Abstract
In this study, we identified culturable microscopic fungi in soil from areas frequented by people (parks, allotments, and other green areas) in the city of Wrocław (Poland). In addition to general species analysis, attention was focused on keratin-degrading fungi. From 60 soil samples (12 study sites), we obtained 75 isolates of keratinophilic and keratinolytic fungi using the hair bait method, and 54 isolates of fungi were isolated on PDA (Potato Dextrose Agar) medium. Based on morphological and molecular analyses, a total of 37 strains were identified, classified into 2 phyla, 11 families, 17 genera, and 30 filamentous species. The mean values of the Shannon Diversity Index for both experimental variants ranged from 0.074 to 0.117. The most common species was the Penicillium genus, which accounted for 33.33% of all fungal species obtained in these studies. These fungi are common in both indoor and outdoor environments. However, particularly noteworthy in this study are the species belonging to the group of dermatophytes (Arthroderma uncinatum, Keratinophyton wagnerii, Nannizzia gypsea, and Paraphyton cookei), which may pose a real biological threat to humans and animals due to their well-known potential to cause dermatomycosis.
1. Introduction
Fungi, being cosmopolitan organisms, are common in many parts of the world and can thrive across a wide range of pH and temperatures [1,2]. Soil is one of the ecosystems that serves as their natural reservoir [3]. The diversity and activity of soil fungi are regulated by a variety of biotic (e.g., plant roots, soil fauna) and abiotic (e.g., moisture, temperature) factors [4,5]. Fungi are involved in the transformation of dead organic matter into biomass, carbon dioxide, and organic acids, among other processes [6]. Their ability to produce a wide range of extracellular enzymes allows them to decompose all types of organic matter, breaking down soil components, thereby regulating the carbon and nutrient balance in the soil [1]. As a result, fungi predominate in soils rich in organic matter, while their presence is reduced in soils subjected to intensive mineral exploitation [3]. Moreover, many species of fungi have the ability to biosorb toxic metals by accumulating them in fruiting bodies [7].
A large array of keratinolytic and keratinophilic fungi occur in both natural habitats and those affected by anthropogenic activity. They can be found in soil, sewage, freshwater, and many other samples in either urban areas or outside them [8]. Based on their occurrence, these fungi have been categorized by their preferred habitats: antropophilic (inhabiting and causing mycosis in humans), geophilic (inhabiting soils), and zoophilic (inhabiting and causing mycosis in animals). Their metabolic activity allows them to degrade keratinous material, which is present in skin or hair [9]. There is a specific group among them called dermatophytes. They are an etiological factor causing dermatophytosis both in humans and animals, although primarily they are considered as soil saprophytes [9,10]. They are classified into genera of Microsporum, Epidermophyton, Trichophyton, Lophophyton, Nannizzia, and Arthroderma. On a global scale, approximately 20–25% of the population has experienced mycosis, predominantly dermatophytosis [10].
Keratin, especially important in epidemiological terms, is a component decomposed by fungi. This filamentous polypeptide compound [11] is found in the cells of all of the known mammals [12]. The main function of the keratin is to protect epithelial cells. Damage to keratin may lead to ruptures and the death of epithelial cells. It is also found in appendages like hair or nails [11,13]. There is a group of enzymes called keratinases, capable of degrading keratin due to their proteolytic activity. This group has significant biotechnological potential due to its stability across a broad range of temperatures. Moreover, keratinases are active in alkaline to neutral pH, with optimal activity between pH 7.0 and 9.0. Keratinases can be found in a large quantity of microorganisms. Fungi are being recognized as great keratin decomposers due to their extracellular keratinase production [13].
Despite increasing urbanization, which affects the levels of diversification of human-related fungi found in urban soil, they are not systematically studied [14,15]. Therefore, the study of urban ecology, especially fungal communities, is becoming increasingly important for biodiversity conservation and maintaining biological safety. Relatively only a few studies focusing on fungal communities in soils in green urban areas are available [15,16,17,18]. An interesting study was presented by Abrego et al. [19], who compared fungal communities in the air and soil from natural and urban areas in Finland. In the research, they discovered that fungal abundance and diversity were significantly higher in samples from natural habitats than in urban habitats, both in air and soil [19].
The aim of this research is to present and compare the cultivable fungal diversity present in the soil across various urban locations in the city of Wrocław, Poland, with a particular focus on keratinophilic and keratinolytic species due to their potentially higher health risks.
2. Materials and Methods
2.1. Study Area
The city of Wrocław is located in southwestern Poland (51° N, 17° E) and is situated along the Oder River (Figure 1). The population is about 640,000 in a city area of 293 square kilometers. Most of Wrocław’s area (>60%) is covered by green and agricultural land. Buildings, both residential and industrial, make up about 1/3 of the city’s area [20]. The climate is mild and generally warm and temperate. Wrocław has a significant amount of rainfall during the year (ca. 700 mm). In Wrocław, the average annual temperature is 10.0 °C [21].
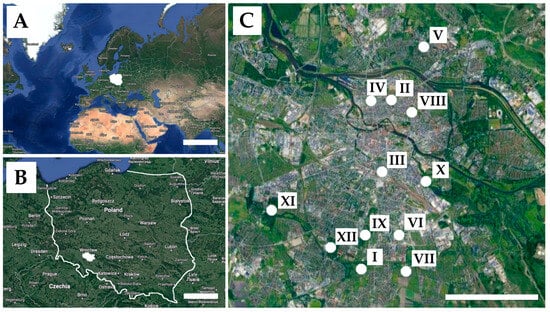
Figure 1.
Geographic location of Poland (A), the city of Wrocław (B), and sampling sites (C): I—“Gaj” Allotment Gardens, II—Słowiańska Hill, III—Dąbrowskiego Street, IV—Staszic Park, V—Bełza Square, VI—Słoneczny Park, VII—Allotment “Pod Topolami”, VIII—Tołpa Park, IX—Skowroni Park, X—Na Niskich Łąkach Park, XI—Grabiszyński Park, XII—Południowy Park. Scale bars: 1000 km (A), 40 km (B), and 2 km (C).
2.2. Sampling
Soil samples for the study were taken in October 2021 from 12 sites frequently used by people (Figure 1, Table 1). For this purpose, 5 soil samples were taken from each research site (parks, allotments, and other green areas), resulting in a total of 60 samples from all study sites. Each sample (0.5–1 kg) was collected from 0 to 30 cm of soil layer with a sterile spoon into appropriately labeled sterile string bags and then stored in a cooler (approximately 10 ± 0.5 °C) until mycological testing.

Table 1.
Basic information about study sites and their geographical coordinates.
2.3. Culture
For the general isolation of microscopic fungi, 3 g of soil separately from each sample was placed in polypropylene test tubes with 12 mL of sterile saline solution (0.85% NaCl). The samples were shaken at room temperature (24 ± 0.5 °C) for 20 min, diluted 50×, 500×, and 5000×, and vortexed; 100 µL of each dilution was spread on plates with PDA medium (Potato Dextrose Agar, BioMaxima, Lublin, Poland) in triplicate with the addition of antibiotics according to Ogórek et al. [22]: ampicillin (50 mg·L−1), chloramphenicol (50 mg·L−1), and cycloheximide (100 mg·L−1). They were incubated at room temperature (24 ± 0.5 °C) for 5 to 21 days in the darkness. Pure cultures were obtained using the single hyphal tip method, which involves cutting out fungal colony with a sterile scalpel and placing it in the center of a Petri dish with PDA using a sterile inoculation needle and incubated for 2 to 5 days at 24 ± 0.5 °C. Then, under aseptic conditions, a plate with a cultured colony was placed under a dissecting microscope to locate the hyphal tips, which were collected with a fungal inoculation loop and placed on the PDA slant for identification.
