Antioxidative and Photoprotective Activity of Pinus nigra, Pinus strobus and Pinus mugo
Abstract
1. Introduction
2. Materials and Methods
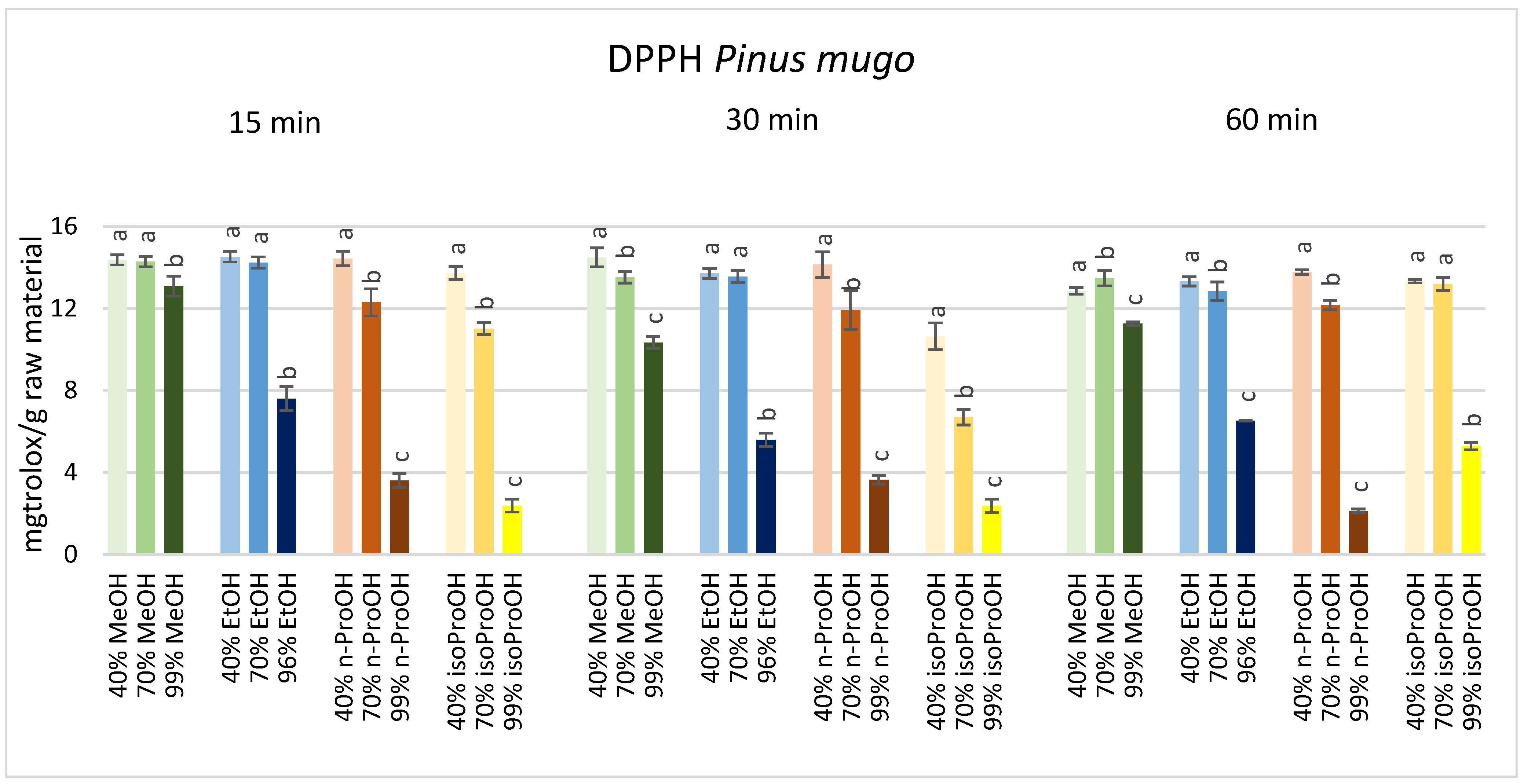
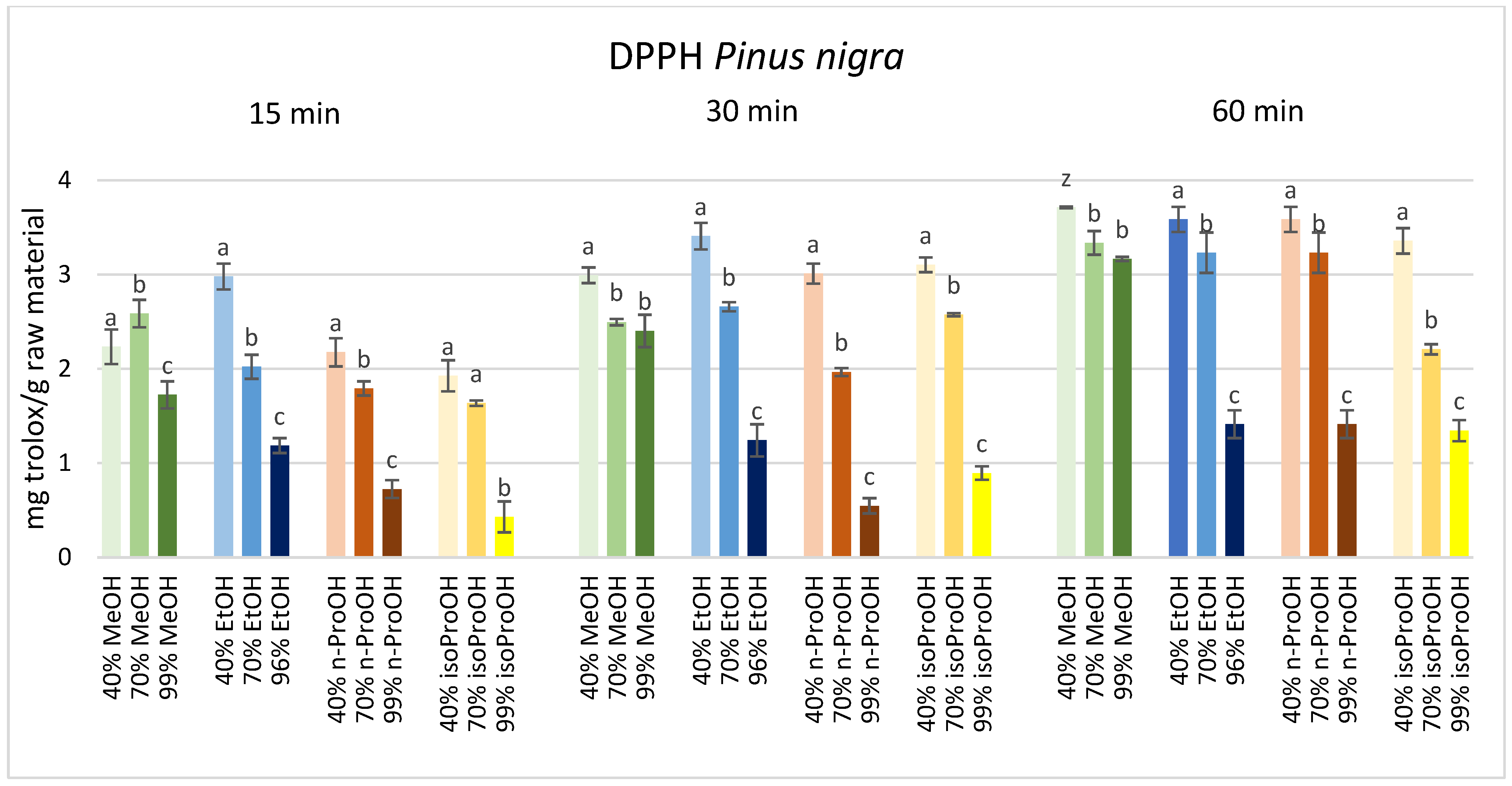
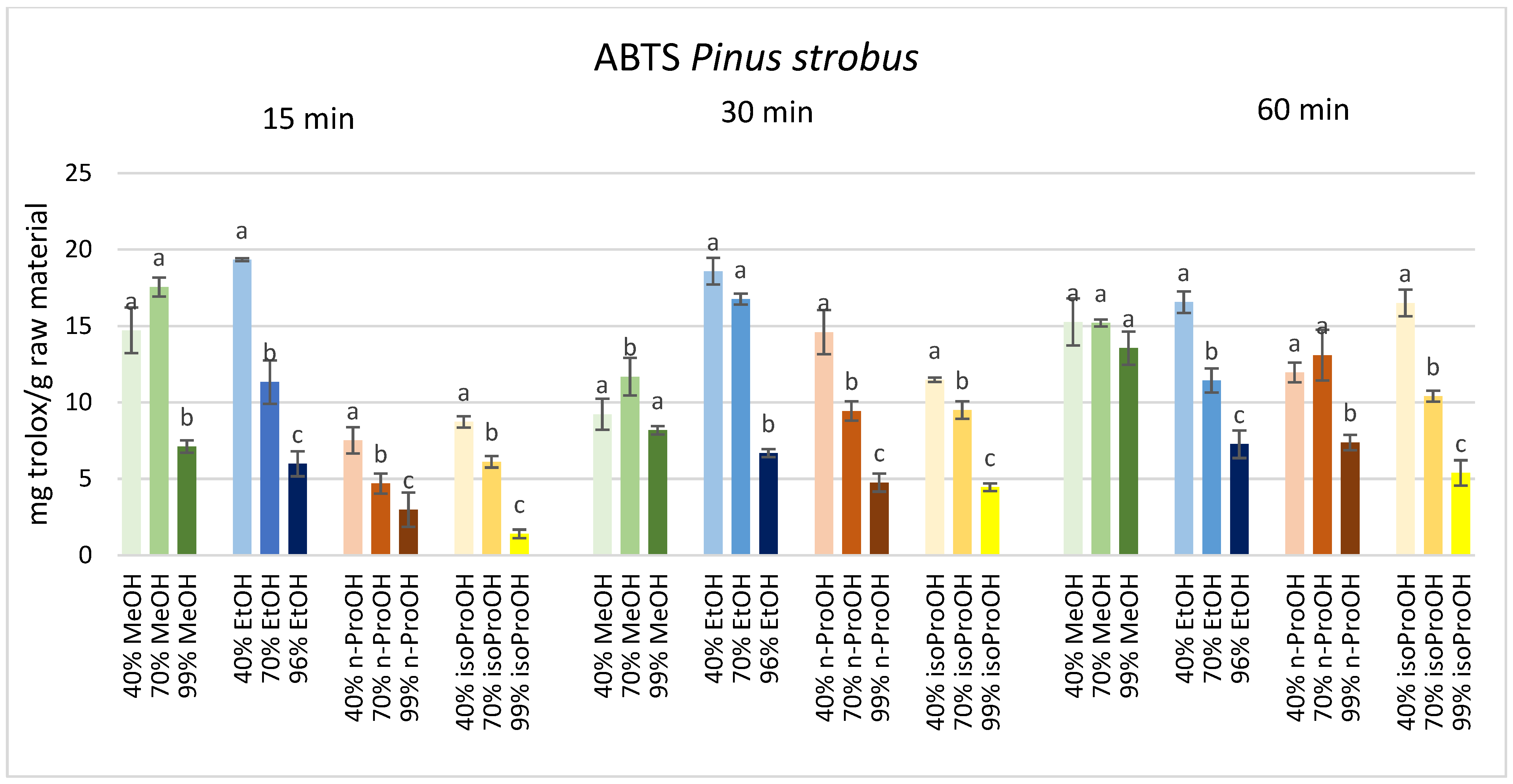
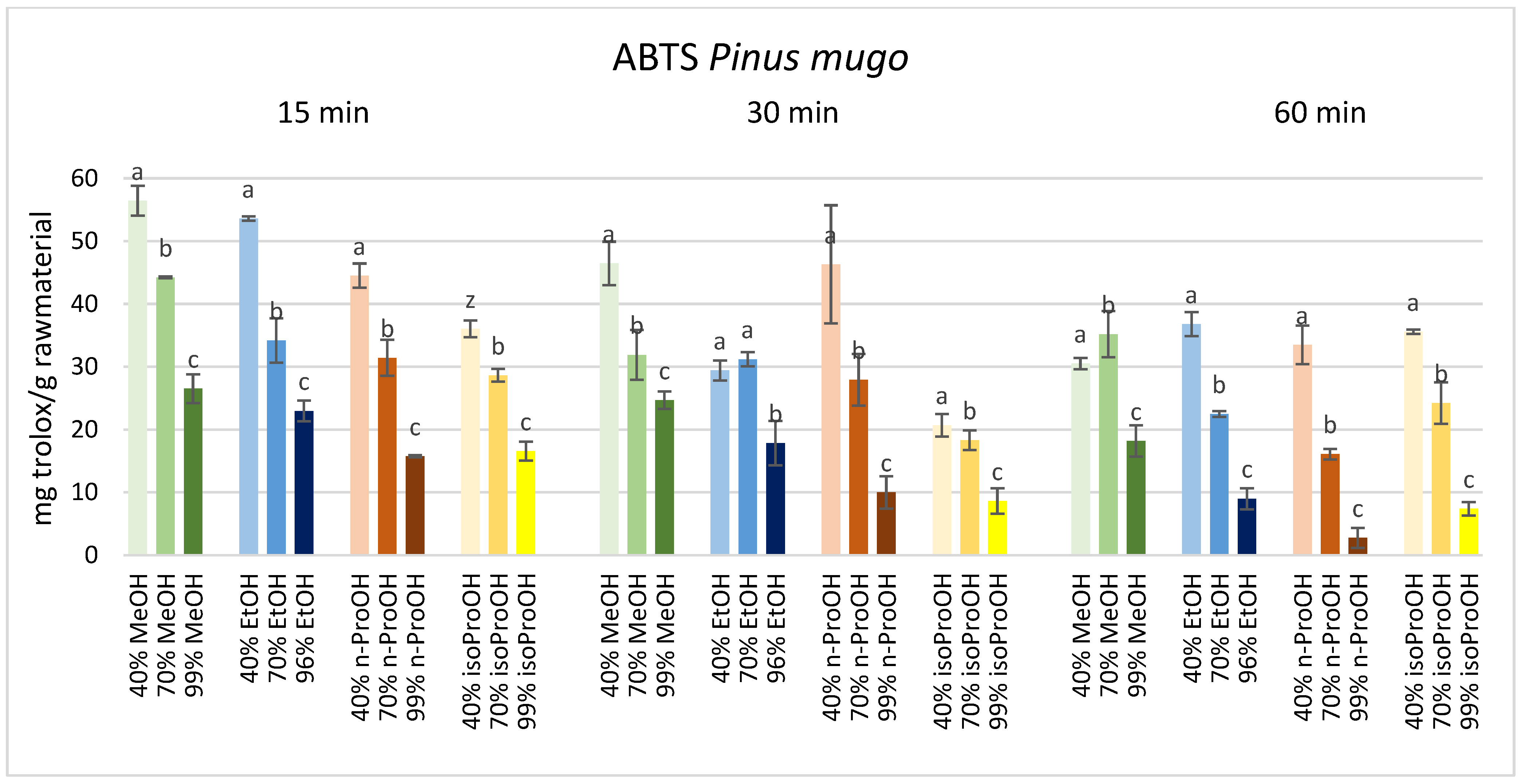
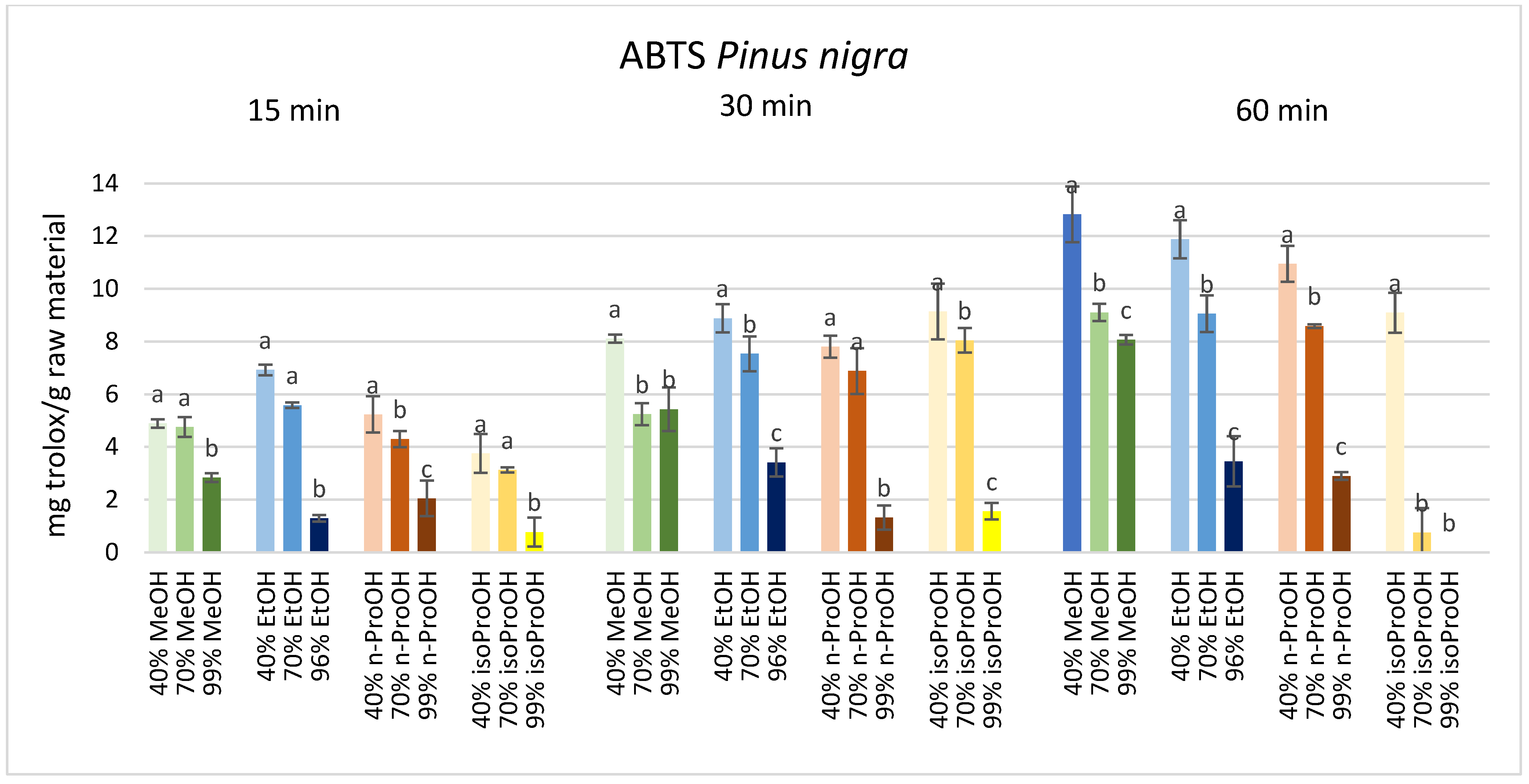
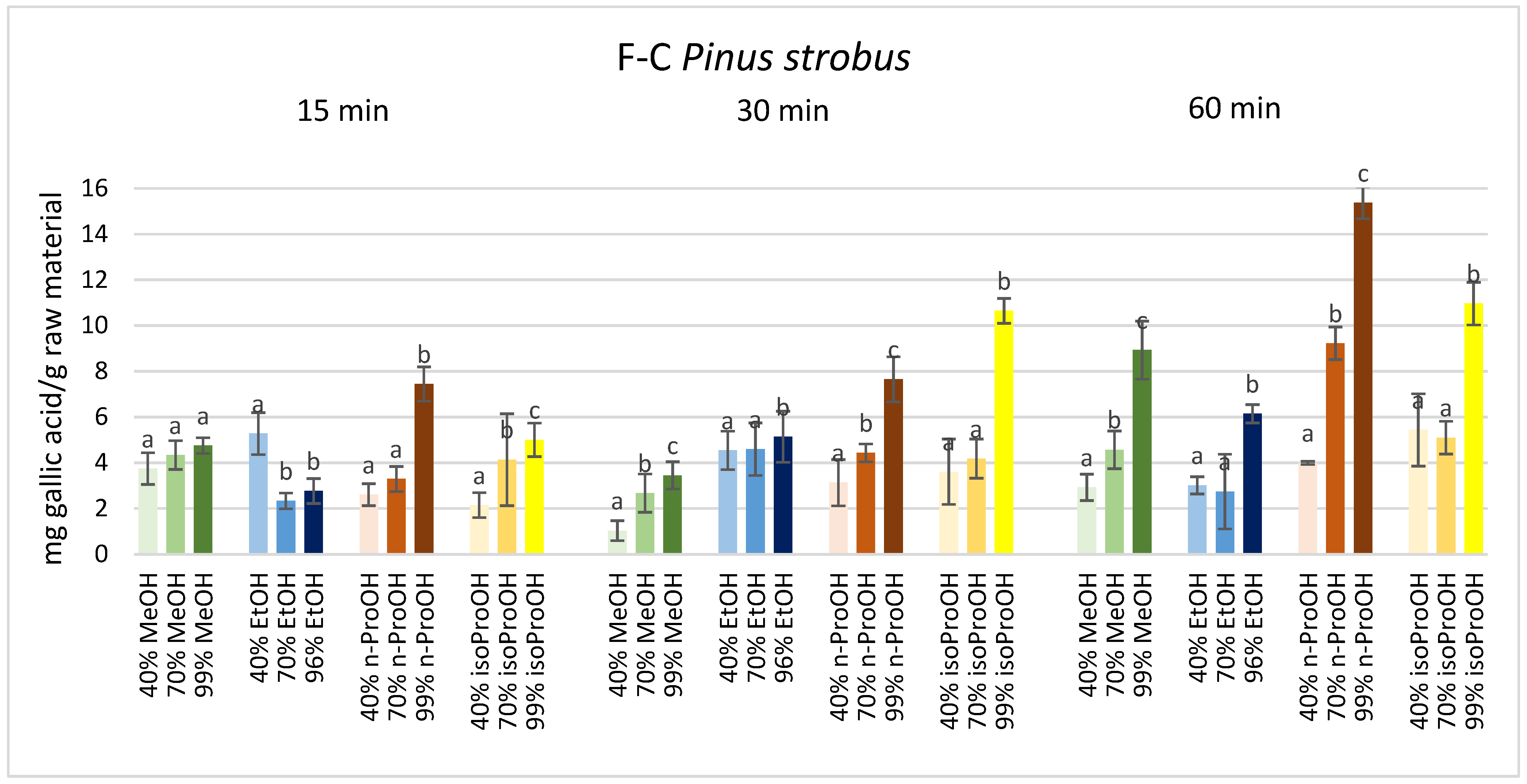
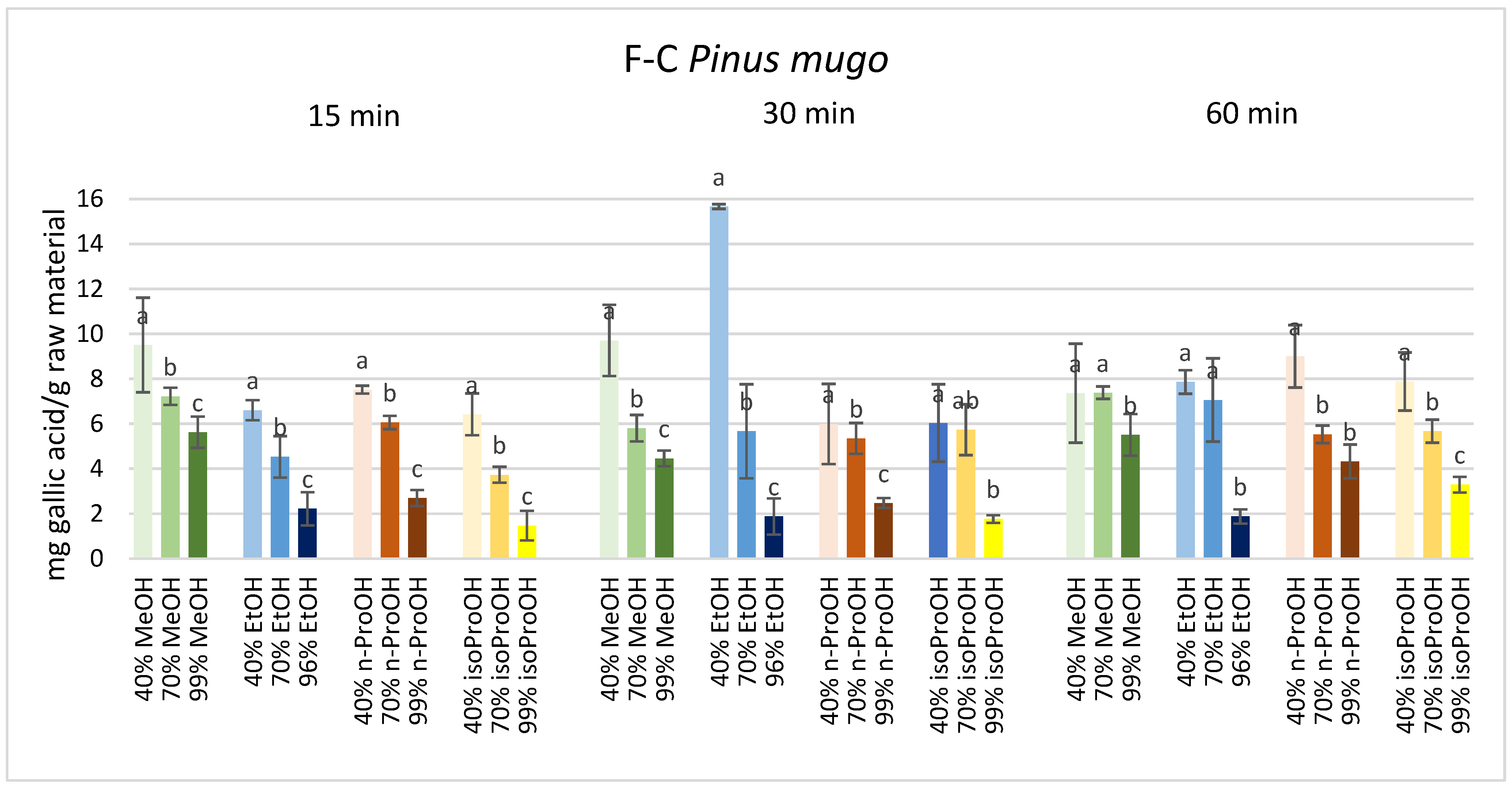
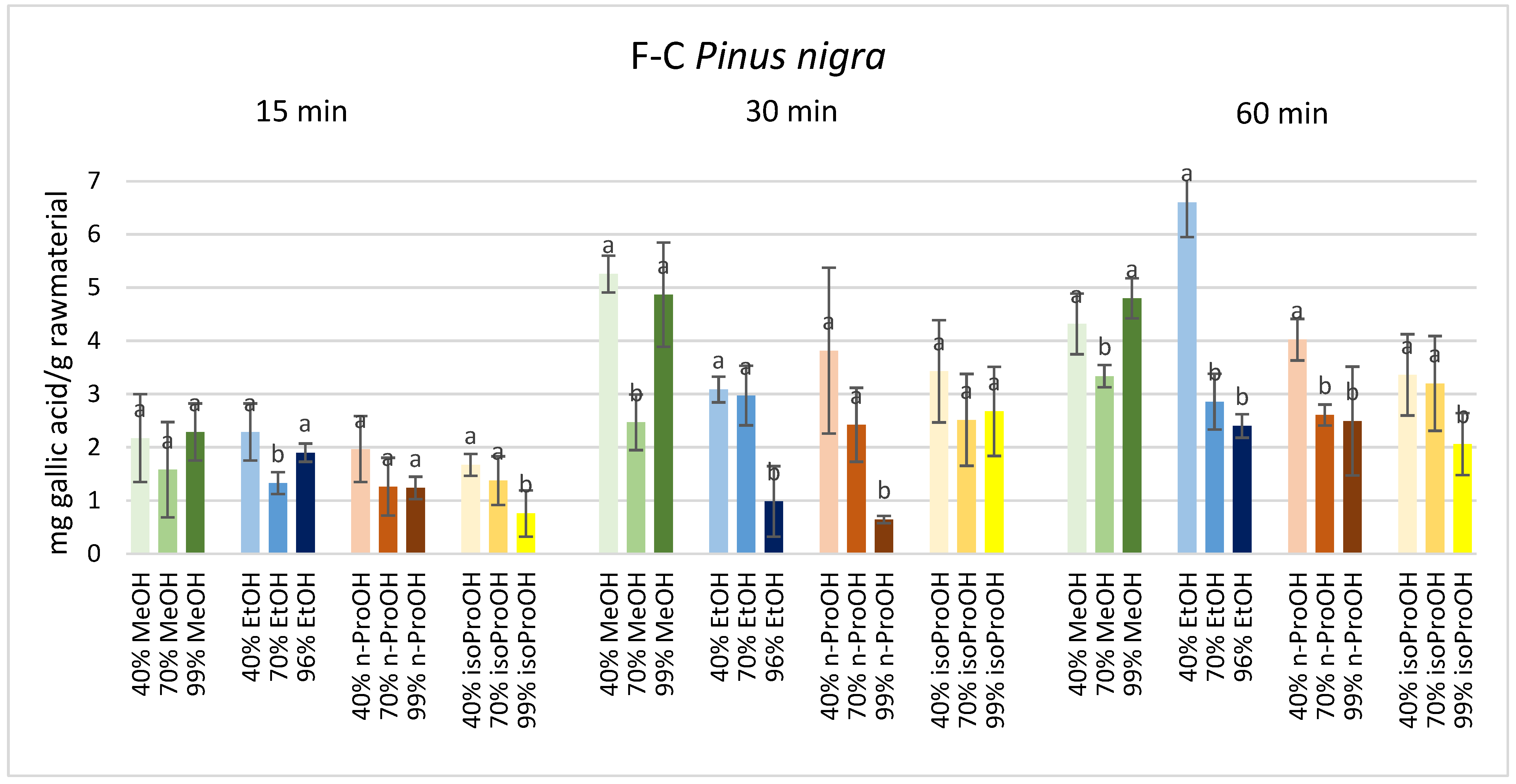
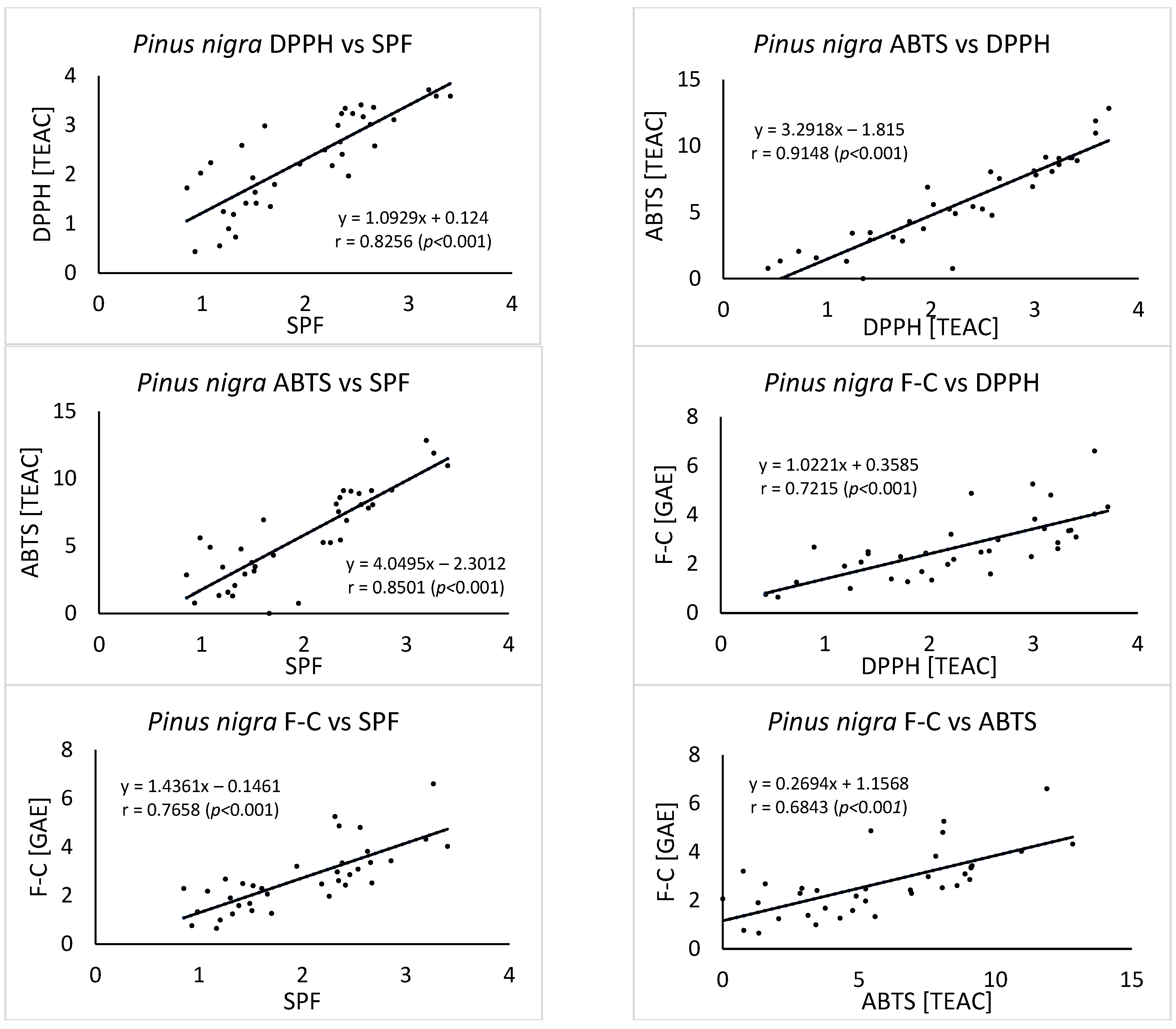
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Characteristics of the aging skin. Adv. Wound Care 2013, 2, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Wąsik, A.; Klimowicz, A. Extracts from Corylus avellana as a source of antioxidants useful in cosmetic preparations. Pomeranian J. Life Sci. 2022, 68, 56–66. [Google Scholar] [CrossRef]
- Moskwa, J.; Bronikowska, M.; Socha, K.; Markiewicz-Żukowska, R. Vegetable as a source of bioactive compounds with photoprotective properties: Implication in the aging process. Nutrients 2023, 15, 3594. [Google Scholar] [CrossRef] [PubMed]
- Jesus, A.; Mota, S.; Torres, A.; Cruz, M.T.; Sousa, E.; Almeida, I.F.; Cidade, H. Antioxidants in sunscreens: Which and what for ? Antioxidants 2023, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, A.; de Beer, J.; James, K.S.; van Wissen, L.; Janssen, F. Comparison of population aging in Europe and Asia using a time-consistent and comparative aging measure. J. Aging Health 2020, 32, 340–351. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Shao, J.; Chen, D.; Xue, E.; Huang, S.; Fu, Y.; Tang, L.; Ye, Z. Healthcare for older adults with multimorbidity: A scoping review of reviews. Clin. Interv. Aging 2023, 2023, 1723–1735. [Google Scholar] [CrossRef]
- Sinikumpu, S.P.; Jokelainen, J.; Keinänen-Kiukaanniemi, S.; Huilaja, L. Skin cancers and their risk factors in older persons: A population-based study. BMC Geriatr. 2022, 22, 269. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.D.; Saraf, S. In vitro sun protection factor determination of herbal oils used in cosmetics. Pharmacogn. Res. 2010, 2, 22–25. [Google Scholar] [CrossRef]
- Ahmed, I.A.; Mikail, M.A.; Zamakshshari, N.; Abdullah, A.S.H. Natural anti-aging skincare: Role and potential. Biogerontology 2020, 21, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant-derived antioxidants: Significance in skin health and the ageing process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Witaszczyk, A.; Klimowicz, A. Usefulness of aloe vera (Aloe vera) as a potential ingredient of cosmetic preparations. Pomeranian J. Life Sci. 2023, 69, 76–87. [Google Scholar] [CrossRef]
