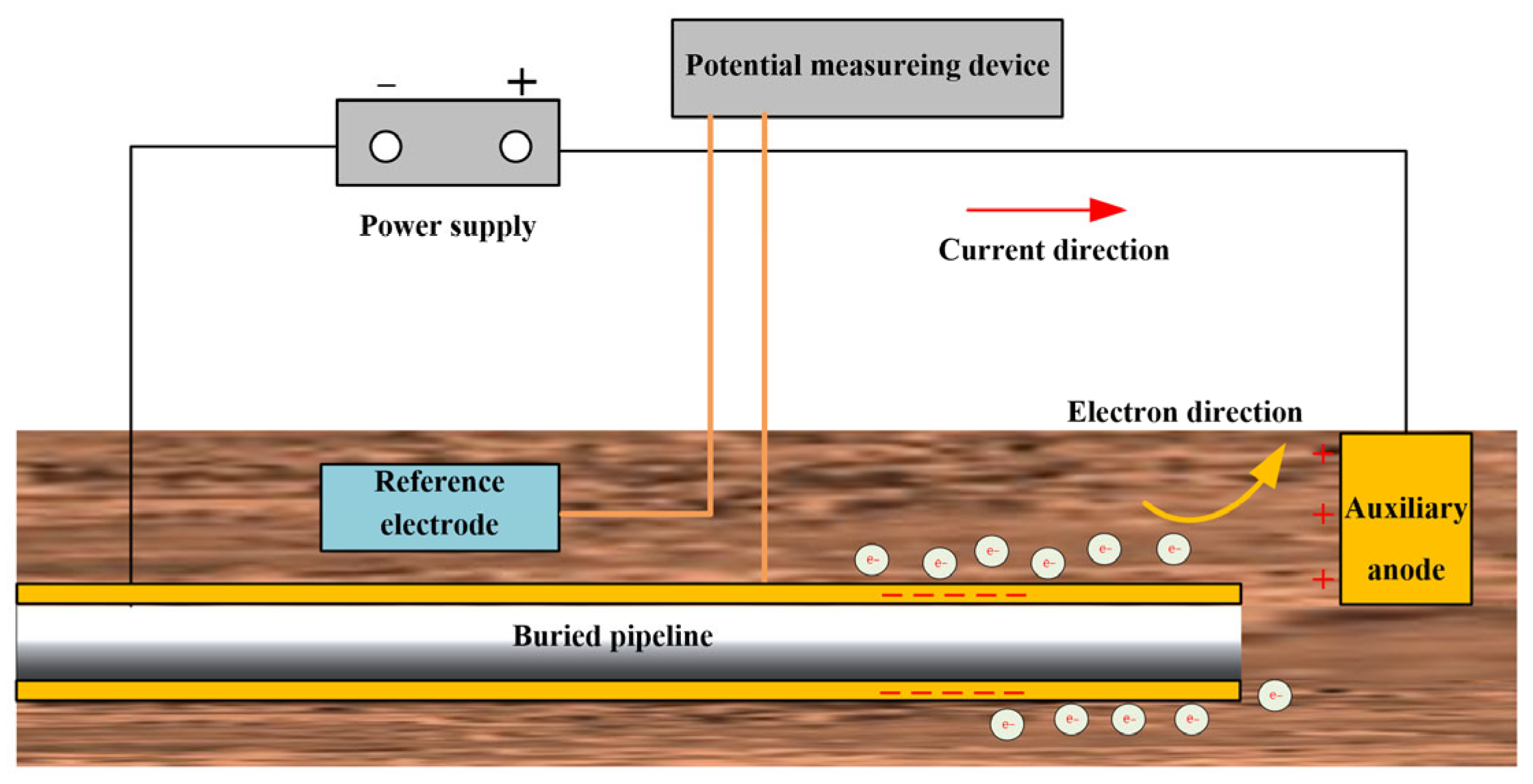
4.2. Cathodic Protection
4.2.1. Sacrificial Anode Method of Cathodic Protection
4.2.2. Impressed Current Method of Cathodic Protection
4.2.3. Drainage Protection of Cathodic Protection
4.3. Internal Anti-Corrosion Technique
4.3.1. Corrosion Inhibitor
4.3.2. Inner Coating Technology
4.3.3. Composite Pipe Technology
5. Common Methods for Studying Corrosion-Resistant Coatings
5.1. Weight-Loss Method
5.2. Advanced Observation Method (SEM, EDS, and AFM)
5.3. Electrochemical Impedance EIS
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, J.; Xiao, S.; Zhang, C.; Luo, Y.; Rao, Y.; Gao, G.; Wu, G.; Sykulski, J.K. Multiobjective Optimization of the Integrated Grounding System for High-Speed Trains by Balancing Train Body Current and Overvoltage. IEEE Trans. Transp. Electrif. 2021, 7, 1712–1723. [Google Scholar] [CrossRef]
- Memon, S.A.; Fromme, P. Stray Current Corrosion and Mitigation: A synopsis of the technical methods used in dc transit systems. IEEE Electrif. Mag. 2014, 2, 22–31. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Xin, G.; Wang, Y.; Xu, S.; Fan, M. Novel method for prediction of corrosion current density of gas pipeline steel under stray current interference based on hybrid LWQPSO-NN model. Measurement 2022, 200, 111592. [Google Scholar] [CrossRef]
- Tang, K. Assessing Stray DC and AC Current-Induced Corrosion in Steel Fibre-Reinforced Concrete (SFRC) in Railway Tunnelling Construction. Int. J. Civ. Eng. 2024. [Google Scholar] [CrossRef]
- Wang, C.; Qin, G. Corrosion of underground infrastructures under metro-induced stray current: A review. Corros. Commun. 2024, 14, 23–38. [Google Scholar] [CrossRef]
- Sibiya, C.; Kusakana, K.; Numbi, B.P. Smart System for Impressed Current Cathodic Protection Running on Hybrid Renewable Energy. In Proceedings of the IEEE Open Innovations Conference, Johannesburg, South Africa, 3–5 October 2018. [Google Scholar] [CrossRef]
- Tan, Z.; Zhu, Z.; Pei, F.; Fu, J.; Tian, X.; Zeng, B. Influence of DC stray current on corrosion behavior of grounding grid materials in soils with different moisture content. Corros. Sci. Prot. Technol. 2013, 25, 207–212. [Google Scholar]
- Peng, X.; Huang, Z.; Chen, B.; Liu, D.; Li, H. On the interference mechanism of stray current generated by DC tram on pipeline corrosion. Eng. Fail. Anal. 2020, 116, 104760. [Google Scholar] [CrossRef]
- Liu, X.; He, Y.; Tian, X. Effect of DC Stray Current on Corrosion Behavior of Q235 Steel in Red Soil. Corros. Prot. 2024, 45, 17–22. (In Chinese) [Google Scholar]
- Cotton, I.; Charalambous, C.; Aylott, P.; Ernst, P. Stray current control in DC mass transit systems. IEEE Trans. Veh. Technol. 2005, 54, 722–730. [Google Scholar] [CrossRef]
- Yu, K.; Ni, Y.; Zeng, X.; Peng, P.; Fan, X.; Leng, Y. Modeling and Analysis of Transformer DC Bias Current Caused by Metro Stray Current. IEEJ Trans. Electr. Electron. Eng. 2020, 15, 1507–1519. [Google Scholar] [CrossRef]
- Du, G.; Liu, N.; Zhang, D.; Li, Q.; Sun, J.; Jiang, X.; Zhu, Z. Grounding Fault Diagnosis of Running Rails Based on a Multi-scale One-Dimensional Convolutional Neural Network in a DC Subway System. Urban Rail Transit 2024, 10, 263–279. [Google Scholar] [CrossRef]
- Szymenderski, J.; Machczyński, W.; Budnik, K. Modeling Effects of Stochastic Stray Currents from D.C. Traction on Corrosion Hazard of Buried Pipelines. Energies 2019, 12, 4570. [Google Scholar] [CrossRef]
- Fangfang, X.; Chengtao, W. Electrochemical Corrosion Study of Buried Pipeline Steel X20 Affected by Stray Current. Am. J. Appl. Ind. Chem. 2019, 3, 9–14. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, L.; Pan, Z.; Bhatti, A.A.; Huang, X.; Zhang, J. Dynamic Diffusion Model of Stray Current in DC Traction Power Supply System. IEEE Trans. Power Deliv. 2023, 38, 2170–2182. [Google Scholar] [CrossRef]