In order to isolate keratinophilic and keratinolytic fungi from the soil, a hair bait method described by Vanbreuseghem was used [23]. For this purpose, in sterile glass Petri dishes, under aseptic conditions, each tested soil sample was placed, onto which sterile human hair was then deposited in triplicate. The material thus prepared was incubated for eight/ten weeks at room temperature (24 ± 0.5 °C), constantly maintaining a moist environment, by systematic watering with sterile deionized water. Macroscopically visible mycelium was transferred to a PDA medium supplemented with ampicillin (50 mg·L−1), chloramphenicol (50 mg·L−1), and cycloheximide (100 mg·L−1) [22]. Pure cultures were obtained as mentioned above, and fungi on PDA slants were also used for identification.
2.4. Fungal Identification
Fungal identification was carried out both by morphological methods according to available keys or monographs [22,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44] and by molecular methods. The collection and comparison of the results obtained from both methods enabled the identification of the tested fungal cultures to the species level.
Overall, preliminary phenotypic identification was performed on PDA [37,45] and in the case of Aspergillus and Penicillium spp. additionally on CYA (Czapek yeast autolysate Agar) and MEA (malt extract agar, BioMaxima, Lublin, Poland) [40,46]. For this purpose, observations were made of the macromorphology of fungal colonies (inter alia colony growth rates and/or growth at various temperatures, texture, degree of sporulation, production of cleistothecia, colors of mycelia, sporulation, soluble pigments, exudates, and colony reverses) [37,40,45,46] and micromorphology (presence or absence of hyphae, spores and their size and appearance) using preparations from cultures on PDA and/or MEA [22,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46]. Moreover, depending on the genus, other micromorphological features were also determined, e.g., the number of branching points between vesicle and phialides, shape of conidial heads, color of stipes, and the dimensions, shapes, and textures of stipes, vesicles, metulae, phialides, and conidia were determined in Aspergillus [46]; the number of branching points between stipe and phialides, dimension, shape and texture of stipes, vesicles, metulae/branches (when present), phialides, and conidia were determined in Penicillium [40]; the absence or presence and appearance of macro- and microconidia and chlamydspores were determined in Fusarium [37]; the absence or presence and appearance of macro- and microconidia were determined in dermatophytes [37]. Then, molecular identification was performed. For this purpose, genomic DNA was isolated from pure cultures using a Bead-Beat Micro AX Gravity kit (A&A Biotechnology, Gdansk, Poland). Fungal internal transcribed spacer regions (ITS) were amplified using primers: ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATGC-3′) [47]. PCR was performed using a T100 thermocycler (Bio-Rad, Berkeley, CA, USA) according to Ogórek et al. [48]. The PCR product was purified using the Clean-Up Kit (A&ABiotechnology, Gdansk, Poland) and sequenced at Macrogen Europe by using high-quality Sanger sequencing.
2.5. Data Analyses
The raw fungal sequence reads were analyzed using the BioEdit Sequence Alignment Editor and compared with those deposited in the GenBank of the National Center for Biotechnology Information (NCBI, Bethesda, MD, USA) using the BLAST algorithm. The criteria according to Zhang et al. [49] were used to interpret sequences from the GenBank database. The acquired sequences were submitted to the GenBank database. The Shannon Diversity Index (H) was used to determine the diversity of fungal species [50,51].
3. Results
Overall, 129 fungal isolates were cultured by using two methods from 60 soil samples obtained from 12 study sites (Table 1). Of the isolates obtained, 72 were isolated using the hair bait method, targeting keratinophilic and keratinolytic fungi. In turn, 57 were cultured on a PDA medium, intended for general isolation without targeting a specific group of fungi. However, keratinophilic and keratinolytic species can also be obtained using this medium (Table 2).

Table 2.
Fungi cultured from the soil of parks and estates in the city of Wrocław. The BLAST analysis was performed on 18 November 2022. All values of query cover were 100%, and all E values were zero. ”V” indicates the fungi isolation by serial dilution on PDA medium and “P” indicates the fungi isolation by Vanbreuseghem hair bait, i.e., targeted mycological analysis for keratinophilic and keratinolytic fungi.
Fungi were divided into groups based on macro- and micromorphological characteristics. Through genetic analyses based on ITS primers, fungi cultured using the hair bait method were classified as 19 strains within 13 species and 11 genera, while fungi obtained on PDA were classified as 18 strains within 17 species and 6 genera. A total of 37 strains were identified from among 30 fungal species. PCR products of the ITS rDNA from fungal cultures obtained in these studies ranged from 358 to 585 bps. All values of query cover for obtained fungal ITS sequences were 100%, and all E values were zero. In turn, the identity of the obtained sequences ranged from 99.49% to 100%. Generated rDNA ITS fungal sequences were submitted to NCBI GenBank under accession numbers from PP034006 to PP034042 (Table 2).
Overall, fungi belonging to the genus Penicillium were the most frequently isolated species in this study (33.33% species—Table 2), and the frequency of their isolation from samples was 21.62%, generally, and 39.50% in the case of fungi cultured on PDA. Conversely, within keratinophilic and keratinolytic species isolated using the hair bait method, the genus Fusarium was the most frequently isolated, constituting 23.88% of all fungal species (Figure 2). Fungi of the genera Arthroderma, Keratinophyton, Metacordyceps, Nannizzia, Paraphyton, Simplicillium, and Trichocladium were isolated only by using the hair bait method. In turn, Clonostachys, Lecanicillium, Linnemannia, Penicillium, Pochonia, and Trichoderma were cultured only using the general culture method with a PDA medium. Species from the genera Aspergillus, Fusarium, Marquandomyces, and Purpureocillium were isolated using both isolation methods—the hair bait method and PDA medium (Figure 2).
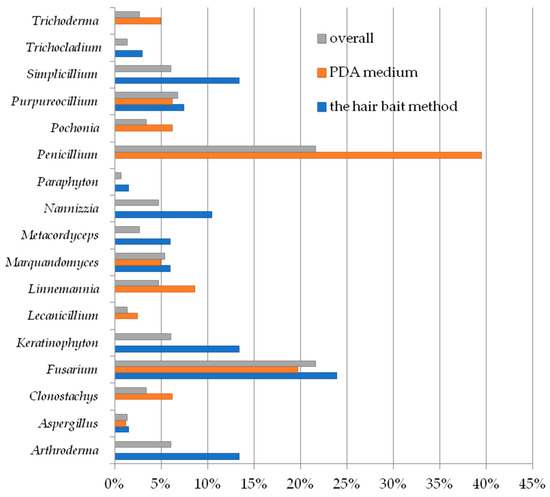
Figure 2.
The percentage contribution of each genus from the soil samples to the total.
The location from which the most keratinophilic and keratinolytic different fungal species (9 different species—the hair bait method) were isolated was location V (Bełza Square), and the least from location VI (Słoneczny Park)—3 different species (Table 3). In turn, the largest number of 13 different species of fungi cultured on PDA was isolated from location XII (Południowy Park), and 4 species were obtained from locations VIII (Tołpa Park) and XI (Grabiszyński Park)—Table 4.

Table 3.
Keratinophilic and keratinolytic fungal species cultured by using the hair bait method in each location. A (+) indicates the fungi were isolated from the study site and a (–) indicates the fungi were not isolated from the study site.

Table 4.
Fungal species cultured on a PDA medium in each location. A (+) indicates the fungi were isolated from the study site and a (–) indicates the fungi were not isolated from the study site.
Overall, the most frequently isolated species in this (16 times) study was Fusarium chlamydosporum and it constituted 10.81% of all cultured fungi. In turn, Paraphyton cookei, Penicillium canescens, Penicillium manginii, Penicillium scabrosum, and Penicillium virgatum were isolated only from one study site, and each of these species represented 0.67% of all fungal species (Figure 3, Table 3 and Table 4).
