Abstract
Background: Knowledge on the effect of heat on recovery is still incomplete. The present study aimed to evaluate the effect of a passive acute hyperthermic stimulus before and after a lactic anaerobic test on the production and oxidation of lactate blood concentrations. In addition, the purpose was to evaluate the effect that the application of this previous hyperthermic stimulus may have on the athletic performance in the test. Methods: For this purpose, a cross-over design through an anaerobic treadmill test in three different situations (normothermia, pre-test hyperthermia, and post-test hyperthermia) was performed. Twelve male subjects participated (age: 21.25 ± 1.64 years; height: 1.76 m ± 0.08; weight: 72.59 ± 9.44 kg). An anthropometric assessment was carried out with weight, height, skinfolds, body perimeters and diameters, and external and internal body temperatures in each of the tests. A nutritional survey was also carried out 48 h prior to each test. Results: The results of the study showed a decrease in blood lactate concentrations when the hyperthermic effect was applied as passive recovery just after the end of the test (p < 0.05). A decrease in lactate concentrations was also achieved when applying the hyperthermic effect just before the start of the test (p < 0.05). However, no significant improvements were obtained from this application of heat on test performance. Conclusions: The results suggest that the application of passive acute hyperthermia has a favourable effect in terms of decreasing blood lactate concentrations in a 5 min recovery period after lactic anaerobic activity.
1. Introduction
Exercising in extreme heat causes a high strain on thermoregulatory mechanisms, increasing the stress induced by physical exertion [1]. Heat can influence energy metabolism due to dehydration and further exacerbation of energy metabolism that occurs during exercise, leading to increased glycogen use [2], which can affect blood lactate concentrations [3].
Physiological responses to exercise above the anaerobic threshold result in the development of metabolic acidosis and reduced exercise endurance [4]. In high-intensity activities, oxygen demand exceeds oxygen supply, so muscles rely on anaerobic glycolysis for ATP production. In anaerobic glycolysis, glucose is broken down within the cell cytoplasm to form pyruvate [5]. Lactate is produced from pyruvate and metabolised by nicotinamide adenine dinucleotide (NAD)-dependent lactate dehydrogenase (LDH) to pyruvate, which is subsequently oxidised in the mitochondria to carbon dioxide and water [6]. Intense muscular exercise increases the use of glycogen through anaerobic glycolysis, resulting in increased lactate production by the active muscle, which in turn leads to lactate accumulation in the muscle and in the blood [7]. Elevated lactate concentration is associated with increased hydrogen ion (H+) concentrations in muscle and blood. Karlsson et al. [7] and MacDougall et al. [8] suggest that increased acidity is directly associated with local muscle fatigue by possibly affecting the contractile mechanism. Blood lactate levels reflect the balance between production, uptake of lactate by tissues, and its elimination. All organs of the body are capable of producing lactate, with muscles and red blood cells being the main culprits [9]. Normally, the ratio of lactate to pyruvate is 10:1; the [NADH]/[NAD+] ratio determines the balance. However, the removal of lactate from muscle and blood and the return of H+ concentrations to resting values is essential for enhanced recovery and successful resumption of subsequent intense exercise. During recovery from intense exercise, blood lactate concentration is reduced mainly through removal and utilisation by the heart, skeletal muscles, and liver [10].
In hot conditions, glycogen metabolism increases. This fact could lead to the assumption that the rise in glycogen metabolism would trigger an increase in lactate production. Nonetheless, several studies have reported no differences in blood lactate concentrations between normothermic and hyperthermic conditions [11]. Similarly, Smolander et al. [12] reported that blood lactate concentrations during and immediately after incremental exercise are similar in both warm and normothermic conditions. Oyono-Enguelle et al. [13] demonstrated that exposure to high temperatures does not adversely affect the rate of lactate clearance during rest. Nonetheless, Falk et al. [14] suggests that heat exposure could improve anaerobic performance. Hyperthermic stimulus could mitigate the lactate production or induce favourable mechanisms to lactate clearance in the organism. Nonetheless, there is scarce literature that can clarify this topic, and it is necessary to understand the effect of heat upon lactate production.
Considering the increase in cardiac output in high temperatures [10], in this study, it is expected that the use of a hyperthermic stimulus during recovery from anaerobic work could lead to a decrease in blood lactate concentrations. Furthermore, previous investigations reported changes after hyperthermic stimulus [14,15]. Therefore, the aim of this study was to evaluate the effect of the application of a passive acute hyperthermic stimulus before and after an anaerobic test on the production and elimination of blood lactate concentrations. In addition, it is proposed to evaluate the effect of the application of this pre-exercise hyperthermic stimulus on performance in the test.
