Abstract
Drying herbs is a crucial method for stabilizing and preserving their essential properties and bioactive compounds. Although freeze drying is the preferred method for most herbs, it is expensive due to high energy consumption and operating costs. Biflavonoids are dimeric flavonoids that have recently been recognized as potential molecules possessing biological activities, such as antiviral and antimicrobial activity, and as effective molecules for the treatment of neurodegenerative and metabolic diseases and for cancer therapies. In this study, we performed a comparative analysis of freeze drying, air drying and oven drying to evaluate their effects on biflavonoid content in yellow ginkgo leaves (Ginkgo biloba L.). After drying, we performed spectrophotometric analysis to determine the browning index, pigments, phenolic compounds and antioxidant activity, while HPLC-DAD was used for the identification and quantification of individual biflavones (amentoflavone, bilobetin, ginkgetin, isoginkgetin and sciadopitysin). The most abundant biflavonoids were isoginkgetin and bilobetin, the amounts of which exceeded 1000 µg/g dw in all leaf samples. They were followed by ginkgetin and sciadopitysin, the amounts of which were about 30% lower. The drying method did not influence biflavone content or the total carotenoids, total polyphenols and total flavonoids. Consequently, our study suggests that all three methods may be used for the preparation of yellow ginkgo leaves as a source of biflavones and other bioactive compounds.
1. Introduction
The ginkgo (Ginkgo biloba L.) is the only member of the Ginkgoaceae family whose fossil remains are over 200 million years old, earning it the nickname “living fossil” [1]. It is characterized by a distinctive crown and trunk, which is most notable for its unique fan-shaped leaves with dichotomously branched veins. This species originates from southeast China and its cultivars are now widespread throughout the world [2]. Ginkgo is often associated with Eastern medicinal and religious traditions and has attracted considerable interest due to its beneficial health effects, particularly in relation to improving memory and the cardiovascular system [3]. These properties are primarily attributed to various bioactive compounds in ginkgo leaves such as flavonoids, phenolic acids and terpenoids [4]. More than a hundred flavonoids have been identified in ginkgo, which occur in two forms—as monomers and dimers in the form of aglycones or glycosides [4]. Biflavonoids, the dimeric forms of flavonoids, are commonly found in ginkgo leaves [5]. They include amentoflavone, bilobetin, ginkgetin, isoginkgetin and sciadopitysin as the predominant biflavones [5,6]. Biflavonoids have recently become of interest due to their antiviral, anticarcinogenic and neuroprotective effects [6,7]. In particular, they are known as antiviral agents against the COVID-19 virus [8,9]. Yellow ginkgo leaves are particularly rich in the biflavones amentoflavone, bilobetin, ginkgetin, isoginkgetin and sciadopitysin, the content of which is higher in yellow leaves than in green leaves [10].
Drying of herbs is a crucial technological process primarily aimed at removing the water content and enabling longer preservation of raw materials [11]. The drying process of plant material facilitates the removal of water, effectively suppresses metabolic processes and ensures the stability of the chemical composition of the plant material [11]. In addition, it helps to minimize the presence of microorganisms so that the full range of physicochemical and sensory properties of the plant product is preserved [12]. Drying processes can take place under natural conditions, such as air drying, or in controlled artificial environments with temperature control (either high or low) and vacuum. Considering the time required and the need to prevent the possible contamination or damage of samples by external factors, artificial drying systems are increasingly preferred, especially drying chambers and freeze drying [13]. As elevated drying temperatures can lead to degradation of and reductions in the desired components, freeze drying is increasingly being used [11,14]. Drying methods have a major impact on the various properties of the final product, including the presence and composition of bioactive components as well as physicochemical and organoleptic properties [15]. In high temperature drying, a hot air circulation system is used to vaporize water, while in freeze drying, also known as vacuum drying, the water is suppressed under low pressure after the plant material has been previously frozen [11]. Freeze drying allows high efficiency in removing water from the plant matrix while keeping all bioactive components stable and preserving their chemical structure. It is often the method of choice to extract flavonoids [13]. Although heat and freeze drying techniques shorten the drying time, they also have their limitations. For example, freeze drying is energy-intensive, while heat drying can affect quality [14]. The use of an appropriate drying method can therefore reduce production costs, preserve the bioactive compounds in the material and improve the quality of the final products. However, the literature shows that the effect of leaf drying depends on the type of leaves. In the case of mulberry leaves, Hu et al. [16] reported that air drying and freeze drying are the best methods to preserve the antioxidant activity of flavonoids. Since air drying has lower operating costs than freeze drying to obtain high flavonoid content and maximum antioxidant activity, they recommended air drying for mulberry leaves. In contrast, freeze drying of leaves was preferred for Carica papaya L. [17], Streblus asper Lour [18], green tea (Camellia sinensis L.) [19] and guava (Psidium guajava L.) [20] leaves to obtain the desired amounts of polyphenols and flavonoids. For nettle (Urtica dioica L.) leaves, the total phenol content and antioxidant activity were higher in oven-dried leaves than in freeze-dried leaves [21], while for Anneslea fragrans leaves [22], shade drying is the preferred drying method. This once again underlines the need to optimize the drying method for each leaf type and for each bioactive compound.