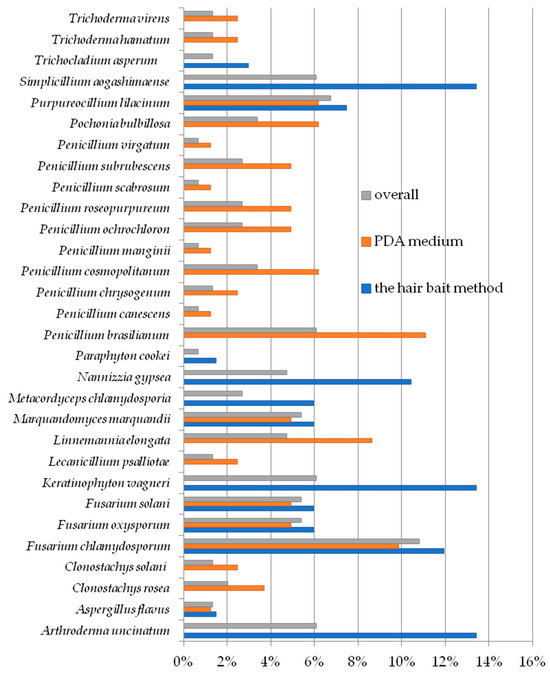
Figure 3.
The percentage contribution of each fungal species from the soil samples to the total.
Arthroderma uncinatum, Keratinophyton wagneri, and Simplicillium aogashimaense were isolated most often as a variant of a hair bait experiment. Each of these species was obtained in nine study locations and represented 13.43% of all fungi (Figure 3, Table 3). In addition, Aspergillus flavus and P. cookei were also the least cultured among the keratinophilic species, each accounting for 1.49% of all of them (Figure 3).
In the case of fungi cultured on PDA, the most frequently isolated species was Penicillium brasilianum, which was obtained in nine study locations and constituted 11.11% of species from this group of fungi. On the other hand, A. flavus, P. canescens, P. manginii, P. scabrosum, and P. virgatum were cultured from one study site, and each of them constituted 1.23% of the fungal species obtained in the variant experimented with PDA (Figure 3, Table 4).
The research sites showed variation in their Shannon Diversity Index values (Figure 4). Overall, the mean Shannon Diversity Index values across all research locations and both fungal isolation methods ranged from 0.074 for study sites VI (Allotment “Pod Topolami”), VIII (Tołpa Park), and XI (Grabiszyński Park) to 0.117 for study site XII (Południowy Park). In the case of fungi isolated using a PDA medium, the greatest species diversity of fungi was recorded also for study site XII (0.128 for Południowy Park) and the least species diversity index (0.065) for study sites VIII (Tołpa Park) and XI (Grabiszyński Park). In turn, the lowest species diversity of fungi was noted for study site VI (Słoneczny Park) for fungi isolated by the hair bait method (0.060), and the greatest species diversity index (0.117) was recorded for study site V (Bełza Square) (Figure 4).
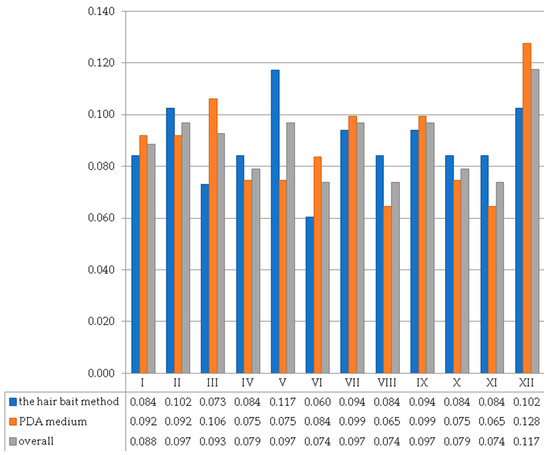
Figure 4.
The values of the Shannon Diversity Index calculated for individual research sites (I–XII) obtained using the hair bait method (keratinophilic and keratinolytic fungi), PDA medium (fungi generally), and overall for both methods (all fungal species).
4. Discussion
In this research, the most commonly isolated genus was Penicillium. Several of them, such as P. brasilianum and P. cosmopolitanum, were isolated from multiple locations, while P. canescens, P. manginii, P. scabrosum, and P. virgatum were isolated from only one location. These data may indicate that while the genus is considered cosmopolitan, not all individual species are ubiquitous. Since the Penicillium genus is one of the most common fungi found in rhizosphere soil, it is suspected to overshadow the results [52], as it interacts positively with crop roots and enhances their growth. Some species exhibit antagonistic activities against plant pathogens by producing antibiotics [53]. For instance, P. ochrlochloron is known for producing antibiotic molecules with high activity against multidrug-resistant bacteria [54]. Most species, however, are of dual environmental importance. On the one hand, the genus contains many species that are considered plant endophytes and are adroit at producing an extensive span of different active compounds that might have conceivable applications in the fields of biotechnology or medicine (such as isolated P. virgatum, P. canescens, P. subrubescens, and P. brasiliensis) [31,55,56,57]. On the other hand, some species are capable of producing harmful mycotoxins, which may cause food spoilage or crop damage [57]. Penicillium spp. spores commonly appearing in the environment in relatively large quantities are known as strong risk factors for asthma [58]. Penicillium spp. are some of the most predominant molds isolated from contaminated buildings with the so-called sick building syndrome (SBS) [59].
The majority of isolated species pertaining to this genus was P. brasilianum. The most of P. brasilianum strains reported in the literature are isolated from soil [57] and can cause losses in onion crops [60]. Additionally, this species can be considered an etiological factor in various human diseases. There is a report of a case of thumbnail infection in a patient living with HIV-1, including P. brasilianum [61]. Other species like P. chrysogenum that were isolated from the soil samples can also cause invasive infections in immunocompromised patients [62,63], including children [64]. Alarmingly, the first case of meningoencephalitis caused by P. chrysogenum was found in a patient previously considered immunocompetent and was recently described [65]. It should be noted that currently there is no antifungal remedy of choice for the management of penicilliosis [64].
Incorporating the general culture method with a PDA medium allowed for the isolation of a greater quantity of keratin-independent species common in the soil. Trichoderma hamatum and T. virens, identified in the study, represent the widely distributed Trichoderma genus in soil. Trichoderma has the ability to colonize plant roots; therefore, it has a promising prospect in the field of agriculture, especially in the context of biocontrol against the wide range of soil phytopathogens [66]. Pochonia bulbillosa, Lecanicillium psalliotae, Clonostachys solani, and C. rosea are recognized as entomopathogenic fungi [67,68,69]. P. bulbillosa can produce urease [70], whereas L. psalliotae has the capability to produce antibiotic and antifungal compounds [71]. On the other hand, Linnemannia elongata (basionym: Mortierella elongata) can decompose various toxic organic substances, potentially improving soil conditions and positively affecting plant development [72,73].
Another group of fungi cultivated and isolated from the soil samples consisted of keratinophilic/keratinolytic species obtained by the hair bait method. These fungi are usually found in the ecosystems near the habitats of animals and humans due to keratin degradation capabilities [74,75,76]. In this study, four species of geophilic dermatophytes were cultivated and identified, i.e., Arthroderma uncinatum, Keratinophyton wagnerii, Nannizzia gypsea, and Paraphyton cookei. Although infections by geophilic species are less common than those caused by anthropophilic and zoophilic species, the inflammatory response is usually more severe, and the duration of infection is generally shorter. Most likely, host-fungal adaptation results in a weakened immune response and a prolonged replication period. The reasoning behind this phenomenon is justified by the fact that geophiles are not adapted to the human host equivalently to anthropophilic species [77,78].