2. Materials and Methods
2.1. Participants
Twelve males participated voluntarily in this investigation. All of them were students from the University of Extremadura (age: 21.25 ± 1.64 years; height: 1.76 m ± 0.08 m; weight: 72.59 ± 9.44 kg). This research was approved by the Biomedical Ethics Committee of the University of Extremadura following the guidelines of the Helsinki ethical declaration (registration number: 196//2022). The participants performed an average of 5.02 ± 4.29 h per week of moderate physical activity. G*Power www.gpower.hhu.de (accessed on 11 January 2024) was used to perform the sample calculation, with a power level of 95% and an α_level of 0.05. It was revealed that a sample size of >12 would be sufficient for the analysis.
For inclusion in the study, participants had to fulfil the following criteria: they had to be male, not modify their lifestyle during the study period, not suffer from any concurrent disease that could affect physical performance, and not take any medication or supplementation. In addition, they were instructed not to take any medication or supplementation for at least 1 month before the experimental period.
Prior to the experimental phase, all participants were contacted to explain the objectives of the study and what their collaboration would consist of, and they all accepted their voluntary participation by signing an informed consent form, in accordance with the ethical guidelines of the World Medical Association’s Declaration of Helsinki (updated at the World Medical Assembly in Fortaleza, 2013) for research involving human subjects, guaranteeing the confidentiality of the data.
2.2. Experimental Design
A randomised cross-over design was carried out. The participants were randomised into three groups. They performed the same physical test at ambient temperature (22 °C ± 2 °C; 20% RH) with different conditions: without sauna before or after the test (normothermia), with 10 min of sauna before the trial (hyperthermic pre), and with 5 min recovery in a sauna after the test (hyperthermic post). Blood lactate and haematocrit were collected in a basal condition, immediately after the trial and after 5 min of recovery. Additionally, in the hyperthermic pre condition, another sample of both haematocrit and blood lactate after the sauna bath was collected. Each trial was separated by one week to avoid fatigue. The consort flow of the study design is shown below. Furthermore, Figure 1 and Figure 2 shows the outline of the study design.
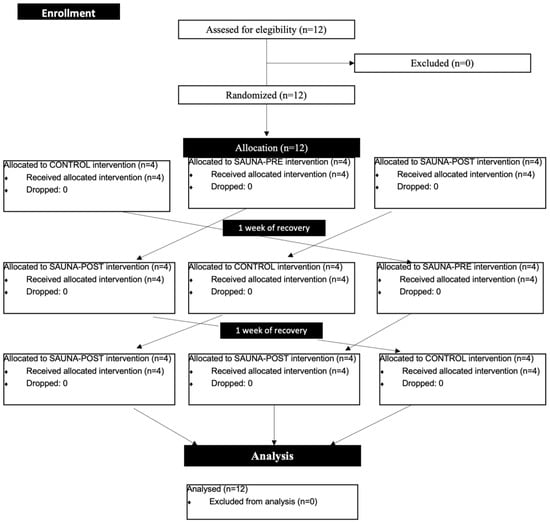
Figure 1.
Consort flow diagram.
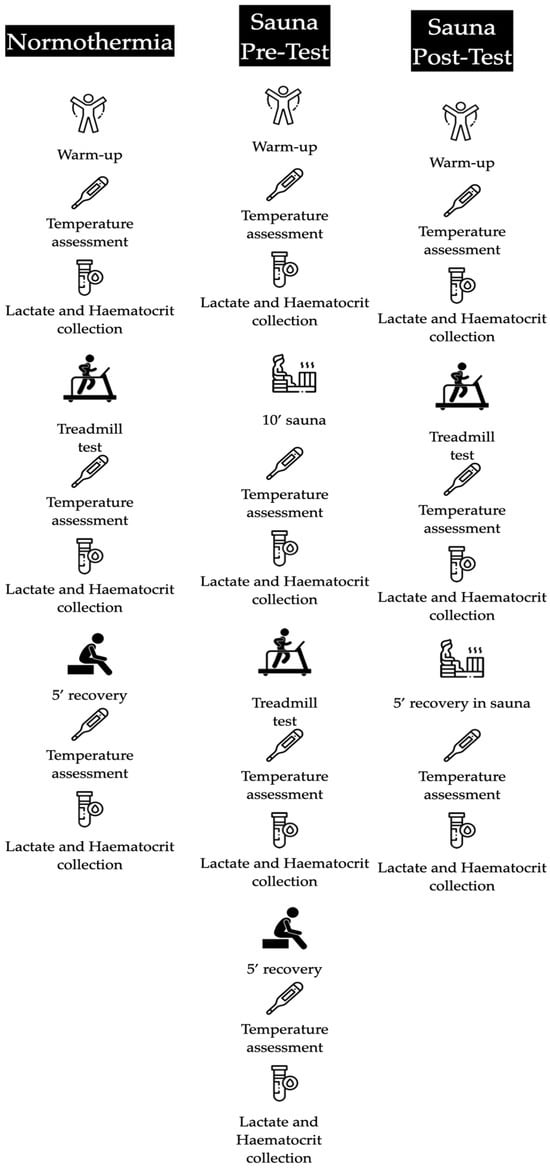
Figure 2.
Conditions of the experimental design.