- Zafar, F.; Asif, H.M.; Shaheen, G.; Ghauri, A.O.; Rajpoot, S.R.; Tasleem, M.W.; Shamim, T.; Hadi, F.; Noor, R.; Ali, T.; et al. A comprehensive review on medicinal plants possessing antioxidant potential. Clin. Exp. Pharmacol. Physiol. 2023, 50, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, M.; Ścibisz, I.; Przybył, J.; Ziarno, M.; Żbikowska, A.; Majewska, E. Phenolic contents and antioxidant activity of extracts of selected fresh and dried herbal materials. Pol. J. Food Nutrit. Sci. 2021, 71, 269–278. [Google Scholar] [CrossRef]
- Koutsaviti, A.; Toutoungy, S.; Saliba, R.; Loupassaki, S.; Tzakou, O.; Roussis, V.; Ioannou, E. Antioxidant potential of pine needles: A systematic study on the essential oils and extracts of 46 species of the genus Pinus. Foods 2021, 10, 142. [Google Scholar] [CrossRef]
- Ancuceanu, R.; Anghel, A.I.; Hovanet, M.V.; Ciobanu, A.-M.; Lascu, B.E.; Dinu, M. Antioxidant activity of essential oils from Pinaceae species. Antioxidants 2024, 13, 286. [Google Scholar] [CrossRef] [PubMed]
- Costanza, K.K.; Whitney, T.D.; McIntire, C.D.; Livingston, W.H.; Gandhi, K.J. A synthesis of emerging health issues of eastern white pine (Pinus strobus) in eastern North America. Forest Ecol. Manag. 2018, 423, 3–17. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Zanuso, E.; Genisheva, Z.; Rocha, C.M.; Teixeira, J.A. Green and sustainable valorization of bioactive phenolic compounds from Pinus by-products. Molecules 2020, 25, 2931. [Google Scholar] [CrossRef] [PubMed]
- Kaundun, S.S.; Lebreton, P. Taxonomy and systematics of the genus Pinus based on morphological, biogeographical and biochemical characters. Plant Syst. Evol. 2010, 284, 1–15. [Google Scholar] [CrossRef]
- Toma, S.; Bertman, S. The atmospheric potential of biogenic volatile organic compounds from needles of white pine (Pinus strobus) in Northern Michigan. Atmos. Chem. Phys. 2012, 12, 2245–2252. [Google Scholar] [CrossRef]
- Silori, G.K.; Kushwaha, N.; Kumar, V. Essential oils from pines: Chemistry and applications. In Essential oil Research: Trends in Biosynthesis, Analytics, Industrial Applications and Biotechnological Production; Malik, S., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 275–297. [Google Scholar] [CrossRef]
- Fkiri, S.; Mezni, F.; Ouarghi, A.; Ghazghazi, H.; Khouja, M.L.; Khaldi, A.; Nasr, Z. Variability of phenolic compounds and antioxidant efficacy in needles extracts of Pinus nigra Arn. J. New Sci. Agricult. Biotechnol. 2018, 53, 3528–3535. [Google Scholar]
- Isajev, V.; Fady, B.; Semerci, H.; Andonovski, V. EUFORGEN Technical Guidelines for Genetic Conservation and Use for European Black Pine (Pinus nigra); Bioversity International: Rome, Italy, 2004. [Google Scholar]
