Abstract
We construct hypergraphs to analyze functional brain connectivity, leveraging event-related coherence in magnetoencephalography (MEG) data during the visual perception of a flickering image. Principal network characteristics are computed for the delta, theta, alpha, beta, and gamma frequency ranges. Employing a coherence measure, a statistical estimate of correlation between signal pairs across frequencies, we generate an edge time series, depicting how an edge evolves over time. This forms the basis for constructing an edge-to-edge functional connectivity network. We emphasize hyperedges as connected components in an absolute-valued functional connectivity network. Our coherence-based hypergraph construction specifically addresses functional connectivity among four brain lobes in both hemispheres: frontal, parietal, temporal, and occipital. This approach enables a nuanced exploration of individual differences within diverse frequency bands, providing insights into the dynamic nature of brain connectivity during visual perception tasks. The results furnish compelling evidence supporting the hypothesis of cortico–cortical interactions occurring across varying scales. The derived hypergraph illustrates robust activation patterns in specific brain regions, indicative of their engagement across diverse cognitive contexts and different frequency bands. Our findings suggest potential integration or multifunctionality within the examined lobes, contributing valuable perspectives to our understanding of brain dynamics during visual perception.
1. Introduction
Understanding the intricacies of brain connectivity in response to diverse stimuli is crucial for unraveling the mechanisms underlying information processing and decision making within the brain. This study delves into three essential forms of brain connectivity: structural, functional, and efficient [1,2,3,4]. Structural connectivity entails the identification of anatomical neural networks, revealing potential pathways for neural communication [5,6]. On the other hand, functional connectivity explores active brain regions exhibiting correlated frequency, phase, and/or amplitude [7]. Finally, effective connectivity utilizes information from functional connectivity to discern the dynamic flow of information within the brain [8,9].
Measurement of effective and functional connectivity can be conducted in both the frequency domain, employing methods such as coherence [10], and in the time domain, utilizing approaches like Granger causality [4] or artificial-neural-network-based functional connectivity [11]. When a sufficiently large population of neurons synchronizes, their electrical and magnetic activities become detectable outside the skull through techniques like electroencephalography (EEG) and magnetoencephalography (MEG) [12]. While EEG measures return or bulk currents outside the neuron (secondary currents), MEG captures ionic currents inside the neuron (primary currents). Notably, MEG holds a distinct advantage over EEG due to its superior spatial resolution, rendering it an exceptional tool for investigating and characterizing interactions between distinct brain regions [13].
To study functional connectivity, some researchers use approaches borrowed from graph theory [14]. Using connections between the biorhythms of the brain in its various parts, a model of a complex network is recreated, in which parts of the brain are considered as nodes, and the connection forces between them are considered as links [15,16]. Using this approach makes it possible to identify not only individual cognitive differences between subjects [17], but also helps to diagnose some diseases at an early stage [18,19,20] and also monitor the aging process [21,22,23,24].
One of the important measures that can be used to quantify neuronal synchrony is event-related coherence [10,25,26]. It examines the frequency domain relationship between two signals, indicating the degree to which their spectral components are synchronized. Essentially, it is an assessment of the constancy of the relative amplitude and phase between two signals within a given frequency range. There is a linear mathematical method that creates a symmetrical matrix, devoid of any directional information. Identical signals produce a coherence value of 1, while the coherence value approaches 0 as the difference between the signals in question increases. Since then, coherence has been used in many brain connectivity studies with both patients and control individuals; these include but are not limited to studies of working memory [27], brain lesions [28], hemiparesis [29], resting state networks [30], schizophrenia [31,32], favorable responses to panic medications [33], and motor imagery [34]. As a result of the unique characteristics of human brains, distinct patterns of coherent neuronal activity were observed among different subjects. For instance, the presentation of flickering visual stimuli induces coherent responses in the visual cortex of subjects at both the flicker frequency and its harmonics, resulting in varied sizes of coherent neural networks [35,36].
In this work, we employ hypergraph analysis, a technique rooted in dynamic graph theory [37], to investigate functional connectivity. We analyze variations in functional connectivity networks using MEG data collected during the observation of flickering images. This approach is an extension of conventional graph theory methods. Specifically, we begin by defining a standard functional network that connects nodes across consecutive time segments. We then generate a set of edge time series, representing the fluctuation of edges over time. We process these edge time series similarly to node time series, creating a network of edge-to-edge functional connectivity. Within this framework, we focus on “hyperedges,” which are the connected components of an absolute-valued end-to-end functional connectivity network.
Conventional graphs are limited in their ability to represent connections between pairs of nodes, while hypergraphs can depict relationships among sets of nodes, especially when complex interactions involve more than two elements. Using a hypergraph allows for a more intuitive interpretation of inter-element relationships, enhancing comprehension and result interpretation. It should be noted that Wang et al. [38] have already demonstrated the utility of hypergraphs in showcasing multiple relationships between vertices by employing Pearson correlation for interaction derivation. However, despite the fact that the hypergraph theory was applied several years ago, our paper introduces a significant advancement in hypergraph data representation by leveraging event-related coherence between brain lobes rather than conventional correlation metrics. We prefer coherence due to its ability to measure signal relationships based on relative phase, thereby capturing temporal synchronization between signals. By using a hypergraph, we can clearly and succinctly visualize the correlation between brain lobes and frequency bands, crucial for unraveling the intricate network of cerebral interactions. Moreover, our approach, centered on visualization with modulation, enriches our research with an additional layer of depth, offering an innovative and pertinent perspective in this burgeoning field.
2. Materials and Methods
2.1. Subjects
In this study, we analyze the MEG data of 15 control subjects (aged 17–64 years; 10 men and 5 women) obtained in the experiment based on a flickering image paradigm [39] at the Center for Biomedical Technology of the Universidad Politécnica de Madrid, Spain. The MEG data have been downloaded from https://zenodo.org/record/4408648#.X-72UdYo-Cc (accessed on 10 August 2023).
2.2. Experimental Paradigm
The experimental protocol, depicted in Figure 1, consists of two stages. During the first stage, subjects were presented with a static (unmodulated) black square with white lines for 120 s. They were instructed to fix their gaze on a red dot located at the center of the square. The MEG of the baseline neuronal activity (B-trial) was recorded during this stage. After a brief break (40–390 s), the second stage of the experiment commenced. During this stage, the brightness of pixels on the square image was periodically modulated with a frequency of Hz and a maximum amplitude of 50% of the RGB color model, oscillating between black (0) and gray (127). This frequency was chosen due to its ability to elicit a prominent spectral response in the visual cortex [35]. The flickering image was presented 2–5 times at intervals of 120 s, with a 30-second break between each presentation, and the MEG was recorded (F-trials). The averaged F-trials were then normalized to the B-trial for each subject and subsequently averaged across all subjects.
