Comparative Analysis of Fatty Acids Concentration in Liver and Muscle Tissues of Rats and Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diet Composition
2.3. Tissue Sample Preparation
2.4. Estimation of Saturated, Monounsaturated and Polyunsaturated Fatty Acid Fractions by GC-MS Approach
2.5. Statistics
3. Results
3.1. Comparative Analysis of Fatty Acid Profiles in Skeletal Muscle: Delineating Species-Specific Differences in Polar and Non-Polar Lipid Fractions between Mice and Rats
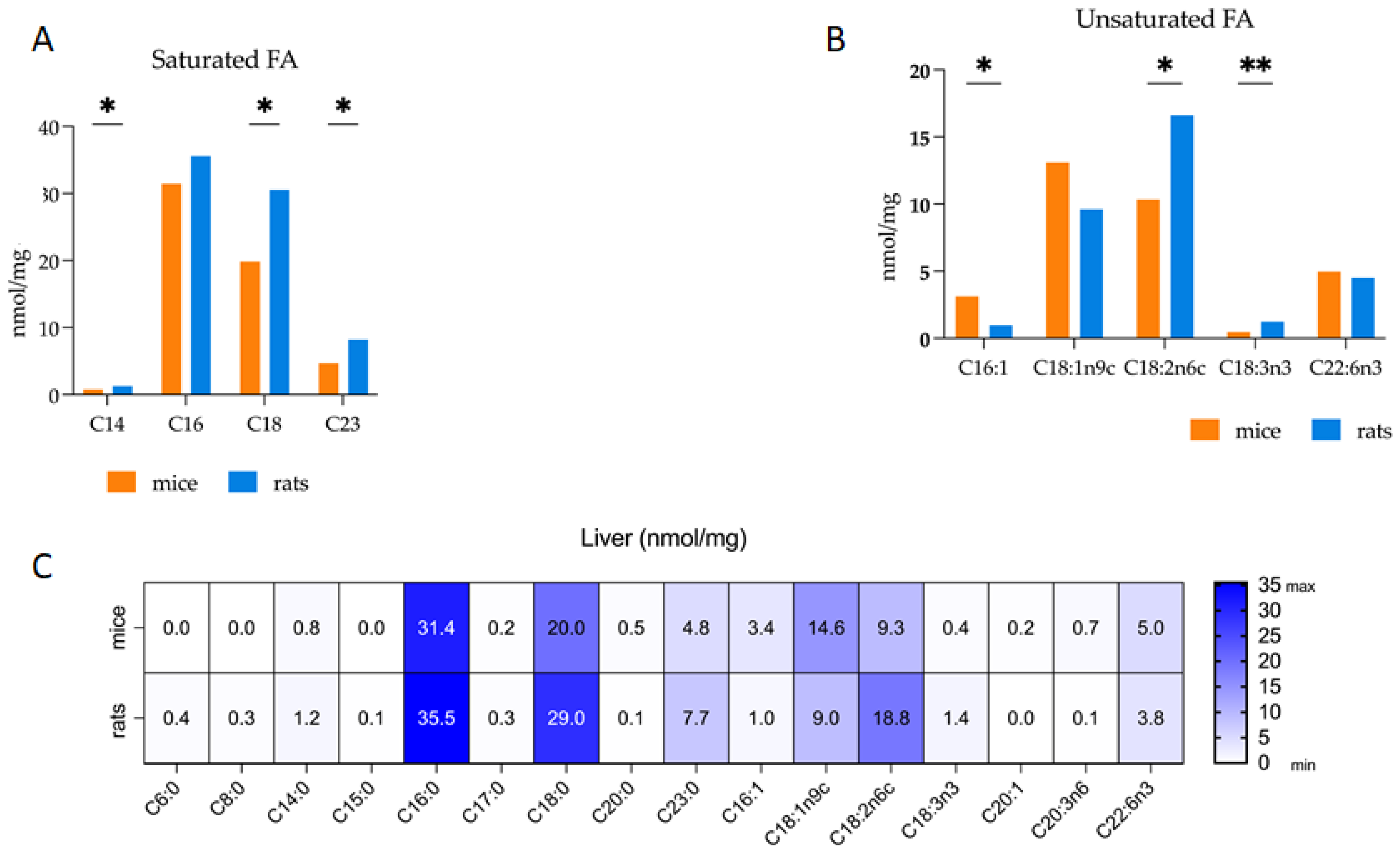
3.2. Comparative Analysis of Fatty Acid Profiles in Liver: Delineating Species-Specific Differences in Polar and Non-Polar Lipid Fractions between Mice and Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagao, K.; Yanagita, T. Bioactive Lipids in Metabolic Syndrome. Prog. Lipid Res. 2008, 47, 127–146. [Google Scholar] [CrossRef]
- Nilsson, S.; Helou, K.; Walentinsson, A.; Szpirer, C.; Nerman, O.; Ståhl, F. Rat-Mouse and Rat-Human Comparative Maps Based on Gene Homology and High-Resolution Zoo-FISH. Genomics 2001, 74, 287–298. [Google Scholar] [CrossRef]
- Whelan, J.; Whelan, J. Conversion of Dietary Polyunsaturated Fats between Humans and Rodents: A Review of Allometric Scaling Models. Prostaglandins Leukot. Essent. Fat. Acids 2020, 158, 102094. [Google Scholar] [CrossRef]
- Hulbert, A.J.; Faulks, S.C.; Buffenstein, R. Oxidation-Resistant Membrane Phospholipids Can Explain Longevity Differences among the Longest-Living Rodents and Similarly-Sized Mice. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1009–1018. [Google Scholar] [CrossRef]
- Xu, L.; Schmitt, M.V.; Ruan, H.; Jiao, Y.; Wang, X.; Wang, Y.; Yang, T.; Lienau, P.; Reichel, A.; Liu, X. Systematic Analysis of the Whole-Body Tissue Distribution and Fatty Acid Compositions of Membrane Lipids in CD1 and NMRI Mice and Wistar Rats. Int. J. Anal. Chem. 2020, 2020, 8819437. [Google Scholar] [CrossRef]
- Krümmel, B.; von Hanstein, A.S.; Plötz, T.; Lenzen, S.; Mehmeti, I. Differential Effects of Saturated and Unsaturated Free Fatty Acids on Ferroptosis in Rat β-Cells. J. Nutr. Biochem. 2022, 106, 109013. [Google Scholar] [CrossRef]
- Avela, H.F.; Sirén, H. Advances in Lipidomics. Clin. Chim. Acta 2020, 510, 123–141. [Google Scholar] [CrossRef]
- Briggs, M.A.; Petersen, K.S.; Kris-Etherton, P.M. Saturated Fatty Acids and Cardiovascular Disease: Replacements for Saturated Fat to Reduce Cardiovascular Risk. Healthcare 2017, 5, 29. [Google Scholar] [CrossRef]
- Lunn, J.; Theobald, H.E. The Health Effects of Dietary Unsaturated Fatty Acids. Nutr. Bull. 2006, 31, 178–224. [Google Scholar] [CrossRef]
