Protective Effects of Hesperetin on Cardiomyocyte Integrity and Cytoskeletal Stability in a Murine Model of Epirubicin-Induced Cardiotoxicity: A Histopathological Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Experimental Design
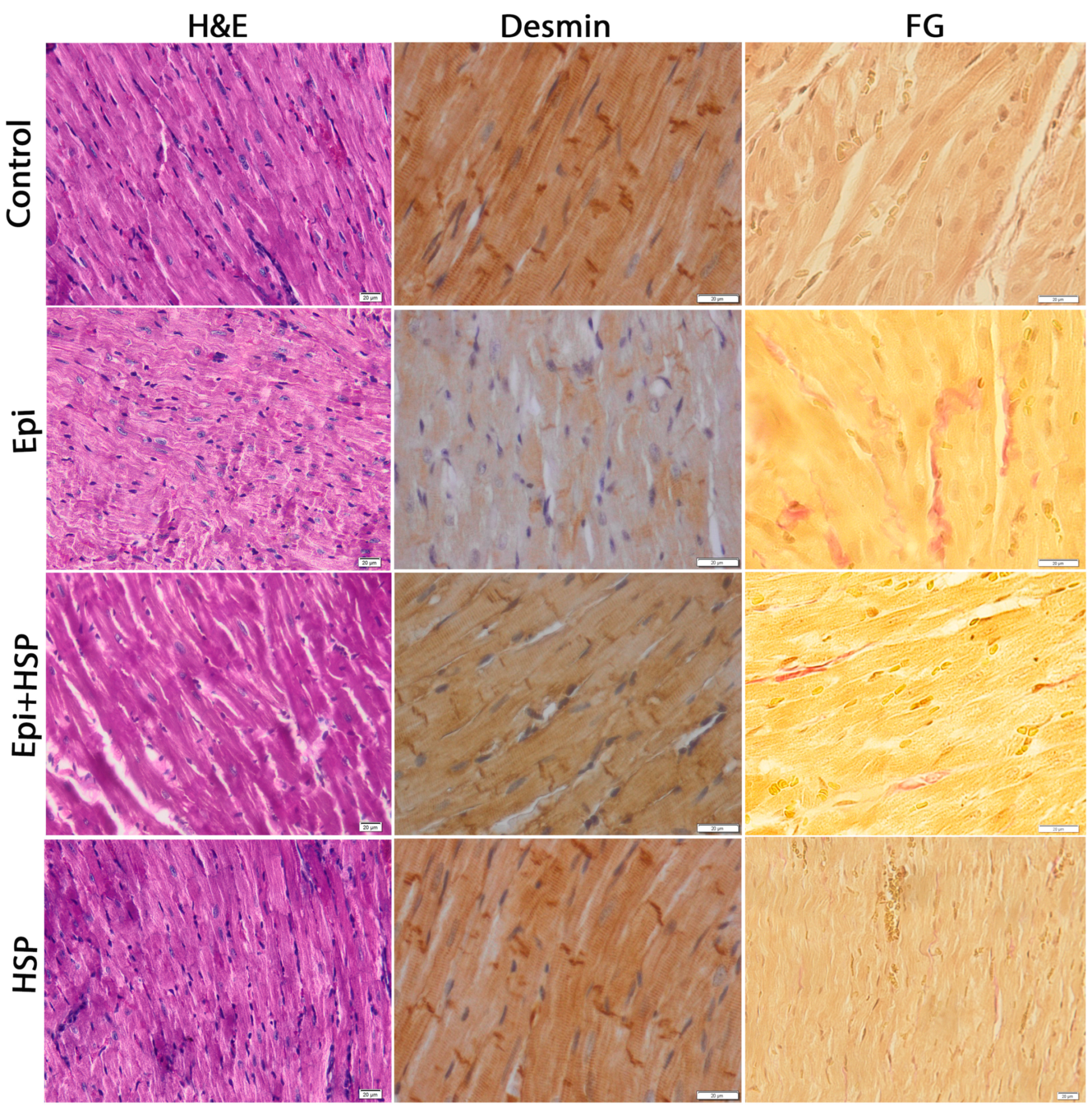
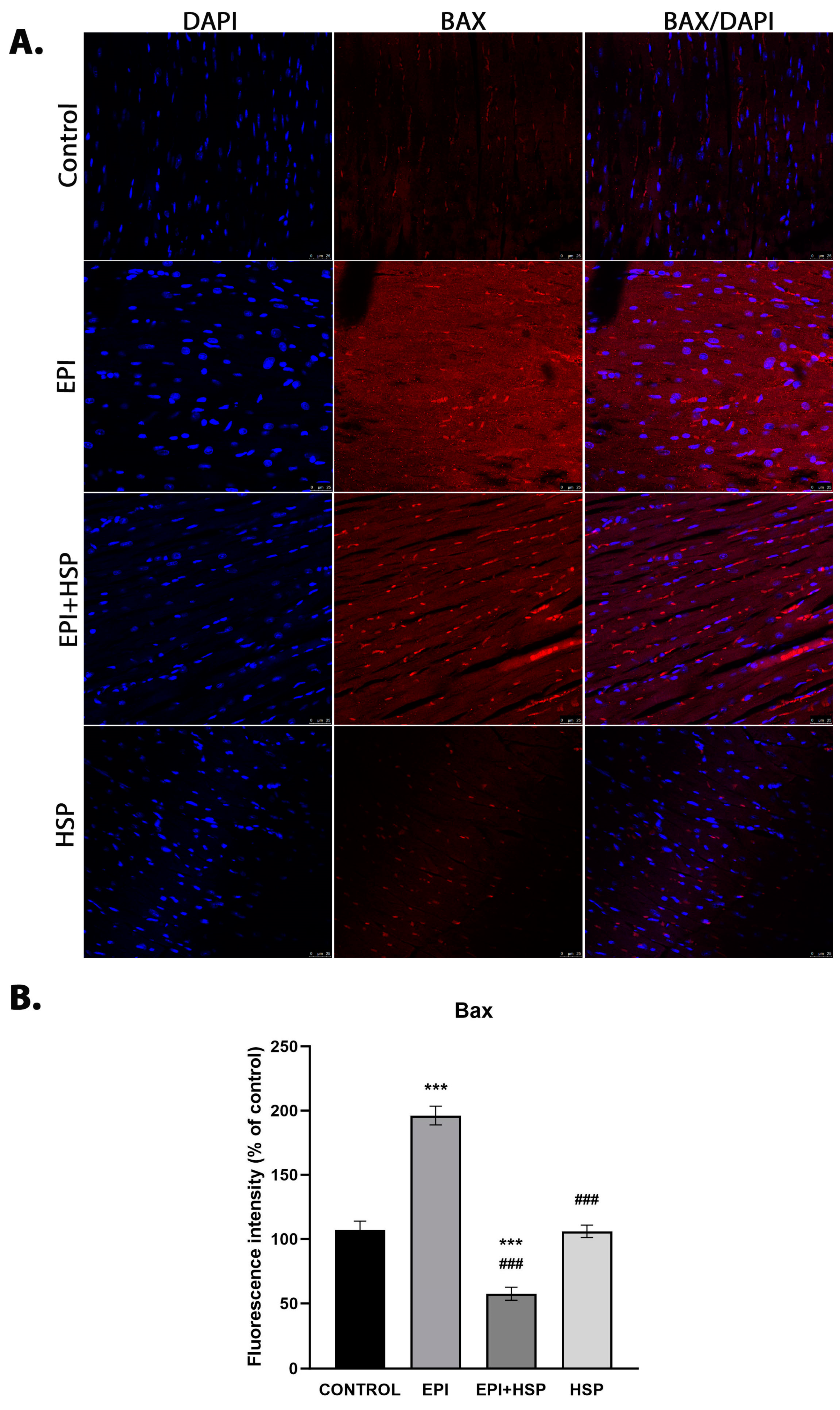
- Control group—received a daily oral administration of 0.7% carboxymethyl cellulose (CMC) solution for 13 consecutive days.
- Epi group—received a total of 12 mg/kg Epi (cumulative dose consistent with human clinical treatments), administered intraperitoneally. This total dose was divided into six equal parts, with each part being 2 mg/kg and given every other day, starting from the second day of the experiment.
- Epi+HSP group—each animal was given 100 mg/kg of HSP orally (dissolved in a 0.7% CMC solution), starting one day before the initial Epi injection and continuing daily up to 13th day of the experiment.