- Nisca, A.; Stefanescu, R.; Stegdrus, D.I.; Mare, A.D.; Farczadi, L.; Tanase, C. Comparative study regarding the chemical composition and biological activity of pine (Pinus nigra and P. sylvestris) bark extracts. Antioxidants 2021, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Papp, N.; Purger, D.; Czigle, S.; Czégényi, D.; Stranczinger, S.; Tóth, M.; Dénes, T.; Kocsis, M.; Takácsi-Nagy, A.; Filep, R. The Importance of Pine Species in the Ethnomedicine of Transylvania (Romania)]. Plants 2022, 11, 2331. [Google Scholar] [CrossRef] [PubMed]
- Demirtas, İ.; Ozen, T.; Marah, S.; Mutlu, D.; Arslan, Ş.; Gül, F. Functional food components and activities of Pinus nigra and Pinus sylvestris barks as food supplements. Int. J. Chem. Technol. 2023, 7, 229–238. [Google Scholar] [CrossRef]
- Milić, N.; Milanović, M.; Četojević-Simin, D.; Malenčić, Đ.; Prvulović, D.; Pavkov, N.; Radulović, Z.; Milošević, N.; Rašković, A.; Mandić, A. Phytochemical characterization and effects on cell proliferation of Pinus nigra Arn. bark. Arch. Pharm. 2021, 354, 2000416. [Google Scholar] [CrossRef] [PubMed]
- Chropeňová, M.; Gregušková, E.K.; Karásková, P.; Přibylová, P.; Kukučka, P.; Baráková, D.; Čupr, P. Pine needles and pollen grains of Pinus mugo Turra—A biomonitoring tool in high mountain habitats identifying environmental contamination. Ecol. Indic. 2016, 66, 132–142. [Google Scholar] [CrossRef]
- Basholli-Salihu, M.; Schuster, R.; Hajdari, A.; Mulla, D.; Viernstein, H.; Mustafa, B.; Mueller, M. Phytochemical composition, anti-Inflammatory activity and cytotoxic effects of essential oils from three Pinus spp. Pharm. Biol. 2017, 55, 1553–1560. [Google Scholar] [CrossRef]
- Popescu, D.I.; Lengyel, E.; Apostolescu, F.G.; Soare, L.C.; Botoran, O.R.; Sutan, N.A. Volatile compounds and antioxidant and antifungal activity of bud and needle extracts from three populations of Pinus mugo Turra growing in Romania. Horticulturae 2022, 8, 952. [Google Scholar] [CrossRef]
- Rigel, D.S.; Lim, H.W.; Draelos, Z.D.; Weber, T.M.; Taylor, S.C. Photoprotection for all: Current gaps and opportunities. J. Amer. Acad. Dermat. 2022, 86, S18–S26. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Passeron, T.; Gilaberte, Y.; Granger, C.; Leone, G.; Narda, M.; Schalka, S.; Trullas, C.; Masson, P.; Lim, H.W. Photoprotection of the future: Challenges and opportunities. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 447–454. [Google Scholar] [CrossRef]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Ozdemir, M.; Gungor, V.; Melikoglu, M.; Aydiner, C. Solvent selection and effect of extraction conditions on ultrasound-assisted extraction of phenolic compounds from galangal (Alpinia officinarum). J. Appl. Res. Med. Aromat. Plants 2024, 38, 100525. [Google Scholar] [CrossRef]