2.3. Familiarisation
Prior to the experimental period, all participants completed a familiarisation week. During this week, each participant visited the laboratory to familiarise themselves with the laboratory equipment and tools and performed the maximal test on the treadmill (Ergoline 900; Bitz, Germany). Additionally, anthropometric measurements took place during this period.
2.4. Experimental Protocol
The tests were performed on a treadmill under laboratory conditions of constant temperature and humidity (22 ± 2 °C; 20% RH). Prior to the test, the participants warmed up for 10 min, which consisted of a continuous run at low intensity, to produce cardiovascular and respiratory activation, along with stretching and joint mobility. Afterward, the treadmill test was performed. Three blood lactate and haematocrit measurements were also taken (pre-test, post-test, and recovery), as explained above. Core temperature (Ct), measured at the buccal mucosa, and skin temperature (St), measured at the frontal region of the head, were monitored using an infrared thermometer [TAT 5000 “Exergen Temporal Scanner” (Corp., EE. UU.)] at the beginning and end of the tests. In the hyperthermic pre condition, a hyperthermic stimulus was applied by means of a sauna session (Harvia C105S Logix Combi Control; 3–15 W; Helsinki, Finland) prior to the treadmill test. This lasted 10 min, with an ambient temperature of 100 °C ± 5 °C; 20% RH). Elsewhere, in the hyperthermic post condition, the heat stimulus was performed after the test under the same temperature conditions (100 °C ± 5 °C; 20% RH).
2.5. Treadmill Test
The protocol used was based on the research of Cunningham and David Faulkner [16] This test is 60 s in duration, with an incline of 18% on the treadmill and a speed of 13 km/h. Our study followed this test, except for the duration, where the subject had to run to exhaustion (approximately 60 s). The performance during the test was evaluated by the time in seconds of the subjects on the treadmill.
2.6. Nutritional Assessment and Weekly Training
In order to keep nutritional control and to avoid contamination of the data evaluated, the participants had to record all the foods and their respective quantities consumed in the 48 h prior to each of the tests, in order to know their macronutrient intake. In addition, participants had to quantify the number of hours of physical exercise performed during the week prior to the test. Both assessments were collected using the app “myfitnesspal®” (V24.10.1) [17].
2.7. Anthropometric Study
The measurements taken were as follows: (1) weight, which was measured in kg—the subject stands on the scales, barefoot with feet parallel, and with as little clothing as possible; (2) height, measured in m—the subject stands fully stretched and barefoot, with feet parallel; (3) 6 skinfolds (abdominal, suprailiac, subscapular, tricipital, thigh, and calf); and (4) three bone diameters (bi-styloid, humeral bicondyloid, and femoral bicondyloid). We also measured arm and leg perimeters (calf).
For the anthropometric assessment, we used a Seca 220 (Hamburg, Germany) measuring rod, with an accuracy of ±1 mm; Seca 769 (Hamburg, Germany) weight scales with digital electronic calibrated scales; a Holtain skinfold compass (Crymych, UK), with an accuracy of ±0.2 mm; a Holtain bone diameter compass (Crymych, UK), with an accuracy of ±1 mm; and a Seca tape measure (Hamburg, Germany), with an accuracy of ±1 mm.
All measurements were taken at the same place, by the same scout, and on the right side of the body. Furthermore, all of them were carried out at the same time and following the recommendations of the Spanish Group of Cineanthropometry [18].
2.8. Haematocrit and Lactate Assessment
Haematocrit was obtained by centrifuging whole blood in a 75 µL glass capillary containing heparin in a Microcen microcentrifuge. Lactate concentrations were obtained from capillary blood samples. The samples were analysed using a Lactate Scout (Biolaster, Guipúzcoa, Spain) with a margin of error of ±0.2 mmol/L. For both measurements (haematocrit and lactate), a puncture was made with a MenaLancetPro lancet in the index finger of each participant, and the samples were drawn together.
2.9. Statistical Analysis
Statistical analyses of the results were performed using the “Statistical Package for the Social Sciences” (SPSS) 20.0 for Windows (SPSS Inc., Chicago, IL, USA). The normality of the distribution of variables was analysed using the Shapiro–Wilk test. The Wilcoxon test was performed to compare differences between assessments. A p < 0.05 was considered statistically significant. Results are expressed as means ± standard deviation.
3. Results
The results obtained in the different tests carried out in the study are shown below.
Table 1 shows the anthropometric data of the subjects in the different tests carried out in the study, and as can be seen, there are no significant differences in any of the values.

Table 1.
Anthropometric characteristics of study participants.
Table 2 displays the macronutrients consumed by the study participants 48 h before each test as recorded in the nutritional survey.

Table 2.
Macronutrient intake of subjects in the 48 h prior to each test.
The data related to internal and external temperature and test performance are shown in Table 3, and at the beginning and end of each test. No significant differences were found.

Table 3.
Temperature and time performance in each test.