In the case of ginkgo, drying methods for ginkgo seeds have been studied [23,24,25,26,27], but little attention has been paid to the influence of the drying method on the bioactive compounds in ginkgo leaves. Considering the growing interest in biflavonoids and the fact that yellow ginkgo leaves are a potential source of biflavonoids [6], we wanted to test different drying methods for yellow ginkgo leaves and investigate how they affect biflavonoids and other bioactive compounds. As far as we know, this is the first report on the influence of drying method on the form of dimeric flavonoids in yellow ginkgo leaves and on biflavones overall. We compared three drying methods for plant material: freeze drying, air drying and oven drying for yellow ginkgo leaves. After drying, we determined the browning index and the total content of phenols, flavonoids, phenolic acids and pigments (chlorophyll a, b, total chlorophylls and carotenoids) spectrophotometrically and the content of the five most abundant biflavonoids in the ginkgo leaves (amentoflavone, bilobetin, ginkgetin, isoginkgetin, sciadopitysin) by HPLC-DAD. We also measured the antioxidant scavenging capacity using the DPPH method.
2. Materials and Methods
2.1. Chemicals, Reagents and Instruments
We used the following chemicals and reagents: ethanol 96% (GRAM-MOL, Zagreb, Croatia), acetone (GRAM-MOL, Zagreb, Croatia), acetonitrile ≥ 99.9% UHPLC gradient-grade (Fischer Scientific, Taipei City, Taiwan), formic acid 98–100% (Sigma Aldrich, Darmstadt, Germany), HPLC grade-standard amentoflavone (PhytoLab, Vestenbergsgreuth, Germany), ginkgetin (PhytoLab, Vestenbergsgreuth, Germany), isoginkgetin (PhytoLab, Vestenbergsgreuth, Germany), bilobetin (PhytoLab, Vestenbergsgreuth, Germany) and sciadopitysin (PhytoLab, Vestenbergsgreuth, Germany), gallic acid 98% (Acros Organics, China), Folin–Ciocalteu’s reagent (Sigma Aldrich, Buchs, Schwitzerland), sodium carbonate (T.T.T., Sveta Nedelja, Croatia), (+)-catechin hydrate (Sigma Aldrich, St. Louis, MO, USA), aluminum chloride hexahydrate (Thermo Fischer Scientific, Kandel, Germany), hydrochloric acid 36.5% (Kemika, Zagreb, Croatia), sodium hydroxide (T.T.T., Sveta Nedelja, Croatia), sodium nitrite (Kemika, Zagreb, Croatia), sodium molybdate (VI) dihydrate (Sigma Aldrich, St. Louis, MO, USA), caffeic acid ≥ 98% HPLC-grade (Sigma Aldrich, St. Louis, MO, USA), 2,2-diphenyl-1-picrylhydrazyl (Sigma Aldrich, Steinheim, Germany), methanol (Kemika, Zagreb, Croatia), (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid 97% (Sigma Aldrich, Buchs, Schwitzerland).