- Barelli, H.; Antonny, B. Lipid Unsaturation and Organelle Dynamics. Curr. Opin. Cell Biol. 2016, 41, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, B.; Kapoor, D.; Gautam, S.; Singh, R.; Bhardwaj, S. Dietary Polyunsaturated Fatty Acids (PUFAs): Uses and Potential Health Benefits. Curr. Nutr. Rep. 2021, 10, 232–242. [Google Scholar] [CrossRef]
- Surma, M.A.; Gerl, M.J.; Klose, C.; Simons, K. Mouse Lipidomics Reveals Inherent Flexibility of a Mammalian Lipidome. Sci. Rep. 2021, 11, 19364. [Google Scholar] [CrossRef]
- Pradas, I.; Huynh, K.; Cabré, R.; Ayala, V.; Meikle, P.J.; Jové, M.; Pamplona, R. Lipidomics Reveals a Tissue-Specific Fingerprint. Front. Physiol. 2018, 9, 387781. [Google Scholar] [CrossRef]
- Sul, H.S.; Wang, D. Nutritional And Hormonal Regulation Of Enzymes In Fat Synthesis: Studies of Fatty Acid Synthase and Mitochondrial Glycerol-3-Phosphate Acyltransferase Gene Transcription. Annu. Rev. Nutr. 1998, 18, 331–351. [Google Scholar] [CrossRef]
- Postic, C.; Dentin, R.; Girard, J. Role of the Liver in the Control of Carbohydrate and Lipid Homeostasis. Diabetes Metab. 2004, 30, 398–408. [Google Scholar] [CrossRef]
- Guo, X.; Xu, K.; Zhang, J.; Li, H.; Zhang, W.; Wang, H.; Lange, A.J.; Chen, Y.E.; Huo, Y.; Wu, C. Involvement of Inducible 6-Phosphofructo-2-Kinase in the Anti-Diabetic Effect of Peroxisome Proliferator-Activated Receptor Gamma Activation in Mice. J. Biol. Chem. 2010, 285, 23711–23720. [Google Scholar] [CrossRef]
- Christie, W. Isolation, Separation, Identification and Structural Analysis of Lipids. In Lipid Analysis, 3rd ed.; Oily Press: Mondovi, WI, USA, 2003. [Google Scholar]
- Christie, W. High-Performance Liquid Chromatography & Lipids: A Practical Guide; Pergamon: Oxford, UK, 1987; pp. 183–208. [Google Scholar]
- Ötles, S.; Ozgoz, S. Health Effects of Dietary Fiber. Acta Sci. Pol. Technol. Aliment. 2014, 13, 191–202. [Google Scholar] [CrossRef]
- Oshida, K.; Shimizu, T.; Takase, M.; Tamura, Y.; Shimizu, T.; Yamashiro, Y. Effects of Dietary Sphingomyelin on Central Nervous System Myelination in Developing Rats. Pediatr. Res. 2003, 53, 589–593. [Google Scholar] [CrossRef]
- Barbosa, A.D.; Siniossoglou, S. Function of Lipid Droplet-Organelle Interactions in Lipid Homeostasis. Biochim. Biophys. Acta-Mol. Cell Res. 2017, 1864, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Albert, B.B.; Derraik, J.G.B.; Brennan, C.M.; Biggs, J.B.; Smith, G.C.; Garg, M.L.; Cameron-Smith, D.; Hofman, P.L.; Cutfield, W.S. Higher Omega-3 Index Is Associated with Increased Insulin Sensitivity and More Favourable Metabolic Profile in Middle-Aged Overweight Men. Sci. Rep. 2014, 4, 6697. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.E. Dietary Sources of Omega-3 Fatty Acids Versus Omega-3 Fatty Acid Supplementation Effects on Cognition and Inflammation. Curr. Nutr. Rep. 2020, 9, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Budd, J.M.; Hucik, B.; Wang, C.; King, A.N.; Sarr, O.; Nakamura, M.T.; Harasim-Symbor, E.; Chabowski, A.; Dyck, D.J.; Mutch, D.M. A Reduction of Skeletal Muscle DHA Content Does Not Result in Impaired Whole Body Glucose Tolerance or Skeletal Muscle Basal Insulin Signaling in Otherwise Healthy Mice. Am. J. Physiol. Endocrinol. Metab. 2023, 324, E241–E250. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated Fatty Acids and Risk of Cardiovascular Disease: Synopsis of the Evidence Available from Systematic Reviews and Meta-Analyses. Nutrients 2012, 4, 1989–2007. [Google Scholar] [CrossRef] [PubMed]
- Piccinin, E.; Cariello, M.; De Santis, S.; Ducheix, S.; Sabbà, C.; Ntambi, J.M.; Moschetta, A. Role of Oleic Acid in the Gut-Liver Axis: From Diet to the Regulation of Its Synthesis via Stearoyl-CoA Desaturase 1 (SCD1). Nutrients 2019, 11, 2283. [Google Scholar] [CrossRef]
- Hidalgo, M.A.; Carretta, M.D.; Burgos, R.A. Long Chain Fatty Acids as Modulators of Immune Cells Function: Contribution of FFA1 and FFA4 Receptors. Front. Physiol. 2021, 12, 668330. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, B.; Pal, P.K.; Chattopadhyay, A.; Bandyopadhyay, D. Oleic Acid Protects against Cadmium Induced Cardiac and Hepatic Tissue Injury in Male Wistar Rats: A Mechanistic Study. Life Sci. 2020, 244, 117324. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. “Cell Membrane Theory of Senescence” and the Role of Bioactive Lipids in Aging, and Aging Associated Diseases and Their Therapeutic Implications. Biomolecules 2021, 11, 241. [Google Scholar] [CrossRef]
- Eitel, K.; Staiger, H.; Brendel, M.D.; Brandhorst, D.; Bretzel, R.G.; Häring, H.U.; Kellerer, M. Different Role of Saturated and Unsaturated Fatty Acids in β-Cell Apoptosis. Biochem. Biophys. Res. Commun. 2002, 299, 853–856. [Google Scholar] [CrossRef]
- Welters, H.J.; Diakogiannaki, E.; Mordue, J.M.; Tadayyon, M.; Smith, S.A.; Morgan, N.G. Differential Protective Effects of Palmitoleic Acid and CAMP on Caspase Activation and Cell Viability in Pancreatic β-Cells Exposed to Palmitate. Apoptosis 2006, 11, 1231–1238. [Google Scholar] [CrossRef]