- Li, Z.; Niasar, M.G.; Kavian, M.; Wild, F.d. Analysis of Stray Current Corrosion on Buried Pipeline due to HVDC Grounding Current. In Proceedings of the 2020 IEEE 29th International Symposium on Industrial Electronics (ISIE), Delft, The Netherlands, 17–19 June 2020; pp. 1233–1238. [Google Scholar]
- Zhang, Y.; Feng, Q.; Yu, L.; Wu, C.-M.L.; Ng, S.-P.; Tang, X. Numerical modelling of buried pipelines under DC stray current corrosion. J. Electrochem. Sci. Eng. 2019, 9, 125–134. [Google Scholar] [CrossRef]
- Du, Y.; Qin, H.; Liu, J.; Tang, D. Research on corrosion rate assessment of buried pipelines under dynamic metro stray current. Mater. Corros. 2021, 72, 1038–1050. [Google Scholar] [CrossRef]
- Zhichao, C.; Cheng, H. Evaluation of metro stray current corrosion based on finite element model. J. Eng. 2019, 2019, 2261–2265. [Google Scholar] [CrossRef]
- Zhu, C.; Du, G.; Ding, Y.; Huang, W.; Wang, J.; Fan, M.; Zhu, Z. Rail potential control with train diagram optimization in multitrain DC traction power system. Int. J. Rail Transp. 2022, 10, 476–496. [Google Scholar] [CrossRef]
- Gu, J.; Yang, X.; Zheng, T.Q.; Shang, Z.; Zhao, Z.; Guo, W. Negative Resistance Converter Traction Power System for Reducing Rail Potential and Stray Current in the Urban Rail Transit. IEEE Trans. Transp. Electrif. 2021, 7, 225–239. [Google Scholar] [CrossRef]
- McCollum, B.; Ahlborn, G.H. The Influence of Frequency of Alternating or Infrequently Reversed Current on Electrolytic Corrosion. Trans. Am. Inst. Electr. Eng. 1916, 35, 301–345. [Google Scholar] [CrossRef]
- Nielsen, L. Role of Alkalization in AC Induced Corrosion of Pipelines and Consequences Hereof in Relation to CP Requirements. In Proceedings of the 59th Annual CorrosionNACExpo, Houston, TX, USA, 3–7 April 2005. [Google Scholar]
- Wang, X.; Xu, C.; Chen, Y.; Tu, C.; Wang, Z.; Song, X. Effects of stray AC on corrosion of 3-layer polyethylene coated X70 pipeline steel and cathodic delamination of coating with defects in 3.5 wt-% NaCl solution. Corros. Eng. Sci. Technol. 2018, 53, 214–225. [Google Scholar] [CrossRef]
- Wang, X.; Tang, X.; Wang, L.; Wang, C.; Zhou, W. Synergistic effect of stray current and stress on corrosion of API X65 steel. J. Nat. Gas Sci. Eng. 2014, 21, 474–480. [Google Scholar] [CrossRef]
- Xu, L.Y.; Cheng, Y.F. Corrosion of X100 pipeline steel under plastic strain in a neutral pH bicarbonate solution. Corros. Sci. 2012, 64, 145–152. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, W.; Pan, Y.; Liu, Z.Y.; Zhou, X.C.; Li, X.G. Electrochemical mechanism of stress corrosion cracking of API X70 pipeline steel under different AC frequencies. Constr. Build. Mater. 2018, 171, 622–633. [Google Scholar] [CrossRef]
- Chin, D.T.; Sachdev, P. Corrosion by Alternating Current: Polarization of Mild Steel in Neutral Electrolytes. J. Electrochem. Soc. 1983, 130, 1714. [Google Scholar] [CrossRef]
- Niu, Q.; Li, Z.; Cui, G.; Wang, B. Effect of Flow Rate on the Corrosion Behavior of N80 Steel in Simulated Oil Field Environment Containing CO2 and HAc. Int. J. Electrochem. Sci. 2017, 12, 10279–10290. [Google Scholar] [CrossRef]
- Panossian, Z.; Filho, S.; Almeida, N.; Filho, M.; Silva, D.; Laurino, E.; Oliver, J.; Pimenta, G.; Albertini, J. Effect of alternating current by high power lines voltage and electric transmission systems in pipelines corrosion. In NACE International Corrosion Conference Series; NACE International: Houston, TX, USA, 2009. [Google Scholar]
- Tang, D.; Du, Y.; Lu, M.; Dong, L.; Jiang, Z. Progress in the mutual effects between AC interference and the cathodic protection of buried pipelines. J. Chin. Soc. Corros. Prot. 2013, 33, 351–356. [Google Scholar]
- Yang, Y.; Zeng, W.; Wang, L.; Liu, Y.; Ji, J. Numerical Simulations of Factors Affecting Stray Current Corrosion on Pipeline. IOP Conf. Ser. Earth Environ. Sci. 2020, 474, 052089. [Google Scholar] [CrossRef]