For drying samples, we used a freeze dryer (LIO-5PLT, KAMBIČ, Ljubljana, Slovenia) and oven dryer (VENTI-Line 180 Prime, VWR, Gdańsk, Poland). For grinding leaves, a bead mill (Bead Ruptor 12, Omni International, Kennesaw, GA, USA) was used. For weighing plant material, an analytical balance (Adam Equipment, Maidstone, UK) was utilized. For preparing extracts, an ultrasonic bath (DU-100, Argo Lab, Carpi, Italy), mechanical rotator (Bio RS-24, Biosan, Riga, Latvia), vortex (V-1 plus, Biosan, Riga, Latvia) and centrifuge (LMC-4200R, Biosan, Riga, Latvia) were employed. For spectrophotometric analyses, a UV-VIS spectrophotometer (ONDA TOUCH UV-21, China) was used. For determining biflavonoids, an Agilent 1260 Infinity II high-performance liquid chromatography system (Agilent, Santa Clara, CA, USA) with a diode array detector (DAD) was employed. System controlling and data analysis was performed using OpenLab CDS Workstation Software version 2.8.
2.2. Plant Material and Drying Experiments
Yellow ginkgo leaves were collected from nine different trees in a ginkgo alley in Koprivnica, Croatia in October 2022. We collected the leaves from each tree (in equal amounts, around 100 leaves). The leaves were immediately brought to the laboratory on ice and then divided into three groups for different drying methods (approximately 100 leaves for each method). This ensured that our sample sizes were homogenized and that the only parameters that could influence the differences were the different drying methods.
The plant material was then immediately subject to different drying methods: freeze drying, air drying and oven drying. All leaves were dried until a constant weight was reached. Leaves were dried in a freeze dryer (LIO-5PLT, KAMBIČ, Ljubljana, Slovenia) under conditions of approximately −102 °C and 0.3303 mBar pressure. Before freeze drying, leaves were frozen at −80 °C for two hours and then transferred to a freeze dryer already operating at freezing temperature. Air drying of the leaves was performed in a laboratory bench at room temperature around 21–23 °C and humidity 30–50% without direct sun exposure but in light for a part of the day. The third type of drying, oven drying (VENTI-Line 180 Prime, Poland) with circulating air, was carried out at 60 °C and with the air flap control set to 50%.
After reaching the constant weight (which was checked by regular leaf weighing) the dried leaves (Figure 1) were ground into fine powder using a bead mill (Bead Ruptor 12, Omni International, USA). Samples dried in a different way were ground together in one run in separated tubes with the addition of a 2.4 mm metal bead at 6 m/s for 3 min, and the resulting powder was used for all subsequent analyses.

Figure 1.
Yellow ginkgo leaves and leaf powder after freeze drying, air drying and oven drying, respectively.
2.3. Browning Index
The browning index (BI) was determined according to the method described by Lee et al. [28]. Ten milligrams of the ginkgo powder were mixed with one millilitre of pure acetone and then centrifuged. The supernatant was decanted and transferred to a plastic cuvette, and the absorption was read at 420 nm. The results are given as values of the measured absorption values.
2.4. Pigments
The measurement of pigments included the quantitative determination of chlorophylls and carotenoids in tissue according to the method of Lichtenthaler and Buschmann [29]. Ten milligrams of ginkgo powder were mixed with one millilitre of pure acetone and then centrifuged. The supernatant was separated and the precipitate was discarded. The absorbance was measured in the supernatant at three wavelengths (661.6, 644.8 and 470 nm), which were used for further calculations for pure acetone as solvent. The results are given in µg/g dw.
2.5. Phenolic Compounds
For the determination of total phenols, flavonoids, phenolic acid and individual biflavonoids, 60 mg of ginkgo leaf powder was weighed and 2 ml of 70% ethanol was added. The samples were then shaken and incubated in an ultrasonic bath for 10 min. The samples were then placed in a rotator for 45 min. The supernatant was separated after centrifugation and used for the analysis.