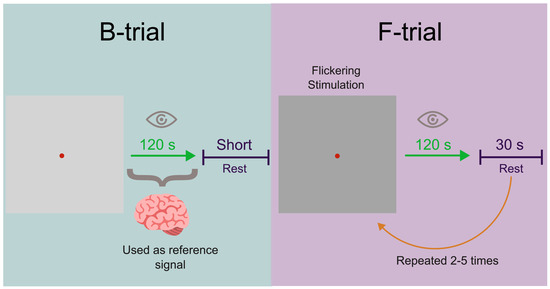
Figure 1.
Experiment protocol. The B-trial corresponds to the baseline neuronal activity induced by the unmodulated visual stimulus and the F-trials correspond to the flickering image.
2.3. Signal Analysis in Brainstorm
The signal analysis was performed using the Brainstorm 3.231017 software, a collaborative, open-source application based on MATLAB R2022a that is dedicated to processing and analyzing brain recordings obtained through different brain imaging techniques [40]. The included tools, along with the interface, facilitated the creation of the scripts used in this article.
2.4. Head Model Adjustment
The default Brainstorm head model was adjusted to the head points recorded using a Polhemus Fastrak system, with 2% deformation and automatic refinement of head points.
2.5. Signal Processing
Signal analysis involved reading MEG data, and applying a Notch filter to eliminate 50 Hz power line frequencies and their harmonics. Artifacts from the electrooculogram (EOG) and electrocardiogram (ECG) signals were automatically identified and manually reviewed to ensure the inclusion of any potentially omitted artifacts. Signal–space projection (SSP) methods were applied to correct the artifacts by order.
2.6. Event Segmentation
The signals were segmented into 120 s epochs for two experimental phases: B-trial and F-trial (Figure 1). The signal recorded during the B section was used as a reference signal. These epochs were further divided into 3 s trials.
2.7. Source Reconstruction
Reconstruction of electrical activity in the brain from MEG measurements was carried out by creating a forward model and a lead field matrix. Brainstorm’s overlapped spheres method was used, maintaining the recommended 15,000 cortical sources. The inverse solution was calculated using standardized low-resolution electromagnetic tomography (sLORETA).
2.8. Fourier Analysis
To analyze the recorded signal, a Fourier analysis technique using a hanning window was employed. First, a Hanning window was generated with an appropriate length corresponding to the duration of the recorded signal (3 s). Subsequently, this window was applied to the signal to mitigate edge effects and enhance frequency domain resolution. Once the frequency spectrum of the processed signals was obtained, the spectrum of the signal of interest was normalized to the reference signal. This was accomplished by computing the fast Fourier transform (FFT)—using MATLAB’s FFT function—of each processed signal and subsequently dividing the frequency spectrum of the signal of interest by that of the reference (baseline) signal.
2.9. Signal Coherence
The brain is known to generate electromagnetic activity in a wide frequency range, from slow waves of 0.5 Hz to fast waves of 500 Hz and higher frequencies [41]. These rhythms are classified according to their frequencies and are assigned Greek letters. In this paper, we consider five frequency bands: delta (0.5–4 Hz), theta (4–7 Hz), alpha (8–12 Hz), beta (13–30 Hz), and gamma (31–90 Hz). Coherence measures the correlation within discrete frequency bands for selected epoch lengths and is independent of signal amplitude [42]. We constructed brain networks based on coherence, a mathematical measure quantifying synchronization patterns between spatially separated sensors or between brain areas [10].
After the Hanning window was applied to the signal, we estimated the strength of network interactions according to coherence between eight brain areas: frontal left—FL; frontal right—FR; occipital left—OL; occipital right—OR; parietal left—PL; parietal right—PR; temporal left—TL; temporal right—TR. The 15,000 brain sources were grouped into these eight lobes using Brainstorm’s segmentation model, PALS-12 Lobes with ten structures, excluding the insula, resulting in eight structures or vertices.
The stored vertices were used to average signals within each lobe, reducing complexity to eight signals. The square magnitude coherence was then calculated between time series of each lobe with the rest, for both F and B trials ( and , respectively). Subsequently, the absolute difference between the coherence values of F and B was obtained and normalized to the B activity, giving event-related coherence () as
2.10. Visualization with BrainNet
The output consisted of a tensor with dimensions . The average matrix of all subjects was calculated for each frequency band, and these matrices were saved in “.edge” text files for visualization with BrainNet Viewer. The latter is a tool that facilitates the visualization of structural and functional connectivity patterns in brain networks [43]. The surface template used was “BrainMesh_ICBM152_smoothed.nv”, included in the “BrainNetViewer_20191031” folder when downloaded.
A “Node.node” text file was created with the format defined by BrainNet Viewer to set the position of nodes in the brain figure. The “.edge” files for each frequency band obtained earlier were used to display interactions.
2.11. Graph Construction
Coherence matrices showed coefficients between 0.1 and 19.16. A threshold was set for graph construction, with its value varying between 0.1 and 1.25. The analysis was conducted to assess how graph characteristics change when including interactions above . Various centrality measures were calculated such as degree centrality (number of edges [44]), betweenness centrality (fraction of shortest paths passing through a node [45]), and eigenvector centrality (importance of a node considering the importance of its neighbors [44]). Connected components of the graphs were explored [45]. The shortest path distances between all node pairs were calculated, and cycles in the graph were identified, and defined as connected graphs in which each vertex has degree 2 [46]. These metrics, with respect to , are presented in Appendix (Figure A1, Figure A2, Figure A3, Figure A4, Figure A5, Figure A6 and Figure A7).
To assess the connectivity of the graphs generated in this study, the ‘conncomp’ function in MATLAB was employed. This function facilitates the identification of connected components in an undirected graph, thus providing a measure of the level of connection within the network. This function assigns each node in the graph a connected component identifier, enabling the determination of the total number of connected components present in the graph. The graph’s connectivity coefficient can be calculated as
where n is the number of connected components. This methodological approach allowed for the analysis of how the variation in the threshold affects the global connectivity of the graphs.
A threshold value of was decided upon, as lower values show coherence interactions that may include noise, and values higher than this completely lose one of our frequency bands.