- Büchler, M.; Schöneich, H.-G. Investigation of Alternating Current Corrosion of Cathodically Protected Pipelines: Development of a Detection Method, Mitigation Measures, and a Model for the Mechanism. Corrosion 2009, 65, 578–586. [Google Scholar] [CrossRef]
- Lu, C.; Liu, C.; Li, N.; Ma, H.; Liu, Z.; Li, X. Effect of alternating current density on corrosion behaviour of X65 steel in weakly alkaline soil environment without cathodic protection. Corros. Eng. Sci. Technol. 2024, 59, 490–501. [Google Scholar] [CrossRef]
- Kou, J.; Fu, Y. Effects of AC Stray Current Density on Corrosion Behavior of X80 Pipeline Steel. Corros. Prot. 2018, 39, 124–128. (In Chinese) [Google Scholar]
- Ding, Q.; Qin, Y.; Shen, T.; Gao, Y. Effect of Alternating Stray Current Density on Corrosion Behavior of X80 Steel under Disbonded Coating. Int. J. Corros. 2021, 2021, 8833346. [Google Scholar] [CrossRef]
- Ding, Q.; Fan, Y. Experimental Study on the Influence of AC Stray Current on the Cathodic Protection of Buried Pipe. Int. J. Corros. 2016, 2016, 5615392. [Google Scholar] [CrossRef][Green Version]
- Kim, D.-K.; Muralidharan, S.; Ha, T.-H.; Bae, J.-H.; Ha, Y.-C.; Lee, H.-G.; Scantlebury, J.D. Electrochemical studies on the alternating current corrosion of mild steel under cathodic protection condition in marine environments. Electrochim. Acta 2006, 51, 5259–5267. [Google Scholar] [CrossRef]
- Lan, W.; Li, Q.; Wei, B.; Bi, W.; Xu, C.; Liu, D. Evaluation of AC corrosion under anodic polarization using microzone pH analysis. Corros. Sci. 2023, 219, 111219. [Google Scholar] [CrossRef]
- Gang, X.; Wen, W.; Wei, J. Failure analysis of sour natural gas gathering and transportation pipeline. Chem. Eng. Oil Gas 2012, 41, 99–101+123–124. (In Chinese) [Google Scholar]
- EN50152; Railway Applications. Fixed Installations. Particular Requirements for a.c. Switchgear. Available online: https://landingpage.bsigroup.com/LandingPage/Series?UPI=BS%20EN%2050152 (accessed on 26 December 2024).
- Cheng, S.; Zhang, L.; Yang, A. Influence of Subway Stray Current Corrosion on Buried Metal Pipeline. Gas Heat 2003, 7, 435–437. (In Chinese) [Google Scholar]
- Chen, D.; Long, Y. A Set of Rapid Combined Testing Technology for Buried Pipeline Leak Detection. In ICPTT 2013; American Society of Civil Engineers: Reston, VA, USA, 2013; pp. 107–114. [Google Scholar] [CrossRef]
- Deng, J. Research on Dynamic Stray Current Detection Method Based on SCM System. Pipeline Technol. Equip. 2022, 1, 56–58. (In Chinese) [Google Scholar]
- Xu, S.; Peng, Q.; Xing, F.; Sun, J. Magnetostrictive current sensor with high sensitivity and a large linear range for the subway. Appl. Opt. 2021, 60, 9741–9747. [Google Scholar] [CrossRef]
- Li, S.; Kim, Y.-G.; Jung, S.; Song, H.-S.; Lee, S.-M. Application of steel thin film electrical resistance sensor for in situ corrosion monitoring. Sens. Actuators B Chem. 2007, 120, 368–377. [Google Scholar] [CrossRef]
- SY/T0087.1-2006; Standard of Steel Pipeline and Tank Corrosion Assessment-Steel Pipeline External Corrosion Direct Assessment. Petroleum Industry Press: Beijing, China, 2006.
- GB50698-2011; Standard for AC Interference Mitigation of Buried Steel Pipelines. China Planning Press: Beijing, China, 2011.
- DIN50925; Corrosion Protection of Metals; Electroplated Coatings; Technical Delivery Conditions. Deutsches Institut für Normung (DIN): Berlin, Germany, 1985.
- CEN/TS15280; Evaluation of a.c. Corrosion Likelihood of Buried Pipelines—Application to Cathodically Protected Pipelines. European Committee for Standardization (CEN): Brussels, Belgium, 2006.
- ISO 15589-1-2003; Petroleum and Natural Gas Industries—Cathodic Protection of Pipeline Transportation Systems—Part 1: On-Land Pipelines. International Organization for Standardization (ISO): Geneva, Switzerland, 2003.
- EN1295; Structural Design of Buried Pipelines Under Various Conditions of Loading—Part 1: General Requirements. European Committee for Standardization (CEN): Brussels, Belgium, 1997.