- HSP group—received a daily oral administration of HSP in 0.7% carboxymethyl cellulose solution, like group 3.
2.4. Histopathology
2.5. Immunofluoresce
2.6. Immunohistochemistry
2.7. Transmission Electron Microscopy (TEM)
2.8. In Situ Detection of DNA Fragmentation
2.9. Statistical Analyses
3. Results
3.1. Hesperetin Protects and Maintains the Structural and Ultrastructural Integrity of Cardiac Tissue Affected by Epirubicin-Induced Damage
3.2. Hesperetin Provides Protection for Cardiomyocytes against Apoptosis Caused by Epirubicin-Induced Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monsuez, J.-J.; Charniot, J.-C.; Vignat, N.; Artigou, J.-Y. Cardiac Side-Effects of Cancer Chemotherapy. Int. J. Cardiol. 2010, 144, 3–15. [Google Scholar] [CrossRef]
- Arola, O.J.; Saraste, A.; Pulkki, K.; Kallajoki, M.; Parvinen, M.; Voipio-Pulkki, L.M. Acute Doxorubicin Cardiotoxicity Involves Cardiomyocyte Apoptosis. Cancer Res. 2000, 60, 1789–1792. [Google Scholar]
- Fisher, P.W.; Salloum, F.; Das, A.; Hyder, H.; Kukreja, R.C. Phosphodiesterase-5 Inhibition with Sildenafil Attenuates Cardiomyocyte Apoptosis and Left Ventricular Dysfunction in a Chronic Model of Doxorubicin Cardiotoxicity. Circulation 2005, 111, 1601–1610. [Google Scholar] [CrossRef]
- Zhang, J. Doxorubicin-Induced Apoptosis in Spontaneously Hypertensive Rats: Differential Effects in Heart, Kidney and Intestine, and Inhibition by ICRF-187. J. Mol. Cell. Cardiol. 1996, 28, 1931–1943. [Google Scholar] [CrossRef]
- Arıca, V.; Demir, İ.H.; Tutanc, M.; Basarslan, F.; Arıca, S.; Karcıoglu, M.; Öztürk, H.; Nacar, A. N-Acetylcysteine Prevents Doxorubucine-Induced Cardiotoxicity in Rats. Hum. Exp. Toxicol. 2013, 32, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Yu, X.; Wang, Y.; Wang, F.; Li, H.; Wang, Y.; Lu, D.; Qi, R.; Wang, H. Berberine Inhibits Doxorubicin-Triggered Cardiomyocyte Apoptosis via Attenuating Mitochondrial Dysfunction and Increasing Bcl-2 Expression. PLoS ONE 2012, 7, e47351. [Google Scholar] [CrossRef]
- Rašković, A.; Stilinović, N.; Kolarović, J.; Vasović, V.; Vukmirović, S.; Mikov, M. The Protective Effects of Silymarin against Doxorubicin-Induced Cardiotoxicity and Hepatotoxicity in Rats. Molecules 2011, 16, 8601–8613. [Google Scholar] [CrossRef] [PubMed]
- Guven, A.; Yavuz, O.; Cam, M.; Ercan, F.; Bukan, N.; Comunoglu, C. Melatonin Protects against Epirubicin-Induced Cardiotoxicity. Acta Histochem. 2007, 109, 52–60. [Google Scholar] [CrossRef]
- Choi, E.J. Antioxidative Effects of Hesperetin against 7,12-Dimethylbenz(a)Anthracene-Induced Oxidative Stress in Mice. Life Sci. 2008, 82, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Pari, L.; Shagirtha, K. Hesperetin Protects against Oxidative Stress Related Hepatic Dysfunction by Cadmium in Rats. Exp. Toxicol. Pathol. 2012, 64, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Shagirtha, K.; Pari, L. Hesperetin, a Citrus Flavonone, Protects Potentially Cadmium Induced Oxidative Testicular Dysfunction in Rats. Ecotoxicol. Environ. Saf. 2011, 74, 2105–2111. [Google Scholar] [CrossRef]
- Trivedi, P.P.; Tripathi, D.N.; Jena, G.B. Hesperetin Protects Testicular Toxicity of Doxorubicin in Rat: Role of NFκB, P38 and Caspase-3. Food Chem. Toxicol. 2011, 49, 838–847. [Google Scholar] [CrossRef]