With the specified threshold, we generated graphs explicitly depicting the mentioned measures using MATLAB. The calculation of the distances between node pairs was carried out without considering the weight of the edges, i.e., treating it as an unweighted graph. Betweenness centrality and eigenvector centrality measures can be found in Appendix A (Figure A6 and Figure A7).
2.12. Hypergraph Construction
To contextualize the analysis of hypergraphs, we define the elements of graph theory used to construct hypergraphs formed by nodes and edges, where nodes denote brain regions, or groups of voxels, and edges denote correlations in activity between pairs of nodes over time. Significant correlation in activity between pairs of edges over time is denoted as links. In this context, we define a hyperedge as a group of links connecting two or more edges with significantly correlated temporal profiles. Finally, a set of hyperedges forms a hypergraph, H, defined as an ordered pair . Here, V is a finite set of nodes or vertices and is a family of nonempty subsets of elements of X, called hyperedges or hyperlinks, showing the interaction between elements of X [14].
The algorithm of [47] was used for part of the hypergraph visualization. The incidence matrix was given as input. The obtained representations included the hypergraph, the incidence matrix in linear form, and the star expansion. Hypergraph characteristics were obtained along with some matrix representations of the same.
2.12.1. Adjacency and Node Stars
Adjacency between vertices is established when at least one hyperedge contains two vertices [14]. This is illustrated by the star of the nodes, which is defined as the collection of hyperedges incident on node i: is referred to as the star of i, where h is a hyperedge.
2.12.2. Degrees of Vertices and Hyperedges
The degree of vertices in the hypergraph is defined as , which corresponds to the size of its stars, i.e., the number of hyperedges incident to i. The degree of a hyperedge, , is the number of vertices it contains, denoted as .
3. Results and Discussion
3.1. Coherence Brain Networks and Matrices
Figure 2 displays brain networks (left column) of the coherence and corresponding coherence matrices (right column) for five frequency bands (delta, theta, alpha, beta, and gamma), presented using the BrainNet Viewer template. The coherence values shown in this figure were computed from the averaged F-trials normalized to the B-trials. One can observe that, in general, the coherence at low frequencies (delta and theta waves) is stronger than that at high frequencies (alpha, beta, and gamma waves). In particular, the strongest coherence at low frequencies occurred between the right and left temporal lobes (Figure 2a,b). At the same time, for alpha waves, the strongest coherence was observed between the right occipital and left parietal lobes (Figure 2c), while for beta waves, the strongest coherence occurred between the right frontal and left occipital lobes (Figure 2d). Finally, for gamma waves, the strongest coherence was observed between the left frontal and right occipital lobes (Figure 2e). The coherence values in both the brain and matrix representations are prominently depicted in the color bar, appearing as a distinct shade of dark red.
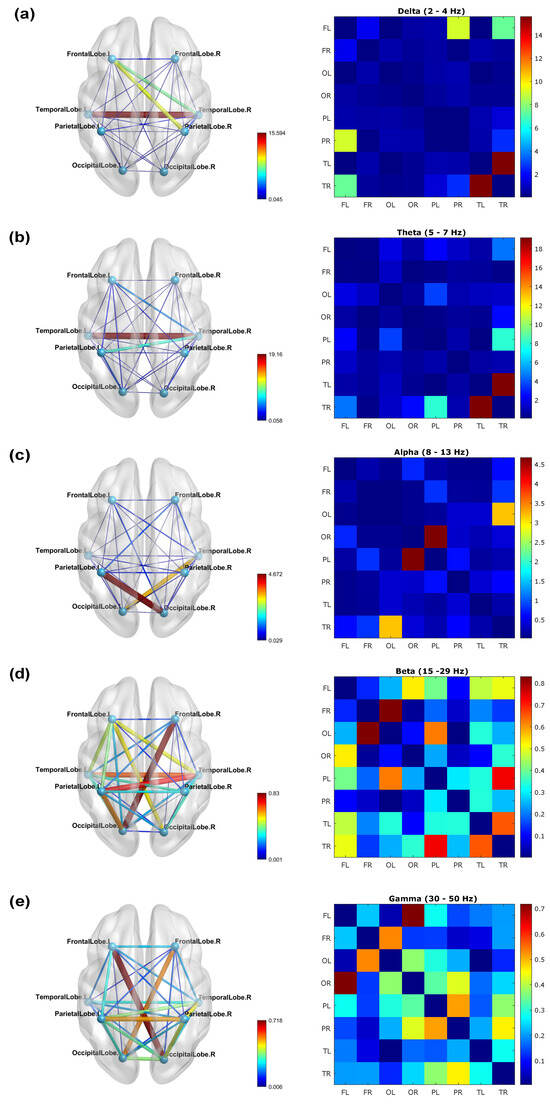
Figure 2.
Coherence networks (left column) with their respective matrices (right column) for (a) delta, (b) theta, (c) alpha, (d) beta, and (e) gamma waves of the normalized F-trials. The stronger coherence is represented by wider lines.
3.2. Frequency Spectra
Figure 3 displays the brain activity in the frequency domain. Here, we present the normalized power spectra in eight lobes, which correspond to the average fast Fourier transform (FFT) of the F-trials, normalized to the FFT of the B-trials.
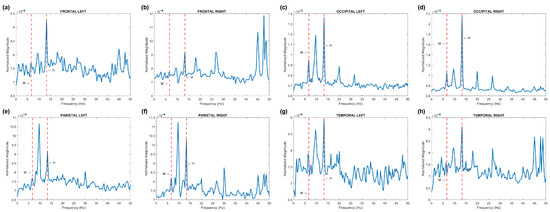
Figure 3.
Power spectra of brain activity in different lobes: (a) left frontal, (b) right frontal, (c) left occipital, (d) right occipital, (e) left parietal, (f) right parietal, (g) left temporal, and (h) right temporal. The dotted lines marked by letters M and H denote the modulation frequency and its second harmonic , respectively.
The modulation frequency Hz (M) is notably visible in the frequency spectrum of most lobes. However, its second harmonic, Hz (H), exhibits the highest amplitude across all lobes. Furthermore, the higher harmonics at and are discernible in the occipital lobes. This observation aligns cohesively with findings reported by Chholak et al. [39].
3.3. Network Characteristics
We computed various network characteristics. As depicted in Figure 2, the coherence coefficient ranges from 0 to 19.16 across different frequency bands, reaching a distinct maximum value for each band. Table 1 displays the maximum coherences observed within each frequency band, along with the lobes between which they were identified.

Table 1.