Arthroderma uncinatum (formerly Trichophyton ajelloi) was the only species isolated from the genus Arthroderma. It was found in 9 out of 12 presented sites. Widespread occurrence of A. uncinatum was also recorded in another Polish city during a study of keratinophilic fungal biodiversity in different soil types in Lublin. Arthroderma uncinatum was one of the four most frequently isolated fungi and the second most frequently isolated geophilic dermatophyte in that study [79,80,81,82,83,84]. Similar results were obtained by Mohanty and Prakash in a study of the pathogenicity of keratinophilic soil fungi against Culex quinquefasciatus mosquito larvae. At that time, A. uncinatum was also the only species isolated within the genus Arthroderma. Although it was not the most commonly isolated fungus, it was the most effective against mosquito larvae [85].
Keratinophyton wagnerii was also commonly found in the samples. This was the only fungus of the genus Keratinophyton that we isolated. It was first described by Labuda et al. [29] who observed that the newly described fungi had keratinolytic properties and the ability to degrade human hair. Ogórek et al. [86] also isolated K. wagnerii in their speleomycological study of three caves in Slovakia in 2022.
Nannizzia gypsea (formerly Microsporum gypseum) was isolated in 7 of the 12 sites tested. This fungus is one of the better-known representatives of the geophilic dermatophytes. Nannizzia gypsea is a cosmopolitan fungus, and it hardly ever causes disease in humans [87]. Scientists from Slovenia conducted a study over a period of 15 years (2000-2015). A total of 14,703 dermatophyte-positive cultures were isolated during the research period, and N. gypsea accounted for 1.5% of them. Almost 40% of those infected were children under the age of nine. It was also found that women were more likely to be infected than men in every age group [88]. Although dermatoses caused by N. gypsea represent only a small part of all dermatophyte mycoses, there are many reports of its isolation from patients in different regions of the world. Soankasina and colleagues described an interesting case of dermatophytosis of the smooth skin (tinea corporis) in a 22-year-old woman from the Madagascar region. A detailed medical history revealed that she probably contracted the infection through contact with free-living cats [89]. Infection with N. gypsea by contact with an infected animal has also been observed in a 2-year-old boy from Italy with signs of infection on the scalp [90]. In contrast, geophyte dermatophyte infections linked to direct contact with the soil were observed in a study conducted in eastern Poland among farmers and non-farmers with suspected fungal infections of the skin or its appendages. It should be emphasized, however, that no significant differences in infection were found between farmers and non-farmers, and the identified geophilic fungi were responsible for superficial mycosis and/or onychomycosis [91].
In this study, the presence of Paraphyton cookei was observed at one of the sites. Paraphyton cookei exhibits hair decomposition and a positive effect in the in vitro hair perforation test, as observed by Ogórek et al. [22] studying a P. cookei strain isolated from a Slovakian cave. The pathogenic activity of P. cookei has also been demonstrated by researchers in Australia who isolated this species from skin lesions on the legs [92].
Several species of fungi that degrade keratin were isolated, although they do not belong to the group of dermatophytes. The Fusarium genus was the most frequently isolated among keratinophilic and keratinolytic fungi. The following species were isolated: Fusarium solani, Fusarium chlamydosporum, and Fusarium oxysporum. Although many Fusarium species are classified as soil saprophytes or plant pathogens, some have the ability to infect humans. Fusarium fungi are often considered as an etiological factor causing fungal keratitis [93]; moreover, there are multiple reports of superficial infections such as onychomycoses caused by Fusarium spp., mostly by F. solani [94]. Segal et al. [95] first described an invasive disease caused by F. chlamydosporum. In that study of an immunocompromised patient, lesions caused by F. chlamydosporum were localized in the nasal auricle. Many reports have focused on the negative effects of Fusarium on crops. Recently, in the Kashmir division of the northern Himalayan region of India, F. chlamydosporum has been observed as a dangerous pathogen decimating crops. While F. oxysporum and F. solani have often been described as pathogens causing chilli and brinjal diseases, F. chlamydosporum has been described for the first time in this region [96].
Fungi of the genus Simplicillium may perform many different functions in the environment whilst being found in many different ecological niches. Some are presenting saprophytic activity in soil [33] or freshwater [97]. Others can cause plant diseases [98] or maybe entomopathogenic [99]. In this study, there was one species of Simplicillium isolated—S. aogashimaense. It was first discovered in the Japanese islands by Nonaka’s team. Besides S. aogashimaense, other Simplicillium species such as S. subtropicum, S. minatense, S. cylindrosporum, and S. sympodiophorum have been isolated [33]. Simplicillium aogashimaense can be a fungal endophyte of plants, as in Brachiaria brizantha [100]. This species can also present mycoparasitic activity. Zhu et al. [101] demonstrated the inhibitory effect of this fungus on wheat powdery mildew, whereas it causes some of the highest crop losses in the world.
Tricholadium asperum is commonly found in the rhizosphere and can positively affect plant growth [102]. It is known for producing various enzymes, such as cellulases [103], elastase [104], or keratinase, so it has already been isolated from soil by the hair trap method [105].
The keratinolytic fungus Purpureocillium lilacinum (Paecilomyces lilacinus) was isolated from five localizations included in the study. Other results have been obtained by scientists studying soils from Slovakian national parks, where P. lilacinum was one of the most frequently isolated fungi with keratinophilic potential [106]. The keratinolytic activity of P. lilacinum was reported in a study by Cavello et al. [107], where the potential of a keratinase isolated from fungi of Argentine has been proved. In turn, Kotwal and Sumbali presented that Purpureocillium lilacinum has the ability to grow on animal (feathers) and human (hair, nails) tissues [108].
Two other species, Metacordyceps chlamydosporia and Marquandomyces marquandii, were isolated from four parks in Wrocław. Similar results were obtained by Javoreková et al. [106], where the isolation of M. chlamydosporia was claimed in soil from only one of the trialed parks [106]. M. chlamydosporia was also isolated from a Korean island of volcanic origin by other researchers. This was the first report of M. chlamydosporia in this area [109]. The ability of M. chlamydosporia to utilize keratin from hair was observed by Scott and Untereiner [110]. Metacordyceps marquandii produces keratinases that present high stability and degradation activity in a broad pH range. Gradišar and his research team studied keratinases isolated from M. marquandii. Tests conducted on said keratinases exhibited strong activity against stratum corneum and nails, while the ability of human and animal hair decomposition was not observed [111]. In contrast, other researchers showed similar degradation of keratin by M. marquandii and fungi of the genera Fusarium, Alternaria, and Lecanicillium in a bioassay using the Keratin Azure reagent [112].
Another study found that of 300 common fungi tested, Aspergillus flavus produced the most potent keratinases [113]. In this study, A. flavus was isolated from only one site—Skowroni Park. In contrast, in the study conducted by Mohanty and Prakash, it was isolated in abundance from soils near ponds. These fungi were isolated using the feather-baiting technique [85]. Aspergillus flavus was shown to grow excellently on feathers and human nails but performed moderate growth on human hair and slow growth on sheep wool [108].
The last species isolated was Clonostachys rosea, which has been observed at three sites. It is a very common species worldwide, in many types of habitats. It is most commonly isolated from soil [114]. Clonostachys rosea has been isolated in national parks in Slovakia [106]. When testing its keratinophilic properties, researchers showed moderate decomposition of feathers, human hair, and nails by C. rosea [108].
As previously mentioned, fungi contribute to soil ecosystems at various levels, including geophilic dermatophytes involved in keratin degradation. Infection with soil pathogens occurs through direct contact with contaminated soil. Therefore, it should be noted that fungi may pose a serious threat to children, the elderly, and immunosuppressed people, not only in the context of superficial dermatomycosis but also invasive infections [115].