- Yang, H.-L.; Chen, S.-C.; Senthil Kumar, K.J.; Yu, K.-N.; Lee Chao, P.-D.; Tsai, S.-Y.; Hou, Y.-C.; Hseu, Y.-C. Antioxidant and Anti-Inflammatory Potential of Hesperetin Metabolites Obtained from Hesperetin-Administered Rat Serum: An Ex Vivo Approach. J. Agric. Food Chem. 2012, 60, 522–532. [Google Scholar] [CrossRef]
- Nalini, N.; Aranganathan, S.; Kabalimurthy, J. Chemopreventive Efficacy of Hesperetin (Citrus Flavonone) against 1,2-Dimethylhydrazine-Induced Rat Colon Carcinogenesis. Toxicol. Mech. Methods 2012, 22, 397–408. [Google Scholar] [CrossRef]
- Cho, J. Antioxidant and Neuroprotective Effects of Hesperidin and Its Aglycone Hesperetin. Arch. Pharm. Res. 2006, 29, 699–706. [Google Scholar] [CrossRef]
- Choi, E.J.; Ahn, W.S. Neuroprotective Effects of Chronic Hesperetin Administration in Mice. Arch. Pharm. Res. 2008, 31, 1457–1462. [Google Scholar] [CrossRef]
- Trivedi, P.P.; Kushwaha, S.; Tripathi, D.N.; Jena, G.B. Cardioprotective Effects of Hesperetin against Doxorubicin-Induced Oxidative Stress and DNA Damage in Rat. Cardiovasc. Toxicol. 2011, 11, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, Y.O.; Sharma, P.K.; Shrivastava, B.; Ojha, S.; Upadhya, H.M.; Arya, D.S.; Goyal, S.N. Hesperidin Produces Cardioprotective Activity via PPAR-γ Pathway in Ischemic Heart Disease Model in Diabetic Rats. PLoS ONE 2014, 9, e111212. [Google Scholar] [CrossRef]
- Anghel, N.; Herman, H.; Balta, C.; Rosu, M.; Stan, M.S.; Nita, D.; Ivan, A.; Galajda, Z.; Ardelean, A.; Hermenean, A.; et al. Acute Cardiotoxicity Induced by Doxorubicin in Right Ventricle Is Associated with Increase of Oxidative Stress and Apoptosis in Rats. Histol. Histopathol. 2018, 33, 365–378. [Google Scholar] [CrossRef]
- Anghel, N.; Cotoraci, C.; Ivan, A.; Suciu, M.; Herman, H.; Balta, C.; Nicolescu, L.; Olariu, T.; Galajda, Z.; Hermenean, A.; et al. Chrysin Attenuates Cardiomyocyte Apoptosis and Loss of Intermediate Filaments in a Mouse Model of Mitoxantrone Cardiotoxicity. Histol. Histopathol. 2015, 30, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Alderton, P.M.; Gross, J.; Green, M.D. Comparative Study of Doxorubicin, Mitoxantrone, and Epirubicin in Combination with ICRF-187 (ADR-529) in a Chronic Cardiotoxicity Animal Model. Cancer Res. 1992, 52, 194–201. [Google Scholar]
- Pereira, G.C.; Silva, A.M.; Diogo, C.V.; Carvalho, F.S.; Monteiro, P.; Oliveira, P.J. Drug-Induced Cardiac Mitochondrial Toxicity and Protection: From Doxorubicin to Carvedilol. Curr. Pharm. Des. 2011, 17, 2113–2129. [Google Scholar] [CrossRef]
- Kuwana, T.; Newmeyer, D.D. Bcl-2-Family Proteins and the Role of Mitochondria in Apoptosis. Curr. Opin. Cell Biol. 2003, 15, 691–699. [Google Scholar] [CrossRef]
- Parrish, A.B.; Freel, C.D.; Kornbluth, S. Cellular Mechanisms Controlling Caspase Activation and Function. Cold Spring Harb. Perspect. Biol. 2013, 5, a008672. [Google Scholar] [CrossRef]
- Dorn, G.W. Molecular Mechanisms That Differentiate Apoptosis from Programmed Necrosis. Toxicol. Pathol. 2013, 41, 227–234. [Google Scholar] [CrossRef]
- Rohrbach, S.; Muller-Werdan, U.; Werdan, K.; Koch, S.; Gellerich, N.F.; Holtz, J. Apoptosis-Modulating Interaction of the Neuregulin/erbB Pathway with Antracyclines in Regulating Bcl-xS and Bcl-xL in Cardiomyocytes. J. Mol. Cell. Cardiol. 2005, 38, 485–493. [Google Scholar] [CrossRef]