Figure 3 comprises three graphs illustrating haematocrit values from various measurements and tests conducted in the study. The “Day 1—Normothermia” graph depicts values from the normothermia test, revealing a significant difference of p < 0.05 between pre-test and post-test measurements. Meanwhile, the “Day 2—Sauna Pre” graph displays values from the hyperthermia pre-test, demonstrating a significance of p < 0.05 between post-sauna and post-test measurements, as well as between pre-test and Rec measurements. Finally, the “Day 3—Sauna Post” graph presents values from the hyperthermia post-test, indicating a significant difference of p < 0.05 between pre-test and post-test, as well as between pre-test and recovery (sauna) measurements.
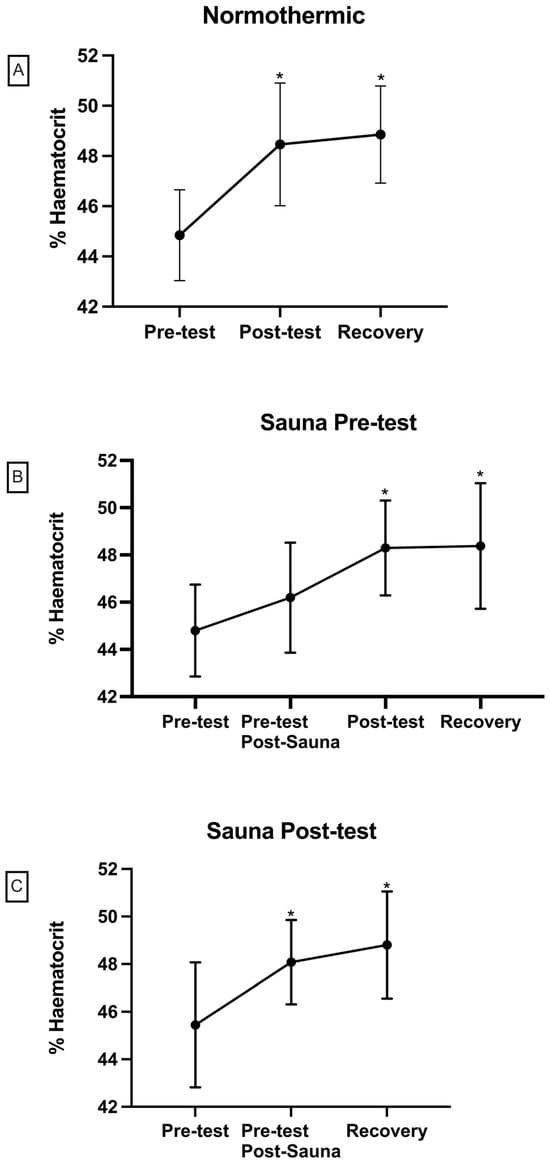
Figure 3.
Haematocrit concentrations in each test ((A) = normothermic; (B) = sauna pre-test; (C) = sauna post-test). Differences with respect to pre-test (* p < 0.05).
Figure 4 illustrates lactate values obtained in various test measurements with and without correction for haemoconcentration. In the “Day 1—Normal” graph, from the normothermia test, a significant difference of p < 0.01 is observed between pre-test and post-test measurements. Additionally, a significance of p < 0.05 is noted between post-test and Rec measurements, and between pre-test and Rec measurements. In the “Day 2—Sauna Pre” graph, representing lactate values from the hyperthermia pre-test, a significance of p < 0.05 is observed between pre-test and post-test measurements, as well as between pre-test and Rec measurements. Finally, the “Day 3—Sauna Post” graph, depicting values from the hyperthermia post-test, shows a significant difference of p < 0.01 between pre-test and post-test measurements. Furthermore, a significant difference of p < 0.05 is evident between post-test values, and between pre-test and recovery (sauna) measurements.
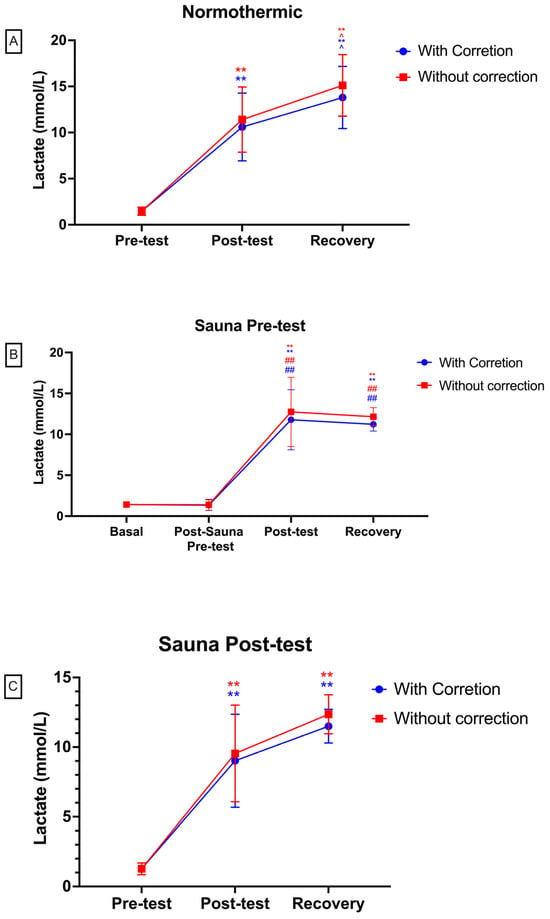
Figure 4.
