Abstract
Every year, over 600,000 new cases of esophageal cancer are registered worldwide. Treatment depends on the stage of the disease. In the early stages, surgical treatment is the basis (T1–T2 lesion < 3 cm, N0M0), while in more advanced stages, surgical treatment is preceded by radiochemotherapy or only radiochemotherapy is used. In the case of generalized disease, the main treatments used are systemic treatments of chemotherapy, immunotherapy and palliative teleradiotherapy or brachytherapy. Brachytherapy can be used at virtually any stage of disease, both as a radical treatment and as a palliative treatment. This paper presents the possibilities of using brachytherapy at various stages of esophageal cancer treatment. Particular attention was paid to the role of combining brachytherapy and immunotherapy and the possibility of an abscopal effect.
1. Introduction
Esophageal cancer (EC) is the ninth most common cancer and the sixth leading cause of cancer death worldwide. More than 600,000 people suffer from esophageal cancer every year, and 10–30% die within a year [1,2]. Esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) are two major histological subtypes with different epidemiological and clinical features. While the global incidence of ESCC is decreasing, the incidence of EAC is increasing in many countries [1].
Early esophageal cancer remains asymptomatic for a long time; therefore, the vast majority of cases are detected in advanced stages of cancer. The main symptom of esophageal cancer is dysphagia, which results in progressive weight loss and cachexia.
Due to the submucosal spread of esophageal cancer, imaging diagnostics is demanding and based on the use of many diagnostic methods, each of which has its limitations. For local assessment, endoscopic examination supported by transesophageal ultrasound is used, and CT examination is used to assess the locoregional advancement, but it plays a minor role in assessing local advancement. PET CT allows the assessment of local advancement and distant metastases, but is characterized by low spatial resolution. Also, in recent years, the role of MRI in the diagnosis of local advancement of esophageal cancer has increased [3].
The treatment of esophageal cancer is complex and depends on both the stage of the cancer and its location [4]. The basic method of treatment is the use of various surgical techniques, both radical, such as esophagectomy, and palliative, such as stent implantation [5]. At the stage of locally advanced disease, radiotherapy, usually combined with chemotherapy, plays an important role. In the case of metastatic disease, the basis of treatment is systemic treatment, which in recent years has been enriched with new drugs whose action is determined by the presence of biomarkers such as the expression of the HER2 receptor, PD-1 (programmed death receptor-1) receptor or the detection of microsatellite instability (MSI) [6]. In the case of advanced disease, in addition to the above-mentioned treatment, an important local treatment plays a role, aimed at reducing the main symptom of the disease, which is dysphagia. One of the basic methods, apart from the previously mentioned stent implantation, is intraductal brachytherapy (BT). This is a widespread method of local irradiation, which involves the intraesophageal administration of a high dose of radiation to the area of the tumor responsible for swallowing problems. Due to the treatment technique, this method is mainly intended for palliative patients, but in special cases, it may play a role in radical treatment, most often in combination with external beam radiotherapy (EBRT) [7].
Brachytherapy in early stages can be used as an independent treatment because it allows the therapeutic dose to cover the area of tumor infiltration with an appropriate margin of healthy tissue, ensuring the local control of the tumor. In higher stages of advancement, due to the risk of cancer spreading beyond the esophageal wall, it is necessary to combine brachytherapy with radio or radiochemotherapy.
The following work presents reports from recent years which show that, in spite of progress in teleradiotherapy techniques and new methods of systemic treatment, brachytherapy plays an important role in the treatment of this type of cancer.
The targets of the manuscript are shown in the Figure 1 below.
Figure 1.
The targets of the manuscript.
2. Brachytherapy as Radical Treatment
2.1. Intraductal Brachytherapy as an Independent Treatment in Early Esophageal Cancer
The role of brachytherapy (BT) alone in the treatment of early esophageal cancer involving only the mucosa or submucosa has not been determined. Currently, the obvious and recommended method is surgery.
Surgical treatment is the standard treatment for early esophageal cancer (T1–T2 lesions, <3 cm, N0M0), but it is associated with a high risk of complications and mortality. For this reason, the endoscopic removal of esophageal tumor is increasingly used as an alternative procedure. But treatment results are ambiguous regarding the effectiveness of this procedure. Meta-analysis compares 15 studies covering a total of 2467 patients after endoscopic treatment and 2264 after surgical treatment. Endoscopic treatment is a safe procedure in early esophageal cancer (we observe fewer side effects—RR 0.46 at 95% CI and lower-treatment-related mortality—RR 0.27 at 95% CI); however, after esophagectomy, we observe fewer relapses, more R0 treatments and longer overall survival [8].
The question that is increasingly being asked is that of whether brachytherapy can be an alternative, especially to esophagectomy in early esophageal cancer.
Few studies from many years ago cover small groups of patients and in a short observation period indicate a local response after the use of independent brachytherapy. In the study by Maingon et al. [9], out of 25 patients, 12 had tumor recurrence, 11 of which were in the irradiated area. mDFS (median disease-free survival) was 14 months and 1-, 2-, and 3-year OS was 76%, 37%, and 14%, respectively. Unfortunately, despite the low stage of advancement, overall survival was very low and did not differ significantly between patients with stage T1 and Tis 20% vs. 24%, p = 0.83. In the study by Murakami et al. [10], 44 patients with stage T1a cancer were treated with intraductal brachytherapy, administering 25–35 Gy in 5–14 fractions. The 5-year locoregional control (LC), cancer specific survival (CSS), and overall survival (OS) rates were 75%, 97%, and 84%, respectively. Hishikawa et al. [11] described six patients who were irradiated to 24 Gy in a 4 × 6 Gy/2× week schedule. All patients had a complete local response, but the follow-up period was insufficient to fully assess the treatment results. Excellent results were described in the analysis of 23 patients with early esophageal cancer in the study by Nemoto et al. [12], which compared various radiotherapy techniques, including HDR brachytherapy alone. The 5-year LC in stage T1a was 100% and T1b was 54%. Based on all the above data, brachytherapy alone cannot be recommended for the radical treatment of early esophageal cancer. The role of brachytherapy in this indication may only be limited to selected patients whose general condition prevents the use of radical surgical treatment, radiochemotherapy or teleradiotherapy, and who do not consent to endoscopic treatment.
2.2. Brachytherapy Combined with Teleradiotherapy in the Treatment of Early Esophageal Cancer
As the degree of local advancement increases, the risk of lymph node metastases increases, e.g., in stage T1b, it is 20% [2,13]. In the case of local advancement in stages above T1a–T2, the combination of brachytherapy with teleradiotherapy is justified (Figure 2). However, such treatment is not the standard of care, and surgical treatment remains the basis of treatment in these stages of advancement [6]. Despite this, the results of treatment using teleradiotherapy with brachytherapy are very good [9,14]. The effectiveness of treatment decreases with an increasing depth of infiltration and is the greatest when the mucous membrane is involved. Studies indicate that the 5-year local control in such cases reaches 100% in patients with mucosa involvement and 75% in those with submucosa involvement [15], although some analyses indicate that the 2-year local control rate is only 63% [16]. In the case of submucosal invasion, the local control rate is slightly lower, usually approximately 10%, compared to stage T1a [12,17].