- Liang, Y.; Du, Y.; Tang, D.; Chen, L.; Zhang, L.; Qiao, L. Research on AC corrosion behavior and corrosion product film evolution of X70 steel under the combined action of AC interference and CP. Corros. Sci. 2022, 197, 110085. [Google Scholar] [CrossRef]
- Shabangu, T.H.; Shrivastava, P.; Abe, B.T.; Olubambi, P.A. Stability Assessment of Pipeline Cathodic Protection Potentials under the Influence of AC Interference. Prog. Electromagn. Res. 2018, 66, 19–28. [Google Scholar] [CrossRef]
- Shabangu, T.H.; Shrivastava, P.; Abe, B.T.; Adedeji, K.B.; Olubambi, P.A. Influence of AC interference on the cathodic protection potentials of pipelines: Towards a comprehensive picture. In Proceedings of the 2017 IEEE AFRICON, Cape Town, South Africa, 18–20 September 2017; pp. 597–602. [Google Scholar] [CrossRef]
- Fu, W.; Zheng, X.Y.; Zhang, Z.L.; Liao, Y.Z.; Li, J.J.; Chen, S.S. The Problems and Countermeasures of Cathodic Protection Accepatnce of The Yili River Crossing by Horizontal Directional Drilling Method. In Proceedings of the 2015 International Conference on Materials Chemistry and Environmental Protection (MEEP-15), Sanya, China, 19–21 December 2015; pp. 168–170, 2016/03. [Google Scholar] [CrossRef]
- Ormellese, M.; Goidanich, S.; Lazzari, L. Effect of AC interference on cathodic protection monitoring. Corros. Eng. Sci. Technol. 2011, 46, 618–623. [Google Scholar] [CrossRef]
- Shamsuddoha, M.; Islam, M.M.; Aravinthan, T.; Manalo, A.; Lau, K.-T. Effectiveness of using fibre-reinforced polymer composites for underwater steel pipeline repairs. Compos. Struct. 2013, 100, 40–54. [Google Scholar] [CrossRef]
- Yan, X. Prevention and Treatment of AC Stray Current in Long Distance Natural Gas Pipeline. Total Corros. Control 2023, 37, 39–42. [Google Scholar]
- Xu, L.Y.; Su, X.; Yin, Z.X.; Tang, Y.H.; Cheng, Y.F. Development of a real-time AC/DC data acquisition technique for studies of AC corrosion of pipelines. Corros. Sci. 2012, 61, 215–223. [Google Scholar] [CrossRef]
- Bukit, F.R.A.; Hidayat, M. Analysis of the Effect of Addition of Bentonite Zeolite Bunch Ash and Coconut Kernel Oil as Earthing Media. In Proceedings of the 2023 7th International Conference on Electrical, Telecommunication and Computer Engineering (ELTICOM), Medan, Indonesia, 13–14 December 2023; pp. 218–223. [Google Scholar] [CrossRef]
- Bai, F.; Wang, S.; Zhao, L.; Cao, F.; Du, Y. Study on the Effect of Temperature and Water Content on Conductivity of Soil in Tibet of China. In Proceedings of the 2023 2nd Asia Power and Electrical Technology Conference (APET), Shanghai, China, 28–30 December 2023; pp. 886–892. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, G. Simulation of Influencing Factors of Buried Pipeline Interfered by Subway Stray Current. Highlights Sci. Eng. Technol. 2023, 35, 140–148. [Google Scholar] [CrossRef]
- Xiaolong, L.; Xiao, L.; Nan, L.; Tao, M.; Jiwang, Z.; Xuyun, Y.; Renyang, H. Numerical simulation of stray current corrosion of buried steel pipelines. In Proceedings of the 3rd International Conference on Optoelectronic Information and Functional Materials (OIFM 2024), Wuhan, China, 19–21 April 2024; p. 131633I. [Google Scholar]
- Dzakyprasetyo, R.; Ferdian, D. Analysis of external corrosion protection performance on buried gas pipeline using CIPS and DCVG methods. ITM Web Conf. 2024, 61, 1022. [Google Scholar] [CrossRef]
- Chung, N.T.; So, Y.-S.; Kim, W.-C.; Kim, J.-G. Evaluation of the Influence of the Combination of pH, Chloride, and Sulfate on the Corrosion Behavior of Pipeline Steel in Soil Using Response Surface Methodology. Materials 2021, 14, 6596. [Google Scholar] [CrossRef]
- Ogunsola, A.; Sandrolini, L.; Mariscotti, A. Evaluation of Stray Current From a DC-Electrified Railway with Integrated Electric–Electromechanical Modeling and Traffic Simulation. IEEE Trans. Ind. Appl. 2015, 51, 5431–5441. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, X.; Cheng, H. Evaluation of DC-Subway Stray Current Corrosion with Integrated Multi-Physical Modeling and Electrochemical Analysis. IEEE Access 2019, 7, 168404–168411. [Google Scholar] [CrossRef]
- Brenna, M.; Dolara, A.; Leva, S.; Zaninelli, D. Effects of the DC stray currents on subway tunnel structures evaluated by FEM analysis. In Proceedings of the IEEE PES General Meeting, Minneapolis, MN, USA, 25–29 July 2010; pp. 1–7. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, Y.; Zhang, J. Influence Factors of Stray-Current Corrosion of Buried Metal Pipeline. Appl. Phys. 2019, 9, 250. (In Chinese) [Google Scholar] [CrossRef]
- Qin, Y.; Shi, X.; Cui, Y. Study on Potential Interference of DC Stray Current on Buried Pipeline. Oil-Gas Field Surf. Eng. 2019, 38, 60–65. (In Chinese) [Google Scholar]
- Guo, Y.; Liu, C.; Wang, D.; Liu, S. Effects of alternating current interference on corrosion of X60 pipeline steel. Pet. Sci. 2015, 12, 316–324. [Google Scholar] [CrossRef]