Total polyphenols were determined by the colorimetric method using Folin–Ciocalteu (FC) reagent [30]. First, 200 µL of the extract was mixed with 1580 µL of distilled water and 100 µL of FC reagent. Then, 300 µL of sodium carbonate was added and after two hours the absorbance was measured at 765 nm. The standard curve was prepared with gallic acid and the results are expressed as gallic acid equivalents per dry weight (µg GAE/mg dw). The determination of total flavonoids was performed according to the method of Zhishen et al. [31]. Initially, 200 µL of the extract was mixed with 800 µL of distilled water. The reaction was started with 60 µL NaNO2 (5% solution). After five minutes, 60 µL of a 10% solution of Al2Cl3 was added. After a further 6 min, 400 µL NaOH (1 M) was added and the absorbance was measured at 510 nm. The standard curve was prepared with catechol and the results are expressed as catechol equivalents per dry weight (µg CAE/mg dw). Total phenolic acids were determined using Arnow’s reagent method [32]. Initially, 300 µL of the extract was mixed with 300 µL distilled water, 100 µL HCl (0.5 M) and 100 µL Arnow’s reagent. Subsequently, 100 µL NaOH (1 M) and 100 µL distilled water were added. The absorbance was measured at 505 nm. The standard curve was prepared with caffeic acid and the results were expressed as caffeic acid equivalent per dry weight (µg CA/mg dw).
Identification and quantification of the individual bioflavonoids (amentoflavone, bilobetin, ginketin, isogingetin and sciadopitysin) was performed using an Agilent 1260 Infinity II high-performance liquid chromatography system (Agilent, Santa Clara, CA, USA) with a diode array detector (DAD). Chromatographic separation was performed using a Zorbax 300Extend-C18 column (Agilent, Santa Clara, CA, USA). Data acquisition and subsequent processing were performed using Agilent OpenLAB CDS software (version 2.6, Agilent, Santa Clara, CA, USA). The extraction, analysis parameters and calibration curves were consistent with our previously published work by Kovač Tomas et al. [5]. The results were expressed as µg/g dw.
2.6. Antioxidant Activity
Antioxidant activity was determined using the DPPH (2,2-diphenyl-1-picrylhydrazyl) assay [33]. A total of 5 µL of the ethanolic extract was mixed with 995 µL of the DPPH solution and the mixture was allowed to stand in the dark for about half an hour. The absorbance was measured at 515 nm. The standard curve was prepared with Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) and so the results are expressed as Trolox equivalents per dry weight (mM TE/g dw).
2.7. Statistical Analysis
All analyses were conducted in triplicate, and the results are expressed as mean ± standard deviation (SD). All statistical analyses were performed using PAST software, version 4.13. One-way ANOVA and subsequent multiple mean comparisons with Tukey’s HSD test were executed, and distinctions between measurements were considered significant at p < 0.05.
3. Results
3.1. Influence of Different Drying Methods on the Browning Index
The influence of different drying methods—freeze drying, air drying and oven drying—on the browning index is shown in Figure 2.
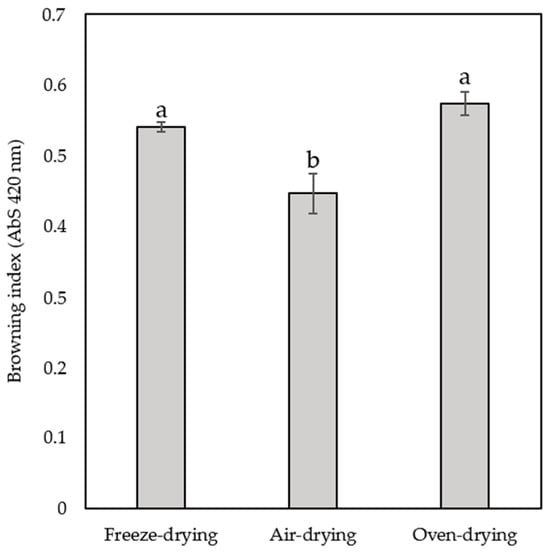
Figure 2.
Browning index for the three types of drying yellow ginkgo leaves. Values marked with different letters are significantly different at p < 0.05.
Oven-dried (0.57 ± 0.02) and freeze-dried (0.54 ± 0.01) leaves showed comparable values, while the significantly lowest values were observed for air-dried leaves (0.45 ± 0.03).
3.2. Influence of Different Drying Methods on the Composition of Chlorophylls and Carotenoids
The content of chlorophyll a and b, total chlorophylls and carotenoids, as well as the ratios of chlorophyll a/b and total chlorophylls to carotenoids in yellow ginkgo leaves dried using three different methods are presented in Table 1.

Table 1.
The content of pigments in yellow ginkgo leaves affected by different drying methods. Values marked with different letters are significantly different at p < 0.05.