Lactate values in each test ((A) = normothermic; (B) = sauna pre-test; (C) = sauna post-test). Red colour: lactate levels without correction for haemoconcentration. Blue colour: values with correction for haemoconcentration. Differences with respect to pre-test or basal: ** p < 0.01. Differences between pre-test and recovery values: ^ p < 0.05. Differences in comparison of post-sauna and pre-test: ## p < 0.01.
Lastly, the comparison between the different days of measurement at the same points can be seen in Figure 5. No significant differences were found.
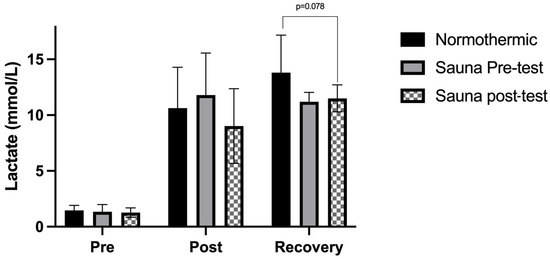
Figure 5.
This figure shows the comparison between three conditions. In the hyperthermic pre-test condition, the pre-test lactate values are after the sauna bath. All values were corrected for haemoconcentration.
4. Discussion
The present study evaluates the acute effect of exposure to high temperatures before and after an anaerobic lactic test in normothermia on lactate concentrations before and after 5 min of recovery. In addition, the effect of this pre-heat application on sports performance in an anaerobic test is evaluated. In our study, it was possible to observe multiple significances in the measurements taken during the tests. Firstly, referring to Figure 3, a significance of p < 0.05 was observed in the haematocrit values obtained between the pre-test and post-test measurements. The haematocrit values increase at the end of the test, which was observed in the three different tests performed. This could be due to haemoconcentration as a result of fluid loss and the reduction in plasma volume that occurs during physical activity, which also increases in warm environments [19]. Therefore, there is an increase in the percentage of red blood cells in whole blood in the early stages of exercise [20]. This haemoconcentration must be taken into account when measuring lactate. For this reason, the lactate values were corrected by the Van Beaumont Equation through haematocrit [21].
Elsewhere, between the lactate measurement pre-test and post-test, and between pre-test and Rec (Figure 4), of the three tests, a significant difference of p < 0.05 can be shown. These significant differences could be due to the fact that, when treating the test as a lactic anaerobic test, a logarithmic increase in lactate is produced by anaerobic glycolysis [4]; therefore, it is logical that these significant differences occur between pre- and post-exercise conditions.
The application of heat just before the test produces an increase in lactate concentrations at the end of the test, but there were no significant changes. It has been reported that when exercising under anaerobic conditions, the use of glycogen via the glycolytic pathway predominates [2]. In addition, this mechanism is exacerbated in situations of heat stress, causing an increase in anaerobic glycolysis. The enzyme lactate dehydrogenase can convert pyruvate to lactate [5]. Therefore, an increase in blood lactate concentrations is expected to occur during intense exercise and even more under heat stress conditions before the test. Furthermore, the high temperatures have a potential to affect substrate supply and metabolite release upon modifying vasodilation [22,23]. However, the application of the acute passive thermal effect of 10 min duration just before the test shows a greater elimination of lactate concentrations in blood when performing a passive 5 min recovery in normothermia. It should be noted that there was no significant difference in lactate concentration between the post-test and after 5 min of recovery in the hyperthermia pre condition (Figure 4B), while in the first test (normothermia; Figure 4A), there was a significant increase after 5 min of recovery. In this respect, it is known that the sweating rate increases after exposure to heat [24]. Hence, it is possible that sweating was greater in the second test and that there was higher lactate elimination through sweating [25]. A recent study reported that heat acclimatization reduces sweat lactate [26]. Another possible reason is the elimination of lactate through urine [27,28]; however, heat inhibits urine production through the action of ADH and the renin angiotensin system [1], so this pathway is implausible.