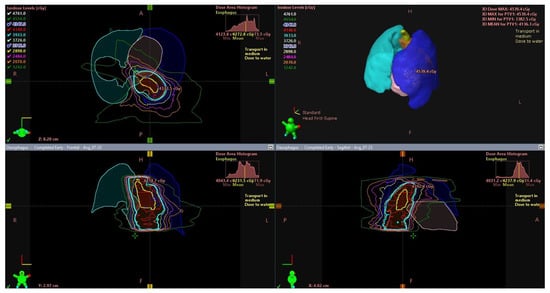
Figure 2.
External beam radiotherapy (EBRT)—radiotherapy target volume in esophageal cancer. Tumor in the upper and middle thoracic esophagus (yellow). Planned target volume (PTV) (cyan).
Five-year survival rates vary in different analyses and range from 31 to 66% [14,18,19,20,21,22,23] (Table 1). There are no studies directly comparing the effectiveness of combined EBRT and BT vs. surgical treatment, but the results seem to be slightly worse than those of surgical treatment (5-OS 77–85.5%) [24,25]. The diversity of results is influenced by the retrospective nature of the studies and the variety of treatment regimens. Teleradiotherapy doses range from 50 to even 70 Gy, conventionally fractionated in the dose range of 1.8–2 Gy [14,18,19,20,21,22,23]. The variety of brachytherapy regimens is even greater when low dose rate (LDR) and high dose rate (HDR) techniques are used. The number of fractions was from 1 to 4, and the fractional dose was from 3 to 7.5 Gy, which gave a different biologically effective dose (EQD2). EQD2 for the alpha/beta = 10 ratio ranged from nearly 10 to even 32 Gy, which gave a total biologically equivalent dose of over 70 Gy. Some studies also used concurrent chemotherapy, mainly based on cisplatin with 5FU. Current indications for radiochemotherapy alone recommend a dose of 50.4 Gy, conventionally fractionated without the use of brachytherapy, which is a much lower dose than in the above analyses. Late complications were related to BT dose and the number of fractions—17% for 12 Gy compared to 80% for 24 Gy [22]. Ulcers occurred in 3.5–20%, depending on the study. Less common complications included fistula, esophageal stricture, radiation pneumonia, and heart failure [10,12,14,15,16,17,18,19,20,21,22,23,26].

Table 1.
Study results of brachytherapy combined with teleradiotherapy in the treatment of early esophageal cancer.
Neoadjuvant radiochemotherapy according to the CROSS regimen is the cornerstone of treatment for more advanced (T2–T3, N0-1, M0) operable esophageal cancer. Pre-operative radiotherapy up to 41.4 Gy concurrent with paclitaxel and carboplatin significantly improved OS and DFS compared to surgery alone in patients with the resectable (T1N1M0, T2–T3, N0-1.M0) cancer of the esophagus or gastroesophageal junction (GEJ).
The median OS in the study group was 5 years, and in the control group, 2 years [27]. Improved OS in patients with gastric adenocarcinoma and GEJ was noted when surgery was combined with subsequent radiochemotherapy in the SWOG/INT 0116 study. The median OS was 36 months in the group treated with chemoradiotherapy compared to 27 months in patients treated with surgery alone [28]. In the CALGB 9781 study, in the group of patients treated with induction radiochemotherapy with cisplatin and 5FU, the median OS was nearly 4.5 years, and in patients treated solely with surgery, almost 1.8 years [29]. Based on the above studies, induction radiochemotherapy has become the standard in the treatment of early cancer of the esophagus and gastroesophageal junction.
The role of surgical treatment in patients who achieve a complete response after radiochemotherapy remains controversial. On the one hand, studies indicate that omitting surgical treatment in such a case is beneficial in terms of OS and quality of life, but on the other hand, the diagnostic method assessing complete response remains a problem. The data indicate that only a combination of histopathological examination with PET-CT and MRI allows for an accurate assessment of complete response and the omission of surgical treatment [30].
3. Brachytherapy Combined with Teleradiotherapy in Advanced, Non-Operative Stages
Most patients with esophageal cancer are in poor general condition and with advanced local disease, where treatment with the intention of surgery is impossible. A multicenter, randomized phase III trial (NROG-001) [31] showed that in the high-dose arm (59.4 Gy) there was no improvement in OS (HR = 0.93, p = 0.54), but an improvement in PFS was demonstrated (29.1 months–20.0 months, HR = 0.77, p = 0.023) compared with the lower dose arm (50.4 Gy). In the study by Herskovitz et al. [32], the median OS was 8.9 months in the group with radiotherapy alone and 12.5 months in patients with radiochemotherapy. In non-operative cases, attempts to improve treatment results were made by escalating the dose using brachytherapy (Table 2). The most frequently used radiotherapy doses were in the range of 40–50 Gy, conventionally fractionated [33,34,35,36,37], although in some studies, the dose administered using EBRT was as high as 60 Gy [38,39,40,41]. The dose from brachytherapy ranged from 10 Gy to 24 Gy hypofractionated in the range of 5–10 Gy/fraction [33,34,35,36,37,38,39,40,41]. Some studies also used chemotherapy. Due to the poor prognosis in this group of patients, some regimens included hypofractionated radiotherapy with a brachytherapy boost, and the aim of the treatment was primarily palliative. Dose escalation using brachytherapy increased the local effectiveness, but at the cost of an increased rate of complications such as ulceration, esophageal stenosis, and bleeding.

Table 2.
Results of brachytherapy combined with teleradiotherapy in advanced, non-operative stages.
Because the results of treatment with radiotherapy alone are poor and the combination with chemotherapy or surgery does not bring satisfactory results, checkpoint inhibitors (CPIs) are introduced. In the PALACE-1 trial [45], pembrolizumab was added to typical chemoradiotherapy from the CROSS trial. At this point, the available data showed good treatment tolerance with a pCR (pathological complete response) rate of 55.6%. Currently, the PALACE 2 phase 2 study is underway. In the ongoing CRISEC study [46], tislelizumab was added to chemoradiotherapy according to the same regimen. The interim analysis of this study showed a high pathological response of 86.7% with good tolerability of the combination of immunotherapy and chemoradiotherapy. Similarly, the atezolizumab trial [47] only analyzed the pCR rate. pCR was 25% and was similar to that in the CROSS study. Also, the addition of toripalimab to induction chemoradiotherapy in the phase II study [48] did not bring any breakthrough. The pCR rate was 50% and 36% in the immunotherapy group and chemoradiotherapy alone group, respectively. Although it differed numerically, this difference was not statistically significant. The first survival data on small groups of patients in combination with Camrelizumab showed 12 m and 24 m OS of 85.0% and 69.6%, and 12 m and 24 m PFS of 80.0% and 65.0%, and in combination with pembrolizumab, 6, 12, and 18 m OS of 89.3%, 80.8%, and 73.1%, respectively [49,50].
Taking into account the still unsatisfactory results of neoadjuvant immunotherapy, further research on adjuvant immunotherapy after chemoradiotherapy was conducted. The study with the addition of durvalumab turned out to be negative, with no differences in DFS or OS [51]. However, the study on nivolumab turned out to be positive [49]. However, not all patients were included in this study, but only those who did not achieve pCR after chemoradiotherapy. The addition of nivolumab extended mDFS from 11.0 to 22.4 months [52].