5. Conclusions
This research has enabled a better understanding of the fungal communities that inhabit soil in urban areas commonly frequented by people. Specifically, this soil, beyond harboring standard saprotrophs, can also be a source of fungi with allergenic and pathogenic potential. The most common genus identified was Penicillium, a cosmopolitan group that constituted 33.33% of all identified fungal species. Particularly noteworthy, however, are species belonging to the group of dermatophytes (Arthroderma uncinatum, Keratinophyton wagnerii, Nannizzia gypsea, and Paraphyton cookei), which may pose a real biological threat to humans and animals due to their well-documented potential to cause dermatomycosis.
Author Contributions
Conceptualization, K.S.; methodology, K.S., K.K., W.S. and R.O.; software, K.S.; validation, K.S. and R.O.; formal analysis, R.O.; investigation, K.S., K.K., W.S. and R.O.; resources, K.S.; data curation, K.S.; writing—original draft preparation, K.S., K.K., W.S. and R.O.; writing—review and editing, K.S. and R.O.; visualization, K.S. and R.O.; supervision, K.S. and R.O.; project administration, K.S.; funding acquisition, K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank Jakub Suchodolski for his help with English correction.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Frąc, M.; Hannula, S.E.; Bełka, M.; Jędryczka, M. Fungal Biodiversity and Their Role in Soil Health. Front. Microbiol. 2018, 9, 707. [Google Scholar] [CrossRef] [PubMed]
- Frąc, M.; Jezierska-Tys, S.; Takashi, Y. Occurrence, detection, and molecular and metabolic characterization of heat-resistant fungi in soils and plants and their risk to human health. Adv. Agron. 2015, 132, 161–204. [Google Scholar]
- Kuzikova, I.L.; Medvedeva, N.G. Long-Chain Alkylphenol Biodegradation Potential of Soil Ascomycota. Dokl. Biol. Sci. 2023, 511, 228–234. [Google Scholar] [CrossRef] [PubMed]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrell, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Collaet, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Žifčáková, L.; Vetrovský, T.; Howe, A.; Baldrian, P. Microbial activity in forest soil reflects the changes in ecosystem properties between summer and winter. Environ. Microbiol. 2016, 18, 288–301. [Google Scholar] [CrossRef]
- Baldrian, P. Interactions of heavy metals with white-rot fungi. Enzym. Microb. Technol. 2003, 32, 78–91. [Google Scholar] [CrossRef]
- Gu, L.; Coulombe, P. Keratin function in skin epithelia: A broadening palette with surprising shades. Curr. Opin. Cell Biol. 2007, 19, 13–23. [Google Scholar] [CrossRef]
- Chu, P.G.; Weiss, L.M. Keratin expression in human tissues and neoplasms. Histopathology 2002, 40, 403–439. [Google Scholar] [CrossRef]
- Nnolim, N.; Udenigwe, C.; Okoh, A.; Nwodo, U. Microbial Keratinase: Next Generation Green Catalyst and Prospective Applications. Front. Microbiol. 2020, 11, 580164. [Google Scholar] [CrossRef]
- Ulfig, K. The occurrence of keratinolytic fungi in waste and waste-contaminated habitats. In Biology of Dermatophytes and Other Keratinophilic Fungi; Kushwaha, R.K.S., Guarro, J., Eds.; Revista Iberoamericana de Micología: Bilbao, Spain, 2000; Volume 17, pp. 44–50. [Google Scholar]
- Jain, N.; Sharma, M. Influence of Temperature and Culture Conditions on the Survival of Keratinophilic and Dermatophytic Fungi. Braz. Arch. Biol. Technol. 2022, 65, e22210337. [Google Scholar] [CrossRef]
- Begum, J.; Mir, N.A.; Lingaraju, M.C.; Buyamayum, B.; Dev, K. Recent advances in the diagnosis of dermatophytosis. J. Basic Microbiol. 2020, 60, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Newbound, M.; Mccarthy, M.A.; Lebel, T. Fungi and the urban environment: A review. Landsc. Urban Plan. 2010, 96, 138–145. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Shao, Q.Y.; Li, X.; Chen, W.H.; Liang, J.D.; Han, Y.F.; Huang, J.Z.; Liang, Z.Q. Culturable Fungi from Urban Soils in China I: Description of 10 New Taxa. Microbiol. Spectr. 2021, 9, e0086721. [Google Scholar] [CrossRef]
- Al-Defiery, M.E.; Al-Shaam, T.J.B.; Al-Husaniy, L.R. Fungal diversity in some soils of Hillah city. AIP Conf. Proc. 2023, 2977, 040016. [Google Scholar] [CrossRef]
- Lykov, I.N.; Pavlova, O.P.; Rudova, S.A. Sanitary and hygienic aspects of urban environment pollution by dog feces. In Proceedings of the IV International Scientific Conference: AGRITECH-IV-2020: Agribusiness, Environmental Engineering and Biotechnologies, Krasnoyarsk, Russia, 18–20 November 2020; IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 677, p. 052033. [Google Scholar] [CrossRef]
- McGuire, K.L.; Payne, S.G.; Palmer, M.I.; Gillikin, C.M.; Keefe, D.; Kim, S.J.; Gedallovich, S.M.; Discenza, J.; Rangamannar, R.; Koshner, J.A.; et al. Digging the New York City Skyline: Soil fungal communities in green roofs and city parks. PLoS ONE 2013, 8, e58020. [Google Scholar] [CrossRef] [PubMed]
- Abrego, N.; Crosier, B.; Somervuo, P.; Ivanova, N.; Abrahamyan, A.; Abdi, A.; Hämäläinen, K.; Junninen, K.; Maunula, M.; Purhonen, J.; et al. Fungal communities decline with urbanization-more in air than in soil. ISME J. 2020, 14, 2806–2815. [Google Scholar] [CrossRef] [PubMed]
- Szymanowski, M.; Kryza, M. GIS-based techniques for urban heatisland spatialization. Clim. Res. 2009, 38, 171–187. [Google Scholar] [CrossRef]
- Wrocław Climate (Poland). Data and graphs for weather & climate in Wrocław. 2023. Available online: https://en.climate-data.org/europe/poland/lower-silesian-voivodeship/wroc%C5%82aw-4531/ (accessed on 30 December 2023).
