Effects of Temperature and Salinity on the LMS (Lysosomal Membrane Stability) Biomarker in Clams Donax trunculus and Chamelea gallina
Abstract
1. Introduction
2. Materials and Methods
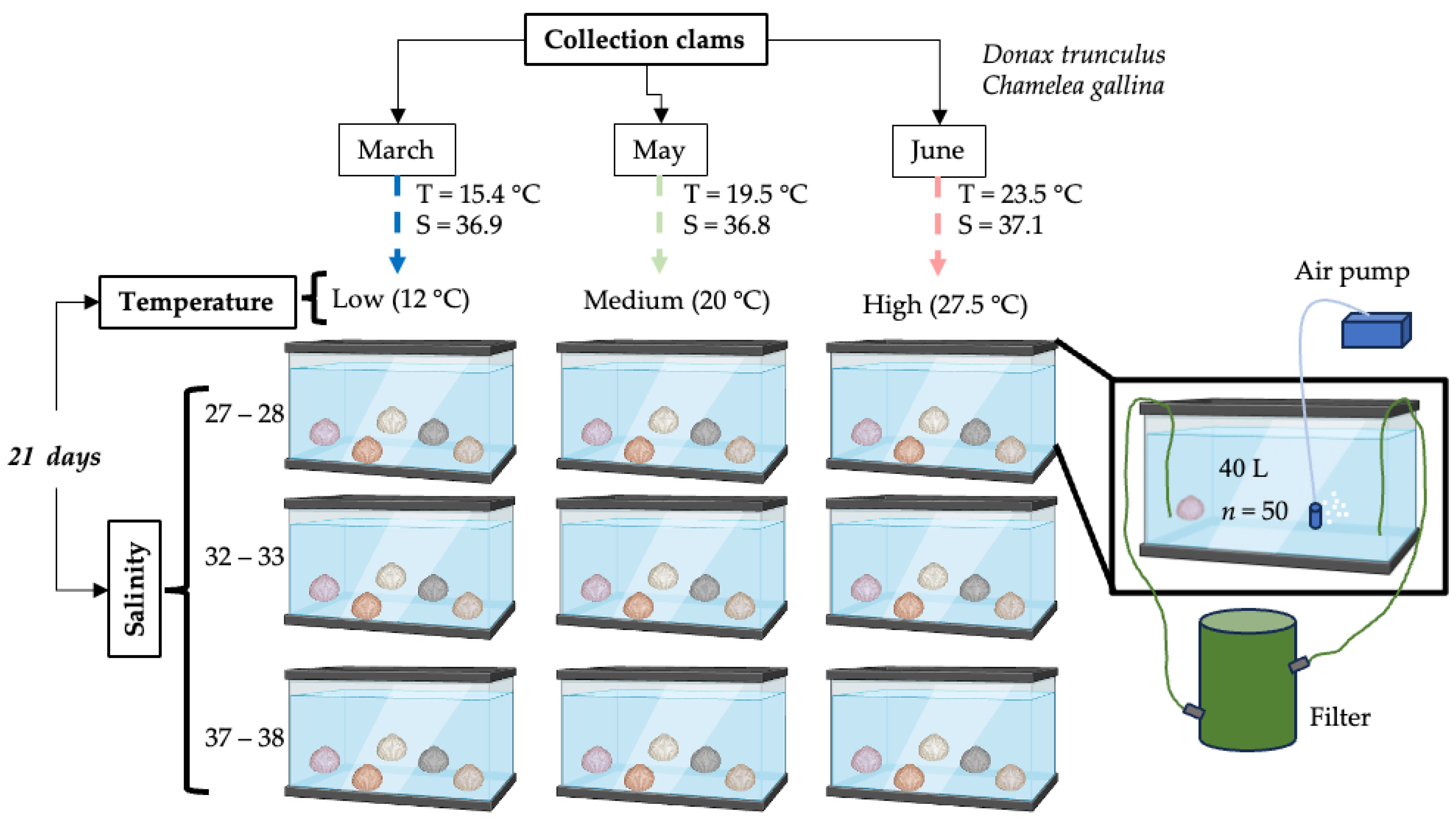
2.1. Collection of Clams and Maintenance
2.2. Exposure to Experimental Temperatures and Salinities
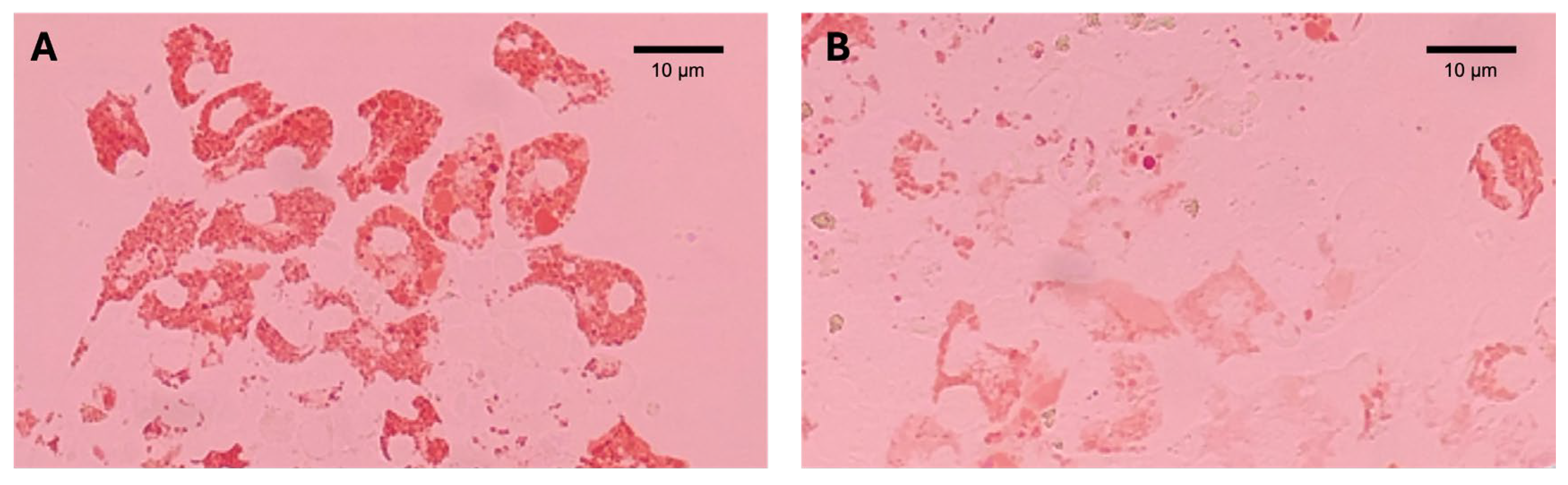
2.3. Lysosomal Membrane Stability (LMS)
2.4. Condition Index (CI)
2.5. Data Analysis
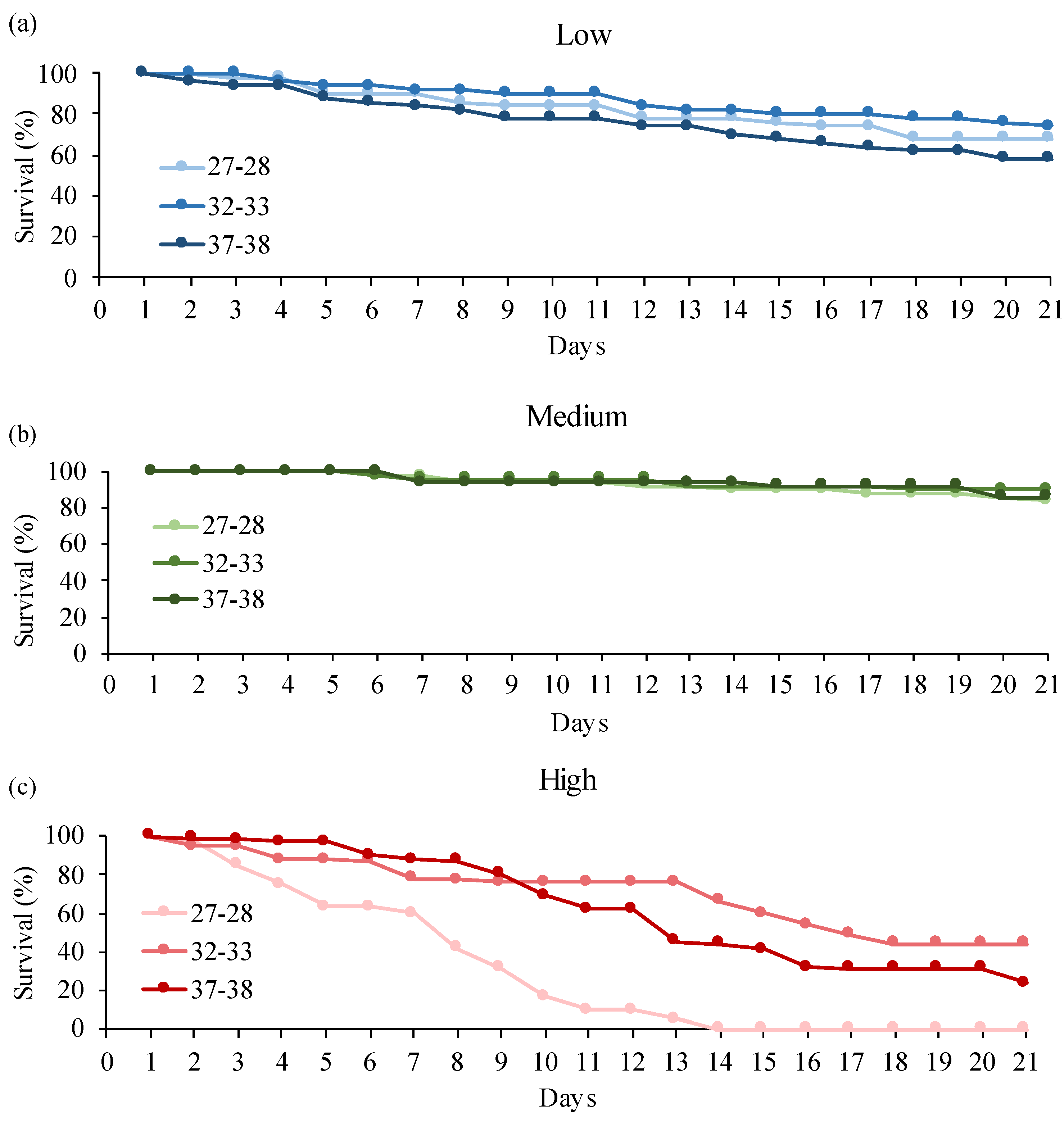
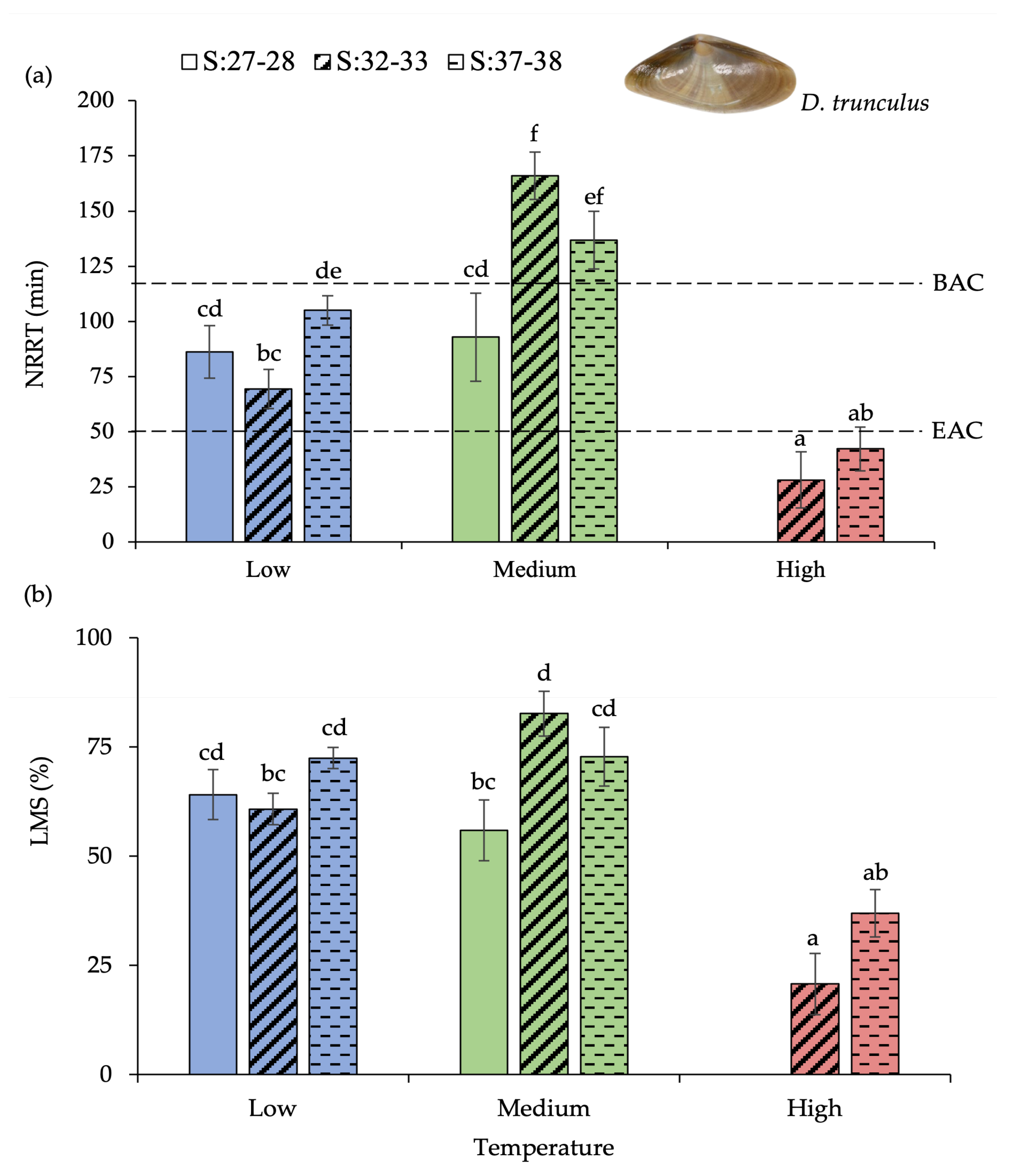
3. Results
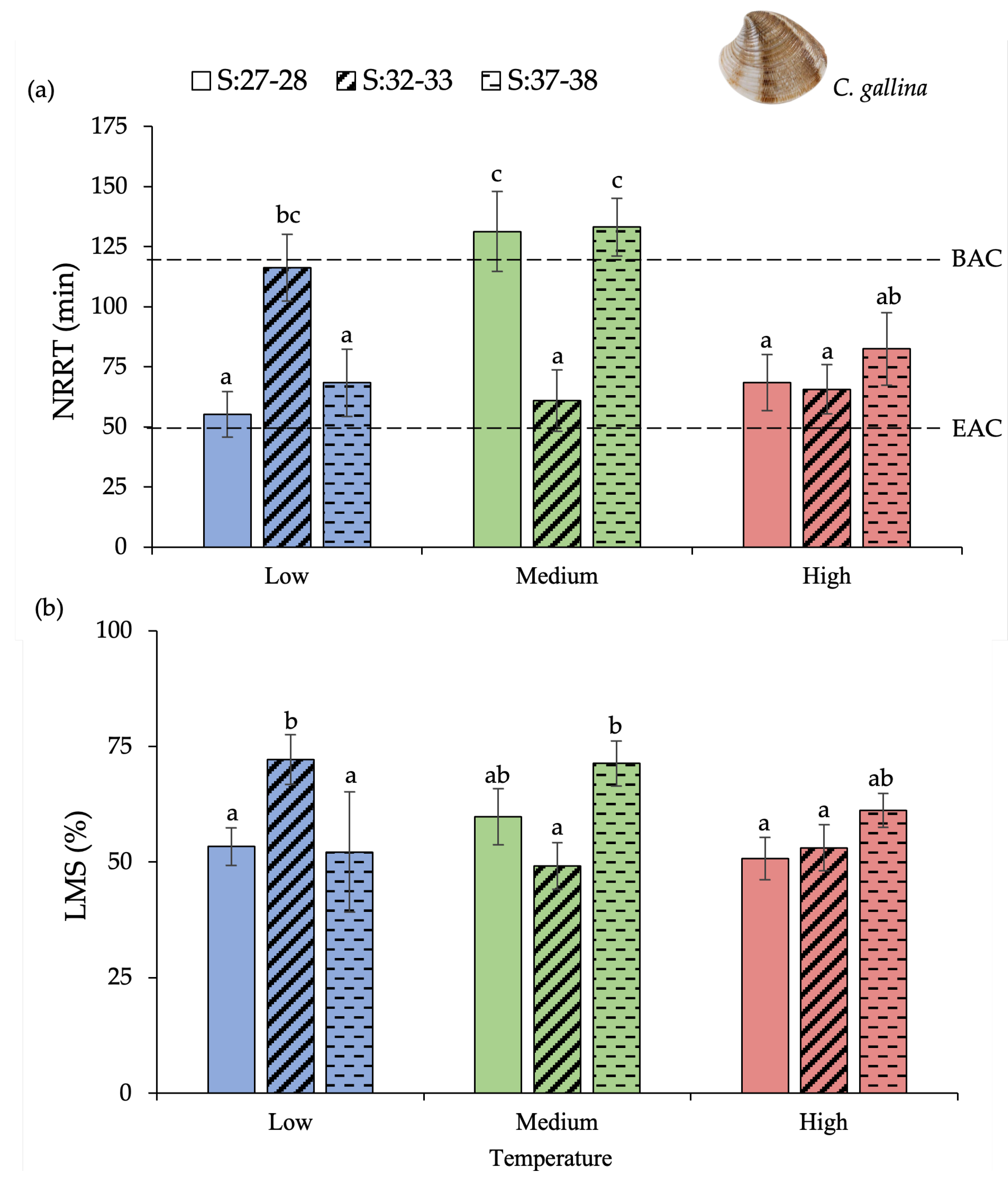
3.1. Chamelea Gallina
3.2. Donax Trunculus
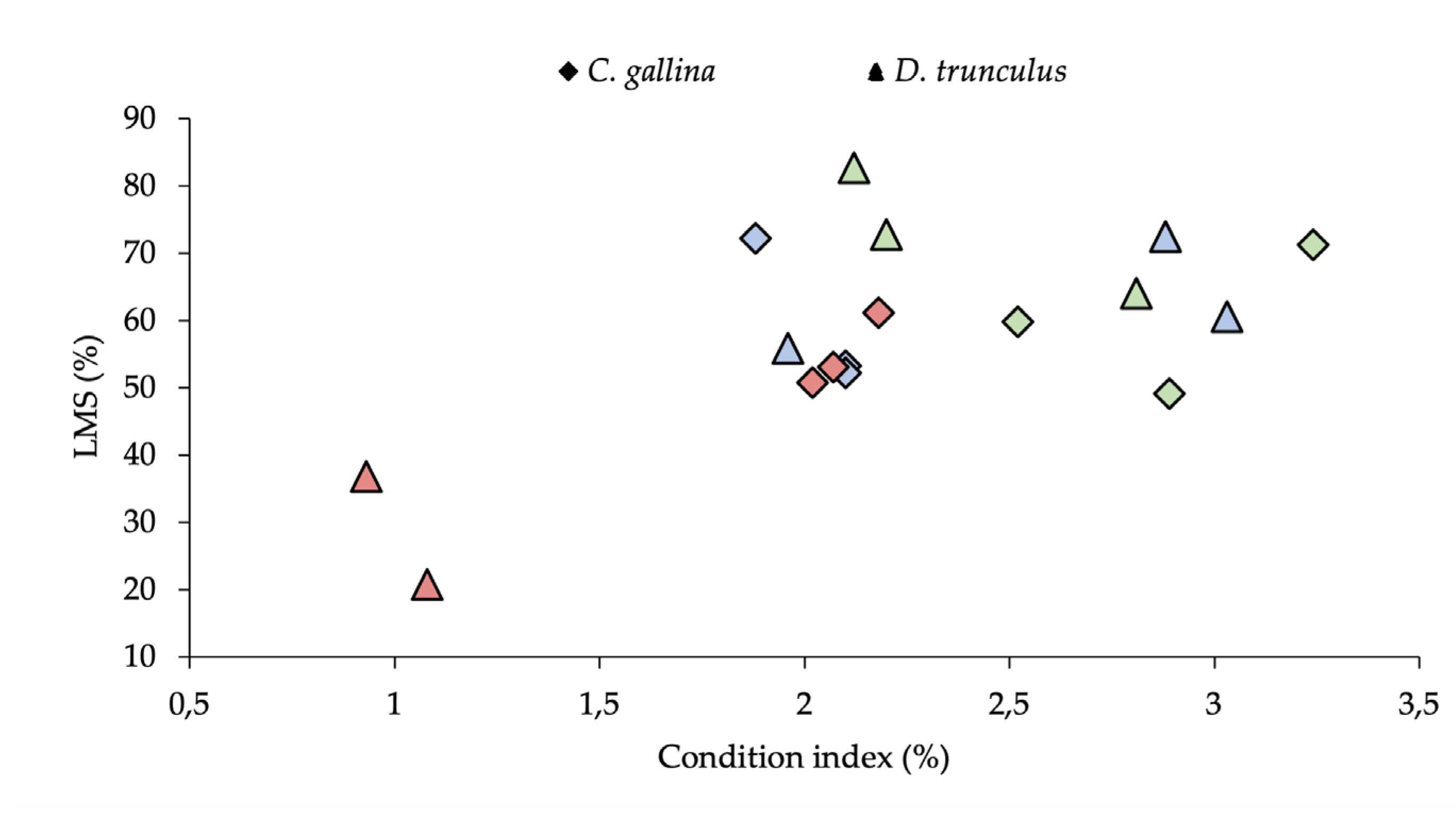
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baeta, M.; Solís, M.A.; Ballesteros, M.; Defeo, O. Long-Term Trends in Striped Venus Clam (Chamelea gallina) Fisheries in the Western Mediterranean Sea: The Case of Ebro Delta (NE Spain). Mar. Policy 2021, 134, 104798. [Google Scholar] [CrossRef]
- Escrivá, J.; Rodilla, M.; Llario, F.; Falco, S. The Collapse of a Wedge Clam Fishery in the Spanish Mediterranean Coast and Recovery Problems. J. Shellfish Res. 2021, 40, 37–47. [Google Scholar] [CrossRef]
- La Valle, P.; Nicoletti, L.; Finoia, M.G.; Ardizzone, G.D. Donax trunculus (Bivalvia: Donacidae) as a Potential Biological Indicator of Grain-Size Variations in Beach Sediment. Ecol. Indic. 2011, 11, 1426–1436. [Google Scholar] [CrossRef]
- Escrivá, J.; Rodilla, M.; Martin-Diaz, J.P.; Estruch, V.D.; Sebastiá-Frasquet, M.T.; Llario, F.; Falco, S. Driving Forces That Structure Sublittoral Macrobenthic Communities in Sandy Beaches along Environmental Gradients. Estuar. Coast. Shelf Sci. 2020, 233, 106517. [Google Scholar] [CrossRef]