4. Brachytherapy in the Palliative Treatment of Esophageal Cancer
The palliative treatment of patients with esophageal cancer is based on both local therapy and systemic treatment. This very heterogeneous group includes patients in good general condition with a small tumor mass and single metastases as well as patients with a large local extension or massive spread in multiple organs. For this reason, the effect of local treatment on overall survival is difficult to demonstrate and local treatment is primarily important for symptom relief. Intraesophageal brachytherapy has a major role in relieving dysphagia. It results in a reduction in dysphagia in 70–90% of cases and achieves a complete endoscopic response in 50% of cases, which, depending on the study, lasts between 2 and 9 months [53]. Studies indicate that both fractional and total dose should be adjusted according to the patient’s general condition and life expectancy, as well as any previously administered dose of external beam irradiation. The most common irradiation schedules range from a single dose of 10–12 Gy through two fractions of 8 Gy to three fractions of 6–7.5 Gy. This allows for an EQD2 of alpha/beta 10 ranging from 16.6 to 32.8 Gy [13].
A comparative analysis between intraesophageal brachytherapy and other palliative techniques indicates a benefit of brachytherapy in relieving dysphagia. This was demonstrated both in a review paper with a meta-analysis of prospective studies by Fuccio et al. [7] and in an Italian literature review by Lancelotta et al. [54]. These studies also highlight the relatively low toxicity of the treatment, which was 8–12% in grades 3 and 4. Studies comparing brachytherapy with other palliative techniques have also been conducted. In 2002, the first randomized study comparing stenting and endobronchial brachytherapy (one fraction—12 Gy) was published. It showed an advantage of brachytherapy over stenting in terms of dysphagia-free time, weight loss, and improved quality of life [55]. There are no randomized trials directly comparing brachytherapy with EBRT. The results of the NTR7397 trial comparing three palliative treatment regimens—brachytherapy 12 Gy once and EBRT five times of 4 Gy up to 20 Gy—have still not been published. Only an indirect comparison is possible. Based on a comparison of the POLDER 1 study in which EBRT (20 Gy/5 fr/4 Gy) was used to relieve dysphagia and the SIREC study in which a single dose of 10 Gy was used, it was shown that both therapies offered similar results in terms of relieving dysphagia at 3 months after treatment, but EBRT was more effective in controlling pain, nausea, vomiting, and loss of appetite [56].
The standard of first-line treatment for patients with advanced or metastatic esophageal cancer is chemotherapy. Various chemotherapy regimens based on platinum derivatives, fluoropyrimidine, and taxanes are used in the treatment of metastatic esophageal cancer. In the first line, mainly a combination of platinum with fluoropyrimidine derivatives, in subsequent lines, taxanes or irinotecan. In the case of the overexpression of the HER 2 receptor in adenocarcinoma, the use of trastuzumab is possible.
Since the prognosis after such treatment remains poor, attempts are made to use immunotherapy alone or in combination with chemotherapy. The indications for its use vary depending on histology. In squamous cell carcinomas, the CheckMate 648 study, in which patients were randomized to three cohorts of nivolumab with ipilimumab, nivolumab with cisplatin-fluorouracil chemotherapy and chemotherapy alone, showed that, in patients with PD-L1 positive status, prolonged survival was achieved in both the immunotherapy alone and chemotherapy with immunotherapy groups compared to chemotherapy alone [57]. Similarly, the benefit of adding immunotherapy to chemotherapy was shown in the PD-L1 CPS 10 or above group after pembrolizumab in the Keynote 590 trial and in the overall patient group after Camrelizumab in the ESCORT first trial [58,59]. The benefit of immunotherapy has also been demonstrated in patients with adenocarcinoma. The CheckMate 649 study showed an OS benefit in CPS greater than or equal to five when nivolumab was added to standard chemotherapy, and the Keynote 590 study showed a benefit with pembrolizumab [58,60]. A benefit in OS was also highlighted for second and subsequent lines of treatment for esophageal cancer with pembrolizumab in the Keynote 181 study (for CPS 10 and above) and for nivolumab in the ATTRACTION-3 study (squamous or adenocarcinoma) [61,62]. The primary predictor of response to immunotherapy in esophageal cancer is the PD-1 (programmed death receptor-1) receptor protein, the high expression of which is responsible for a 25% response rate to immunotherapy in this cancer. Studies indicate the possibility of increasing the expression of the PD-1 molecule under the influence of chemoradiotherapy. In a study by Kelly et al. [63], it was shown that in a tumor burden, there is an increased level of interferon γ and activated CD8+ T cells. The upregulations of PD-L1 and many other immune checkpoints, including TIM3 (T-cell immunoglobulin and mucin domain 3), GITR (glucocorticoid-induced tumor necrosis factor receptor family-related protein IDO1(Indoleamine 2,3-dioxygenase 1), LAG3 (lymphocyte-activation gene 3), OX40 and KIRs (killer immunoglobulin-like receptors), have also been demonstrated. The upregulation of PD-L1 is dose-dependent and transiently elevated after radiation exposure. Similar conclusions come from the study by Osipov et al. [64] in which 28 patients with stomach and gastrointestinal junction cancer were analyzed in terms of PD1 and PDL1 expression before and after chemoradiotherapy, as well as the impact of PD1 expression on prognosis. Increased PD1 expression has been shown to be associated with poor OS in gastroesophageal (GE) cancer patients. Neoadjuvant chemoradiotherapy increases both PDL1 and PD1 levels. In patients with stomach cancer, this led to significantly poorer survival.
In general, higher doses of radiation appear to have more immunostimulatory effects, while lower doses of radiation (approximately 2 Gy) may produce greater immunosuppressive and anti-inflammatory effects [65]. It is believed that brachytherapy may have a potential advantage over EBRT in terms of stimulating the immune response within the tumor. Whereas in EBRT radiation, beams pass through a significant volume of healthy tissue to achieve appropriate dose distribution in the target volume, brachytherapy allows for a highly conformal dose distribution, with a rapid dose decay outside the irradiated target volume and a high but also heterogeneous radiation dose in the target volume (Figure 3). This dose heterogeneity depends on the distance of the source from the target volume [66]. Patel et al. [65] assumed that a highly conformal dose distribution in the target volume allows the sparing of healthy tissue, especially the circulating components of the immune system and lymphatic tissue, which minimizes the immunological effect outside the target volume. The dose gradient resulting from the inverse square law produces multiple immunological mechanisms, ranging from immunogenic cell death and the release of tumor-specific antigens in the highest dose regions, to increased cytokine release in the mid-dose region, and finally to the short-term depletion of tumor-infiltrating suppressive lymphocytes in the surrounding cancer tissues [65].
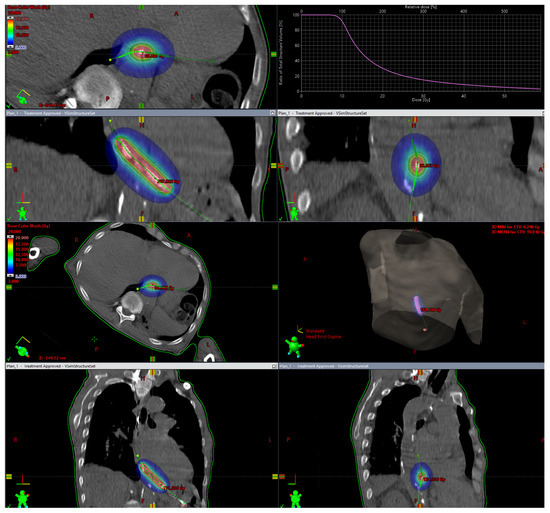
Figure 3.
Brachytherapy. Tumor in the lower esophagus. Target volume is relatively small, and one can observe rapid dose decay outside the irradiated target volume and a high radiation dose in the target volume.