Maximum coherence of each frequency band.
In Figure 4, we plot the global connectivity coefficient found by Equation (2) for each frequency band with respect to the threshold value . A general decrease in the coherence coefficient between nodes was observed with increasing frequency.
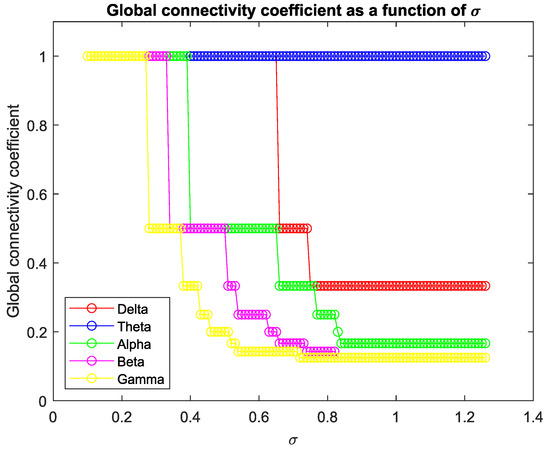
Figure 4.
Global connectivity coefficient for different frequency bands as a function of the threshold value.
Additionally, it is noteworthy that our representation emphasizes the heightened connectivity of low-frequency components compared to high frequencies, aligning with previous observations by Salvador et al. [48]. This depiction offers a clearer insight into the variations in connectivity between lobes within each frequency band.
One can see that, for each frequency range, there is a coherence threshold value at which centrality measures, shortest-path distances, and degree of nodes undergo significant changes (Figure A1, Figure A2, Figure A3, Figure A4 and Figure A5). This threshold value depends on the wave frequency. Specifically, as seen in Figure 4, for delta waves and for theta waves, the global connectivity appears to remain constant at 1 at all these values: for alpha waves, this is , for beta waves, this is , and for gamma waves, this is . This means that decreases as the wave frequency increases, i.e., the brain network of functional connectivity is more stable at low frequencies.
A connectivity threshold serves as a crucial parameter in delineating genuine connections within a functional network while filtering out spurious ones. This approach enables a focused examination of network properties. Analyzing a graph necessitates setting a connectivity threshold to discern valid connections among nodes and discard erroneous ones. The choice of threshold value is somewhat arbitrary, with increasing thresholds excluding weaker, potentially noisy connections. However, setting the threshold too high risks eliminating important frequency bands like beta and gamma, resulting in a connectivity coefficient of 0 for the resulting graph. Our chosen threshold value of ensures a minimum coherence of this magnitude between lobes’ signals. Despite its strictness, this threshold preserves all connections across various frequency bands.
Figure 5 illustrates the results of the analysis of degree centrality in the brain network of the eight lobes for different frequency ranges. The node sizes indicate their importance as a function of edge weights. Centrality, as extensively documented in the electrophysiological literature, has consistently underscored the non-uniform distribution of coherence across frequencies [49]. It is well established that different systems of brain regions may exhibit varying levels of coherence at distinct frequencies [48].
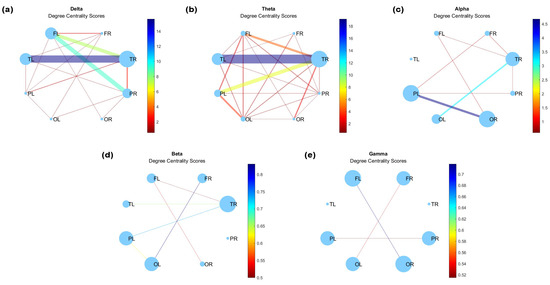
Figure 5.
Degree centrality for (a) delta, (b) theta, (c) alpha, (d) beta, and (e) gamma waves at .
The centrality patterns also demonstrate frequency-specific nuances. Specifically, at low frequencies, centrality predominantly manifests in temporal lobes, with noteworthy lateralization observed in delta and theta waves (Figure 5a,b). For alpha frequencies (Figure 5c), the coherence reveals a shift in centrality, now prominently observed in the right occipital and left parietal lobes, whereas in the case of beta and gamma waves (Figure 5d,e), the centrality is very weak and homogeneous between lobes. Contralateral coherences are observed between frontal and occipital lobes in the high-frequency bands.
Figure 6 presents another representation of the node degrees for the lobes. It is evident that the nodes exhibit larger coherence-related connections in the low-frequency bands (Figure 6a,b). However, in the higher-frequency bands (Figure 6c–e), the connections are less pronounced. Notably, the connections in the delta band (Figure 6a) are smaller compared to those in the theta network (Figure 6b), which shows nodes with degrees of 7. A more evident alteration is observed for alpha waves (Figure 6c), where the left temporal lobe is completely disconnected, while the other lobes experience a reduction in connections. In the beta graph (Figure 6d), the right parietal lobe ceases to participate entirely. Meanwhile, for gamma waves (Figure 6e), engagement diminishes for the temporal lobes, while the rest show a degree of 1.
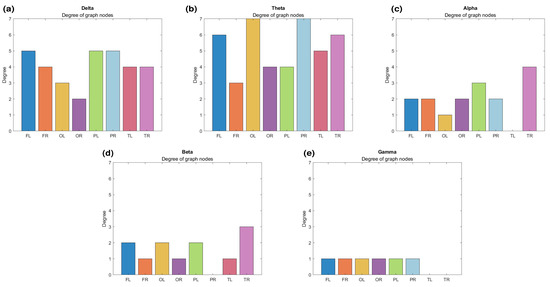
Figure 6.
Node degrees for (a) delta, (b) theta, (c) alpha, (d) beta, and (e) gamma waves. FL—left frontal; FR—right frontal; OL—left occipital; OR—right occipital; PL—left parietal; PR—right parietal; TL—left temporal; TR—right temporal.
Figure 7 illustrates the connected graph components, which are the subset of network nodes where there is a path from each node in the subset to any other node in the same subset [45]. This representation allows us to observe the formation of groups as connections begin to dissolve. Identifying connected components within an undirected graph provides insight into the network’s level of connectivity and aids in the extraction of coefficient (Equation (2)). Table 2 presents the global connectivity coefficients for .
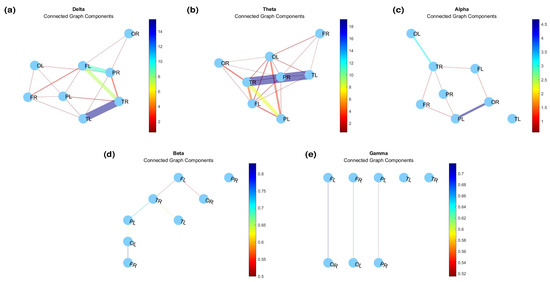
Figure 7.