- Picard, J. Recherches qualitatives sur les biocoenoses marines des substrats meubles dragables de la región marseillaise. Recl. Trav. Stn. Mar. Endoume 1965, 36, 1–160. [Google Scholar]
- de la Huz, R.; Lastra, M.; López, J. The Influence of Sediment Grain Size on Burrowing, Growth and Metabolism of Donax trunculus L. (Bivalvia: Donacidae). J. Sea Res. 2002, 47, 85–95. [Google Scholar] [CrossRef]
- Pérès, J.M.; Picard, J. Nouveau Manuel de Bionomie Benthique de la Mer Méditerranée; Station Marine d’Endoume: Marseille, France, 1964; Volume 31. [Google Scholar]
- Morello, E.B.; Froglia, C.; Atkinson, R.J.; Moore, P.G. Impacts of Hydraulic Dredging on a Macrobenthic Community of the Adriatic Sea, Italy. Can. J. Fish. Aquat. Sci. 2005, 62, 2076–2087. [Google Scholar] [CrossRef]
- Zeichen, M.M.; Agnesi, S.; Mariani, A.; Maccaroni, A.; Ardizzone, G.D. Biology and Population Dynamics of Donax trunculus L. (Bivalvia: Donacidae) in the South Adriatic Coast (Italy). Estuar. Coast. Shelf Sci. 2002, 54, 971–982. [Google Scholar] [CrossRef]
- Baeta, M.; Solís, M.A.; Frias-Vidal, S.; Claramonte, L.; Sepouna, A.; Ballesteros, M. The Wedge Clam (Donax trunculus) Hand-Operated Fishery in the NW Mediterranean Sea: Landings, Catch Composition, Damage Rates and Impact of Fishing Activity. Ocean Coast. Manag. 2023, 237, 106534. [Google Scholar] [CrossRef]
- Martínez-Patiño, D.; Otero, J.; Louzán, A.; Ojea, J.; Nóvoa, S.; Álvarez-Salgado, X.A. Wedge Clam (Donax trunculus Linnaeus, 1758) Reproduction: Reproductive Traits and Environmental Influence in the NW Iberian Coast and Contrast across Atlantic and Mediterranean Waters. Hydrobiologia 2021, 848, 1347–1366. [Google Scholar] [CrossRef]
- Poloczanska, E.S.; Brown, C.J.; Sydeman, W.J.; Kiessling, W.; Schoeman, D.S.; Moore, P.J.; Brander, K.; Bruno, J.F.; Buckley, L.B.; Burrows, M.T.; et al. Global Imprint of Climate Change on Marine Life. Nat. Clim. Chang. 2013, 3, 919–925. [Google Scholar] [CrossRef]