Current progress in radiotherapy largely relies on the precise administration of high fractional doses. However, the effect of damaging cancer cells outside the irradiation area has long been described [66]. The so-called abscopal effect results from the activation of the body’s immune system caused by radiation. The mechanism of the abscopal effect is based on several phenomena. In addition to double-strand DNA breaks, the tumor microenvironment is damaged by the action of reactive oxygen species on the endothelium of tumor blood vessels [67]. Moreover, damaged cancer cells release tumor antigens and damage-associated molecular patterns (DAMPs) into the environment [68,69]. Additionally, Calreticulin is released, which increases the ability to present tumor antigens on dendritic cells. As a result, the release of antigens stimulates the immune system and produces CD8+ cytotoxic lymphocytes against cancer cells [70]. Low and intermediate doses of radiation can modulate the inflammatory environment by releasing pro-inflammatory cytokines from irradiated tumor cells, such as tumor necrosis factor-α (TNF-α), interferon-α (IFN-α), IFN-β, and IFN-γ [71,72]. Furthermore, in the cytoplasm of irradiated cells, cyclic GMP-AMP synthase (cGAS) binds to translocated double-stranded DNA (dsDNA) fragments induced by irradiation and activates a signaling cascade via the second messenger 2′3′-cGAMP (cGAMP) and the stimulator of interferon gene (STING), which leads to the increased production of type 1 interferon (IFN 1) in the tumor microenvironment [73,74]. INF 1 plays an important role in radiation-induced tumor destruction by activating the production of cytotoxic lymphocytes with the upregulation of PD1 and PD-L1 [72,74]. A similar mechanism of action has been demonstrated for pro-inflammatory chemokines such as CCL5, CXCL16, and CXCL10 [75,76].
Between 1969 and 2018, only 47 cases of metastasis reduction outside the irradiation field were described, which could be explained by the abscopal effect [77]. This effect did not depend on the type of cancer, dose, or fractionation method. Only Postow et al. [78] described the effect of the slight remission of lung cancer metastases treated with irradiation and immunotherapy against CTLA 4 (immune checkpoint inhibitors—ICIs)—Ipilimumab. Since then, there has been an increase in data regarding the abscopal effect of radiotherapy induced by immunotherapy [79]. The question arises whether the abscopal effect occurs when radiotherapy is administered alone. The addition of immune checkpoint inhibitors such as anti-CTLA4 and anti-PD1/PD-L1 act synergistically with radiotherapy to promote an effective anti-tumor cytotoxic lymphocyte response [80]. Experience from studies in other cancers indicated the benefit of combining high fractional doses of radiotherapy with immunotherapy, but the optimal dose has not been determined [81]. Studies have shown the benefits of various radiation regimens. For example, the study by Shaue et al. [82] showed that fractionated radiotherapy with a mean radiation dose of 7.5 Gy/fraction in 2 fractions provided the best immunogenic effect and the highest tumor control. This effect was better compared to both a single dose of 15 Gy and the 5 × 3 Gy or 3 × 5 Gy regimens. Similarly, in a previous study by Dewan et al. [83], it was found that the abscopal effect caused by anti-CTLA 4 immunotherapy is stronger in hypofractionated radiotherapy with 10 × 3 Gy than in the case of radiosurgery with 1 × 20 Gy.
The abscopal effect in esophageal cancer has only been found in a few case reports. In the oldest of these, a study by Rees et al. [84] in a 49-year-old patient reported an abcopal effect of the regression of lung metastases after the irradiation of the primary focus with a conventional (40 Gy/20 fr/2 Gy) regimen that persisted 6 months after treatment [84]. A shorter abscopal effect lasting 1.5–2 months was obtained in more recent studies using a hypofractionated radiotherapy regimen of 30 Gy/10 fr/3 Gy to the primary site. In the study by Bruton et al. [85], it concerned retroperitoneal lymph nodes, and in the study by Biswas et al. [86], it concerned retroperitoneal and mediastinal lymph nodes. The study by Zhao et al. [87], a combination of docetaxel and cisplatin chemotherapy was used in a 65-year-old man treated for advanced, poorly differentiated squamous cell carcinoma of the middle part of the esophagus with metastases to numerous lymph nodes. After four cycles of chemotherapy, pembrolizumab was administered as maintenance therapy. Despite an early good response to treatment detected in the PET-CT scan, after a month of immunotherapy, progression in the retroperitoneal lymph nodes and metastases to numerous pelvic lymph nodes were observed. After applying stereotactic radiotherapy using the Cyberknife technique in the 42 Gy/6 fr/7 Gy regimen to the retroperitoneal lymph nodes, the complete regression of all metastases was achieved lasting 8 months. Throughout the entire period, the patient received maintenance treatment with pembrolizumab [87].
There are only isolated reports of the abscopal effect in patients treated with immunotherapy and brachytherapy. In a single-center analysis of a series of three cases of patients with advanced adrenocortical carcinoma, combined treatment was used—brachytherapy and pembrolizumab. Two patients had a complete response and one had a partial response, which persisted at the last follow-up after 23, 45, and 4 months, respectively. Two patients experienced immune-related adverse events such as colitis (grade 3), gastroduodenal inflammation (grade 3), pneumonia (grade 2), and thyroiditis (grade 1) [88]. Similarly, an abscopal effect was also found after the brachytherapy and nivolumab treatment of iliac metastases from renal cell carcinoma [89]. Single data also concern the occurrence of the abscopal effect in the case of brachytherapy using permanent implants. In the study by Belia et al. [90], permanent implants of the alpha emitter Ra 224 were used in the treatment of multifocal skin cancer. One year after the treatment, the complete remission of all cancer lesions was observed. The intention of the authors was to further monitor the concentration of activated T lymphocytes, the increase in which would explain the immunological effect of this phenomenon. Also, an increase in the level of T lymphocytes (CD3+HLA-DR+, CD4+HLA-DR+, and CD8+HLA-DR+), a decrease in CD8+ memory T lymphocytes and a gradual increase in the ratio of activated T lymphocytes to regulatory T lymphocytes in peripheral blood after the LDR brachytherapy of the prostate induced effective immune response in patients, which may consequently reduce the frequency of relapses and the spread of the disease in this case [91].
Brachytherapy in esophageal cancer is a valuable method of treatment, and is primarily palliative. It is used primarily as a treatment for a primary lesion to reduce dysphagia; however, there are data available for the treatment of metastases, e.g., liver metastases [92].
There are no studies confirming the abscopal effect after esophageal brachytherapy, but available data from other cancers indicate the possibility of this phenomenon occurring in esophageal cancer, especially in the increasingly common combination of irradiation with immunotherapy.
5. Conclusions
The role of brachytherapy in esophageal cancer is not fully established. It seems that it should have wider application than it does in practice. There are few papers, especially randomized trials, on this topic. Brachytherapy can be a complement to teletherapy if it is necessary to increase the dose due to local control. At the same time, brachytherapy may be an alternative to surgery in some cases in the early stages of the disease. Increasing the dose may contribute to the radicalization of treatment and thus prevent local recurrence and treatment failure in patients not qualified for surgical treatment. In recent years, the role of immunotherapy has significantly increased, mainly in the treatment of metastatic esophageal cancer. Radiobiological mechanisms such as the abscopal effect provide hope for the benefit of combining brachytherapy with immunotherapy.
The conducted studies show that combining brachytherapy with immunotherapy may also bring satisfactory results in metastatic disease due to the possibility of an abscopal effect.