- Usman, M.; Nakagawa, M.; Cheng, S. Emerging Trends in Green Extraction Techniques for Bioactive Natural Products. Processes 2023, 11, 3444. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and drawbacks of ultrasound-assisted extraction for the recovery of bioactive compounds from marine algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Chiari-Andréo, B.G.; Almeida, F.B.D.; Yamasaki, P.R.; Santos, J.L.D.; Corrêa, M.A.; Chin, C.M.; Isaac, V.L.B. Can natural products improve skin photoprotection? Rodriguésia 2020, 71, e00672019. [Google Scholar] [CrossRef]
- Lewandowska, N.; Klimowicz, A. Antioxidant properties of selected parts of Syringa vulgaris L. Pomeranian J. Life Sci. 2022, 68, 64–74. [Google Scholar] [CrossRef]
- Gajewska, S.; Siemak, J.; Bilska, J.; Nowak, A.; Klimowicz, A. Effect of storage on the antioxidant properties of Plantago lanceolata L. and Plantago major L. alcoholic extracts. Pomeranian J. Life Sci. 2021, 67, 52–56. [Google Scholar] [CrossRef]
- Nowak, A.; Florkowska, K.; Zielonka-Brzezicka, J.; Duchnik, W.; Muzykiewicz, A.; Klimowicz, A. The effects of extraction techniques on the antioxidant potential of extracts of different parts of milk thistle (Silybum marianum L.). Acta Sci. Pol. Technol. Aliment. 2021, 20, 37–46. [Google Scholar] [CrossRef]
- Grzeszczak, J.; Wróblewska, A.; Klimowicz, A.; Gajewska, S.; Kucharski, Ł.; Koren, Z.C.; Janda-Milczarek, K. Antioxidant activities of ethanolic extracts obtained from α-pinene-containing plants and their use in cosmetic emulsions. Antioxidants 2024, 13, 811. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Duchnik, W.; Muzykiewicz-Szymańska, A.; Kucharski, Ł.; Zielonka-Brzezicka, J.; Nowak, A.; Klimowicz, A. The changes of antioxidant activity of three varieties of ‘Nalewka’, a traditional Polish fruit alcoholic beverage during long-term storage. Appl. Sci. 2023, 13, 1114. [Google Scholar] [CrossRef]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The chemistry behind the Folin–Ciocalteu method for the estimation of (poly) phenol content in food: Total phenolic intake in a mediterranean dietary pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef]
- Kucharski, Ł.; Cybulska, K.; Kucharska, E.; Nowak, A.; Pełech, R.; Klimowicz, A. Biologically active preparations from the leaves of wild plant species of the genus Rubus. Molecules 2022, 27, 5486. [Google Scholar] [CrossRef]
- Madanowska, A.; Kowalska-Baron, A. Application trial of a simple spectrophotometric method in determination of sun protection parameters of selected sunscreen cosmetics. Biotechnol. Food Sci. 2023, 85, 55–62. [Google Scholar] [CrossRef]
- Herzog, B.; Mongiat, S.; Deshayes, C.; Neuhaus, M.; Sommer, K.; Mantler, A. In vivo and in vitro assessment of UVA protection by sunscreen formulations containing either butyl methoxy dibenzoyl methane, methylene bis-benzotriazolyl tetramethylbutylphenol, or microfine ZnO. Int. J. Cosmet. Sci. 2002, 24, 170–185. [Google Scholar] [CrossRef]