- Tlili, S.; Mouneyrac, C. The Wedge Clam Donax trunculus as Sentinel Organism for Mediterranean Coastal Monitoring in a Global Change Context. Reg. Environ. Chang. 2019, 19, 995–1007. [Google Scholar] [CrossRef]
- Giangrande, A.; Pierri, C.; Del Pasqua, M.; Gravili, C.; Gambi, M.C.; Gravina, M.F. The Mediterranean in Check: Biological Invasions in a Changing Sea. Mar. Ecol. 2020, 41, e12583. [Google Scholar] [CrossRef]
- Lejeusne, C.; Chevaldonné, P.; Pergent-Martini, C.; Boudouresque, C.F.; Pérez, T. Climate Change Effects on a Miniature Ocean: The Highly Diverse, Highly Impacted Mediterranean Sea. Trends Ecol. Evol. 2010, 25, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Gissi, E.; Manea, E.; Mazaris, A.D.; Fraschetti, S.; Almpanidou, V.; Bevilacqua, S.; Coll, M.; Guarnieri, G.; Lloret-Lloret, E.; Pascual, M.; et al. A Review of the Combined Effects of Climate Change and Other Local Human Stressors on the Marine Environment. Sci. Total Environ. 2021, 755, 142564. [Google Scholar] [CrossRef]
- Cheng, L.; von Schuckmann, K.; Abraham, J.P.; Trenberth, K.E.; Mann, M.E.; Zanna, L.; England, M.H.; Zika, J.D.; Fasullo, J.T.; Yu, Y.; et al. Past and Future Ocean Warming. Nat. Rev. Earth Environ. 2022, 3, 776–794. [Google Scholar] [CrossRef]
- Doney, S.C.; Fabry, V.J.; Feely, R.A.; Kleypas, J.A. Ocean Acidification: The Other CO2 Problem. Annu. Rev. Mar. Sci. 2009, 1, 169–192. [Google Scholar] [CrossRef]
- Harvey, B.P.; Gwynn-Jones, D.; Moore, P.J. Meta-Analysis Reveals Complex Marine Biological Responses to the Interactive Effects of Ocean Acidification and Warming. Ecol. Evol. 2013, 3, 1016–1030. [Google Scholar] [CrossRef]