- Moran, A.J.; Lillard, R.S. A Modeling Approach to Understanding the Interrelated Nature of Cathodic Protection Current and AC Stray Current on Pipelines. Corrosion 2023, 79, 526–538. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Z.; Huang, H.; Wang, D.; He, R. Research on the stress corrosion and cathodic protection of API X80 steel under AC stray current interference. Anti-Corros. Methods Mater. 2021, 68, 346–353. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Yan, M.; Shi, L.; Wang, J.; Zhao, W.; Li, X. Corrosion Behavior of Pipeline Steel with Stray Current Interference at Coating Defects. In Proceedings of the ASME 2020 Pressure Vessels & Piping Conference, Online, 3 August 2020. [Google Scholar] [CrossRef]
- Chen, L.; Du, Y.; Guo, L.; Zhu, Z.; Liang, Y.; Zhang, L. Corrosion Behavior and Evaluation Method of Pipeline Steel Under Dynamic AC Interference: A Study. Mater. Corros. 2024. [Google Scholar] [CrossRef]
- Feng, J.; Chen, Z.; Xie, Y.; Wu, C.; Qin, C. Influence of Urban Rail Transit on Corrosion of Buried Steel Gas Pipeline. Int. J. Electrochem. Sci. 2021, 16, 210617. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, J.; Liu, X.; Zhang, X.; Cai, Z.; Chen, X. Study on Distribution Characteristics of Metro Stray Current and Evaluation of Cumulative Corrosion Effect. Adv. Civ. Eng. 2022, 2022, 6845847. [Google Scholar] [CrossRef]
- Guo, Y.; Meng, T.; Wang, D.; Tan, H.; He, R. Experimental research on the corrosion of X series pipeline steels under alternating current interference. Eng. Fail. Anal. 2017, 78, 87–98. [Google Scholar] [CrossRef]
- Wang, X.; Song, X.; Chen, Y.; Wang, Z. Effect of Alternating Stray Current and Stress on the Corrosion Behavior of X80 Pipeline Steel in Soil Simulated Solution. Int. J. Electrochem. Sci. 2018, 13, 5654–5666. [Google Scholar] [CrossRef]
- Xu, L.Y.; Su, X.; Cheng, Y.F. Effect of alternating current on cathodic protection on pipelines. Corros. Sci. 2013, 66, 263–268. [Google Scholar] [CrossRef]
- Chao, Y.; Jianliang, L.; Zili, L.; Shouxin, Z.; Long, D.; Chengbin, Z. Study on interference and protection of pipeline due to high-voltage direct current electrode. Corros. Rev. 2019, 37, 273–281. [Google Scholar] [CrossRef]
- Farahani, E.M.; Su, Y.; Chen, X.; Wang, H.; Laughorn, T.R.; Onesto, F.; Zhou, Q.; Huang, Q. AC corrosion of steel pipeline under cathodic protection: A state-of-the-art review. Mater. Corros. 2024, 75, 290–314. [Google Scholar] [CrossRef]
- CSAZ245.1-2002; Steel Pipes for the Transport of Fluids—Part 1: Technical Delivery Conditions. Chinese Standardization Administration (CSA): Beijing, China, 2002.
- Liao, D.; Zhang, L.; Tao, G. Study on corrosion rate of buried gas steel pipeline in Nanjing based on the GM(1, N) optimization model. IOP Conf. Ser. Mater. Sci. Eng. 2019, 490, 022025. [Google Scholar] [CrossRef]
- Ekperi, R.; Ajieh, M.U.; Ikpeseni, S.C.; Owamah, H.I.; Edomwonyi-Otu, L.C.; Okafor, I.F. Application of solar photovoltaic electricity in unravelling the effects of coating defects on cathodic protection parameters of buried pipeline. IOP Conf. Ser. Earth Environ. Sci. 2023, 1178, 012002. [Google Scholar] [CrossRef]
- Liu, S.; Zuo, Y.; Zhang, Z. A New Detecting Technology for External Anticorrosive Coating Defects of Pipelines Based on Ultrasonic Guided Wave. IOP Conf. Ser. Earth Environ. Sci. 2018, 108, 022073. [Google Scholar] [CrossRef]
- Farh, H.M.H.; Ben Seghier, M.E.A.; Zayed, T. A comprehensive review of corrosion protection and control techniques for metallic pipelines. Eng. Fail. Anal. 2023, 143, 106885. [Google Scholar] [CrossRef]
- Fix, D.; Andreeva, D.; Lvov, Y.; Shchukin, D.; Moehwald, H. Application of Inhibitor-Loaded Halloysite Nanotubes in Active Anti-Corrosive Coatings. Adv. Funct. Mater. 2009, 19, 1720–1727. [Google Scholar] [CrossRef]
- Verachtert, E.; Van Den Eeckhaut, M.; Poesen, J.; Deckers, J. Spatial interaction between collapsed pipes and landslides in hilly regions with loess-derived soils. Earth Surf. Process. Landf. 2013, 38, 826–835. [Google Scholar] [CrossRef]
- Li, H.; He, W.; Li, S. Characteristics and causes of landslide deformation along Guizhou Section of Myanmar-China Oil and Gas Pipelines. Oil Gas Storage Transp. 2023, 42, 178–187. (In Chinese) [Google Scholar]
- Xu, Q.; Liu, H.; Ran, J.; Li, W.; Sun, X. Field monitoring of groundwater responses to heavy rainfalls and the early warning of the Kualiangzi landslide in Sichuan Basin, southwestern China. Landslides 2016, 13, 1555–1570. [Google Scholar] [CrossRef]
- Yang, Z.; Dong, M. A new measurement method for radial permeability and porosity of shale. Pet. Res. 2017, 2, 178–185. [Google Scholar] [CrossRef]
- Samimi, A. Use of Polyurethane Coating to Prevent Corrosion in Oil and Gas Pipelines Transfer. Int. J. Innov. Appl. Stud. 2012, 1, 186–193. Available online: https://hal.science/hal-00772349 (accessed on 26 December 2024).