6. Future Direction
Randomized studies are needed to determine whether and when the use of brachytherapy to radicalize radiochemotherapy may be an alternative to the surgical treatment of esophageal cancer. Research on the occurrence of the abscopal effect in the case of brachytherapy and the possibility of using it with immunotherapy also seems to be justified.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Soni, K.K.; Arora, V.; Chaudhary, A.; Kumar, H.S.; Tanwar, R.K.; Sharma, N.; Jakhar, S.L.; Purohit, B.N. Comparative Study between conventional EBRT alone and EBRT followed by intraluminal brachytherapy in local advanced cancer esophagus. J. Radiat. Cancer Res. 2023, 14, 28–32. [Google Scholar]
- Lancellotta, V.; Cellini, F.; Fionda, B.; De Sanctis, V.; Vidali, C.; Fusco, V.; Frassine, F.; Tomasini, D.; Vavassori, A.; Gambacorta, M.A.; et al. The role of interventional radiotherapy (brachytherapy) in stage I esophageal cancer: An AIRO (Italian Association of Radiotherapy and Clinical Oncology) systematic review. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7589–7597. [Google Scholar]
- Pellat, A.; Dohan, A.; Soyer, P.; Veziant, J.; Coriat, R.; Barret, M. The Role of Magnetic Resonance Imaging in the Management of Esophageal Cancer. Cancers 2022, 14, 1141. [Google Scholar] [CrossRef] [PubMed]
- Dudzic, W.; Płatkowski, C.; Folwarski, M.; Meyer-Szary, J.; Kaźmierczak-Siedlecka, K.; Ekman, M.; Wojciechowicz, T.; Dobosz, M. Nutritional Status and the Outcomes of Endoscopic Stenting in Benign and Malignant Diseases of Esophagus. Nutrients 2023, 15, 1524. [Google Scholar] [CrossRef]
- Tai, P.; Yu, E. Esophageal cancer management controversies: Radiation oncology point of view. World J. Gastrointest. Oncol. 2014, 6, 263–274. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Farjah, F.; Gerdes, H.; et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 393–422. [Google Scholar] [CrossRef] [PubMed]
- Fuccio, L.; Mandolesi, D.; Farioli, A.; Hassan, C.; Frazzoni, L.; Guido, A.; de Bortoli, N.; Cilla, S.; Pierantoni, C.; Violante, F.S.; et al. Brachytherapy for the palliation of dysphagia owing to esophageal cancer: A systematic review and metaanalysis of prospective studies. Radiother. Oncol. 2017, 122, 332–339. [Google Scholar] [CrossRef]
- Zheng, H.; Kang, N.; Huang, Y.; Zhao, Y.; Zhang, R. Endoscopic resection versus esophagectomy for early esophageal cancer: A meta-analysis. Transl. Cancer Res. 2021, 10, 2653–2662. [Google Scholar] [CrossRef]
- Maingon, P.; d’Hombres, A.; Truc, G.; Barillot, I.; Michiels, C.; Bedenne, L.; Horiot, J.C. High dose rate brachytherapy for superficial cancer of the esophagus. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 71–76. [Google Scholar] [CrossRef]
- Murakami, Y.; Nagata, Y.; Nishibuchi, I.; Kimura, T.; Kenjo, M.; Kaneyasu, Y.; Okabe, T.; Hashimoto, Y.; Akagi, Y. Longterm outcomes of intraluminal brachytherapy in combination with external beam radiotherapy for superficial esophageal cancer. Int. J. Clin. Oncol. 2012, 17, 263–271. [Google Scholar] [CrossRef]
- Hishikawa, Y.; Kurisu, K.; Taniguchi, M.; Kamikonya, N.; Miura, T. High-dose-rate intraluminal brachytherapy (HDRIBT) for esophageal cancer. IJROBP 1991, 21, 1133–1135. [Google Scholar] [CrossRef]
- Nemoto, K.; Yamada, S.; Hareyama, M.; Nagakura, H.; Hirokawa, Y. Radiation therapy for superficial esophageal cancer: A comparison of radiotherapy methods. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 639–644. [Google Scholar] [CrossRef]
- Rovirosa, Á.; Tagliaferri, L.; Chicheł, A.; Lancellotta, V.; Zhang, Y.; Antelo, G.; Hoskin, P.; Steen-Banasik, E.V.; Biete, A.; Kovács, G. Why is a very easy, useful, old technique underused? An overview of esophageal brachytherapy—Interventional radiotherapy. J. Contemp. Brachytherapy 2022, 14, 299–309. [Google Scholar] [CrossRef]
- Murakami, M.; Kuroda, Y.; Nakajima, T.; Okamoto, Y.; Mizowaki, T.; Kusumi, F.; Hajiro, K.; Nishimura, S.; Matsusue, S.; Takeda, H. Comparison between chemoradiation protocol intended for organ preservation and conventional surgery for clinical T1-T2 esophageal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 277–284. [Google Scholar] [CrossRef]
- Ishikawa, H.; Sakurai, H.; Tamaki, Y.; Nonaka, T.; Yamakawa, M.; Saito, Y.; Kitamoto, Y.; Higuchi, K.; Hasegawa, M.; Nakano, T. Radiation therapy alone for stage I (UICC T1N0M0) squamous cell carcinoma of the esophagus: Indications for surgery or combined chemoradiotherapy. J. Gastroenterol. Hepatol. 2006, 21, 1290–1296. [Google Scholar] [CrossRef]
- Pasquier, D.; Mirabel, X.; Adenis, A.; Rezvoy, N.; Hecquet, G.; Fournier, C.; Coche-Dequeant, B.; Prevost, B.; Castelain, B.; Lartigau, E. External beam radiation therapy followed by high-dose-rate brachytherapy for inoperable superficial esophageal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1456–1461. [Google Scholar] [CrossRef]
- Tamaki, T.; Ishikawa, H.; Takahashi, T.; Tamaki, Y.; Kitamoto, Y.; Okamoto, M.; Noda, S.E.; Katoh, H.; Shirai, K.; Sakurai, H.; et al. Comparison of efficacy and safety of low-dose-rate vs. high-dose-rate intraluminal brachytherapy boost in patients with superficial esophageal cancer. Brachytherapy 2012, 11, 130–136. [Google Scholar] [CrossRef]
- Sai, H.; Mitsumori, M.; Araki, N.; Mizowaki, T.; Nagata, Y.; Nishimura, Y.; Hiraoka, M. Long-term results of definitive radiotherapy for stage I esophageal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 1339–1344. [Google Scholar] [CrossRef]
- Yamada, K.; Murakami, M.; Okamoto, Y.; Okuno, Y.; Nakajima, T.; Kusumi, F.; Takakuwa, H.; Matsusue, S. Treatment results of chemoradiotherapy for clinical stage I (T1N0M0) esophageal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1106–1111. [Google Scholar] [CrossRef]
- Shioyama, Y.; Nakamura, K.; Sasaki, T.; Ooga, S.; Urashima, Y.; Kimura, M.; Uehara, S.; Terashima, H.; Honda, H. Clinical results of radiation therapy for stage I esophageal cancer: A single institutional experience. Am. J. Clin. Oncol. 2005, 28, 75–80. [Google Scholar] [CrossRef]
- Nishimura, Y.; Okuno, Y.; Ono, K.; Mitsumori, M.; Nagata, Y.; Hiraoka, M. External beam radiation therapy with or without high-dose-rate intraluminal brachytherapy for patients with superficial esophageal carcinoma. Cancer 1999, 86, 220–228. [Google Scholar] [CrossRef]