- Kruft Welton, R.A.; Hoppit, G.; Schmidt, D.N.; Witts, J.D.; Moon, B.C. Reviews and Syntheses: The Clam before the Storm—A Meta-Analysis Showing the Effect of Combined Climate Change Stressors on Bivalves. Biogeosciences 2024, 21, 223–239. [Google Scholar] [CrossRef]
- Laubier, L. Mediterranean Sea and Humans: Improving a Conflictual Partnership. In The Mediterranean Sea; Saliot, A., Ed.; Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2005; Volume 5K, pp. 3–27. [Google Scholar] [CrossRef]
- Rivetti, I.; Fraschetti, S.; Lionello, P.; Zambianchi, E.; Boero, F. Global Warming and Mass Mortalities of Benthic Invertebrates in the Mediterranean Sea. PLoS ONE 2014, 9, e115655. [Google Scholar] [CrossRef]
- Pastor, F.; Khodayar, S. Marine Heat Waves: Characterizing a Major Climate Impact in the Mediterranean. Sci. Total Environ. 2023, 861, 160621. [Google Scholar] [CrossRef] [PubMed]
- Moschino, V.; Marin, M.G. Seasonal Changes in Physiological Responses and Evaluation of “Well-Being” in the Venus Clam Chamelea gallina from the Northern Adriatic Sea. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 145, 433–440. [Google Scholar] [CrossRef]
- Widdows, J.; Staff, F. Biological Effects of Contaminants: Measurement of Scope for Growth in Mussels; Report; International Council for the Exploration of the Sea (ICES): Copenhagen, Denmark, 2006. [Google Scholar] [CrossRef]
- Froglia, C. II Contributo della Ricerca Scientifica Alia Gestio-Ne della Pesca dei Molluschi Bivalvi con Draghe Idrauliche. Biol. Mar. Medit. 2000, 7, 71–82. [Google Scholar]
- Ansell, A.D.; Barnett, P.R.O.; Bodoy, A.; Massé, H. Upper Temperature Tolerances of Some European Molluscs II. Donax vittatus, D. semistriatus and D. trunculus. Mar. Biol. 1980, 58, 41–46. [Google Scholar] [CrossRef]