- Sayre, R.M.; Agin, P.P.; Levee, G.J.; Marlowe, E. Comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Zduńska-Pęciak, K.; Kołodziejczak, A.; Rotszein, H. Two superior antioxidants: Ferulic acid and ascorbic acid in reducing signs of photoaging—A split-face comparative study. Dermatol. Ther. 2022, 35, e15254. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.D.; Seow, L.J.; Sekar, M.; Rani, N.N.I.M.; Lum, P.T. Ten commonly available medicinal plants in Malaysia with potential sun protection factor and antioxidant properties—A review. Pharmacogn. J. 2022, 14, 444–455. [Google Scholar] [CrossRef]
- Mejía-Giraldo, J.C.; Gallardo, C.; Puertas-Mejía, M.A. Selected extracts from high mountain plants as potential sunscreens with antioxidant capacity. Photochem. Photobiol. 2022, 98, 211–219. [Google Scholar] [CrossRef]
- Pniewska, A.; Kalinowska-Lis, U. A Survey of UV Filters Used in Sunscreen Cosmetics. Appl. Sci. 2024, 14, 3302. [Google Scholar] [CrossRef]
- Cefali, L.C.; Franco, J.G.; Nicolini, G.F.; Ataide, J.A.; Mazzola, P.G. In vitro antioxidant activity and solar protection factor of blackberry and raspberry extracts in topical formulation. J. Cosmet. Dermatol. 2019, 18, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhao, J.; Yang, Z.; Xiong, L.; Li, L.; Gu, Z.; Li, Y. Polyphenolic sunscreens for photoprotection. Green Chem. 2022, 24, 3605–3622. [Google Scholar] [CrossRef]
- He, H.; Li, A.; Li, S.; Tang, J.; Li, L.; Xiong, L. Natural components in sunscreens: Topical formulations with sun protection factor (SPF). Biomed. Pharmacother. 2020, 134, 111161. [Google Scholar] [CrossRef]
- Ali, Z.S.; Muhammad, D.; Zrieki, A. In vitro assessment of sun protection factor (SPF) and antioxidant activity of Viola odorata extracts. Res. J. Pharm. Technol. 2022, 15, 655–660. [Google Scholar] [CrossRef]
- Aguilera, J.; Gracia-Cazaña, T.; Gilaberte, Y. New developments in sunscreens. Photochem. Photobiol. Sci. 2023, 22, 2473–2482. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Alves Filho, E.G.; Sousa, V.M.; Rodrigues, S.; de Brito, E.S.; Fernandes, F.A. Green ultrasound-assisted extraction of chlorogenic acids from sweet potato peels and sonochemical hydrolysis of caffeoylquinic acids derivatives. Ultrason. Sonochem. 2020, 63, 104911. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, W.; Xie, Q. Chemical composition, total phenolic content, and antioxidant activity of the essential oils extracted from the needle of ten Pinus Taxa. J. Chem. 2022, 2022, 7440906. [Google Scholar] [CrossRef]
- Kang, Y.H.; Howard, L.R. Phenolic composition and antioxidant activities of different solvent extracts from pine needles in Pinus species. Prev. Nutr. Food Sci. 2010, 15, 36–43. [Google Scholar] [CrossRef][Green Version]