- Yorozu, A.; Dokiya, T.; Oki, Y.; Suzuki, T. Curative radiotherapy with high-dose-rate brachytherapy boost for localized esophageal carcinoma: Dose-effect relationship of brachytherapy with the balloon type applicator system. Radiother. Oncol. 1999, 51, 133–139. [Google Scholar] [CrossRef]
- Gaspar, L.E.; Winter, K.; Kocha, W.I.; Coia, L.R.; Herskovic, A.; Graham, M. A phase I/II study of external beam radiation, brachytherapy, and concurrent chemotherapy for patients with localized carcinoma of the esophagus (Radiation Therapy Oncology Group Study 9207): Final report. Cancer 2000, 88, 988–995. [Google Scholar] [CrossRef]
- Kato, K.; Ito, Y.; Nozaki, I.; Daiko, H.; Kojima, T.; Yano, M.; Ueno, M.; Nakagawa, S.; Takagi, M.; Tsunoda, S.; et al. Parallel-Group Controlled Trial of Surgery Versus Chemoradiotherapy in Patients with Stage I Esophageal Squamous Cell Carcinoma. Gastroenterology 2021, 161, 1878–1886.e2. [Google Scholar] [CrossRef]
- Haneda, R.; Booka, E.; Ishii, K.; Kikuchi, H.; Hiramatsu, Y.; Kamiya, K.; Ogawa, H.; Yasui, H.; Takeuchi, H.; Tsubosa, Y. Evaluation of Definitive Chemoradiotherapy Versus Radical Esophagectomy in Clinical T1bN0M0 Esophageal Squamous Cell Carcinoma. World J. Surg. 2021, 45, 1835–1844. [Google Scholar] [CrossRef]
- Okawa, T.; Dokiya, T.; Nishio, M.; Hishikawa, Y.; Morita, K. Multi-institutional randomized trial of external radiotherapy with and without intraluminal brachytherapy for esophageal cancer in Japan. Japanese Society of Therapeutic Radiology and Oncology (JASTRO) Study Group. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 623–628. [Google Scholar] [CrossRef]
- van Hagen, P.; Hulshof, M.C.; van Lanschot, J.J.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.; Richel, D.J.; Nieuwenhuijzen, G.A.; Hospers, G.A.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef]
- Macdonald, J.S.; Smalley, S.R.; Benedetti, J.; Hundahl, S.A.; Estes, N.C.; Stemmermann, G.N.; Haller, D.G.; Ajani, J.A.; Gunderson, L.L.; Jessup, J.M.; et al. Chemoradiotherapy after Surgery Compared with Surgery Alone for Adenocarcinoma of the Stomach or Gastroesophageal Junction. N. Engl. J. Med. 2001, 345, 725–730. [Google Scholar] [CrossRef]
- Tepper, J.; Krasna, M.J.; Niedzwiecki, D.; Hollis, D.; Reed, C.E.; Goldberg, R.; Kiel, K.; Willett, C.; Sugarbaker, D.; Mayer, R. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J. Clin. Oncol. 2008, 26, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Yea, J.W.; Oh, S.A.; Park, J.W. Omitting surgery in esophageal cancer patients with complete response after neoadjuvant chemoradiotherapy: A systematic review and meta-analysis. Radiat. Oncol. 2021, 16, 219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, M.; Zhang, K.; Zheng, A.; Li, G.; Chen, S.; Chen, X.; Li, X.; Sheng, Y.; Sun, X.; et al. Concurrent Chemoradiation of Different Doses (50.4 Gy vs. 59.4 Gy) and Different Target Field (ENI vs. IFI) for Locally Advanced Esophageal Squamous Cell Carcinoma: Results from a Randomized, Multicenter Phase Ⅲ Clinical. IJROBP 2022, 114, S15. [Google Scholar] [CrossRef]
- Herskovic, A.; Martz, K.; Sarraf, M.; Leichman, L.; Brindle, J.; Vaitkevicius, V.; Cooper, J.; Byhardt, R.; Davis, L.; Emami, B. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N. Engl. J. Med. 1992, 326, 1593–1598. [Google Scholar] [CrossRef]
- Hujala, K.; Sipilä, J.; Minn, H.; Ruotsalainen, P.; Grenman, R. Combined external and intraluminal radiotherapy in the treatment of advanced oesophageal cancer. Radiother. Oncol. 2002, 64, 41–45. [Google Scholar] [CrossRef]
- Vuong, T.; Szego, P.; David, M.; Evans, M.; Parent, J.; Mayrand, S.; Corns, R.; Burtin, P.; Faria, S.; Devic, S. The safety and usefulness of high-dose-rate endoluminal brachytherapy as a boost in the treatment of patients with esophageal cancer with external beam radiation with or without chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 758–764. [Google Scholar] [CrossRef]
- López Carrizosa, M.C.; Samper Ots, P.M.; Rodríguez Pérez, A.; Sotoca, A.; Sáez Garrido, J.; de Miguel, M.M. High dose rate brachytherapy (HDR-BT) in locally advanced oesophageal cancer. Clinic response and survival related to biological equivalent dose (BED). Clin. Transl. Oncol. 2007, 6, 385–391. [Google Scholar] [CrossRef]
- Taal, B.G.; Aleman, B.M.; Koning, C.C.; Boot, H. High dose rate brachytherapy before external beam irradiation in inoperable oesophageal cancer. Br. J. Cancer 1996, 74, 1452–1457. [Google Scholar] [CrossRef][Green Version]
- Ye, M.; Han, D.; Mao, Z.; Cheng, G. A prospective study of radical external beam radiotherapy versus external beam radiotherapy combined with intraluminal brachytherapy for primary esophageal cancer. Brachytherapy 2022, 5, 703–711. [Google Scholar] [CrossRef]
- Muijs, C.T.; Beukema, J.C.; Mul, V.E.; Plukker, J.T.; Sijtsema, N.M.; Langendijk, J.A. External beam radiotherapy combined with intraluminal brachytherapy in esophageal carcinoma. Radiother. Oncol. 2012, 2, 303–308. [Google Scholar] [CrossRef]
- Someya, M.; Sakata, K.; Saito, A.; Nagakura, H.; Oouchi, A.; Hareyama, M. Results of external irradiation and low-dose-rate intraluminal brachytherapy for esophageal cancer. Acta Oncol. 2002, 41, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Calais, G.; Dorval, E.; Louisot, P.; Bourlier, P.; Klein, V.; Chapet, S.; Reynaud-Bougnoux, A.; Huten, N.; De Calan, L.; Aget, H.; et al. Radiotherapy with high dose rate brachytherapy boost and concomitant chemotherapy for Stages IIB and III esophageal carcinoma: Results of a pilot study. Int. J. Radiat. Oncol. Biol. Phys. 1997, 38, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Mangesius, J.; Hörmandinger, K.; Jäger, R.; Skvortsov, S.; Plankensteiner, M.; Maffei, M.; Seppi, T.; Dejaco, D.; Santer, M.; Sarcletti, M.; et al. Chemoradiotherapy Combined with Brachytherapy for the Definitive Treatment of Esophageal Carcinoma. Cancers 2023, 15, 3594. [Google Scholar] [CrossRef]
- Laskar, S.G.; Lewis, S.; Agarwal, J.P.; Mishra, S.; Mehta, S.; Patil, P. Combined brachytherapy and external beam radiation: An effective approach for palliation in esophageal cancer. J. Contemp. Brachytherapy 2015, 7, 453–461. [Google Scholar] [CrossRef]