According to the data presented in Table 1, the highest content of chlorophyll a and b and total chlorophyll was observed in the samples dried by oven drying, followed by freeze drying and air drying. However, the total carotenoid content showed no statistical differences between the three drying methods and all leaf samples showed values in the range of 159–174 µg/g dw.
The ratio of chlorophyll a to chlorophyll b was lower in the air-dried samples and comparable in the freeze-dried and oven-dried samples. Similarly, the chlorophyll/carotenoid content was also much lower in air-dried leaves. The low chlorophyll/carotenoid content indicates a higher carotenoid content, which is to be expected in yellow leaves as we used in this experiment.
3.3. Influence of Different Drying Methods on the Content of the Total Polyphenols, Flavonoids, Phenolic Acids and Individual Biflavonoids
The influence of the different drying methods on the content of total polyphenols, total flavonoids and total phenolic acids is shown in Figure 3.
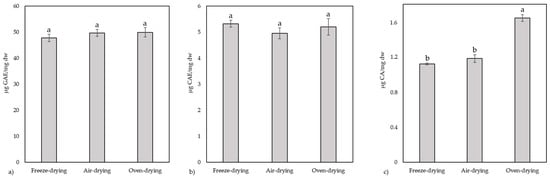
Figure 3.
Total polyphenols (a), total flavonoids (b) and total phenolic acids (c) for the three types of drying used on yellow ginkgo leaves. Values marked with different letters are significantly different at p < 0.05.
The total content of polyphenols and flavonoids did not differ significantly in the leaf samples dried by different methods. Non-significantly, the highest levels of total flavonoids were found in the freeze-dried samples (5.32 ± 0.13 µg CAE/mg dw), while the oven- dried samples had the highest levels of total polyphenols (49.92 ± 1.81 µg GAE/mg dw). Oven-dried leaves had the highest content of total phenolic acids (1.64 ± 0.04 µg CA/mg dw), and this value was significantly higher.
The influence of different drying methods on the individual composition of biflavonoids in yellow ginkgo leaves is shown in Figure 4.
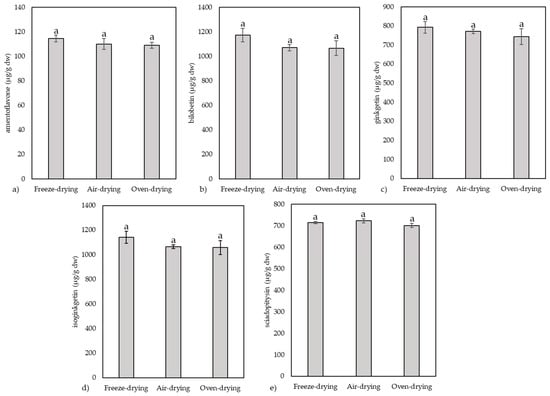
Figure 4.
The individual biflavonoid composition, including amentoflavone (a), bilobetin (b), ginkgetin (c), isoginkgetin (d) and sciadopitysin (e), for the three types of drying used on yellow ginkgo leaves. Values marked with different letters are significantly different at p < 0.05.
The most abundant biflavonoids were isoginkgetin and bilobetin, the amount of which exceeded 1000 µg/g dw in all leaf samples. They were followed by ginkgetin and sciadopitysin, the amounts of which were about 30% lower, in the range of 700–800 µg/g dw. The least abundant was amentoflavone, which was just over 100 µg/g dw, 10 times less than isoginkgetin and bilobetin. Freeze-dried samples showed slightly higher average values, but these were not significant, so our results indicate that the drying method had no significant effect on the content of individual biflavonoids.
3.4. Influence of Different Drying Methods on Antioxidant Activity
The influence of the different drying methods on the antioxidant activity of the yellow ginkgo leaves is shown in Figure 5.
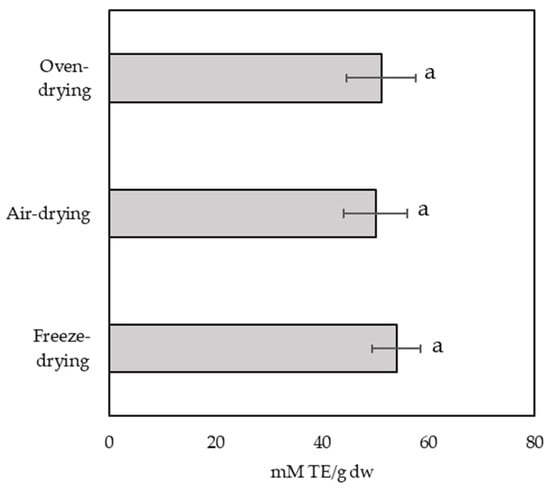
Figure 5.