- Lupi, R.; Dotta, F.; Marselli, L.; Del Guerra, S.; Masini, M.; Santangelo, C.; Patané, G.; Boggi, U.; Piro, S.; Anello, M.; et al. Prolonged Exposure to Free Fatty Acids Has Cytostatic and Pro-Apoptotic Effects on Human Pancreatic Islets: Evidence That β-Cell Death Is Caspase Mediated, Partially Dependent on Ceramide Pathway, and Bcl-2 Regulated. Diabetes 2002, 51, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid Peroxidation in Cell Death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and Function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Pope, L.E.; Dixon, S.J. Regulation of Ferroptosis by Lipid Metabolism. Trends Cell Biol. 2023, 33, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Olzmann, J.A. The Cell Biology of Ferroptosis. Nat. Rev. Mol. Cell Biol. 2024. [Google Scholar] [CrossRef]
- Bergen, W.G.; Mersmann, H.J. Comparative Aspects of Lipid Metabolism: Impact on Contemporary Research and Use of Animal Models. J. Nutr. 2005, 135, 2499–2502. [Google Scholar] [CrossRef]
- Aviram, R.; Manella, G.; Kopelman, N.; Neufeld-Cohen, A.; Zwighaft, Z.; Elimelech, M.; Adamovich, Y.; Golik, M.; Wang, C.; Han, X.; et al. Lipidomics Analyses Reveal Temporal and Spatial Lipid Organization and Uncover Daily Oscillations in Intracellular Organelles. Mol. Cell 2016, 62, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Hardy, S.; Langelier, Y.; Prentki, M. Oleate Activates Phosphatidylinositol 3-Kinase and Promotes Proliferation and Reduces Apoptosis of MDA-MB-231 Breast Cancer Cells, Whereas Palmitate Has Opposite Effects. Cancer Res. 2000, 60, 6353–6358. [Google Scholar]
- Listenberger, L.L.; Ory, D.S.; Schaffer, J.E. Palmitate-Induced Apoptosis Can Occur through a Ceramide-Independent Pathway. J. Biol. Chem. 2001, 276, 14890–14895. [Google Scholar] [CrossRef]
- De Vries, J.E.; Vork, M.M.; Roemen, T.H.M.; De Jong, Y.F.; Cleutjens, J.P.M.; Van Der Vusse, G.J.; Van Bilsen, M. Saturated but Not Mono-Unsaturated Fatty Acids Induce Apoptotic Cell Death in Neonatal Rat Ventricular Myocytes. J. Lipid Res. 1997, 38, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Maedler, K.; Spinas, G.A.; Dyntar, D.; Moritz, W.; Kaiser, N.; Donath, M.Y. Distinct effects of saturated and monounsaturated Fatty Acids on β-Cell Turnover and Function. Diabetes 2021, 50, 69–76. [Google Scholar] [CrossRef] [PubMed]



| Macronutrients | Dose | Minerals | Dose | Vitamins | Dose | Amino Acids | Dose |
|---|---|---|---|---|---|---|---|
| Raw protein | 175.0 g | Calcium | 9.5 g | Vitamin A | 12,000.0 IU | Lysine | 9.0 g |
| Raw fat | 2.8 g | Total phosphorus | 6.5 g | Vitamin D3 | 800.0 IU | Methionine + Cyst. | 6.3 g |
| Crude fiber | 70.0 g | Magnesium | 3.0 g | Vitamin E | 78.0 mg | Tryptophan | 2.0 g |
| Starch | 330.0 g | Potassium | 7.5 g | Vitamin K3 | 2.4 mg | Threonine | 6.0 g |
| Ash | 57.0 g | Sodium | 1.9 g | Vitamin B1 | 8.0 mg | Isoleucine | 6.0 g |
| Sulfur | 1.9 g | Vitamin B2 | 7.0 mg | Leucine | 12.0 g | ||
| Iron | 144.0 mg | Vitamin B6 | 11.0 mg | Valine | 8.0 g | ||