- Sokolov, E.P.; Sokolova, I.M. Compatible Osmolytes Modulate Mitochondrial Function in a Marine Osmoconformer Crassostrea Gigas (Thunberg, 1793). Mitochondrion 2019, 45, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Solan, M.; Whiteley, N. Stressors in the Marine Environment: Physiological and Ecological Responses; Societal Implications; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Parada, J.M.; Molares, J.; Otero, X. Multispecies Mortality Patterns of Commercial Bivalves in Relation to Estuarine Salinity Fluctuation. Estuaries Coasts 2012, 35, 132–142. [Google Scholar] [CrossRef]
- Reyes-Martínez, M.J.; Martínez-Pita, I.; Soler-Navarro, D.; García-García, F.J. The Impact of Salinity Changes Associated with Size on the Wedge Clam Donax trunculus Linnaeus, 1758 (Mollusca: Bivalvia): A Laboratory Assay. Estuar. Coast. Shelf Sci. 2020, 241, 106838. [Google Scholar] [CrossRef]
- Matozzo, V.; Monari, M.; Foschi, J.; Serrazanetti, G.P.; Cattani, O.; Marin, M.G. Effects of Salinity on the Clam Chamelea gallina. Part I: Alterations in Immune Responses. Mar. Biol. 2007, 151, 1051–1058. [Google Scholar] [CrossRef]
- Pantea, E.-D.; Coatu, V.; Damir, N.-A.; Oros, A.; Lazar, L.; Rosoiu, N. Lysosomal Membrane Stability of Mussel (Mytilus galloprovincialis Lamarck, 1819) as a Biomarker of Cellular Stress for Environmental Contamination. Toxics 2023, 11, 649. [Google Scholar] [CrossRef]
- Castro, M.; Santos, M.M.; Monteiro, N.M.; Vieira, N. Measuring Lysosomal Stability as an Effective Tool for Marine Coastal Environmental Monitoring. Mar. Environ. Res. 2004, 58, 741–745. [Google Scholar] [CrossRef]
- OSPAR Commission. Protecting and Conserving the North-East Atlantic and Its Resources. Available online: https://www.ospar.org/ (accessed on 23 January 2024).