- Ogórek, R.; Piecuch, A.; Višňovská, Z.; Cal, M.; Niedźwiecka, K. First report on the occurence of dermatophytes of Microsporum cookei clade and close affinities to Paraphyton cookei in the Harmanecká Cave (Veľká Fatra Mts., Slovakia). Diversity 2019, 11, 191. [Google Scholar] [CrossRef]
- Vanbreuseghem, R. Technique biologique pour l’isolement des dermatophytes du sol [Biological technique for isolating dermatophytes from soil]. Ann. Soc. Belg. Med. Trop. 1952, 32, 173–178. [Google Scholar]
- Dukik, K.; de Hoog, G.S.; Stielow, J.B.; Freeke, J.; van den Ende, B.G.; Vicente, V.A.; Menken, S.B.J.; Ahmed, S.A. Molecular and Phenotypic Characterization of Nannizzia (Arthrodermataceae). Mycopathologia 2020, 185, 9–35. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Samson, R.A.; Stolk, A.C. A new species of Penicillium, P. scabrosum. Persoonia 1990, 14, 177–182. [Google Scholar]
- Hafizi, R.; Salleh, B.; Latiffah, Z. Morphological and molecular characterization of Fusarium solani and F. oxysporum associated with crown disease of oil palm. Braz. J. Microbiol. 2014, 44, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Houbraken, J.; Frisvad, J.C.; Samson, R.A. Taxonomy of Penicillium section Citrina. Stud. Mycol. 2011, 70, 53–138. [Google Scholar] [CrossRef] [PubMed]
- Kwasna, H.; Nirenberg, H.I. Delimitation of Penicillium virgatum sp. nov. and P. daleae on the basis of morphological and molecular characters. Mycol. Res. 2005, 109, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Labuda, R.; Bernreiter, A.; Hochenauer, D.; Kubátová, A.; Kandemir, H.; Schüller, C. Molecular systematics of Keratinophyton: The inclusion of species formerly referred to Chrysosporium and description of four new species. IMA Fungus 2012, 12, 17. [Google Scholar] [CrossRef]
- Luangsa-Ard, J.J.; Houbraken, J.; van Doorn, T.; Hong, S.B.; Borman, A.M.; Hywel-Jones, N.L.; Samson, R.A. Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS Microbiol. Lett. 2011, 321, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, S.; Houbraken, J.; Samson, R.A.; Frisvad, J.C.; Christensen, M.; Tuthill, D.E.; Koutaniemi, S.; Hatakka, A.; Lankinen, P. A new Penicillium species efficiently producing inulinase. Antonie Van Leeuwenhoek 2013, 103, 1343–1357. [Google Scholar] [CrossRef] [PubMed]
- Mongkolsamrit, S.; Khonsanit, A.; Thanakitpipattana, D.; Tasanathai, K.; Noisripoom, W.; Lamlertthon, S.; Himaman, W.; Houbraken, J.; Samson, R.A.; Luangsa-Ard, J. Revisiting Metarhizium and the description of new species from Thailand. Stud. Mycol. 2020, 95, 171–251. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, K.; Kaifuchi, S.; Masuma, R.; Omura, S. Five new species of Simplicillium from soil in Tokyo, Japan. Mycoscience 2013, 54, 42–53. [Google Scholar] [CrossRef]
- Schroers, H.J. A monograph of Bionectria (Ascomycota, Hypocreales, Bionectriaceae) and its Clonostachys anamorphs. Stud. Mycol. 2001, 46, 1–214. [Google Scholar]
- Schroers, H.-J.; Samuels, G.J.; Seifert, K.A.; Gams, W. Classification of the mycoparasite Gliocladium roseum in Clonostachys as C. rosea, its relationship to Bionectria ochroleuca, and notes on other Gliocladium-like fungi. Mycologia 1999, 91, 365–385. [Google Scholar] [CrossRef]
- Siddiquee, S. Morphology-Based Characterization of Trichoderma Species. In Practical Handbook of the Biology and Molecular Diversity of Trichoderma Species from Tropical Regions. Fungal Biology; Springer: Cham, Switzerland, 2017; pp. 41–73. [Google Scholar] [CrossRef]
- Summerell, B.A.; Salleh, B.; Leslie, J.F. A Utilitarian Approach to Fusarium Identification. Plant Dis. 2003, 87, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Thom, C. Cultural Studies of Species of Penicillium; Wentworth Press: Sydney, Australia, 1910; Volume 118, pp. 1–107. [Google Scholar]
- Vandepol, N.; Liber, J.; Desirò, A.; Na, H.; Kennedy, M.; Barry, K.; Grigoriev, I.V.; Miller, A.N.; O’Donnell, K.; Stajich, J.E.; et al. Resolving the Mortierellaceae phylogeny through synthesis of multi-gene phylogenetics and phylogenomics. Fungal Divers. 2020, 104, 267–289. [Google Scholar] [CrossRef] [PubMed]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.B.; Klaassen, C.H.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and nomenclature of the genus Penicillium. Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Yang, F.Y.; Meijer, M.; Kraak, B.; Sun, B.D.; Jiang, Y.L.; Wu, Y.M.; Bai, F.Y.; Seifert, K.A.; Crous, P.W.; et al. Redefining Humicola sensu stricto and related genera in the Chaetomiaceae. Stud. Mycol. 2019, 93, 65–153. [Google Scholar] [CrossRef]
- Zare, R.; Gams, W.; Evans, H.C. A revision of Verticillium sect. Prostrata. V. The genus Pochonia, with notes on Rotiferophthora. Nova Hedwig. 2001, 73, 51–86. [Google Scholar]
- Zare, R.; Gams, W. A revision of Verticillium section Prostrata. IV. The genera Lecanicillium and Simplicillium. Nova Hedwig. 2001, 73, 1–50. [Google Scholar] [CrossRef]
- Piecuch, A.; Ogórek, R. Quantitative and qualitative assessment of mycological air pollution in a dormitory bathroom with high humidity and fungal stains on the ceiling. Case Study. Pol. J. Environ. Stud. 2021, 30, 1955–1960. [Google Scholar] [CrossRef]
- Krzyściak., P.; Skóra, M.; Macura, A.B. Atlas of Human Pathogenic Fungi; MedPharm Polska: Wrocław, Poland, 2010. [Google Scholar]
- Samson, R.A.; Visagie, C.M.; Houbraken, J.; Hong, S.B.; Hubka, V.; Klaassen, C.H.; Perrone, G.; Seifert, K.A.; Susca, A.; Tanney, J.B.; et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014, 78, 141–173. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar] [CrossRef]
- Ogórek, R.; Dyląg, M.; Kozak, B. Dark stains on rock surfaces in Driny Cave (Little Carpathian Mountains, Slovakia). Extremophiles 2016, 20, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wei, X.L.; Zhang, Y.Q.; Liu, H.-Y.; Yu, L.-Y. Diversity and distribution of lichen-associated fungi in the Ny-Ålesund Region (Svalbard, High Arctic) as revealed by pyrosequencing. Sci. Rep. 2015, 14, 14850. [Google Scholar] [CrossRef]
- Spellerberg, I.F.; Fedor, P. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’ Index. Glob. Ecol. Biogeogr. 2003, 12, 177–179. [Google Scholar] [CrossRef]
- Shannon, C.E.; Wiener, W. The Mathematical Theory of Communication; University Illinois Press: Urbana, IL, USA, 1963; p. 360. [Google Scholar]
- Workneh, F.; Van Bruggen, A.H.C. Microbial density, composition, and diversity in organically and conventionally managed rhizosphere soil in relation to suppression of corky root of tomatoes. Appl. Soil Ecol. 1994, 1, 219–230. [Google Scholar] [CrossRef]
- Srinivasan, R.; Prabhu, G.; Prasad, M.; Mishra, M.; Chaudhary, M.; Srivastava, R. Penicillium. In Beneficial Microbes in Agro-Ecology, 1st ed.; Amaresan, N., Kumar, M.S., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Elservier: Amsderdam, The Netherlands, 2020; pp. 651–667. [Google Scholar] [CrossRef]