Antioxidant activity analysed by DPPH for the three types of drying used on yellow ginkgo leaves. Values marked with different letters are significantly different at p < 0.05.
The radical scavenging capacity ranged from 50.13 ± 5.98 mM TE/g dw in air-dried samples to 54.03 mM TE/g dw in freeze-dried samples. Nevertheless, there were no statistically significant differences depending on the drying method.
4. Discussion
During drying, undesirable colour changes may occur in dried plant materials due to non-enzymatic browning, which may affect consumer preference for the products, but also decrease bioactive compounds [34]. Therefore, we compared the browning index of our samples and found a comparable value in the oven-dried and freeze-dried samples, while the browning index was lowest in the air-dried samples. In general, according to our results, all three methods did not lead to the formation of brown pigments in large quantities. In contrast, for pine needles, hot air drying and freeze drying were reported to increase the browning index four-fold compared to freeze drying [35]. Yilmaz and Alibas [36] reported that higher drying temperature leads to a higher browning index in basil leaves, which is consistent with our results, in which air-dried samples without high temperature had the lowest browning index compared to the oven-dried ones.
The colour of the leaves is also influenced by pigments. Green leaves are dominated by chlorophylls, the amount of which decreases as the leaves age, and yellow leaf colour is due to the presence of carotenoids (yellow pigments) or other flavonoids, depending on the leaf [37]. Although we used yellow leaves, they still contained some chlorophylls, as can be seen from the results in Table 1. The ratio between total chlorophyll and total carotenoids was low, indicating a low chlorophyll content, which is characteristic of yellow leaves. According to our results, the oven-dried samples had significantly higher chlorophyll a, b and total chlorophyll content compared to the air-dried or freeze-dried samples. It is known that chlorophylls are sensitive to light, so these results were to be expected. The air-dried samples were exposed to light, as were the freeze-dried samples, as the design of our freeze-dryer allows the samples to be exposed to light. In contrast, the samples in the oven were protected from light and had the highest chlorophyll content. Similar to our experiments, Shittu et al. [38] reported significantly lower chlorophyll content in air-dried (in the shade) than in heat-dried mint leaves. In our experiment, the chlorophyll a/chlorophyll b ratio was lowest in the air-dried samples, which is probably due to the rapid degradation of chlorophyll a. A similar finding was observed in peppermint leaves, where a lower chlorophyll a/chlorophyll b ratio was found in leaves dried at a temperature of 22 ± 2 °C in air than in leaves dried with hot air or infrared, convection or microwave dryers [39]. In our experiments, all drying methods had an effect on the green chlorophylls, but in contrast the carotenoids were stable and their content did not differ between the samples dried by different methods. Carotenoids are associated with numerous biological activities, such as anticancer, immunomodulatory, anti-inflammatory, antibacterial, antidiabetic and neuroprotective activities [40].
From the point of view of potential pharmaceutical use, the important compounds in ginkgo leaves are those from the polyphenol group [4]. The total polyphenol content in our samples was about 50 µg GAE/mg dw and did not differ significantly from the leaves dried in different ways. The measured amount is higher than in other medicinal plants, such as Hypericum perforatum L. [41,42], Gynostemma pentaphyllum L. [43] or Micromeria croatica (Pers.) Schott [44], which were previously measured using similar protocols. The total flavonoid content was also not significantly different in the ginkgo samples and was around 5 µg CE/mg live weight, while the total phenolic acid content was highest in the oven-dried samples and lowest in the freeze-dried and air-dried samples. The higher amount in the oven-dried samples could be due to the higher temperature, which is reported as a favourable parameter for the extraction of phenolic acids [45]. We also performed quantification of individual biflavonoids and identified five biflavonoids, similar to a previous study on ginkgo leaves [5]. We identified isoginkgetin and bilobetin as the most abundant biflavonoids in yellow ginkgo leaves, which is consistent with previously published studies [46]. Isoginkgetin is known as a general pre-mRNA splicing inhibitor and, as such, has been implicated in research as a new avenue for the development of novel anticancer drugs [47]. Our results show that yellow ginkgo leaves may be an important source of isoginkgetin. Isoginkgetin has a yellow colour, as does ginkgetin, so the characteristic yellow colour of the leaves is probably also due to the presence of biflavonoids. The content of individual biflavonoids in ginkgo leaves may differ at different stages of development or be influenced by growing conditions and other environmental conditions (reviewed by [6]). According to our study, the drying method had no effect on the biflavonoid content, suggesting that air drying can also be used as an alternative to more expensive methods for drying yellow ginkgo leaves as a source of biflavonoids.