- Hagger, J.A.; Lowe, D.; Dissanayake, A.; Jones, M.B.; Galloway, T.S. The Influence of Seasonality on Biomarker Responses in Mytilus edulis. Ecotoxicology 2010, 19, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.N. 9 Background Document: Lysosomal Stability as a Global Health Status Indicator in Biomonitoring. In Integrated Marine Environmental Monitoring of Chemicals and Their Effects; ICES: Copenhagen, Denmark, 2012; p. 68. [Google Scholar]
- Nacef, L.; Bachari, N.E.I.; Bouda, A.; Boubnia, R. Variability and Decadal Evolution of Temperature and Salinity in the Mediterranean Sea Surface. Int. J. Eng. Geosci. 2016, 1, 24–33. [Google Scholar] [CrossRef]
- Martínez-Gómez, C.; Bignell, J.P.; Lowe, D. Lysosomal Membrane Stability in Mussels. ICES Tech. Mar. Environ. Sci. 2015, 56, 1–46. [Google Scholar]
- Lowe, D.M.; Moore, M.N.; Evans, B.M. Contaminant Impact on Interactions of Molecular Probes with Lysosomes in Living Hepatocytes from Dab Limanda limanda. Mar. Ecol. Prog. Ser. 1992, 91, 135–140. [Google Scholar] [CrossRef]
- Guerrero-Rios, R.J.; Hernández, N.T.; Espinoza-Rodríguez, N.; Barrios-Garrido, H.; Montiel, M.; Morales, F. Composición de tallas e Índice de Condición de la Almeja Estuarina en Curarire, Estado Zulia, Venezuela. Zootec. Trop. 2015, 33, 327–336. [Google Scholar]
- Hansson, T.; Thain, J.E.; Martínez-Gómez, C.; Hylland, K.; Gubbins, M.J.; Balk, L. Supporting Variables for Biological Effects Measurements in Fish and Blue Mussel; ICES Techniques in Marine Environmental Sciences: Denmark, Copenhagen, 2017; No. 60. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, H. Review of Molluscan Bivalve Condition Index Calculations and Application in Northern Quahogs Mercenaria mercenaria. Aquac. Res. 2021, 52, 23–36. [Google Scholar] [CrossRef]
- Boscolo, R.; Cornello, M.; Giovanardi, O. Condition Index and Air Survival Time to Compare Three Kinds of Manila Clam Tapes philippinarum (Adams & Reeve) Farming Systems. Aquac. Int. 2003, 11, 243–254. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Ser. B Methodol. 1964, 26, 211–243. [Google Scholar] [CrossRef]
- de Almeida, J.M.B.; Gaspar, M.B.; Castro, M.; Rufino, M.M. Influence of Wind, Rainfall, Temperature, and Primary Productivity, on the Biomass of the Bivalves Spisula solida, Donax trunculus, Chamelea gallina and Ensis siliqua. Fish. Res. 2021, 242, 106044. [Google Scholar] [CrossRef]
- Munari, M.; Matozzo, V.; Chemello, G.; Riedl, V.; Pastore, P.; Badocco, D.; Marin, M.G. Seawater Acidification and Emerging Contaminants: A Dangerous Marriage for Haemocytes of Marine Bivalves. Environ. Res. 2019, 175, 11–21. [Google Scholar] [CrossRef]
- Fisher, M.R. Effect of Temperature and Salinity on Size at Maturity of Female Blue Crabs. Trans. Am. Fish. Soc. 1999, 128, 499–506. [Google Scholar] [CrossRef]
- Pourmozaffar, S.; Tamadoni Jahromi, S.; Rameshi, H.; Sadeghi, A.; Bagheri, T.; Behzadi, S.; Gozari, M.; Zahedi, M.R.; Abrari Lazarjani, S. The Role of Salinity in Physiological Responses of Bivalves. Rev. Aquac. 2020, 12, 1548–1566. [Google Scholar] [CrossRef]
- Camus, L.; Grøsvik, B.E.; Børseth, J.F.; Jones, M.B.; Depledge, M.H. Stability of Lysosomal and Cell Membranes in Haemocytes of the Common Mussel (Mytilus edulis): Effect of Low Temperatures. Mar. Environ. Res. 2000, 50, 325–329. [Google Scholar] [CrossRef]
- Hauton, C.; Hawkins, L.E.; Hutchinson, S. Response of Haemocyte Lysosomes to Bacterial Inoculation in the Oysters Ostrea edulis L. and Crassostrea gigas (Thunberg) and the Scallop Pecten maximus (L.). Fish Shellfish Immunol. 2001, 11, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Carducci, F.; Biscotti, M.A.; Trucchi, E.; Giuliani, M.E.; Gorbi, S.; Coluccelli, A.; Barucca, M.; Canapa, A. Omics Approaches for Conservation Biology Research on the Bivalve Chamelea gallina. Sci. Rep. 2020, 10, 19177. [Google Scholar] [CrossRef] [PubMed]
- Hauton, C.; Hawkins, L.E.; Hutchinson, S. The Use of the Neutral Red Retention Assay to Examine the Effects of Temperature and Salinity on Haemocytes of the European Flat Oyster Ostrea edulis (L). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998, 119, 619–623. [Google Scholar] [CrossRef]
- Munari, M.; Matozzo, V.; Marin, M.G. Combined Effects of Temperature and Salinity on Functional Responses of Haemocytes and Survival in Air of the Clam Ruditapes philippinarum. Fish Shellfish Immunol. 2011, 30, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Maher, W.A.; Ubrihien, R.P. Mortality, Condition Index and Cellular Responses of Anadara trapezia to Combined Salinity and Temperature Stress. J. Exp. Mar. Biol. Ecol. 2017, 497, 172–179. [Google Scholar] [CrossRef]