- Kumar, B.; Gupta, S.K.; Srinivasan, B.P.; Nag, T.C.; Srivastava, S.; Saxena, R.; Jha, K.A. Hesperetin Rescues Retinal Oxidative Stress, Neuroinflammation and Apoptosis in Diabetic Rats. Microvasc. Res. 2013, 87, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Meyer, E.; Friesen, C.; Susin, S.A.; Kroemer, G.; Debatin, K.-M. Cell Type Specific Involvement of Death Receptor and Mitochondrial Pathways in Drug-Induced Apoptosis. Oncogene 2001, 20, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, L.C.; Theophilidis, G.; Thomopoulos, G.N.; Tsiftsoglou, A.S. Structural and Functional Impairment of Mitochondria in Adriamycin-Induced Cardiomyopathy in Mice: Suppression of Cytochrome c Oxidase II Gene Expression. Biochem. Pharmacol. 1999, 57, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.L.; Escaleira, R.; Cataldo, A.; Oliveira, F.; Mermelstein, C.S. Desmin: Molecular Interactions and Putative Functions of the Muscle Intermediate Filament Protein. Braz. J. Med. Biol. Res. 2004, 37, 1819–1830. [Google Scholar] [CrossRef]
- Weisleder, N.; Taffet, G.E.; Capetanaki, Y. Bcl-2 Overexpression Corrects Mitochondrial Defects and Ameliorates Inherited Desmin Null Cardiomyopathy. Proc. Natl. Acad. Sci. USA 2004, 101, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Dinsdale, D.; Lee, J.C.; Dewson, G.; Cohen, G.M.; Peter, M.E. Intermediate Filaments Control the Intracellular Distribution of Caspases During Apoptosis. Am. J. Pathol. 2004, 164, 395–407. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop Moldovan, A.; Dumitra, S.; Popescu, C.; Lala, R.; Anghel, N.Z.; Nisulescu, D.; Nicoras, A.; Cotoraci, C.; Puticiu, M.; Hermenean, A.; et al. Protective Effects of Hesperetin on Cardiomyocyte Integrity and Cytoskeletal Stability in a Murine Model of Epirubicin-Induced Cardiotoxicity: A Histopathological Study. Appl. Sci. 2024, 14, 2560. https://doi.org/10.3390/app14062560
Pop Moldovan A, Dumitra S, Popescu C, Lala R, Anghel NZ, Nisulescu D, Nicoras A, Cotoraci C, Puticiu M, Hermenean A, et al. Protective Effects of Hesperetin on Cardiomyocyte Integrity and Cytoskeletal Stability in a Murine Model of Epirubicin-Induced Cardiotoxicity: A Histopathological Study. Applied Sciences. 2024; 14(6):2560. https://doi.org/10.3390/app14062560
Chicago/Turabian StylePop Moldovan, Adina, Simona Dumitra, Cristina Popescu, Radu Lala, Nicoleta Zurbau Anghel, Daniel Nisulescu, Ariana Nicoras, Coralia Cotoraci, Monica Puticiu, Anca Hermenean, and et al. 2024. "Protective Effects of Hesperetin on Cardiomyocyte Integrity and Cytoskeletal Stability in a Murine Model of Epirubicin-Induced Cardiotoxicity: A Histopathological Study" Applied Sciences 14, no. 6: 2560. https://doi.org/10.3390/app14062560
APA StylePop Moldovan, A., Dumitra, S., Popescu, C., Lala, R., Anghel, N. Z., Nisulescu, D., Nicoras, A., Cotoraci, C., Puticiu, M., Hermenean, A., & Marti, D. T. (2024). Protective Effects of Hesperetin on Cardiomyocyte Integrity and Cytoskeletal Stability in a Murine Model of Epirubicin-Induced Cardiotoxicity: A Histopathological Study. Applied Sciences, 14(6), 2560. https://doi.org/10.3390/app14062560