- Vrabl, P.; Siewert, B.; Winkler, J.; Schöbel, H.; Schinagl, C.W.; Knabl, L.; Orth-Höller, D.; Fiala, J.; Meijer, M.S.; Bonnet, S.; et al. Xanthoepocin, a photolabile antibiotic of Penicillium ochrochloron CBS 123823 with high activity against multiresistant gram-positive bacteria. Microb. Cell Factories 2022, 21, 1. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Sang, Z.; Qiu, K.; Wei, S.; Duan, F.; Zou, Z.; Tan, H. Two new secondary metabolites isolated from the fungus Penicillium virgatum T49-A. Fitoterapia 2023, 168, 105513. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Gong, Y.; Shi, Z.; Qi, C.; Chen, C.; Tong, Q.; Liu, J.; Wang, J.; Zhu, H.; Zhang, Y. Multioxidized aromatic polyketides produced by a soil-derived fungus Penicillium canescens. Phytochemistry 2022, 193, 113012. [Google Scholar] [CrossRef]
- Bazioli, J.M.; Amaral, L.D.S.; Fill, T.P.; Rodrigues-Filho, E. Insights into Penicillium brasilianum Secondary Metabolism and Its Biotechnological Potential. Molecules 2017, 22, 858. [Google Scholar] [CrossRef]
- Licorish, K.; Novey, H.S.; Kozak, P.; Fairshter, R.D.; Wilson, A.F. Role of Alternaria and Penicillium spores in the pathogenesis of asthma. J. Allergy Clin. Immunol. 1985, 76, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.J.; Straus, D.C. The roles of Penicillium and Aspergillus in sick building syndrome. Adv. Appl. Microbiol. 2004, 55, 215–238. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.K.; Han, G.D.; Oh, J.Y.; Chun, S.C.; Kim, K.D. Penicillium brasilianum as a novel pathogen of onion (Allium cepa L.) and other fungi predominant on market onion in Korea. Crop Prot. 2014, 65, 138–142. [Google Scholar] [CrossRef]
- Kaplun, O.; Kekatos, P.; Creed, M.; Psevdos, G. Penicillium brasilianum Fungal Infection of Thumb Nail in a Patient Living with HIV-1. Infect. Dis. Clin. Pract. 2019, 27, e11. [Google Scholar] [CrossRef]
- Barcus, A.L.; Burdette, S.D.; Herchline, T.E. Intestinal invasion and disseminated disease associated with Penicillium chrysogenum. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Shokouhi, S.; Tehrani, S.; Hemmatian, M. Mixed Pulmonary Infection with Penicillium notatum and Pneumocystis jiroveci in a Patient with Acute Myeloid Leukemia. Tanaffos 2016, 15, 53–56. [Google Scholar] [PubMed]
- Avilés-Robles, M.; Gómez-Ponce, C.; Reséndiz-Sánchez, J.; Rodríguez-Tovar, A.V.; Ceballos-Bocanegra, A.; Martínez-Rivera, Á. Disseminated penicilliosis due to Penicillium chrysogenum in a pediatric patient with Henoch–Schönlein syndrome. Int. J. Infect. Dis. 2016, 51, 78–80. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.V.M.; Corrêa-Moreira, D.; Mendes, T.V.; da Costa, G.L.; Vieira, R.M.; Buchele, C.M.N.; Lins, R.S.; Ferreira, A.B.T.B.C.; Veira, D.B.; Pedroso, R.S.A.; et al. First report of fungal meningoencephalitis by Penicillium chrysogenum in Brazil. Int. Infect. Dis. 2023, 126, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Xie, W.; Zhang, J.; Hu, Q. Biodiversity of Entomopathogenic Fungi in the Soils of South China. Microorganisms 2019, 7, 311. [Google Scholar] [CrossRef] [PubMed]
- Razinger, J.; Lutz, M.; Schroers, H.J.; Urek, G.; Grunder, J. Evaluation of insect associated and plant growth promoting fungi in the control of cabbage root flies. J. Econ. Entomol. 2014, 107, 1348–1354. [Google Scholar] [CrossRef]
- Iqbal, M.; Dubey, M.; McEwan, K.; Menzel, U.; Franko, M.A.; Viketoft, M.; Jensen, D.F.; Karlsson, M. Evaluation of Clonostachys rosea for Control of Plant-Parasitic Nematodes in Soil and in Roots of Carrot and Wheat. Phytopathology 2018, 108, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, H.; Kandula, D.R.W.; Hampton, J.G.; Stewart, A.; Leung, D.W.M.; Edwards, Y.; Smith, C. Urease producing microorganisms under dairy pasture management in soils across New Zealand. Geoderma Reg. 2017, 11, 78–85. [Google Scholar] [CrossRef]
- Harm, G.F.S.; Papanicolaou, A.; Cuddy, W.S.; Park, R.F.; Moffitt, M.C. Draft Genome Sequence of the Fungus Lecanicillium psalliotae Strain HWLR35, Isolated from a Wheat Leaf Infected with Leaf Rust (Caused by Puccinia triticina). Genome Announc. 2018, 6, e01442-17. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Redmile-Gordon, M.; Zhang, J.; Zhang, C.; Ning, Q.; Li, W. Mortierella elongata’s roles in organic agriculture and crop growth promotion in a mineral soil. Land Degrad. Dev. 2018, 29, 1642–1651. [Google Scholar] [CrossRef]
- Vandepol, N.; Liber, J.; Yocca, A.; Matlock, J.; Edger, P.; Bonito, G. Linnemannia elongata (Mortierellaceae) stimulates Arabidopsis thaliana aerial growth and responses to auxin, ethylene, and reactive oxygen species. PLoS ONE 2022, 17, e0261908. [Google Scholar] [CrossRef]
- Verekar, S.A.; Deshmukh, S.K. Keratinophilic fungi distribution, pathogenicity and biotechnological potentials. In Developments in Fungal Biology and Applied Mycology; Satyanarayana, T., Deshmukh, S., Johri, B., Eds.; Springer: Singapore, 2017; pp. 75–97. [Google Scholar]
- Călin, M.; Constantinescu-Aruxandei, D.; Alexandrescu, E.; Răut, I.; Doni, M.B.; Arsene, M.L.; Oancea, F.; Jecu, L.; Lazăr, V. Degradation of keratin substrates by keratinolytic fungi. Electron. J. Biotechnol. 2017, 28, 101–112. [Google Scholar] [CrossRef]
- Ali-Shtayeh, M.S.; Jamous, R.M. Keratinophilic fungi and related dermatophytes in polluted soil and water habitats. In Biology of Dermatophytes and other Keratinophilic Fungi; Kushwaha, R.K.S., Guarro, J., Eds.; Revista Iberoamericana de Micología: Bilbao, Spain, 2000; Volume 17, pp. 51–59. [Google Scholar]
- Ali-Shtayeh, M.S.; Arda, H.M. Isolation of keratinophilic fungi from floor dust in Arab elementary and preparatory schools in the West Bank of Jordan. Mycopathologia 1989, 106, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Moskaluk, A.E.; VandeWoude, S. Current topics in Dermatophyte classification and clinical diagnosis. Pathogens 2022, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- De Hoog, G.S.; Dukik, K.; Monod, M.; Packeu, A.; Stubbe, D.; Hendrickx, M.; Kupsch, C.; Stielow, J.B.; Freeke, J.; Göker, M.; et al. Toward a Novel Multilocus Phylogenetic Taxonomy for the Dermatophytes. Mycopathologia 2017, 182, 5–31. [Google Scholar] [CrossRef] [PubMed]
- Weitzman, I.; Summerbell, R.C. The dermatophytes. Clin. Microbiol. Rev. 1995, 8, 240–259. [Google Scholar] [CrossRef]
- Shtayeh, M.S.A.; Arda, H.M. Incidence of dermatophytosis in Jordan with special reference to tinea capitis. Mycopathologia 1985, 92, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Cortez, A.C.A.; de Souza, J.V.B.; Sadahiro, A.; de Oliveira, J.A.A. Frequency and aetiology of dermatophytosis in children age 12 and under in the state of Amazonas, Brazil. Rev. Iberoam. Micol. 2012, 29, 223–226. [Google Scholar] [CrossRef]
- Mantovani, A. The role of animals in the epidemiology of the mycoses. Mycopathologia 1978, 65, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Bohacz, J.; Korniłłowicz-Kowalska, T. Species diversity of keratinophilic fungi in various soil types. Cent. Eur. J. Biol. 2012, 7, 259–266. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Prakash, S. Comparative efficacy and pathogenicity of keratinophilic soil fungi against Culex quinquefasciatus larvae. Indian J. Microbiol. 2010, 50, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Ogórek, R.; Suchodolski, J.; Piecuch, A.; Przywara, K.; Višňovská, Z. Keratinophilic and Keratinolytic Fungi in Cave Ecosystems: A Culture-Based Study of Brestovská Cave and Demänovská Ľadová and Slobody Caves (Slovakia). Appl. Sci. 2022, 12, 1455. [Google Scholar] [CrossRef]