Connected graph components for (a) delta, (b) theta, (c) alpha, (d) beta, and (e) gamma bands.

Table 2.
Connectivity coefficients for different frequency bands.
Figure 8 shows cycles formed in the networks for three frequency bands: delta, theta, and alpha. A cycle is a connected graph, in which each vertex has degree 2 [46]. The total number of cycles found for each graph is indicated in the figure. The theta graph (Figure 8b) displays the greatest connectivity and the most edges, resulting in a higher number of cycles within the network. In contrast, both the alpha and delta graphs (Figure 8a–c, respectively) exhibit fewer connections, with the alpha graph revealing only three cycles. Furthermore, no cycles were observed in the beta or gamma graphs. This observation is consistent with the broader literature, which suggests that slower rhythmic patterns typically exhibit a more widespread network configuration compared to faster ones [50,51].
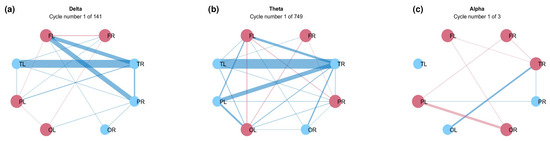
Figure 8.
Cycles of the graphs for (a) delta, (b) theta, and (c) alpha bands.
Distances between nodes are represented as matrices (, , , , ), showing the shortest path distances. When the nodes are not connected, the distance is infinite. The disconnection of the networks is also noticeable in these matrices. The largest value is a five-node distance, only seen in the beta wave.
3.4. Hypergraphs
Figure 9 represents the hypergraph constructed based on a chosen threshold () in different forms with colors corresponding to different frequency bands. In particular, the hypergraph is shown as a network in Figure 9a, a star expansion in Figure 9b with connections for each node, and as its incidence matrix in Figure 9c. It is observed that all eight lobes are coupled in the low-frequency ranges of the delta and theta bands, seven lobes (FL, FR, OL, OR, TR, PL, and PR) are coupled for the alpha band, eight lobes (FL, FR, TL, TR, OL, OR, and PL) for the beta band, and six lobes (FL, FR, OL, OR, PL, and PR) for the gamma band.
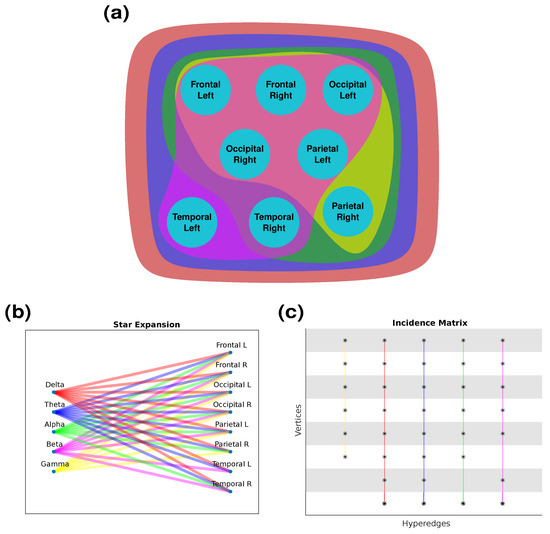
Figure 9.
Hypergraph representation as (a) network, (b) star expansion, and (c) adjacency matrix. Colors are maintained between hypergraph representations. Delta (red), Theta (blue), Alpha (green), Beta (magenta), Gamma (yellow).
The analysis was carried out following the basic properties of hypergraphs. The degrees of the vertices and hyperedges are given in Table 3, where is the number of vertices incident on i, and the degree of a hyperedge is the number of vertices it contains.

Table 3.
Vertices and hyperedges degrees.
A star, as mentioned earlier, denotes a pattern where a central vertex—in this case, representing a cerebral lobe—is intricately connected to multiple peripheral vertices, symbolizing different brain frequency ranges. This graphical representation is valuable as it effectively portrays the intricate relationship between a specific cerebral lobe and its involvement across diverse frequency bands. The presence of stars within the graph signifies that the central cerebral lobe exhibits activation across various cognitive conditions or mental states, indicative of its multifunctional nature.
The stars of each lobe are the following:
- -
- Left frontal lobe (node 1): Delta, theta, alpha, beta, and gamma.
- -
- Right frontal lobe (node 2): Delta, theta, alpha, beta, and gamma.
- -
- Left occipital lobe (node 3): Delta, theta, alpha, beta, and gamma.
- -
- Right occipital lobe (node 4): Delta, theta, alpha, beta, and gamma.
- -
- Left parietal lobe (node 5): Delta, theta, alpha, beta, and gamma.
- -
- Right parietal lobe (node 6): Delta, theta, alpha, and gamma.
- -
- Left temporal lobe (node 7): Delta, theta, and beta.
- -
- Right temporal lobe (node 8): Delta, theta, alpha, and beta.
Although correlations between frequencies have been observed in previous studies [52], and biophysical models have been proposed to explain interactions among different frequency bands, such as theta and gamma [53], further research, similar to the current study, is necessary to elucidate the potential coupling between the mechanisms generating these distinct frequencies.
4. Conclusions
In this study, we conducted a comprehensive hypergraph analysis of functional connectivity using MEG data acquired from a modulated visual stimulus. By focusing on differences within distinct frequency bands, we constructed a coherence-based hypergraph to explore functional connectivity among the frontal, parietal, temporal, and occipital brain lobes. Our findings suggest that each frequency band has a specific coherence threshold; beyond this, significant changes occur in network characteristics, such as centrality, shortest-path distances, and node degree. Interestingly, this threshold is lower for higher-frequency bands, indicating stronger connectivity between lobes at lower frequencies and greater stability in the brain network of functional connectivity. Specifically, we observed strong coherence among all eight lobes in the delta and theta bands; this was only observed among six lobes in the gamma band.
Furthermore, we noted variations in global connectivity with coherence thresholds across different frequency bands. Strong coherence was observed between temporal right and left lobes for delta and theta waves, occipital right and parietal left lobes for alpha waves, frontal right and occipital left lobes for beta waves, and frontal and occipital lobes for gamma waves. These findings collectively contribute to our understanding of brain network dynamics across different frequency bands. Our results lend credence to the idea that cortico–cortical interactions can manifest at multiple levels. The resultant hypergraph exposes robust activation patterns in select brain regions across varied cognitive contexts associated with various frequency bands, hinting at possible integration or multifunctionality within these lobes.