- Matozzo, V.; Chinellato, A.; Munari, M.; Finos, L.; Bressan, M.; Marin, M.G. First Evidence of Immunomodulation in Bivalves under Seawater Acidification and Increased Temperature. PLoS ONE 2012, 7, e33820. [Google Scholar] [CrossRef]
- Soon, T.K.; Ransangan, J. Extrinsic Factors and Marine Bivalve Mass Mortalities: An Overview. J. Shellfish Res. 2019, 38, 223–232. [Google Scholar] [CrossRef]
- Matozzo, V.; Monari, M.; Foschi, J.; Papi, T.; Cattani, O.; Marin, M.G. Exposure to Anoxia of the Clam Chamelea gallina: I. Effects on Immune Responses. J. Exp. Mar. Biol. Ecol. 2005, 325, 163–174. [Google Scholar] [CrossRef]
- Andreyeva, A.Y.; Kladchenko, E.S.; Gostyukhina, O.L. Effect of Hypoxia on Immune System of Bivalve Molluscs. Mar. Biol. J. 2022, 7, 3–16. [Google Scholar]
- Lowe, D.M.; Soverchia, C.; Moore, M.N. Lysosomal Membrane Responses in the Blood and Digestive Cells of Mussels Experimentally Exposed to Fluoranthene. Aquat. Toxicol. 1995, 33, 105–112. [Google Scholar] [CrossRef]
- Zhao, C.; Li, X.; Luo, S.; Chang, Y. Assessments of Lysosomal Membrane Responses to Stresses with Neutral Red Retention Assay and Its Potential Application in the Improvement of Bivalve Aquaculture. Afr. J. Biotechnol. 2011, 10, 13968–13973. [Google Scholar]
- Vethaak, A.D.; Davies, I.M.; Thain, J.E.; Gubbins, M.J.; Martínez-Gómez, C.; Robinson, C.D.; Moffat, C.F.; Burgeot, T.; Maes, T.; Wosniok, W. Integrated Indicator Framework and Methodology for Monitoring and Assessment of Hazardous Substances and Their Effects in the Marine Environment. Mar. Environ. Res. 2017, 124, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Monari, M.; Matozzo, V.; Foschi, J.; Cattani, O.; Serrazanetti, G.P.; Marin, M.G. Effects of High Temperatures on Functional Responses of Haemocytes in the Clam Chamelea gallina. Fish Shellfish Immunol. 2007, 22, 98–114. [Google Scholar] [CrossRef]
- Yu JinHa, Y.J.; Song JaeHee, S.J.; Choi MinChul, C.M.; Park SungWoo, P.S. Effects of Water Temperature Change on Immune Function in Surf Clams, Mactra veneriformis (Bivalvia: Mactridae). J. Invertebr. Pathol. 2009, 102, 30–35. [Google Scholar]
- Moore, M.N. Autophagy as a Second Level Protective Process in Conferring Resistance to Environmentally-Induced Oxidative Stress. Autophagy 2008, 4, 254–256. [Google Scholar] [CrossRef]
- Osada, M.; Matsumoto, T. Endocrine Control of Gametogenesis and Spawning in Bivalves. In Advances in Invertebrate (Neuro) Endocrinology. Volume 1: Phyla Other Than Arthropoda; Apple Academic Press: Palm Bay, FL, USA, 2021; pp. 179–219. [Google Scholar]
- Saucedo, P.E.; Southgate, P.C. Reproduction, Development and Growth. In Pearl Oyster; Elsevier: Oxford, UK, 2008; pp. 131–186. [Google Scholar]
- Moore, M.N.; Viarengo, A.G.; Somerfield, P.J.; Sforzini, S. Linking Lysosomal Biomarkers and Ecotoxicological Effects at Higher Biological Levels. In Ecological Biomarkers: Indicators of Ecotoxicological Effects; CRC Press Ltd.: Oxford, UK, 2012; pp. 107–130. [Google Scholar]
- Davies, I.M.; Gubbins, M.; Hylland, K.; Thain, J.; Maes, T.; Martínez-Gómez, C.; Giltrap, M.; Burgeot, T.; Wosniok, W.; Lang, T. 30 Technical Annex: Assessment Criteria for Biological Effects Measurements. In Integrated Marine Environmental Monitoring of Chemicals and Their Effects; ICES: Copenhagen, Denmark, 2012; p. 209. [Google Scholar]
- Bayne, B.L. Aspects of Reproduction in Bivalve Molluscs. In Estuarine Processes; Wiley, M., Ed.; Academic Press: Cambridge, MA, USA, 1976; pp. 432–448. [Google Scholar] [CrossRef]
- Song, L.; Li, X.; Clarke, S.; Wang, T.; Bott, K. The Application of Neutral Red Retention Assay to Evaluate the Differences in Stress Responses to Sexual Maturation and Spawning between Different Sizes of Pacific Oyster, Crassostrea gigas (Thunberg). J. Shellfish Res. 2007, 26, 493–499. [Google Scholar] [CrossRef]
- Harding, J.M.; Couturier, C.; Parsons, G.J.; Ross, N.W. Evaluation of the Neutral Red Retention Assay as a Stress Response Indicator in Cultivated Mussels (Mytilus Spp.) in Relation to Seasonal and Environmental Conditions. J. Shellfish Res. 2004, 23, 745–752. [Google Scholar]
- Ramón, M. Estudio de Las Poblaciones de Chamelea gallina (Linnaeus, 1758) y Donax trunculus Linnaeus, 1758 (Mollusca: Bivalvia) En El Golfo de Valencia (Mediterráneo Occidental); Universidad de Barcelona: Barcelona, Spain, 1993. [Google Scholar]