In contrast, when applying the acute passive thermal stimulus for 5 min immediately after the end of the test (post hyperthermia) and looking at Figure 4C, we can observe that there is a significant decrease in Rec lactate concentrations compared to the post-test measurement. This is something that we cannot observe in the other two tests carried out in the study (normothermia and hyperthermia pre; Figure 4A and Figure 4B, respectively), so it could be concluded that passive recovery in post-test hyperthermia conditions favours the elimination of lactate concentrations in blood. This could be since, under hyperthermia conditions, a higher cardiac output is produced [29], and this fact during recovery could favour a decrease in blood lactate concentrations through extraction and utilisation by the heart, skeletal muscles, and liver. In this respect, it is known that heat increases enzyme activity [30]. Therefore, it could be hypothesised that the lactate/H+ cotransporter (monocarboxylate transporter, MCT) increases its activity, leading to increased lactate movement through the sarcolemma into adjacent cells. Heat would also increase the enzymatic activity of pyruvate carboxylase, which would trigger the generation of acetyl CoA from pyruvic acid for the Krebs cycle. In addition to these facts, the haemoglobin and myoglobin curve shifts to the right with increasing body temperature [31], enhancing this Krebs cycle process in the mitochondria. Alternatively, the increased cardiac output due to the thermoregulatory process could result in an enhanced Cori cycle, where lactate is cleared for conversion to glucose, occurring to a greater extent due to the higher blood flow. Conversely, blood flow to the liver is reduced in hot conditions compared to normothermic conditions [32]. Hence, this explanation is not plausible. Finally, it is important to consider what has been mentioned in the sauna pre case, where sweat elimination could potentially play a strong role. Thus, exposure to heat at 100 °C in recovery could increase this lactate elimination through sweating [26]. In addition, increased oxygen debt or excess post-exercise oxygen consumption (EPOC) in hot conditions would trigger an increased action of bicarbonate as a buffer for lactate clearance and subsequent CO2 elimination through ventilation [33]. Conversely, these results obtained in our study disagree with the research of Folk et al. [10], who show that passive recovery in hyperthermic conditions at 5 min does not produce a greater decrease in lactate concentrations compared to passive recovery in normothermic conditions; however, the temperatures used were different. Elsewhere, researchers have suggested that there could be less lactate production during intense exercise in heat. This is because heat in prolonged exercise triggers central fatigue [34]. It has likewise been argued that the hyperthermia-prompted decline in cycling execution is presumably connected with cardiovascular and perceptual constraints, as opposed to a physiological failure to produce motor drive [35,36]. Nonetheless, performance did not decrease after the application of heat before exercise in this research.
Finally, as for the effect of the application of the passive acute hyperthermic stimulus before the test, no significant differences in performance can be concluded. Although a non-significant improvement of a few seconds is observed, this could be due to a series of cardiovascular, metabolic, and enzymatic adaptations. However, studies have shown that long-term heat acclimatisation training (≥8 heat exposures) improves physical performance [37]. These results agree with those obtained in the research by Cheuvront et al. [38]; however, they do not agree with the results of the study by Falk et al. [14]. Although the latter suggests that exposure to heat can improve anaerobic performance, in that study, the session was carried out in an environment with a temperature of 35 °C, unlike ours, where we applied the thermal stimulus before, and not during, the test, and at a temperature of 100 °C ± 5 °C. Furthermore, the aforementioned study utilised a series of shorter time intervals (15 s), so that intense exercise in a warm environment could lead to an improvement in anaerobic performance in short periods of time (15 s).
Based on the results obtained, a pre- or post-exercise heat stimulus could be applied to a competition situation, especially in those where the athlete competes at several times throughout the day or in different events, such as in the Olympic Games.
This study’s outcomes should be interpreted within the context of several limitations. Firstly, the relatively modest sample size and potential homogeneity of the participants might restrict the broader applicability of the findings to diverse populations. Secondly, while efforts were made to maintain a controlled environment at a constant temperature of 22 °C, other external factors, such as individual responses to testing conditions, could introduce variability. Furthermore, variations in individual responses to the sauna, including differences in heat tolerance, hydration levels, or familiarity with sauna use, could introduce inconsistencies in the observed physiological responses, challenging the uniformity of the findings.
5. Conclusions
The application of a 10 min acute hyperthermic stimulus before a lactic anaerobic test decreases blood lactate concentrations during recovery.
Furthermore, the application of this same stimulus, with a duration of 10 min just before the test, could help to reduce lactate concentrations after 5 min of recovery, compared to normothermic conditions.
However, the application of this hyperthermic stimulus did not lead to a significant improvement in test performance.