While previous studies have noted correlations between frequencies and proposed biophysical models to explain interactions, our results highlight the importance of further research to unravel potential connections between the mechanisms generating different frequencies and with a modulated visual stimulus, using coherence instead of traditional correlation. Additionally, we argue that exploring hypergraph visualizations with a focused examination of finer-grained neural ensembles or smaller regions of interest (ROIs) can reveal compelling dynamics that merit further scholarly investigation.
Author Contributions
N.P.S.: investigation, formal analysis; R.J.-R.: resources, project administration, original draft preparation; A.N.P.: conceptualization, methodology, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Technical University of Madrid (protocol No. 19, 3 February 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A. Network Characteristics as a Function of Coherence Threshold
The dependencies of the main network characteristics on the coherence threshold for different brain waves are illustrated in Figure A1, Figure A2, Figure A3, Figure A4 and Figure A5 (delta in Figure A1; theta in Figure A2; alpha in Figure A3; beta in Figure A4; gamma in Figure A5). The lobes are represented with different colors. The shortest path distances and numbers of cycles depict averages in the case of distances and totals in the case of cycles.
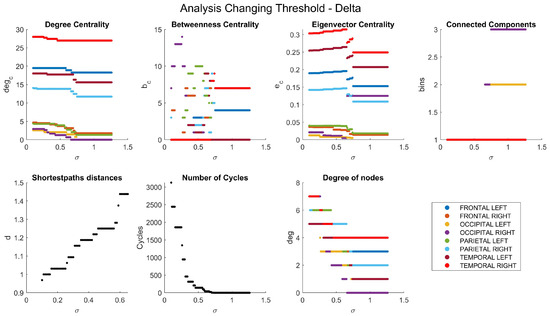
Figure A1.
Network characteristics versus coherence threshold value for delta band: degree centrality (degc), betweenness centrality (bc), eigenvector centrality (ec), connected components (bins), shortest-path distances (d), number of cycles (Cycles), and degree of nodes (deg).
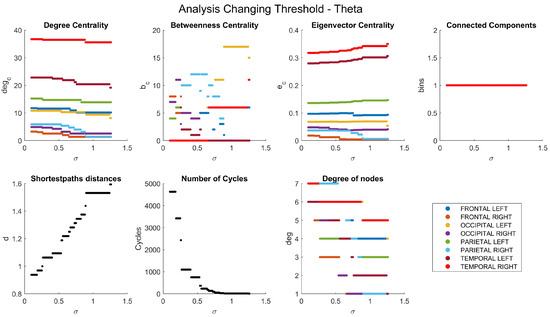
Figure A2.
Network characteristics for theta band.
Figure A6 illustrates the results of the analysis of the betweenness centrality coefficient, which measures the importance of a node in terms of the number of shortest paths that pass through it. Once again, the connections are less pronounced at higher frequencies (Figure A6c,d) compared to lower frequencies (Figure A6a,b). Furthermore, the nodes with the highest betweenness centrality vary across different frequency bands. In the gamma band (Figure A6d), the nodes show a uniform and reduced size, whereas in the delta band (Figure A6a), the nodes with the highest betweenness centrality coefficient are the left parietal and left frontal lobes. In contrast, in the theta band (Figure A6b), a notable enlargement was observed in the right parietal lobe compared to other lobes, presenting a striking contrast to the sizes depicted in the degree centrality graph (Figure 5b), where it initially appeared to be one of the smallest.
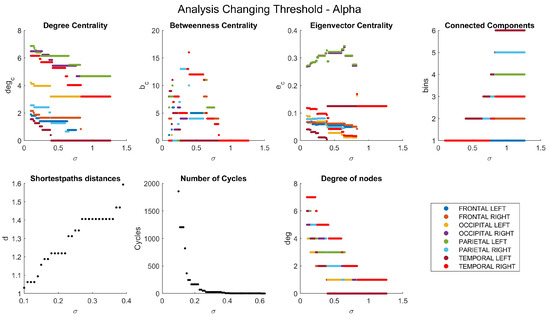
Figure A3.
Network characteristics for alpha band.
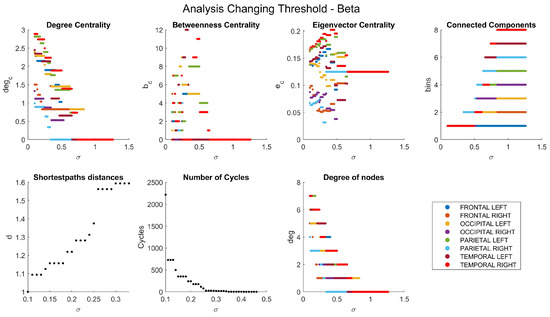
Figure A4.
Network characteristics for beta band.
The eigenvector centrality, depicting the significance of a node based on the importance of its neighboring nodes, is illustrated in Figure A7. Node sizes reflect their respective importance, mirroring the patterns observed in Figure 5 across most graphs. However, in the case of gamma waves, node sizes remain consistent. Conversely, nodes associated with alpha waves, particularly in the right temporal region, exhibit notably diminished sizes compared to their prominence in degree centrality (Figure 5c).
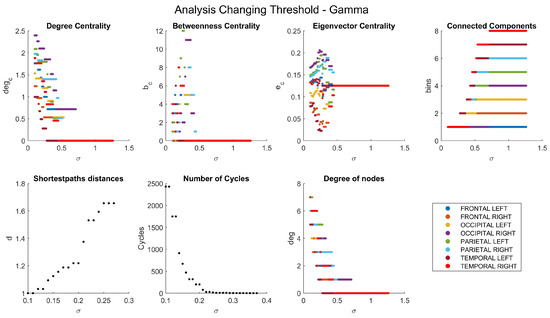
Figure A5.
Network characteristics for gamma band.
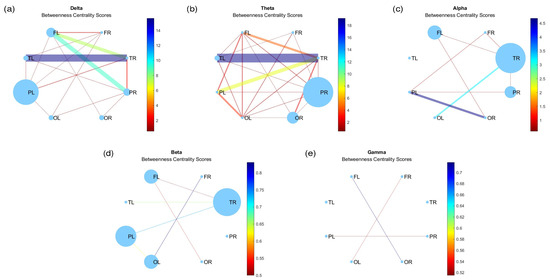
Figure A6.
Betweenness centrality for (a) delta, (b) theta, (c) alpha, (d) beta, and (e) gamma bands.