- Yan, M.; Yang, S.; Xu, J.; Sun, C.; Wu, T.; Yu, C.; Ke, W. Stress corrosion cracking of X80 pipeline steelat coating defect in acidic soil. Acta Metall. Sin. 2016, 52, 1133–1141. [Google Scholar] [CrossRef]
- Miley, N.E. Application of Plastic Tape to NATURAL GAS PIPELINE. Anti-Corros. Methods Mater. 1958, 5, 85–86. [Google Scholar] [CrossRef]
- Alsaiari, H.; Sayed, M.; Reddy, B.; Metouri, S.; Al-Taie, I. The Importance of the Stability of Cement Sheaths: Interaction Between Cement, Acid, Carbon Steel and Formation and Treatment Fluids. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 13–16 November 2017. [Google Scholar] [CrossRef]
- Lyu, Y.; Sun, W.; Feng, T.; Li, W.; Jiang, Y.; Zuo, C.; Wang, S. Anticorrosion Performance of Waterborne Coatings with Modified Nanoscale Titania under Subtropical Maritime Climate. Polymers 2024, 16, 1919. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, X.; Wang, Y.; Zhang, H.; Cao, Q.; Cheng, X. Corrosion Behavior of Al2O3-40TiO2 Coating Deposited on 20MnNiMo Steel via Atmospheric Plasma Spraying in Hydrogen Sulfide Seawater Stress Environments. Coatings 2024, 14, 588. [Google Scholar] [CrossRef]
- Allison, P.G.; Moser, R.D.; Weiss, C.A.; Malone, P.G.; Morefield, S.W. Nanomechanical and chemical characterization of the interface between concrete, glass–ceramic bonding enamel and reinforcing steel. Constr. Build. Mater. 2012, 37, 638–644. [Google Scholar] [CrossRef]
- Song, D.; Tang, R.; Yang, F.; Qiao, Y.; Sun, J.; Jiang, J.; Ma, A. Development of High-Performance Enamel Coating on Grey Iron by Low-Temperature Sintering. Materials 2018, 11, 2183. [Google Scholar] [CrossRef]
- Shafaamri, A.; Kasi, R.; Balakrishnan, V.; Subramaniam, R.; Arof, A.K. Amelioration of anticorrosion and hydrophobic properties ofepoxy/PDMS composite coatings containing nano ZnO particles. Prog. Org. Coat. 2016, 92, 54–65. [Google Scholar] [CrossRef]
- Kongparakul, S.; Kornprasert, S.; Suriya, P.; Le, D.; Samart, C.; Chantarasiri, N.; Prasassarakich, P.; Guan, G. Self-healing hybrid nanocomposite anticorrosive coating from epoxy/modified nanosilica/perfluorooctyl triethoxysilane. Prog. Org. Coat. 2017, 104, 173–179. [Google Scholar] [CrossRef]
- Popov, B.N.; Kumaraguru, S.P. Chapter 24—Cathodic protection of pipelines. In Handbook of Environmental Degradation of Materials; Kutz, M., Ed.; William Andrew Publishing: New York, NY, USA, 2005; pp. 503–521. [Google Scholar]
- Tzeng, Y.-C.; Chen, R.-Y. The effect of the Zn content on the electrochemical performance of Al-Zn-Sn-Ga alloys. Mater. Chem. Phys. 2023, 299, 127510. [Google Scholar] [CrossRef]
- Kong, L.; Tang, X.; Du, X.; Xie, Z.; Wang, X.; Xie, Q.; Wang, J.; Cai, J. Surface engineering of TiO2@SrTiO3 heterojunction with Ni2S3 for efficient visible-light-driven photoelectrochemical cathodic protection. J. Alloys Compd. 2022, 927, 166861. [Google Scholar] [CrossRef]
- Christodoulou, C.; Glass, G.; Webb, J.; Austin, S.; Goodier, C. Assessing the long term benefits of Impressed Current Cathodic Protection. Corros. Sci. 2010, 52, 2671–2679. [Google Scholar] [CrossRef]
- Law, D.W.; Nicholls, P.; Christodoulou, C. Residual protection of steel following suspension of Impressed Current Cathodic Protection system on a wharf structure. Constr. Build. Mater. 2019, 210, 48–55. [Google Scholar] [CrossRef]
- Cain, T.W.; Melia, M.A.; Fitz-Gerald, J.M.; Scully, J.R. Evaluation of the Potential Range for Sacrificial Mg Anodes for the Cathodic Protection of Mg Alloy AZ31B-H24. Corrosion 2017, 73, 544–562. [Google Scholar] [CrossRef]
- Angst, U.; Büchler, M.; Martin, B.; Schöneich, H.G.; Haynes, G.; Leeds, S.; Kajiyama, F. Cathodic protection of soil buried steel pipelines—A critical discussion of protection criteria and threshold values. Mater. Corros. 2016, 67, 1135–1142. [Google Scholar] [CrossRef]
- Li, L.; Mahmoodian, M.; Li, C.-Q.; Robert, D. Effect of corrosion and hydrogen embrittlement on microstructure and mechanical properties of mild steel. Constr. Build. Mater. 2018, 170, 78–90. [Google Scholar] [CrossRef]
- Zvirko, O.I.; Savula, S.F.; Tsependa, V.M.; Gabetta, G.; Nykyforchyn, H.M. Stress corrosion cracking of gas pipeline steels of different strength. Procedia Struct. Integr. 2016, 2, 509–516. [Google Scholar] [CrossRef][Green Version]
- Zhu, M.; Yuan, Y.; Yin, S.; Guo, S. Synergistic Effect of AC and Cl- on Stress Corrosion Cracking Behavior of X80 Pipeline Steel in Alkaline Environment. Int. J. Electrochem. Sci. 2018, 13, 10527–10538. [Google Scholar] [CrossRef]
- Tang, C.; van Asch, T.W.J.; Chang, M.; Chen, G.Q.; Zhao, X.H.; Huang, X.C. Catastrophic debris flows on 13 August 2010 in the Qingping area, southwestern China: The combined effects of a strong earthquake and subsequent rainstorms. Geomorphology 2012, 139–140, 559–576. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, J.; Du, Y. Limit load prediction analysis of X80 pipeline containing corrosion in mountainous landslide section. Geoenergy Sci. Eng. 2023, 229, 212107. [Google Scholar] [CrossRef]
- Sun, F.; Han, P.; He, B. An analysis of electrochemical corrosion on pipeline steel in silty soil under salt-temperature coupling environments. Chem. Eng. Sci. 2023, 274, 118704. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, P.; Dan, Y.; Liu, G.; Xiang, R.; Zou, J. A Simulation Study of the Direct Current Corrosion Characteristics of Carbon Steel Grounding Electrode with Ground Lead. Int. J. Electrochem. Sci. 2018, 13, 11974–11985. [Google Scholar] [CrossRef]
- Bairagi, H.; Vashishth, P.; Ji, G.; Shukla, S.K.; Ebenso, E.E.; Mangla, B. Polymers and their composites for corrosion inhibition application: Development, advancement, and future scope—A critical review. Corros. Commun. 2024, 15, 79–94. [Google Scholar] [CrossRef]
- Nie, X.; Meletis, E.I.; Jiang, J.C.; Leyland, A.; Yerokhin, A.L.; Matthews, A. Abrasive wear/corrosion properties and TEM analysis of Al2O3 coatings fabricated using plasma electrolysis. Surf. Coat. Technol. 2002, 149, 245–251. [Google Scholar] [CrossRef]
- Fujiyama, H.; Tokitu, Y.; Uchikawa, Y.; Kuwahara, K.; Miyake, K.; Kuwahara, K.; Doi, A. Ceramics inner coating of narrow tubes by a coaxial magnetron pulsed plasma. Surf. Coat. Technol. 1998, 98, 1467–1472. [Google Scholar] [CrossRef]
- GB/T 16545-2015; Cathodic Protection of Steel Structures. Standardization Administration of the People’s Republic of China (SAC): Beijing, China, 2015.