- Karapandzova, M.; Stefkov, G.; Karanfilova, I.C.; Panovska, T.K.; Stanoeva, J.P.; Stefova, M.; Kulevanova, S. Chemical characterization and antioxidant activity of mountain pine (Pinus mugo Turra, Pinaceae) from Republic of Macedonia. Rec. Nat. Prod. 2019, 13, 50–63. [Google Scholar] [CrossRef]



| Solvent | Time | SPF | UVA/UVB | Critical Wavelength | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| 40% methanol | 15 min | 2.408 | 0.153 | 0.380 | 0.010 | 295 |
| 70% methanol | 15 min | 3.006 | 0.029 | 0.409 | 0.004 | 295 |
| 99% methanol | 15 min | 1.273 | 0.111 | 0.441 | 0.008 | 295 |
| 40% ethanol | 15 min | 3.212 | 0.025 | 0.402 | 0.002 | 295 |
| 70% ethanol | 15 min | 1.813 | 0.020 | 0.422 | 0.001 | 295 |
| 96% ethanol | 15 min | 1.323 | 0.022 | 0.488 | 0.003 | 296 |
| 40% n-propanol | 15 min | 1.700 | 0.021 | 0.379 | 0.002 | 295 |
| 70% n-propanol | 15 min | 1.020 | 0.036 | 0.395 | 0.008 | 295 |
| 99% n-propanol | 15 min | 0.794 | 0.193 | 0.425 | 0.010 | 295 |
| 40% isopropanol | 15 min | 1.568 | 0.020 | 0.402 | 0.005 | 295 |
| 70% isopropanol | 15 min | 1.200 | 0.017 | 0.427 | 0.004 | 295 |
| 99% isopropanol | 15 min | 0.466 | 0.006 | 0.489 | 0.009 | 296 |
| 40% methanol | 30 min | 1.133 | 0.027 | 0.349 | 0.001 | 295 |
| 70% methanol | 30 min | 1.620 | 0.017 | 0.382 | 0.006 | 295 |
| 99% methanol | 30 min | 1.644 | 0.039 | 0.448 | 0.011 | 295 |
| 40% ethanol | 30 min | 3.098 | 0.071 | 0.409 | 0.040 | 295 |
| 70% ethanol | 30 min | 2.834 | 0.034 | 0.433 | 0.003 | 295 |
| 96% ethanol | 30 min | 0.849 | 0.024 | 0.480 | 0.008 | 296 |
| 40% n-propanol | 30 min | 2.522 | 0.118 | 0.414 | 0.009 | 295 |
| 70% n-propanol | 30 min | 1.492 | 0.040 | 0.416 | 0.008 | 295 |
| 99% n-propanol | 30 min | 0.624 | 0.009 | 0.445 | 0.006 | 295 |
| 40% isopropanol | 30 min | 1.915 | 0.051 | 0.403 | 0.003 | 295 |
| 70% isopropanol | 30 min | 1.452 | 0.030 | 0.457 | 0.009 | 296 |
| 99% isopropanol | 30 min | 0.609 | 0.064 | 0.461 | 0.027 | 296 |
| 40% methanol | 60 min | 2.138 | 0.049 | 0.382 | 0.002 | 295 |
| 70% methanol | 60 min | 2.646 | 0.159 | 0.414 | 0.006 | 295 |
| 99% methanol | 60 min | 3.299 | 0.027 | 0.485 | 0.003 | 295 |
| 40% ethanol | 60 min | 2.380 | 0.038 | 0.408 | 0.006 | 295 |
| 70% ethanol | 60 min | 1.851 | 0.040 | 0.432 | 0.004 | 295 |
| 96% ethanol | 60 min | 1.093 | 0.095 | 0.452 | 0.011 | 296 |
| 40% n-propanol | 60 min | 2.417 | 0.015 | 0.384 | 0.003 | 295 |
| 70% n-propanol | 60 min | 2.182 | 0.025 | 0.432 | 0.006 | 295 |
| 99% n-propanol | 60 min | 1.183 | 0.043 | 0.463 | 0.005 | 296 |
| 40% isopropanol | 60 min | 2.371 | 0.152 | 0.427 | 0.010 | 295 |
| 70% isopropanol | 60 min | 1.683 | 0.036 | 0.448 | 0.013 | 296 |
| 99% isopropanol | 60 min | 0.974 | 0.047 | 0.500 | 0.023 | 296 |
| Solvent | Time | SPF | UVA/UVB | Critical Wavelength | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| 40% methanol | 15 min | 1.084 | 0.490 | 0.314 | 0.011 | 294 |