| Manganese | 50.0 mg | Vitamin B12 | 42.0 mcg | Histidine | 4.0 g | ||
| Zinc | 50.0 mg | Pantothenic acid | 25.0 mg | Arginine | 10.0 g | ||
| Copper | 11.0 mg | Folic acid | 2.0 mg | Phenylalanine | 7.0 g | ||
| Iodine | 0.2 mg | Biotin | 0.3 mg | Tyrosine | 5.0 g | ||
| Selenium | 0.4 mg | Nicotinic acid | 94.0 mg | Betaine | 17.0 g | ||
| Choline | 1900.0 mg |
| Muscle FA | Mice | Rat | ||
|---|---|---|---|---|
| nmol/mg | Q25–Q75 | nmol/mg | Q25–Q75 | |
| TFA | 83.5 * | 9.9–31.9 | 51.7 * | 2.6–7.7 |
| PL | 12.5 * | 0.8–1.8 | 10.7 * | 2.1–0.3 |
| NPL | 67.8 | 7.1–33.3 | 41.0 | 2.8–7.0 |
| SFA | 46.2 | 6.6–12.3 | 40.9 | 2.8–4.4 |
| UFA | 38.5 * | 6.7–7.7 | 11.0 * | 0.3–0.2 |
| MUFA | 22.3 * | 2.6–7.8 | 4.1 * | 0.4–0.4 |
| PUFA | 15.3 * | 3.2–2.4 | 6.9 * | 0.5–0.5 |
| NL/PL | 5.1 | 1.5–2.0 | 4.0 | 0.3–0.3 |
| UFA/TFA | 0.46 * | 0.03–0.02 | 0.21 * | 0.02–0.02 |
| PUFA/UFA | 0.39 | 0.04–0.05 | 0.61 | 0.03–0.04 |
| Liver FA | Mice | Rat | ||
|---|---|---|---|---|
| nmol/mg | Q25–Q75 | nmol/mg | Q25–Q75 | |
| TFA | 90.0 | 10.5–20.5 | 108.7 | 20.1–28.6 |
| PL | 33.1 | 4.3–5.0 | 32.4 | 8.4–2.1 |
| NPL | 55.5 | 3.9–10.5 | 80.6 | 30.0–22.2 |
| SFA | 56.4 | 2.1–9.1 | 74.6 | 7.1–16.5 |
| UFA | 33.5 | 8.4–11.4 | 34.2 | 13.0–12.1 |
| MUFA | 15.8 | 1.6–6.5 | 10.6 | 5.3–2.8 |
| PUFA | 16.5 | 5.6–1.3 | 22.9 | 7.1–11. 5 |
| NL/PL | 1.45 | 0.10–1.29 | 3.16 | 0.74–0.29 |
| UFA/TFA | 0.37 | 0.06–0.03 | 0.31 | 0.07–0.04 |
| PUFA/UFA | 0.51 * | 0.07–0.05 | 0.72 * | 0.06–0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kłosok, M.; Gendosz de Carrillo, D.; Łaszczyca, P.; Płociniczak, T.; Jędrzejowska-Szypułka, H.; Sawczyn, T. Comparative Analysis of Fatty Acids Concentration in Liver and Muscle Tissues of Rats and Mice. Appl. Sci. 2024, 14, 3192. https://doi.org/10.3390/app14083192
Kłosok M, Gendosz de Carrillo D, Łaszczyca P, Płociniczak T, Jędrzejowska-Szypułka H, Sawczyn T. Comparative Analysis of Fatty Acids Concentration in Liver and Muscle Tissues of Rats and Mice. Applied Sciences. 2024; 14(8):3192. https://doi.org/10.3390/app14083192
Chicago/Turabian StyleKłosok, Marcin, Daria Gendosz de Carrillo, Piotr Łaszczyca, Tomasz Płociniczak, Halina Jędrzejowska-Szypułka, and Tomasz Sawczyn. 2024. "Comparative Analysis of Fatty Acids Concentration in Liver and Muscle Tissues of Rats and Mice" Applied Sciences 14, no. 8: 3192. https://doi.org/10.3390/app14083192
APA StyleKłosok, M., Gendosz de Carrillo, D., Łaszczyca, P., Płociniczak, T., Jędrzejowska-Szypułka, H., & Sawczyn, T. (2024). Comparative Analysis of Fatty Acids Concentration in Liver and Muscle Tissues of Rats and Mice. Applied Sciences, 14(8), 3192. https://doi.org/10.3390/app14083192