- Ginter, G. Ecology, epidemiology and clinical symptomatology of Microsporum gypseum infections. Mycoses 1989, 32, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Dolenc-Voljč, M.; Gasparič, J. Human infections with Microsporum gypseum complex (Nannizzia gypsea) in Slovenia. Mycopathologia 2017, 182, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Soankasina, A.H.; Rakotozandrindrainy, N.; Andrianteloasy, S.; Zafindraibe, N.J.; Rasamoelina, T.; Rafalimanana, C.; Cornet, M.; Razanakolona, L.R.; Rasamindrakotroka, A.; Andrianarivelo, M.R. Dermatophyte infection caused by Nannizzia gypsea: A rare case report from Madagascar. Med. Mycol. Case Rep. 2018, 20, 7–9. [Google Scholar] [CrossRef]
- Cruciani, D.; Papini, M.; Broccatelli, S.; Agnetti, F.; Spina, S.; Natalini, Y.; Crotti, S. Presumptive zoonotic kerion by Nannizzia gypsea: Case report. Front. Vet. Sci. 2021, 8, 718766. [Google Scholar] [CrossRef] [PubMed]
- Spiewak, R.; Szostak, W. Zoophilic and geophilic dermatophytoses among farmers and non-farmers in Eastern Poland. Ann. Agric. Environ. Med. 2000, 7, 125–129. [Google Scholar] [PubMed]
- Muir, D.B.; Pritchard, R.C.; Gregory, J.D. Dermatophytes identified at the Australian National Reference Laboratory in medical mycology 1966–1982. Pathology 1984, 16, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.Y.; Yao, Y.F.; Zhu, Y.F.; Zhang, Y.M.; Zhou, P.; Jin, Y.Q.; Zhang, B. Fungal spectrum identified by a new slide culture and in vitro drug susceptibility using Etest in fungal keratitis. Curr. Eye Res. 2005, 30, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Castro Lopez, N.; Casas, C.; Sopo, L.; Rojas, A.; Del Portillo, P.; Cepero de Garcia, M.C.; Restrepo, S. Fusarium species detected in onychomycosis in Colombia. Mycoses 2009, 52, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Segal, B.H.; Walsh, T.J.; Liu, J.M.; Wilson, J.D.; Kwon-Chung, K.J. Invasive infection with Fusarium chlamydosporum in a patient with aplastic anemia. J. Clin. Microbiol. 1998, 36, 1772–1776. [Google Scholar] [CrossRef] [PubMed]
- Parihar, T.J.; Sofi, M.Y.; Rasool, R.S.; Khursheed, S.; Bhat, Z.A.; Hussain, K.; Dhekale, B.; Zargar, S.M.; Hakak, A.S.; Shah, M.D.; et al. Fusarium chlamydosporum, causing wilt disease of chili (Capsicum annum L.) and brinjal (Solanum melongena L.) in Northern Himalayas: A first report. Sci. Rep. 2022, 12, 20392. [Google Scholar] [CrossRef]
- Liu, F.; Cai, L. Morphological and molecular characterization of a novel species of Simplicillium from China. Cryptogam. Mycol. 2012, 33, 137–144. [Google Scholar] [CrossRef]
- Chen, R.S.; Huang, C.C.; Li, J.C.; Tsay, J.G. First report of Simplicillium lanosoniveum causing brown spot on Salvinia auriculata and S. molesta in Taiwan. Plant Dis. 2008, 92, 1589. [Google Scholar] [CrossRef]
- Chen, W.H.; Han, Y.F.; Liang, J.D.; Liang, Z.Q. Taxonomic and phylogenetic characterizations reveal four new species of Simplicillium (Cordycipitaceae, Hypocreales) from Guizhou, China. Sci. Rep. 2021, 11, 15300. [Google Scholar] [CrossRef]
- Teasdale, S.E.; Caradus, J.R.; Johnson, L.J. Fungal endophyte diversity from tropical forage grass Brachiaria. Plant Ecol. Divers. 2018, 11, 611–624. [Google Scholar] [CrossRef]
- Zhu, M.; Duan, X.; Cai, P.; Li, Y.F.; Qiu, Z. Deciphering the genome of Simplicillium aogashimaense to understand its mechanisms against the wheat powdery mildew fungus Blumeria graminis f. sp. tritici. Phytopathol. Res. 2022, 4, 16. [Google Scholar] [CrossRef]
- Mazurkiewicz-Zapałowicz, K.; Wróbel, J.; Janowicz, K. Influence of Selected Soil Saprotrophes on Gas Exchange, Growth and Yield of Solanum Tuberosum. Acta Physiol. Plant 2004, 26, 157–164. [Google Scholar] [CrossRef]
- Semenov, A.M.; Batomunkueva, B.P.; Nizovtseva, D.V.; Panikov, N.S. Method of determination of cellulase activity in soils and in microbial cultures, and its calibration. J. Microbiol. Methods 1996, 24, 259–267. [Google Scholar] [CrossRef]
- Hopsu-Havu, V.K.; Sonck, C.E.; Tunnela, E. Production of Elastase by Pathogenic and Non-Pathogenic Fungi. Mycoses 2009, 15, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Ali-Shtayeh, M.S. Keratinophilic fungi isolated from children’s sandpits in the Nablus area, West Bank of Jordan. Mycopathologia 1988, 103, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Javoreková, S.; Labuda, R.; Maková, J.; Novák, J.; Medo, J.; Majerčíková, K. Keratinophilic fungi isolated from soils of long-term fold-grazed, degraded pastures in national parks of Slovakia. Mycopathologia 2012, 174, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Cavello, I.A.; Hours, R.A.; Cavalitto, S.F. Bioprocessing of “hair waste” by Paecilomyces lilacinus as a source of a bleach-stable, alkaline, and thermostable keratinase with potential application as a laundry detergent additive: Characterization and wash performance analysis. Biotechnol. Res. Int. 2012, 2012, 369308. [Google Scholar] [CrossRef] [PubMed]
- Kotwal, S.; Sumbali, G. Preferential utilization and colonization of keratin baits by different myco-keratinophiles. Springerplus 2016, 5, 1–6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, H.S.; Adhikari, M.; Yadav, D.R.; Kim, S.W.; Um, Y.H.; Lee, H.B.; Lee, Y.S. First report of Metacordyceps chlamydosporia (Cordyceps chlamydosporia) isolated from soil in Korea. Korean J. Mycol. 2016, 44, 48–50. [Google Scholar] [CrossRef]
- Scott, J.A.; Untereiner, W.A. Determination of keratin degradation by fungi using keratin azure. Med. Mycol. 2004, 42, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Gradišar, H.; Friedrich, J.; Krizaj, I.; Jerala, R. Similarities and specificities of fungal keratinolytic proteases: Comparison of keratinases of Paecilomyces marquandii and Doratomyces microsporus to some known proteases. Appl. Environ. Microbiol. 2005, 71, 3420–3426. [Google Scholar] [CrossRef] [PubMed]
- Hamm, P.S.; Mueller, R.C.; Kuske, C.R.; Porras-Alfaro, A. Keratinophilic fungi: Specialized fungal communities in a desert ecosystem identified using cultured-based and Illumina sequencing approaches. Microbiol. Res. 2020, 239, 126530. [Google Scholar] [CrossRef]
- Friedrich, J.; Gradišar, H.; Mandin, D.; Chaumont, J.P. Screening fungi for synthesis of keratinolytic enzymes. Lett. Appl. Microbiol. 1999, 28, 127–130. [Google Scholar] [CrossRef]
- Sun, Z.B.; Li, S.D.; Ren, Q.; Xu, J.L.; Lu, X.; Sun, M.H. Biology and applications of Clonostachys rosea. J. Appl. Microbiol. 2020, 129, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Kuzikova, I.L.; Medvedeva, N.G. Opportunistic Fungi as Contaminants of Human Environment and Their Potential Pathogenicity. Ekol. Cheloveka (Hum. Ecol.) 2021, 3, 4–14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).