- Aggarwal, A.; Harrison, M.; Glynne-Jones, R.; Sinha-ray, R.; Cooper, D.; Hoskin, P.J. Combination external beam radiotherapy and intraluminal brachytherapy for non-radical treatment of oesophageal carcinoma in patients not suitable for surgery or chemoradiation. Clin. Oncol. 2015, 27, 56–64. [Google Scholar] [CrossRef]
- Kissel, M.; Chirat, E.; Annede, P.; Burtin, P.; Fumagalli, I.; Bronsart, E.; Mignot, F.; Schernberg, A.; Dumas, I.; Haie-Meder, C.; et al. Esophageal brachytherapy: Institut Gustave Roussy’s experience. Brachytherapy 2020, 19, 499–509. [Google Scholar] [CrossRef]
- Li, C.; Zhao, S.; Zheng, Y.; Han, Y.; Chen, X.; Cheng, Z.; Wu, Y.; Feng, X.; Qi, W.; Chen, K.; et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur. J. Cancer 2021, 144, 232–241. [Google Scholar] [CrossRef]
- Yang, J.; Huang, A.; Yang, K.; Jiang, K. Neoadjuvant chemoradiotherapy plus tislelizumab followed by surgery for esophageal carcinoma (CRISEC study): The protocol of a prospective, single-arm, phase II trial. BMC Cancer 2023, 23, 249. [Google Scholar] [CrossRef]
- van den Ende, T.; de Clercq, N.C.; van Berge Henegouwen, M.I.; Gisbertz, S.S.; Geijsen, E.D.; Verhoeven, R.H.A.; Meijer, S.L.; Schokker, S.; Dings, M.P.G.; Bergman, J.J.G.H.M.; et al. Neoadjuvant Chemoradiotherapy Combined with Atezolizumab for Resectable Esophageal Adenocarcinoma: A Single-arm Phase II Feasibility Trial (PERFECT). Clin. Cancer Res. 2021, 27, 3351–3359. [Google Scholar] [CrossRef]
- Chen, R.; Liu, Q.; Li, Q.; Zhu, Y.; Zhao, L.; Liu, S.; Chen, B.; Liu, M.; Hu, Y.; Lin, T.; et al. A phase II clinical trial of toripalimab combined with neoadjuvant chemoradiotherapy in locally advanced esophageal squamous cell carcinoma (NEOCRTEC1901). eClin. Med. 2023, 62, 102118. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, C.; Zhang, T.; Chen, X.; Dong, J.; Zhao, J.; Han, D.; Wang, J.; Zhao, G.; Cao, F.; et al. Addition of camrelizumab to docetaxel, cisplatin, and radiation therapy in patients with locally advanced esophageal squamous cell carcinoma: A phase 1b study. Oncoimmunology 2021, 10, 1971418. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.H.; Kim, H.; Park, S.Y.; Kim, D.J.; Lee, C.G.; Cho, J.; Kim, J.H.; Kim, H.R.; Kim, Y.-H.; Park, S.R.; et al. A phase II trial of preoperative chemoradiotherapy and pembrolizumab for locally advanced esophageal squamous cell carcinoma (ESCC). Ann. Oncol. 2019, 30, 754. [Google Scholar] [CrossRef]
- Park, S.; Sun, J.M.; Choi, Y.L.; Oh, D.; Kim, H.K.; Lee, T.; Chi, S.A.; Lee, S.H.; Choi, Y.S.; Jung, S.H.; et al. Adjuvant durvalumab for esophageal squamous cell carcinoma after neoadjuvant chemoradiotherapy: A placebo-controlled, randomized, double-blind, phase II study. ESMO Open 2022, 7, 100385. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; Van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.; Motoyama, S.; Lièvre, A.; et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N. Engl. J. Med. 2021, 384, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Burchardt, W.; Chyrek, A.; Burchardt, E.; Bielęda, G.; Trojanowski, M.; Chicheł, A. Reducing dysphagia with palliative 2D high-dose-rate brachytherapy improves survival in esophageal cancer. J. Contemp. Brachytherapy 2019, 11, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Lancellotta, V.; Cellini, F.; Fionda, B.; De Sanctis, V.; Vidali, C.; Fusco, V.; Barbera, F.; Gambacorta, M.A.; Corvò, R.; Magrini, S.M.; et al. The role of palliative interventional radiotherapy (brachytherapy) in esophageal cancer: An AIRO (Italian Association of Radiotherapy and Clinical Oncology) systematic review focused on dysphagia-free survival. Brachytherapy 2020, 19, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Homs, M.Y.; Steyerberg, E.W.; Eijkenboom, W.M.; Tilanus, H.W.; Stalpers, L.J.; Bartelsman, J.F.; van Lanschot, J.J.; Wijrdeman, H.K.; Mulder, C.J.; Reinders, J.G.; et al. Single-dose brachytherapy versus metals stent placement for the palliation of dysphagia from oesophageal cancer: Multicenter randomized trial. Lancet 2004, 364, 1497–1504. [Google Scholar] [CrossRef]
- Van Rossum, P.S.N.; Jeene, P.M.; Rozema, T. Patient-reported outcomes after external beam radiotherapy versus brachytherapy for palliation of dysphagia in esophageal cancer: A matched comparison of two prospective trials. Radiother. Oncol. 2021, 155, 73–79. [Google Scholar] [CrossRef]
- Doki, Y.; Ajani, J.A.; Kato, K.; Xu, J.; Wyrwicz, L.; Motoyama, S.; Ogata, T.; Kawakami, H.; Hsu, C.H.; Adenis, A.; et al. CheckMate 648 Trial Investigators. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N. Engl. J. Med. 2022, 386, 449–462. [Google Scholar] [CrossRef]
- Sun, J.M.; Shen, L.; Shah, M.A.; Enzinger, P.; Adenis, A.; Doi, T.; Kojima, T.; Metges, J.P.; Li, Z.; Kim, S.B.; et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet 2021, 398, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Lu, J.; Bai, Y.; Mao, T.; Wang, J.; Fan, Q.; Zhang, Y.; Zhao, K.; Chen, Z.; Gao, S.; et al. ESCORT-1st Investigators. Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients with Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA 2021, 326, 916–925. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Kojima, T.; Shah, M.A.; Muro, K.; Francois, E.; Adenis, A.; Hsu, C.H.; Doi, T.; Moriwaki, T.; Kim, S.B.; Lee, S.H.; et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J. Clin. Oncol. 2020, 38, 4138–4148. [Google Scholar] [CrossRef]
- Kato, K.; Cho, B.C.; Takahashi, M.; Okada, M.; Lin, C.Y.; Chin, K.; Kadowaki, S.; Ahn, M.J.; Hamamoto, Y.; Doki, Y.; et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Zaidi, A.H.; Smith, M.A.; Omstead, A.N.; Kosovec, J.E.; Matsui, D.; Martin, S.A.; DiCarlo, C.; Werts, E.D.; Silverman, J.F.; et al. The Dynamic and Transient Immune Microenvironment in Locally Advanced Esophageal Adenocarcinoma Post Chemoradiation. Ann. Surg. 2018, 268, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Osipov, A.; Li, Q.; Thomassian, S.; Annamalai, L.; Yearley, J.H.; Rutgers, J.K.; Hendifar, A.E.; Tuli, R. Impact of chemoradiotherapy on PD1/PDL1 expression and clinical outcomes in gastroesophageal cancers. J. Clin. Oncol. 2017, 35, 4031. [Google Scholar] [CrossRef]