- Zhong, Y.; Zhang, Y.; Jingwei, D.; Qingsong, Y.; Yawei, S.; Yanqiu, W.; Guozhe, M. Effect of phytic acid on corrosion performance of epoxy coating on rust Q235 carbon steel. Corros. Sci. Prot. Technol. 2015, 27, 183–187. [Google Scholar]
- Shi, X.-B.; Yan, W.; Yan, M.-C.; Wang, W.; Yang, Z.-G.; Shan, Y.-Y.; Yang, K. Effect of Cu Addition in Pipeline Steels on Microstructure, Mechanical Properties and Microbiologically Influenced Corrosion. Acta Metall. Sin. 2017, 30, 601–613. [Google Scholar] [CrossRef]
- Yadav, M.; Sinha, R.R.; Sarkar, T.K.; Bahadur, I.; Ebenso, E.E. Application of new isonicotinamides as a corrosion inhibitor on mild steel in acidic medium: Electrochemical, SEM, EDX, AFM and DFT investigations. J. Mol. Liq. 2015, 212, 686–698. [Google Scholar] [CrossRef]
- Mahdavian, M.; Attar, M.M. Another approach in analysis of paint coatings with EIS measurement: Phase angle at high frequencies. Corros. Sci. 2006, 48, 4152–4157. [Google Scholar] [CrossRef]
- Ehsani, A.; Mahjani, M.G.; Hosseini, M.; Safari, R.; Moshrefi, R.; Mohammad Shiri, H. Evaluation of Thymus vulgaris plant extract as an eco-friendly corrosion inhibitor for stainless steel 304 in acidic solution by means of electrochemical impedance spectroscopy, electrochemical noise analysis and density functional theory. J. Colloid Interface Sci. 2017, 490, 444–451. [Google Scholar] [CrossRef] [PubMed]

| Factor Categories | Specific Influencing Factors | Impact Descriptions | References |
|---|---|---|---|
| Environmental factors | Soil resistivity | Lower resistivity soil allows stray current to pass through more easily, leading to increased corrosion. High-resistivity soil effectively inhibits stray current corrosion. | [62] |
| Soil salt concentration | Higher salt concentration increases conductivity, promoting the spread of stray current, which leads to more severe corrosion. | [64] | |
| Soil oxygen concentration | Higher oxygen concentration in the soil promotes localized chemical reactions, accelerating corrosion. | [16] | |
| Soil porosity | Higher porosity allows stray currents to penetrate the pipeline surface more easily, exacerbating corrosion. | [64] | |
| Buried depth | The burial depth of the pipeline can reduce the impact of stray currents, but soil properties still influence corrosion. | [63] | |
| Temperature, humidity, pH value | High temperature, high humidity, and low pH soil will accelerate the transmission of stray current to the buried pipeline surface and make metal ions more easily dissolved, aggravating corrosion. | [61,64] | |
| Electrical factors | Traction current size | Higher traction current increases the corrosion rate, especially near the track. | [67] |
| Track insulation | Poor insulation of the track causes stray currents to leak into the surrounding environment, increasing the risk of corrosion. | [68] | |
| Grounding system design | The design of the grounding system affects the distribution and intensity of stray currents. Poor design may lead to more concentrated stray currents impacting pipelines. | [64] | |
| Applied voltage | Higher applied voltage results in stronger stray currents, impacting the corrosion rate of the buried pipeline. | [71] | |
| AC current frequency | Higher AC current frequency shifts the corrosion potential positively, reducing the corrosion rate. Low frequency increases corrosion rate. | [72] | |
| AC current direction | Changes in the direction of the AC current lead to asymmetry, increasing the corrosion rate and reducing the effectiveness of cathodic protection. | [73] | |
| AC current waveform | Triangular waveform leads to a higher corrosion rate compared to sine and square waves due to higher peak voltage. | [28] | |
| Potential fluctuations | Potential fluctuations cause local negative shifts, promoting anode dissolution and increasing corrosion rate. | [76] | |
| Other factors | Stress factors | Stress concentration areas (such as welds, joints, and pipeline bends) are more susceptible to stray current interference, leading to increased corrosion and possibly stress corrosion cracking. | [24,78] |
| Quality of anti-corrosion coating and effectiveness of cathodic protection system | High-quality coatings and effective cathodic protection systems significantly reduce corrosion. Damaged coatings exacerbate corrosion. A well-designed cathodic protection system reduces stray current corrosion. | [74,80] | |
| Pipe material factors | Higher-strength steels (e.g., X80) offer better resistance to stray current corrosion but may be more susceptible to hydrogen embrittlement and fatigue cracking. Proper material selection and protection measures can reduce corrosion. | [24,74] |
| Type of Coating | Classification | Characteristics | Advantages | Disadvantages | Applications |
|---|---|---|---|---|---|
| Asphalt-based coating | Petroleum asphalt or enamel | Rust removal requirements are not high; Poor resistance to mechanical damage; Resistance to soil surplus stress differences; | Good electrical insulation, water resistance, and chemical resistance. | Low cohesive force, easily eroded by soil bacteria and penetrated by plant roots and relatively short service life. | Non-cohesive soil with low humidity |
| Coal tar pitch or enamel | Its performance is better than that of petroleum asphalt, its adhesiveness and mechanical strength with steel pipelines are improved, and it will not be damaged by microorganisms | There are still weaknesses, such as high-temperature softening and low-temperature hardness and brittleness. | It is suitable for swampy, underwater, seabed, saline–alkali soils and other environments, but not for gravel and cohesive soil sections. | ||