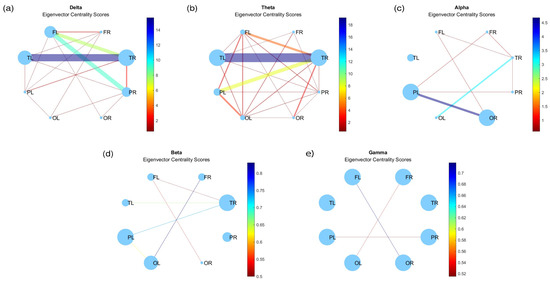
Figure A7.
Eigenvector centrality for (a) delta, (b) theta, (c) alpha, (d) beta, and (e) gamma bands.
References
- Friston, K.J.; Frith, C.D.; Liddle, P.F.; Frackowiak, R.S. Functional connectivity: The principal-component analysis of large (PET) data sets. J. Cereb. Blood Flow Metab. 1993, 13, 5–14. [Google Scholar] [CrossRef]
- Greenblatt, R.E.; Pflieger, M.E.; Ossadtchi, A.E. Connectivity measures applied to human brain electrophysiological data. J. Neurosci. Methods 2012, 207, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, R.E.; Pflieger, M.E.; Ossadtchi, A.E. Review of advanced techniques for the estimation of brain connectivity measured with EEG/MEG. Comput. Biol. Med. 2011, 41, 1110–1117. [Google Scholar]
- Hramov, A.E.; Frolov, N.S.; Maksimenko, V.A.; Kurkin, S.A.; Kazantsev, V.B.; Pisarchik, A.N. Functional networks of the brain: From connectivity restoration to dynamic integration. Phys. Uspekhi 2021, 64, 584–616. [Google Scholar] [CrossRef]
- Le Bihan, D.; Mangin, J.F.; Poupon, C.; Clark, C.A.; Pappata, S.; Molko, N.; Chabriat, H. Diffusion tensor imaging: Concepts and applications. J. Magn. Reson. Imaging JMRI 2001, 13, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Wedeen, V.J.; Wang, R.P.; Schmahmann, J.D.; Benner, T.; Tseng, W.Y.I.; Dai, G.; Pandya, D.N.; Hagmann, P.; D’Arceuil, H.; de Crespigny, A.J. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. NeuroImage 2008, 41, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Towle, V.L.; Hunter, J.D.; Edgar, J.C.; Chkhenkeli, S.A.; Castelle, M.C.; Frim, D.M.; Kohrman, M.; Hecox, K. Frequency domain analysis of human subdural recordings. J. Clin. Neurophysiol. 2007, 24, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Cabral, J.; Kringelbach, M.L.; Deco, G. Exploring the network dynamics underlying brain activity during rest. Prog. Neurobiol. 2014, 114, 102–131. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, B. The elusive concept of brain connectivity. NeuroImage 2003, 19, 466–470. [Google Scholar] [CrossRef]
- Bowyer, S. Coherence a measure of the brain networks: Past and present. Neuropsychiatr. Electrophysiol. 2016, 2, 1. [Google Scholar] [CrossRef]
- Frolov, N.; Maksimenko, V.; Lüttjohann, A.; Koronovskii, A.; Hramov, A. Feed-forward artificial neural network provides data-driven inference of functional connectivity. Chaos 2019, 29, 091101. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Hari, R.; Ilmoniemi, R.J.; Knuutila, J.; Lounasmaa, O.V. Magnetoencephalography—Theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev. Mod. Phys. 1993, 65, 413. [Google Scholar] [CrossRef]
- Burgess, R.C. Magnetoencephalography for localizing and characterizing the epileptic focus. Handb. Clin. Neurol. 2019, 160, 203–214. [Google Scholar]
- Boccaletti, S.; De Lellis, P.; del Genio, C.; Alfaro-Bittner, K.; Criado, R.; Jalan, S.; Romance, M. The structure and dynamics of networks with higher order interactions. Phys. Rep. 2023, 1018, 1–64. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev, Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J. Functional and effective connectivity: A review. Brain Connectivity 2011, 1, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Tavor, I.; Jones, O.P.; Mars, R.; Smith, S.; Behrens, T.; Jbabdi, S. Task-free MRI predicts individual differences in brain activity during task performance. Science 2016, 352, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Raichle, M.E. Disease and the brain’s dark energy. Nat. Rev. Neurol. 2010, 6, 15–28. [Google Scholar] [CrossRef]
- Greicius, M. Resting-state functional connectivity in neuropsychiatric disorders. Curr. Opin. Neurol. 2008, 21, 424–430. [Google Scholar] [CrossRef]
- Dennis, E.L.; Thompson, P.M. Functional brain connectivity using fMRI in aging and Alzheimer’s disease. Neuropsychol. Rev. 2014, 24, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, D.; Volkow, N.D. Aging and functional brain networks. Mol. Psychiatry 2012, 17, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Contreras, J.A.; Goñi, J.; Risacher, S.L.; Sporns, O.; Saykin, A.J. The structural and functional connectome and prediction of risk for cognitive impairment in older adults. Curr. Behav. Neurosci. Rep. 2015, 2, 234–245. [Google Scholar] [CrossRef]
- Sala-Llonch, R.; Bartrés-Faz, D.; Junqué, C. Reorganization of brain networks in aging: A review of functional connectivity studies. Front. Psychol. 2015, 6, 663. [Google Scholar] [CrossRef] [PubMed]
- Davison, E.N.; Turner, B.O.; Schlesinger, K.J.; Miller, M.B.; Grafton, S.T.; Bassett, D.S.; Carlson, J.M. Individual differences in dynamic functional brain connectivity across the human lifespan. PLoS Comput. Biol. 2016, 12, e1005178. [Google Scholar] [CrossRef] [PubMed]
- Andrew, C.; Pfurtscheller, G. Event-related coherence as a tool for studying dynamic interaction of brain regions. Electroencephalogr. Clin. Neurophysiol. 1996, 98, 144–148. [Google Scholar] [CrossRef]
- Pisarchik, A.; Hramov, A. Coherence resonance in neural networks: Theory and experiments. Phys. Rep. 2023, 1000, 1–57. [Google Scholar] [CrossRef]
- Gross, J.; Schmitz, F.; Schnitzler, I.; Kessler, K.; Shapiro, K.; Hommel, B.; Schnitzler, A. Modulation of long-range neural synchrony reflects temporal limitations of visual attention in humans. Proc. Natl. Acad. Sci. USA 2004, 101, 13050–13055. [Google Scholar] [CrossRef]