Author Contributions
Conceptualisation, V.T.-R. and J.M.F.; methodology, M.M.-M.; software, F.J.G.; validation, I.B. and J.S.-C.; formal analysis, J.M.F. and J.S.-C.; investigation, V.T.-R. and J.M.F.; resources, M.M.-M.; data curation, I.B. and J.M.F.; writing—original draft preparation, J.M.F. and J.S.-C.; writing—review and editing, J.S.-C. and M.M.-M.; visualisation, F.J.G.; supervision, M.M.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the University of Extremadura (registration number: 196//2022) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The authors declare that the data are available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kenney, W.L.; Wilmore, J.; Costill, D. Physiology of Sport and Exercise, 6th ed.; Human Kinetics: Champaign, IL, USA, 2015; ISBN 1450477674. [Google Scholar]
- Febbraio, M.A.; Carey, M.F.; Snow, R.J.; Stathis, C.G.; Hargreaves, M. Influence of Elevated Muscle Temperature on Metabolism during Intense, Dynamic Exercise. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1996, 271, R1251–R1255. [Google Scholar] [CrossRef] [PubMed]
- Toro-Román, V.; Prieto-González, I.; Siquier-Coll, J.; Bartolomé, I.; Grijota, F.J.; Maynar-Mariño, M. Effects of High Temperature Exposure on the Wingate Test Performance in Male University Students. Int. J. Environ. Res. Public Health 2023, 20, 4782. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, K. The Anaerobic Threshold: Definition, Physiological Significance and Identification. Adv. Cardiol. 1986, 35, 1–23. [Google Scholar] [PubMed]
- Melkonian, E.A.; Schury, M.P. Biochemistry, Anaerobic Glycolysis; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Leverve, X.M.; Mustafa, I.; Péronnet, F. Pivotal Role of Lactate in Aerobic Energy Metabolism. In Yearbook of Intensive Care and Emergency Medicine 1998; Springer: Berlin/Heidelberg, Germany, 1998; pp. 588–596. [Google Scholar]
- Karlsson, J.; Bonde-Petersen, F.; Henriksson, J.; Knuttgen, H.G. Effects of Previous Exercise with Arms or Legs on Metabolism and Performance in Exhaustive Exercise. J. Appl. Physiol. 1975, 38, 763–767. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, J.D.; Reddan, W.G.; Layton, C.R.; Dempsey, J.A. Effects of Metabolic Hyperthermia on Performance during Heavy Prolonged Exercise. J. Appl. Physiol. 1974, 36, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Sekine, N.; Cirulli, V.; Regazzi, R.; Brown, L.J.; Gine, E.; Tamarit-Rodriguez, J.; Girotti, M.; Marie, S.; MacDonald, M.J.; Wollheim, C.B. Low Lactate Dehydrogenase and High Mitochondrial Glycerol Phosphate Dehydrogenase in Pancreatic Beta-Cells. Potential Role in Nutrient Sensing. J. Biol. Chem. 1994, 269, 4895–4902. [Google Scholar] [CrossRef]
- Folk, B.; Einbinder, M.; Weinstein, Y.; Epstein, S.; Kami, Y.; Yarom, Y.; Rotstein, A. Blood Lactate Concentration Following Exercise: Effects of Heat Exposure and of Active Recovery in Heat-Acclimatized Subjects. Int. J. Sports Med. 1995, 16, 7–12. [Google Scholar] [CrossRef]
- Maxwell, N.S.; Aitchison, T.C.; Nimmo, M.A. The Effect of Climatic Heat Stress on Intermittent Supramaximal Running Performance in Humans. Exp. Physiol. Transl. Integr. 1996, 81, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Smolander, J.; Kolari, P.; Korhonen, O.; Ilmarinen, R. Aerobic and Anaerobic Responses to Incremental Exercise in a Thermoneutral and a Hot Dry Environment. Acta Physiol. Scand. 1986, 128, 15–21. [Google Scholar] [CrossRef]
- Oyono-Enguelle, S.; Heitz, A.; Marbach, J.; Ott, C.; Pape, A.; Freund, H. Heat Stress Does Not Modify Lactate Exchange and Removal Abilities during Recovery from Short Exercise. J. Appl. Physiol. 1993, 74, 1248–1255. [Google Scholar] [CrossRef]
- Falk, B.; Radom-Isaac, S.; Hoffmann, J.R.; Wang, Y.; Yarom, Y.; Magazanik, A.; Weinstein, Y. The Effect of Heat Exposure on Performance of and Recovery from High-Intensity, Intermittent Exercise. Int. J. Sports Med. 1998, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, I.; Toro-Román, V.; Siquier-Coll, J.; Muñoz, D.; Robles-Gil, M.C.; Maynar-Mariño, M. Acute Effect of Exposure to Extreme Heat (100 ± 3 °C) on Lower Limb Maximal Resistance Strength. Int. J. Environ. Res. Public Health 2022, 19, 10934. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D. The Effect of Training on Aerobic and Anaerobic Metabolism during a Short Exhaustive Run. Med. Sci. Sports Exerc. 1969, 1, 65–69. [Google Scholar] [CrossRef]