- Fisher, W.S. Environmental Influence on Host Response. Am. Fish. Soc. Spec. Publ. 1988, 18, 225–237. [Google Scholar]
| Test Treatment | Donax trunculus | Chamelea gallina | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T | S | T C) | S | DO (mg/L) | DO (% sat.) | T C) | S | DO (mg/L) | DO (% sat.) | |
| 1 | Low | 27–28 | 12.30 ± 0.05 | 27.09 ± 0.06 | 9.06 ± 0.01 | 104.79 ± 1.41 | 12.13 ± 0.03 | 27.32 ± 0.04 | 9.12 ± 0.01 | 106.01 ± 0.09 |
| 2 | 32–33 | 12.00 ± 0.03 | 32.56 ± 0.14 | 9.12 ± 0.01 | 104.75 ± 1.41 | 12.02 ± 0.04 | 32.72 ± 0.08 | 9.14 ± 0.01 | 106.10 ± 0.11 | |
| 3 | 37–38 | 12.17 ± 0.03 | 38.12 ± 0.05 | 9.09 ± 0.02 | 103.26 ± 0.98 | 12.26 ± 0.04 | 37.93 ± 0.04 | 9.10 ± 0.01 | 106.19 ± 0.15 | |
| 4 | Medium | 27–28 | 20.11 ± 0.05 | 27.35 ± 0.03 | 7.79 ± 0.01 | 105.82 ± 0.82 | 20.23 ± 0.01 | 27.33 ± 0.01 | 7.76 ± 0.00 | 105.64 ± 0.24 |
| 5 | 32–33 | 19.95 ± 0.04 | 32.70 ± 0.04 | 7.64 ± 0.04 | 102.38 ± 0.66 | 20.16 ± 0.01 | 32.78 ± 0.01 | 7.77 ± 0.00 | 103.49 ± 0.73 | |
| 6 | 37–38 | 20.34 ± 0.05 | 37.63 ± 0.06 | 7.72 ± 0.02 | 102.29 ± 0.87 | 20.07 ± 0.01 | 37.47 ± 0.01 | 7.78 ± 0.00 | 105.51 ± 0.40 | |
| 7 | High | 27–28 | 27.13 ± 0.10 | 27.59 ± 0.05 | 6.91 ± 0.01 | 104.77 ± 0.25 | 27.58 ± 0.12 | 27.55 ± 0.18 | 6.79 ± 0.02 | 105.96 ± 0.09 |
| 8 | 32–33 | 26.94 ± 0.09 | 32.73 ± 0.09 | 6.91 ± 0.01 | 105.70 ± 0.06 | 27.40 ± 0.12 | 32.57 ± 0.07 | 6.86 ± 0.01 | 105.97 ± 0.13 | |
| 9 | 37–38 | 26.98 ± 0.11 | 37.71 ± 0.10 | 6.93 ± 0.01 | 105.03 ± 0.13 | 27.44 ± 0.15 | 37.57 ± 0.08 | 6.82 ± 0.02 | 105.95 ± 0.10 | |
| Factor | NRRT | %LMS | %CI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| df | F | p-Value | df | F | p-Value | df | F | p-Value | ||
| Chamelea gallina | T | 2 | 4.28 | 0.016 | 2 | 1.01 | ns | 2 | 29.08 | 0.000 |
| S | 2 | 0.65 | ns | 2 | 1.47 | ns | 2 | 2.29 | ns | |
| T × S | 4 | 6.75 | 0.000 | 4 | 4.92 | 0.001 | 4 | 1.24 | ns | |
| Donax trunculus | T | 2 | 35.92 | 0.000 | 2 | 33.37 | 0.000 | 2 | 135.27 | 0.000 |
| S | 2 | 4.57 | 0.012 | 2 | 3.67 | 0.029 | 2 | 2.20 | ns | |
| T × S | 4 | 4.49 | 0.005 | 4 | 3.69 | 0.014 | 4 | 0.83 | ns | |
| Reference (Day 0) | Salinity (Day 21) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 27–28 | 32–33 | 37–38 | ||||||||||
| T (°C)/S | Size (mm) | CI (%) | Size (mm) | CI (%) | Size (mm) | CI (%) | Size (mm) | CI (%) | CI | |||
| Chamelea gallina | Temperature | Low | 15.4/36.9 | – | – | 14.45 ± 0.11 | 2.10 a ± 0.16 | 13.95 ± 0.13 | 1.88 a ± 0.07 | 14.10 ± 0.11 | 2.10 a ± 0.08 | ns |
| Medium | 19.5/36.8 | 17.93 ± 0.23 | 2.78 ab ± 0.10 | 18.91 ± 0.35 | 2.52 a ± 0.16 | 19.03 ± 0.29 | 2.89 ab ± 0.24 | 18.86 ± 0.25 | 3.24 ab ± 0.32 | 0.006 | ||
| High | 23.5/37.1 | 17.85 ± 0.18 | 2.61 a ± 0.08 | 18.33 ± 0.23 | 2.02 b ± 0.26 | 19.01 ± 0.22 | 2.07 b ± 0.09 | 18.93 ± 0.29 | 2.18 b ± 0.15 | 0.007 | ||
| Donax trunculus | Low | 15.4/36.9 | – | – | 22.16 ± 0.14 | 2.81 a ± 0.09 | 22.15 ± 0.17 | 3.03 a ± 0.08 | 22.04 ± 0.16 | 2.88 a ± 0.20 | ns | |
| Medium | 19.5/36.8 | 20.66 ± 0.20 | 2.34 a ± 0.08 | 21.51 ± 0.15 | 1.96 c ± 0.07 | 22.08 ± 0.11 | 2.12 bc ± 0.08 | 22.00 ± 0.20 | 2.20 ab ± 0.06 | 0.007 | ||
| High | 23.5/37.1 | 20.21 ± 0.19 | 1.70 a ± 0.07 | X | X | 20.68 ± 0.52 | 1.08 b ± 0.07 | 20.00 ± 0.10 | 0.93 b ± 0.17 | 0.000 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soms-Molina, P.; Martínez-Gómez, C.; Zuñiga, E.; Rodilla, M.; Falco, S. Effects of Temperature and Salinity on the LMS (Lysosomal Membrane Stability) Biomarker in Clams Donax trunculus and Chamelea gallina. Appl. Sci. 2024, 14, 2712. https://doi.org/10.3390/app14072712
Soms-Molina P, Martínez-Gómez C, Zuñiga E, Rodilla M, Falco S. Effects of Temperature and Salinity on the LMS (Lysosomal Membrane Stability) Biomarker in Clams Donax trunculus and Chamelea gallina. Applied Sciences. 2024; 14(7):2712. https://doi.org/10.3390/app14072712
Chicago/Turabian StyleSoms-Molina, Paula, Concepción Martínez-Gómez, Esther Zuñiga, Miguel Rodilla, and Silvia Falco. 2024. "Effects of Temperature and Salinity on the LMS (Lysosomal Membrane Stability) Biomarker in Clams Donax trunculus and Chamelea gallina" Applied Sciences 14, no. 7: 2712. https://doi.org/10.3390/app14072712
APA StyleSoms-Molina, P., Martínez-Gómez, C., Zuñiga, E., Rodilla, M., & Falco, S. (2024). Effects of Temperature and Salinity on the LMS (Lysosomal Membrane Stability) Biomarker in Clams Donax trunculus and Chamelea gallina. Applied Sciences, 14(7), 2712. https://doi.org/10.3390/app14072712