Ginkgo leaf extracts are generally reported to have antioxidant activity [48,49], and according to our results, the antioxidant activity measured by the DPPH method was not affected by the drying method. This is probably due to the similar content of polyphenols and flavonoids in the leaves dried by different methods, which are recognized as potent antioxidants [50]. However, our recent study showed that biflavonoids are less potent antioxidants than monomeric flavonoids [51], but their antioxidant activity remains contradictory.
5. Conclusions
In this study, we investigated the effects of three different drying methods on the browning index, pigment content, polyphenolic components, especially biflavonoids, and antioxidant activity. The most abundant biflavonoids in yellow ginkgo leaves are isoginkgetin and bilobetin. The drying methods affected the browning index, chlorophyll content and total phenolic acid content, while the total carotenoid content, total polyphenols, total flavonoids and individual biflavonoids remained the same. This work represents the first comparison of different drying methods on the individual composition of biflavonoids in yellow ginkgo leaves, one of the major specialized metabolites contributing to the medicinal properties of ginkgo. Our results suggest that any of the three drying methods can be used for drying ginkgo leaves, maximizing the preservation of biflavonoids.
Author Contributions
Conceptualization, D.Š. methodology, D.Š. and I.J.Š.; validation, I.J.Š.; formal analysis, I.J.Š. and L.P.; investigation, I.J.Š., L.P. and D.Š.; resources, D.Š.; writing—original draft preparation, D.Š. and I.J.Š.; writing—review and editing, D.Š.; visualization, I.J.Š.; supervision, D.Š.; project administration, D.Š.; funding acquisition, D.Š. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Croatian Science Foundation project “Biflavonoids role in plants: Ginkgo biloba L. as a model system” under Project No. UIP-2019-04-1018.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gong, W.; Chen, C.; Dobeš, C.; Fu, C.-X.; Koch, M.A. Phylogeography of a Living Fossil: Pleistocene Glaciations Forced Ginkgo biloba L. (Ginkgoaceae) into Two Refuge Areas in China with Limited Subsequent Postglacial Expansion. Mol. Phylogenet. Evol. 2008, 48, 1094–1105. [Google Scholar] [CrossRef]
- Tang, C.Q.; Yang, Y.; Ohsawa, M.; Yi, S.-R.; Momohara, A.; Su, W.-H.; Wang, H.-C.; Zhang, Z.-Y.; Peng, M.-C.; Wu, Z.-L. Evidence for the Persistence of Wild Ginkgo biloba (Ginkgoaceae) Populations in the Dalou Mountains, Southwestern China. Am. J. Bot. 2012, 99, 1408–1414. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, H.; Zhang, Y.; Che, F.; Shen, N.; Cui, Y. Leaves, Seeds and Exocarp of Ginkgo biloba L. (Ginkgoaceae): A Comprehensive Review of Traditional Uses, Phytochemistry, Pharmacology, Resource Utilization and Toxicity. J. Ethnopharmacol. 2022, 298, 115645. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Zhang, J.; Wang, S. Advances in the Chemical Constituents and Chemical Analysis of Ginkgo biloba Leaf, Extract, and Phytopharmaceuticals. J. Pharm. Biomed. Anal. 2021, 193, 113704. [Google Scholar] [CrossRef]