- O’Loughlin, E.K.; Marashi, M.; Sabiston, C.M.; Lucibello, K.M.; Sylvestre, M.-P.; O’Loughlin, J.L. Predictors of Food and Physical Activity Tracking Among Young Adults. Health Educ. Behav. 2023, 50, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Esparza, F.; Alvero, J.R.; Aragonés, M.T.; Cabañas, M.D.; Canda, A.; Casajús, J.A.; Chamorro, M.; Galiano, D. Manual de Cineantropometría; GREC-FEMEDE: Pamplona, Spain, 1993. [Google Scholar]
- Yanchatuña Agualongo, M.N. Determinación de Electrolitos, Glucosa, Hematocrito Pre y Post Entrenamiento En La División Formativa Sub 12 y Sub 14 Del Club Deportivo Mushuc Runa S.C. y Su Relación Con El Tiempo de Actividad Física. Bachelor’s Thesis, Universidad Técnica de Ambato, Ambato, Ecuador, 2017. [Google Scholar]
- Garcia-Vega, O.; Ramos, M.; Mancera, E. Perfiles Hematológicos e Hidroelectrolíticos En Sujetos Sedentarios Durante Ejercicio de Resistencia: Efecto de La Hidratación. Rev. Med. Fac. Med. 2007, 15, 26–39. [Google Scholar]
- Van Beaumont, W. Evaluation of Hemoconcentration from Hematocrit Measurements. J. Appl. Physiol. 1972, 32, 712–713. [Google Scholar] [CrossRef] [PubMed]
- Karvonen, J. Warming up and Its Physiological Effects. Pharmacol. Physiol. 1978, 6, 31–39. [Google Scholar]
- Racinais, S.; Cocking, S.; Périard, J.D. Sports and Environmental Temperature: From Warming-up to Heating-Up. Temperature 2017, 4, 227–257. [Google Scholar] [CrossRef] [PubMed]
- Cotter, J.D.; Patterson, M.J.; Taylor, N.A.S. Sweat Distribution before and after Repeated Heat Exposure. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 76, 181–186. [Google Scholar] [CrossRef]
- Buono, M.J.; Lee, N.V.L.; Miller, P.W. The Relationship between Exercise Intensity and the Sweat Lactate Excretion Rate. J. Physiol. Sci. 2010, 60, 103–107. [Google Scholar] [CrossRef]
- Weller, R.S.; Buono, M.J. The Effect of Heat Acclimation on the Sweat Lactate Concentration vs. Sweat Rate Relationship. J. Therm. Biol. 2022, 109, 103325. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, S.; Kosmidis, I.; Sougioultzis, M.; Kabasakalis, A.; Mougios, V. Diurnal Variation and Reliability of the Urine Lactate Concentration after Maximal Exercise. Chronobiol. Int. 2018, 35, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, S.; Karpouzi, C.; Tsalis, G.; Kabasakalis, A.; Papaioannou, K.G.; Mougios, V. Reliability of Urine Lactate as a Novel Biomarker of Lactate Production Capacity in Maximal Swimming. Biomarkers 2016, 21, 328–334. [Google Scholar] [CrossRef]
- Crandall, C.G.; Gonzalez-Alonso, J. Cardiovascular Function in the Heat-stressed Human. Acta Physiol. 2010, 199, 407–423. [Google Scholar] [CrossRef] [PubMed]
- Marchant, E.D.; Nelson, W.B.; Hyldahl, R.D.; Gifford, J.R.; Hancock, C.R. Passive Heat Stress Induces Mitochondrial Adaptations in Skeletal Muscle. Int. J. Hyperth. 2023, 40, 2205066. [Google Scholar] [CrossRef]
- McCutcheon, L.J.; Geor, R.J.; Hinchcliff, K.W. Effects of Prior Exercise on Muscle Metabolism during Sprint Exercise in Horses. J. Appl. Physiol. 1999, 87, 1914–1922. [Google Scholar] [CrossRef][Green Version]
- Rowell, L.B.; Brengelmann, G.L.; Blackmon, J.R.; Twiss, R.D.; Kusumi, F. Splanchnic Blood Flow and Metabolism in Heat-Stressed Man. J. Appl. Physiol. 1968, 24, 475–484. [Google Scholar] [CrossRef]
- Martin, D.E. The Effect of Heat Stress on Excess Post Exercise Oxygen Consumption; Microform Publications, University of Oregon: Eugene, OR, USA, 1994. [Google Scholar]
- Nybo, L.; Nielsen, B. Hyperthermia and Central Fatigue during Prolonged Exercise in Humans. J. Appl. Physiol. 2001, 91, 1055–1060. [Google Scholar] [CrossRef]
- Périard, J.D.; Cramer, M.N.; Chapman, P.G.; Caillaud, C.; Thompson, M.W. Neuromuscular Function Following Prolonged Intense Self-Paced Exercise in Hot Climatic Conditions. Eur. J. Appl. Physiol. 2011, 111, 1561–1569. [Google Scholar] [CrossRef]
- Racinais, S.; Girard, O. Neuromuscular Failure Is Unlikely to Explain the Early Exercise Cessation in Hot Ambient Conditions. Psychophysiology 2012, 49, 853–865. [Google Scholar] [CrossRef]
- Chalmers, S.; Esterman, A.; Eston, R.; Bowering, K.J.; Norton, K. Short-Term Heat Acclimation Training Improves Physical Performance: A Systematic Review, and Exploration of Physiological Adaptations and Application for Team Sports. Sports Med. 2014, 44, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Cheuvront, S.N.; Carter, R.; Haymes, E.M.; Sawka, M.N. No Effect of Moderate Hypohydration or Hyperthermia on Anaerobic Exercise Performance. Med. Sci. Sports Exerc. 2006, 38, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).