| Epoxy powder coating | (fusion-bonded epoxy, FBE) | Epoxy resin can generate a strong chemical bond with steel pipe and hardly generate volatile matter during installation, thus causing no pollution. | Strong adhesion, firm anticorrosive coating, corrosion resistance, solvent resistance, and soil stress resistance. | FEB has a large water absorption rate, weak damp–heat resistance, and limited impact damage resistance. | It is suitable for most soil environments but not for anti-corrosion coating of metal pipelines conveying mediums with too high temperatures. |
| Double-layer powder structure anticorrosive coating | DPS has better impact resistance than FEB | Cost is higher than for FEB | It is often used as an anticorrosive coating for steel elbows. | ||
| Polyolefin-based anticorrosive material | Polyethylene powder | Polyolefin-based anti-corrosion materials include mainly polyethylene and polypropylene. The former has stable performance and a strong ability to isolate corrosive medium, but it is nonpolar and needs a mixture to be bonded to steel pipelines. | A multi-purpose spraying method is used to spray molten polyethylene powder onto heated steel pipes. The equipment is simple and can be sprayed simultaneously. | Polyethylene is poorly bonded to steel and has limited water vapor permeability resistance. | It is mainly used for anti-corrosion spraying of small-caliber steel pipelines in urban public buildings and factories. |
| (2-Layer Polyethylene) | Bottom layer binder, top layer polyethylene | It has excellent mechanical properties, low water vapor permeability, good anti-corrosion performance, resistance to soil stress, and low cost of anti-corrosion materials and coating. | Bonding ability with steel is not strong, especially at high temperatures. The coordination with the cathode protection is poor, and under the action of the protection current, the viscous viscosity may be lost, and the current may be shielded. | Commonly used in low-temperature environments, small-diameter steel pipes | |
| 3PE (3-Layer Polyethylene) | Low-layer epoxy powder, intermediate adhesive layer, outer polyolefin layer | Combining the advantages of FBE and polyolefin, it has excellent anti-corrosion performance and good mechanical-damage resistance. | It is difficult to construct, repair damages on site, and prevent corrosion of pipe fittings. | ||
| polypropylene | The characteristics are similar to polyethylene, but the use temperature is higher. | Similar to polyethylene | Similar to polyethylene | It has not been applied on a large scale, but it has been exported, for example, the three-layer polypropylene anticorrosive coating exported to Sudan. |
| Protection Type | Classification | Material | Advantages | Disadvantages | Applicable Place |
|---|---|---|---|---|---|
| Traditional sacrificial anode–cathode protection type | sacrificial anode | Mg and magnesium alloy, Zn and Zinc alloy, AI and AI alloy, etc. | The self-corrosion is small, and the long-term discharge process rarely polarizes; large discharge per unit weight, uniform output current, good mechanical properties, low price, wide source | High requirements for anticorrosive coating after metal consumption to replace regularly | Not suitable for high-resistance environments |
| Shallow anode bed | Scrap steel, magnetic iron oxide, high silicon cast iron, platinum-plated anode | Convenient construction, easy maintenance and replacement, low cost | High grounding resistance. It has great influence on the surrounding buildings, and it is difficult to evenly distribute the protection current of complex structures | Shallow surface soils with low resistivity and where the protected object is relatively simple | |
| Deep-well anode bed | The anode grounding resistance is small, the current distribution is even, and the interference with other buildings is small | Construction is more complex, maintenance and replacement difficulties, high cost | Areas with low resistivity or high soil resistivity underground; Landmark metal complex with regional cathodic protection | ||
| New type of sacrificial anode protection | Flexible anode based on conductive polymer | Mostly doped conductive polymer materials, such as graphite, are added to polyethylene medium | Close to the anode; can protect objects with complex shapes; high current utilization; low current loss; when used for pipeline protection, the potential distribution is relatively uniform. The cost is low | Current reliability and life are lower than MMO under high currents | It is not suitable for cases with abnormally large discharge densities |
| MMO Flexible anode | Noble metal oxide | Inherits the advantages of high discharge density and long service life of MMO anode | The cost is higher than the flexible anode of conductive polymer | It can be used in almost any situation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, H.; Wu, Y.; Han, B.; Lin, N.; Wang, J.; Zhang, Z.; Guo, Y. Corrosion of Buried Pipelines by Stray Current in Electrified Railways: Mechanism, Influencing Factors, and Protection. Appl. Sci. 2025, 15, 264. https://doi.org/10.3390/app15010264
Liang H, Wu Y, Han B, Lin N, Wang J, Zhang Z, Guo Y. Corrosion of Buried Pipelines by Stray Current in Electrified Railways: Mechanism, Influencing Factors, and Protection. Applied Sciences. 2025; 15(1):264. https://doi.org/10.3390/app15010264
Chicago/Turabian StyleLiang, Haiming, Yuxi Wu, Bin Han, Nan Lin, Junqiang Wang, Zheng Zhang, and Yanbao Guo. 2025. "Corrosion of Buried Pipelines by Stray Current in Electrified Railways: Mechanism, Influencing Factors, and Protection" Applied Sciences 15, no. 1: 264. https://doi.org/10.3390/app15010264
APA StyleLiang, H., Wu, Y., Han, B., Lin, N., Wang, J., Zhang, Z., & Guo, Y. (2025). Corrosion of Buried Pipelines by Stray Current in Electrified Railways: Mechanism, Influencing Factors, and Protection. Applied Sciences, 15(1), 264. https://doi.org/10.3390/app15010264