| 70% methanol | 15 min | 1.386 | 0.015 | 0.403 | 0.003 | 294 |
| 99% methanol | 15 min | 0.854 | 0.031 | 0.572 | 0.019 | 296 |
| 40% ethanol | 15 min | 1.607 | 0.057 | 0.384 | 0.003 | 295 |
| 70% ethanol | 15 min | 0.987 | 0.121 | 0.464 | 0.026 | 295 |
| 96% ethanol | 15 min | 1.304 | 0.017 | 0.467 | 0.004 | 294 |
| 40% n-propanol | 15 min | 2.260 | 0.063 | 0.341 | 0.004 | 294 |
| 70% n-propanol | 15 min | 1.703 | 0.036 | 0.403 | 0.003 | 294 |
| 99% n-propanol | 15 min | 1.325 | 0.063 | 0.500 | 0.017 | 295 |
| 40% isopropanol | 15 min | 1.492 | 0.071 | 0.278 | 0.006 | 293 |
| 70% isopropanol | 15 min | 1.514 | 0.041 | 0.393 | 0.006 | 294 |
| 99% isopropanol | 15 min | 0.932 | 0.124 | 0.449 | 0.044 | 294 |
| 40% methanol | 30 min | 2.316 | 0.456 | 0.241 | 0.007 | 293 |
| 70% methanol | 30 min | 2.188 | 0.046 | 0.303 | 0.004 | 293 |
| 99% methanol | 30 min | 2.356 | 0.028 | 0.464 | 0.003 | 295 |
| 40% ethanol | 30 min | 2.539 | 0.035 | 0.312 | 0.003 | 294 |
| 70% ethanol | 30 min | 2.338 | 0.116 | 0.369 | 0.007 | 294 |
| 96% ethanol | 30 min | 1.207 | 0.021 | 0.459 | 0.012 | 294 |
| 40% n-propanol | 30 min | 2.630 | 0.194 | 0.375 | 0.016 | 294 |
| 70% n-propanol | 30 min | 2.417 | 0.284 | 0.426 | 0.032 | 294 |
| 99% n-propanol | 30 min | 1.171 | 0.073 | 0.568 | 0.039 | 295 |
| 40% isopropanol | 30 min | 2.857 | 0.332 | 0.340 | 0.021 | 294 |
| 70% isopropanol | 30 min | 2.672 | 0.052 | 0.435 | 0.007 | 295 |
| 99% isopropanol | 30 min | 1.257 | 0.062 | 0.484 | 0.013 | 294 |
| 40% methanol | 60 min | 3.195 | 0.030 | 0.281 | 0.005 | 293 |
| 70% methanol | 60 min | 2.386 | 0.070 | 0.309 | 0.009 | 294 |
| 99% methanol | 60 min | 2.560 | 0.163 | 0.472 | 0.012 | 295 |
| 40% ethanol | 60 min | 3.268 | 0.086 | 0.326 | 0.007 | 294 |
| 70% ethanol | 60 min | 2.459 | 0.090 | 0.372 | 0.009 | 294 |
| 96% ethanol | 60 min | 1.524 | 0.076 | 0.502 | 0.009 | 295 |
| 40% n-propanol | 60 min | 3.403 | 0.071 | 0.393 | 0.005 | 294 |
| 70% n-propanol | 60 min | 2.351 | 0.459 | 0.421 | 0.004 | 294 |
| 99% n-propanol | 60 min | 1.425 | 0.046 | 0.527 | 0.015 | 295 |
| 40% isopropanol | 60 min | 2.660 | 0.111 | 0.353 | 0.003 | 294 |
| 70% isopropanol | 60 min | 1.947 | 0.114 | 0.423 | 0.015 | 294 |
| 99% isopropanol | 60 min | 1.662 | 0.039 | 0.525 | 0.027 | 295 |
| Solvent | Time | SPF | UVA/UVB | Critical Wavelength | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| 40% methanol | 15 min | 6.718 | 0.020 | 0.438 | 0.047 | 295 |
| 70% methanol | 15 min | 3.662 | 0.074 | 0.407 | 0.006 | 295 |
| 99% methanol | 15 min | 2.664 | 0.090 | 0.479 | 0.003 | 295 |
| 40% ethanol | 15 min | 4.663 | 0.271 | 0.438 | 0.047 | 295 |
| 70% ethanol | 15 min | 2.250 | 0.330 | 0.450 | 0.045 | 295 |
| 96% ethanol | 15 min | 0.942 | 0.287 | 0.461 | 0.002 | 295 |