- Guggisberg, A.G.; Honma, S.M.; Findlay, A.M.; Dalal, S.S.; Kirsch, H.E.; Berger, M.S.; Nagarajan, S.S. Mapping functional connectivity in patients with brain lesions. Ann. Neurol. 2008, 63, 193–203. [Google Scholar] [CrossRef]
- Belardinelli, P.; Ciancetta, L.; Staudt, M.; Pizzella, V.; Londei, A.; Birbaumer, N.; Romani, G.L.; Braun, C. Cerebro-muscular and cerebro-cerebral coherence in patients with pre- and perinatally acquired unilateral brain lesions. NeuroImage 2007, 37, 1301–1314. [Google Scholar] [CrossRef]
- de Pasquale, F.; Della Penna, S.; Snyder, A.Z.; Lewis, C.; Mantini, D.; Marzetti, L.; Belardinelli, P.; Ciancetta, L.; Pizzella, V.; Romani, G.L.; et al. Temporal dynamics of spontaneous MEG activity in brain networks. Proc. Natl. Acad. Sci. USA 2010, 107, 6040–6045. [Google Scholar] [CrossRef]
- Kim, J.S.; Shin, K.S.; Jung, W.H.; Kim, S.N.; Kwon, J.S.; Chung, C.K. Power spectral aspects of the default mode network in schizophrenia: An MEG study. BMC Neurosci. 2014, 15, 104. [Google Scholar] [CrossRef]
- Bowyer, S.M.; Gjini, K.; Zhu, X.; Kim, L.; Moran, J.E.; Rizvi, S.U.; Gumenyuk, N.T.; Tepley, N.; Boutros, N.N. Potential biomarkers of schizophrenia from MEG resting-state functional connectivity networks: Preliminary data. J. Behav. Brain Sci. 2015, 5, 1–11. [Google Scholar] [CrossRef]
- Boutros, N.N.; Galloway, M.P.; Ghosh, S.; Gjini, K.; Bowyer, S.M. Abnormal coherence imaging in panic disorder: A magnetoencephalography investigation. Neuroreport 2013, 24, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Chholak, P.; Niso, G.; Maksimenko, V.A.; Kurkin, S.A.; Frolov, N.S.; Pitsik, E.N.; Hramov, A.E.; Pisarchik, A.N. Visual and kinesthetic modes affect motor imagery classification in untrained subjects. Sci. Rep. 2019, 9, 9838. [Google Scholar] [CrossRef] [PubMed]
- Pisarchik, A.N.; Chholak, P.; Hramov, A.E. Brain noise estimation from MEG response to flickering visual stimulation. Chaos Solitons Fractals X 2019, 1, 100005. [Google Scholar] [CrossRef]
- Chholak, P.; Maksimenko, V.A.; Hramov, A.E.; Pisarchik, A.N. Voluntary and involuntary attention in bistable visual perception: A MEG study. Front. Hum. Neurosci. 2020, 14, 555. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Gao, Y. Hypergraph Computation; Springer: Singapore, 2023. [Google Scholar]
- Wang, Z.; Liu, J.; Zhong, N.; Qin, Y.; Zhou, H.; Yang, J.; Li, K. A naive hypergraph model of brain networks. In Proceedings of the Brain Informatics: International Conference, BI 2012, Macau, China, 4–7 December 2012; Springer: Berlin/Heidelberg, Germany, 2012; pp. 119–129. [Google Scholar]
- Chholak, P.; Kurkin, S.A.; Hramov, A.E.; Pisarchik, A.N. Event-related coherence in visual cortex and brain noise: An MEG study. Appl. Sci. 2021, 11, 375. [Google Scholar] [CrossRef]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011, 2011, 1–13. [Google Scholar] [CrossRef]
- Bear, M.; Connors, B.; Paradiso, M.A. Neuroscience: Exploring the Brain, Enhanced Edition: Exploring the Brain; Jones & Bartlett Learning: Burlington, MA, USA, 2020. [Google Scholar]
- French, C.C.; Beaumont, J.G. A critical review of EEG coherence studies of hemisphere function. Int. J. Psychophysiol. 1984, 1, 241–254. [Google Scholar] [CrossRef]
- Xia, M.; Wang, J.; He, Y. BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS ONE 2013, 8, e68910. [Google Scholar] [CrossRef]
- Golbeck, J. Analyzing the Social Web; Morgan Kaufmann: Boston, MA, USA, 2013. [Google Scholar]
- Zinoviev, D. Complex Network Analysis in Python: Recognize-Construct-Visualize-Analyze-Interpret; Pragmatic Bookshelf: Raleigh, NC, USA, 2018. [Google Scholar]
- Voloshin, V.I. Introduction to Graph and Hypergraph Theory; Nova Science Publishers: Hauppauge, NY, USA, 2009. [Google Scholar]
- Pickard, J.; Chen, C.; Salman, R.; Stansbury, C.; Kim, S.; Surana, A.; Bloch, A.; Rajapakse, I. HAT: Hypergraph analysis toolbox. PLoS Comput. Biol. 2023, 19, e1011190. [Google Scholar] [CrossRef] [PubMed]
- Salvador, R.; Suckling, J.; Schwarzbauer, C.; Bullmore, E. Undirected graphs of frequency-dependent functional connectivity in whole brain networks. Philos. Trans. R. Soc. B 2005, 360, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.T.; Miller, L.M.; D’esposito, M. Measuring interregional functional connectivity using coherence and partial coherence analyses of fMRI data. Neuroimage 2004, 21, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Von Stein, A.; Sarnthein, J. Different frequencies for different scales of cortical integration: From local gamma to long range alpha/theta synchronization. Int. J. Psychophysiol. 2000, 38, 301–313. [Google Scholar] [CrossRef]
- Buzsaki, G.; Draguhn, A. Neuronal oscillations in cortical networks. Science 2004, 304, 1926–1929. [Google Scholar] [CrossRef]
- Furl, N.; Coppola, R.; Averbeck, B.B.; Weinberger, D.R. Cross-frequency power coupling between hierarchically organized face-selective areas. Cereb. Cortex 2014, 24, 2409–2420. [Google Scholar] [CrossRef][Green Version]
- Pastoll, H.; Solanka, L.; van Rossum, M.C.; Nolan, M.F. Feedback inhibition enables theta-nested gamma oscillations and grid firing fields. Neuron 2013, 77, 141–154. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).