- Patel, R.B.; Baniel, C.C.; Sriramaneni, R.N.; Bradley, K.; Markovina, S.; Morris, Z.S. Combining brachytherapy and immunotherapy to achieve in situ tumor vaccination: A review of cooperative mechanisms and clinical opportunities. Brachytherapy 2018, 17, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Z. Nonlinear dose-response relationship in the immune system following exposure to ionizing radiation: Mechanisms and implications. Nonlinearity Biol. Toxicol. Med. 2003, 1, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.Q.; Dar, I.A.; Khan, T.; Lone, M.M.; Afroz, F. Radiation Therapy and its Effects beyond the Primary Target: An Abscopal Effect. Cureus 2019, 11, e4100. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liang, H.; Xu, M.; Yang, X.; Burnette, B.; Arina, A.; Li, X.D.; Mauceri, H.; Beckett, M.; Darga, T.; et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 2014, 41, 843–852. [Google Scholar] [CrossRef]
- Harding, S.M.; Benci, J.L.; Irianto, J.; Discher, D.E.; Minn, A.J.; Roger, A. Greenberg Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 2017, 548, 4. [Google Scholar] [CrossRef]
- Gameiro, S.R.; Jammeh, M.L.; Wattenberg, M.M.; Tsang, K.Y.; Ferrone, S.; Hodge, J.W. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget 2014, 5, 403–416. [Google Scholar] [CrossRef]
- Burnette, B.C.; Liang, H.; Lee, Y.; Chlewicki, L.; Khodarev, N.N.; Weichselbaum, R.R.; Fu, Y.X.; Auh, S.L. The efficacy of radiotherapy relies upon induction of type i interferon- dependent innate and adaptive immunity. Cancer Res. 2011, 71, 2488–2496. [Google Scholar] [CrossRef] [PubMed]
- Lugade, A.A.; Sorensen, E.W.; Gerber, S.A.; Moran, J.P.; Frelinger, J.G.; Lord, E.M. Radiation- induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J. Immunol. 2008, 180, 3132–3139. [Google Scholar] [CrossRef] [PubMed]
- Ablasser, A.; Goldeck, M.; Cavlar, T.; Deimling, T.; Witte, G.; Röhl, I.; Hopfner, K.-P.; Ludwig, J.; Hornung, V. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 2013, 498, 380–384. [Google Scholar] [CrossRef]
- Ishikawa, H.; Ma, Z.; Barber, G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 2009, 461, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Wang, B.; Kawashima, N.; Braunstein, S.; Badura, M.; Cameron, T.O.; Babb, J.S.; Schneider, R.J.; Formenti, S.C.; Dustin, M.L.; et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J. Immunol. 2008, 181, 3099–3107. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Demaria, S. Up-regulation of the pro-inflammatory chemokine CXCL16 is a common response of tumor cells to ionizing radiation. Radiat. Res. 2010, 173, 418–425. [Google Scholar] [CrossRef]
- Link, B.; Torres Crigna, A.; Hölzel, M.; Giordano, F.A.; Golubnitschaja, O. Abscopal effects in Metastatic Cancer: Is a Predictive Approach Possible to Improve Individual Outcomes? J. Clin. Med. 2021, 31, 5124. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef]
- Dagoglu, N.; Karaman, S.; Caglar, H.B.; Oral, E.N. Abscopal Effect of Radiotherapy in the Immunotherapy Era: Systematic Review of Reported Cases. Cureus 2019, 11, e4103. [Google Scholar] [CrossRef]
- Davis, A.A.; Patel, V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 278. [Google Scholar] [CrossRef]
- Kang, J.; Demaria, S.; Formenti, S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J. Immunother. Cancer 2016, 4, 51. [Google Scholar] [CrossRef]
- Schaue, D.; Ratikan, J.A.; Iwamoto, K.S.; McBride, W.H. Maximizing tumor immunity with fractionated radiation. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1306. [Google Scholar] [CrossRef]
- Dewan, M.Z.; Galloway, A.E.; Kawashima, N.; Dewyngaert, J.K.; Babb, J.S.; Formenti, S.C.; Demaria, S. Fractionated but Not Single-Dose Radiotherapy Induces an Immune-Mediated Abscopal Effect when Combined with Anti-CTLA-4 Antibody. Clin. Cancer Res. 2009, 15, 5379–5388. [Google Scholar] [CrossRef]
- Rees, G.J.; Ross, C.M. Abscopal regression following radiotherapy for adenocarcinoma. Br. J. Radiol. 1983, 56, 63–66. [Google Scholar] [CrossRef]
- Bruton Joe, M.; Truong, P.T. Abscopal effect after palliative radiation therapy for metastatic adenocarcinoma of the esophagus. Cureus 2018, 10, e3089. [Google Scholar] [CrossRef]
- Biswas, R.; Jindel, R.; Halder, A.; Sen, K.; Kabasi, A. Abscopal effect of radiation in metastatic esophageal carcinoma: Fourth reported case. Int. Cancer Conf. J. 2023, 12, 200–204. [Google Scholar] [CrossRef]
- Zhao, X.; Kang, J.; Zhao, R. Abscopal effect of radiation on lymph node metastasis in esophageal carcinoma: A case report and literature review. Oncol. Lett. 2018, 16, 3555–3560. [Google Scholar] [CrossRef]
- Schwarzlmueller, P.; Corradini, S.; Seidensticker, M.; Zimmermann, P.; Schreiner, J.; Maier, T.; Triebig, A.; Knösel, T.; Pazos, M.; Pfluger, T.; et al. High-Dose Rate Brachytherapy Combined with PD-1 Blockade as a Treatment for Metastatic Adrenocortical Carcinoma—A Single Center Case Series. Horm. Metab. Res. 2024, 56, 30–37. [Google Scholar] [CrossRef]
- Suzuki, G.; Masui, K.; Yamazaki, H.; Takenaka, T.; Asai, S.; Taniguchi, H.; Nakamura, T.; Ukimura, O.; Yamada, K. Abscopal effect of high-dose-rate brachytherapy on pelvic bone metastases from renal cell carcinoma: A case report. J. Contemp. Brachytherapy 2019, 11, 458–461. [Google Scholar] [CrossRef]
- Bellia, S.R.; Feliciani, G.; Duca, M.D.; Monti, M.; Turri, V.; Sarnelli, A.; Romeo, A.; Kelson, I.; Keisari, Y.; Popovtzer, A.; et al. Clinical evidence of abscopal effect in cutaneous squamous cell carcinoma treated with diffusing alpha emitters radiation therapy: A case report. J. Contemp. Brachytherapy 2019, 11, 449–457. [Google Scholar] [CrossRef]
- Kubo, M.; Satoh, T.; Ishiyama, H.; Tabata, K.I.; Tsumura, H.; Komori, S.; Iwamura, M.; Baba, S.; Hayakawa, K.; Kawamura, T.; et al. Enhanced activated T cell subsets in prostate cancer patients receiving iodine-125 low-dose-rate prostate brachytherapy. Oncol. Rep. 2018, 39, 417–424. [Google Scholar] [CrossRef]
- Omari, J.; Heinze, C.; Wilck, A.; Hass, P.; Seidensticker, M.; Damm, R.; Fischbach, K.; Ricke, J.; Pech, M.; Powerski, M. Image-guided interstitial high-dose-rate brachytherapy in the treatment of metastatic esophageal squamous cell carcinoma. J. Contemp. Brachytherapy 2018, 10, 439–445. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).