| 40% n-propanol | 15 min | 2.664 | 1.587 | 0.423 | 0.002 | 295 |
| 70% n-propanol | 15 min | 2.605 | 0.126 | 0.429 | 0.003 | 295 |
| 99% n-propanol | 15 min | 1.070 | 0.030 | 0.556 | 0.006 | 296 |
| 40% isopropanol | 15 min | 2.812 | 0.076 | 0.413 | 0.006 | 295 |
| 70% isopropanol | 15 min | 1.775 | 0.187 | 0.413 | 0.021 | 295 |
| 99% isopropanol | 15 min | 0.460 | 0.031 | 0.540 | 0.036 | 296 |
| 40% methanol | 30 min | 4.356 | 0.040 | 0.381 | 0.006 | 295 |
| 70% methanol | 30 min | 2.522 | 0.097 | 0.367 | 0.001 | 295 |
| 99% methanol | 30 min | 1.730 | 0.091 | 0.464 | 0.005 | 295 |
| 40% ethanol | 30 min | 2.605 | 0.075 | 0.385 | 0.005 | 295 |
| 70% ethanol | 30 min | 2.652 | 0.119 | 0.418 | 0.029 | 295 |
| 96% ethanol | 30 min | 0.966 | 0.041 | 0.480 | 0.008 | 296 |
| 40% n-propanol | 30 min | 5.206 | 0.094 | 0.454 | 0.006 | 295 |
| 70% n-propanol | 30 min | 2.408 | 0.038 | 0.445 | 0.003 | 295 |
| 99% n-propanol | 30 min | 0.730 | 0.098 | 0.506 | 0.024 | 296 |
| 40% isopropanol | 30 min | 1.215 | 0.772 | 0.415 | 0.001 | 295 |
| 70% isopropanol | 30 min | 1.039 | 0.084 | 0.404 | 0.011 | 295 |
| 99% isopropanol | 30 min | 0.462 | 0.040 | 0.477 | 0.025 | 296 |
| 40% methanol | 60 min | 3.743 | 0.211 | 0.407 | 0.009 | 295 |
| 70% methanol | 60 min | 4.214 | 0.229 | 0.465 | 0.032 | 295 |
| 99% methanol | 60 min | 2.710 | 0.097 | 0.517 | 0.006 | 296 |
| 40% ethanol | 60 min | 4.431 | 0.035 | 0.423 | 0.001 | 295 |
| 70% ethanol | 60 min | 2.996 | 0.104 | 0.451 | 0.004 | 295 |
| 96% ethanol | 60 min | 1.545 | 0.040 | 0.455 | 0.004 | 295 |
| 40% n-propanol | 60 min | 4.098 | 1.507 | 0.447 | 0.005 | 295 |
| 70% n-propanol | 60 min | 2.895 | 0.059 | 0.449 | 0.002 | 295 |
| 99% n-propanol | 60 min | 0.767 | 0.210 | 0.532 | 0.016 | 296 |
| 40% isopropanol | 60 min | 3.930 | 0.660 | 0.428 | 0.016 | 295 |
| 70% isopropanol | 60 min | 3.096 | 0.079 | 0.446 | 0.003 | 295 |
| 99% isopropanol | 60 min | 1.244 | 0.020 | 0.485 | 0.007 | 296 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oshetkova, D.; Klimowicz, A. Antioxidative and Photoprotective Activity of Pinus nigra, Pinus strobus and Pinus mugo. Appl. Sci. 2025, 15, 209. https://doi.org/10.3390/app15010209
Oshetkova D, Klimowicz A. Antioxidative and Photoprotective Activity of Pinus nigra, Pinus strobus and Pinus mugo. Applied Sciences. 2025; 15(1):209. https://doi.org/10.3390/app15010209
Chicago/Turabian StyleOshetkova, Daria, and Adam Klimowicz. 2025. "Antioxidative and Photoprotective Activity of Pinus nigra, Pinus strobus and Pinus mugo" Applied Sciences 15, no. 1: 209. https://doi.org/10.3390/app15010209
APA StyleOshetkova, D., & Klimowicz, A. (2025). Antioxidative and Photoprotective Activity of Pinus nigra, Pinus strobus and Pinus mugo. Applied Sciences, 15(1), 209. https://doi.org/10.3390/app15010209