- Kovač Tomas, M.; Jurčević, I.; Šamec, D. Tissue-Specific Profiling of Biflavonoids in Ginkgo (Ginkgo biloba L.). Plants 2022, 12, 147. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Dahija, S.; Hassan, S.T.S. Biflavonoids: Important Contributions to the Health Benefits of Ginkgo (Ginkgo biloba L.). Plants 2022, 11, 1381. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yang, F.; Huang, X. Proceedings of Chemistry, Pharmacology, Pharmacokinetics and Synthesis of Biflavonoids. Molecules 2021, 26, 6088. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.P.; Bhadane, R.N.; Panigrahi, D.; Amawi, H.A.; Asbhy, C.R.; Tiwari, A.K. The Interaction of the Bioflavonoids with Five SARS-CoV-2 Proteins Targets: An In Silico Study. Comput. Biol. Med. 2021, 134, 104464. [Google Scholar] [CrossRef] [PubMed]
- Abdizadeh, R.; Hadizadeh, F.; Abdizadeh, T. Evaluation of Apigenin-Based Biflavonoid Derivatives as Potential Therapeutic Agents against Viral Protease (3CLpro) of SARS-CoV-2 via Molecular Docking, Molecular Dynamics and Quantum Mechanics Studies. J. Biomol. Struct. Dyn. 2023, 41, 5915–5945. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-T.; Fan, X.-H.; Jian, Y.; Dong, M.-Z.; Yang, Q.; Meng, D.; Fu, Y.-J. A Sensitive and Selective Multiple Reaction Monitoring Mass Spectrometry Method for Simultaneous Quantification of Flavonol Glycoside, Terpene Lactones, and Biflavonoids in Ginkgo biloba Leaves. J. Pharm. Biomed. Anal. 2019, 170, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Thamkaew, G.; Sjöholm, I.; Galindo, F.G. A Review of Drying Methods for Improving the Quality of Dried Herbs. Crit. Rev. Food Sci. Nutr. 2021, 61, 1763–1786. [Google Scholar] [CrossRef]
- Rahimmalek, M.; Goli, S.A.H. Evaluation of Six Drying Treatments with Respect to Essential Oil Yield, Composition and Color Characteristics of Thymys Daenensis Subsp. Daenensis. Celak Leaves. Ind. Crops Prod. 2013, 42, 613–619. [Google Scholar] [CrossRef]
- Jurinjak Tušek, A.; Šamec, D.; Šalić, A. Modern Techniques for Flavonoid Extraction—To Optimize or Not to Optimize? Appl. Sci. 2022, 12, 11865. [Google Scholar] [CrossRef]
- Krakowska-Sieprawska, A.; Kiełbasa, A.; Rafińska, K.; Ligor, M.; Buszewski, B. Modern Methods of Pre-Treatment of Plant Material for the Extraction of Bioactive Compounds. Molecules 2022, 27, 730. [Google Scholar] [CrossRef] [PubMed]
- Belwal, T.; Cravotto, C.; Prieto, M.A.; Venskutonis, P.R.; Daglia, M.; Devkota, H.P.; Baldi, A.; Ezzat, S.M.; Gómez-Gómez, L.; Salama, M.M.; et al. Effects of Different Drying Techniques on the Quality and Bioactive Compounds of Plant-Based Products: A Critical Review on Current Trends. Dry. Technol. 2022, 40, 1539–1561. [Google Scholar] [CrossRef]
- Hu, L.; Wang, C.; Guo, X.; Chen, D.; Zhou, W.; Chen, X.; Zhang, Q. Flavonoid Levels and Antioxidant Capacity of Mulberry Leaves: Effects of Growth Period and Drying Methods. Front. Plant Sci. 2021, 12, 684974. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.Y.; Hii, C.L.; Ong, S.P.; Lim, K.H.; Abas, F.; Pin, K.Y. Effects of Drying on Total Polyphenols Content and Antioxidant Properties of Carica papaya Leaves. J. Sci. Food Agric. 2020, 100, 2932–2937. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Mat, I.; Lim, V.; Ahmad, R. Antioxidant Activity and Phenolic Content of Streblus Asper Leaves from Various Drying Methods. Antioxidants 2013, 2, 156–166. [Google Scholar] [CrossRef]
- Roshanak, S.; Rahimmalek, M.; Goli, S.A.H. Evaluation of Seven Different Drying Treatments in Respect to Total Flavonoid, Phenolic, Vitamin C Content, Chlorophyll, Antioxidant Activity and Color of Green Tea (Camellia sinensis or C. assamica) Leaves. J. Food Sci. Technol. 2016, 53, 721–729. [Google Scholar] [CrossRef]
- Nguyen, Q.-V.; Doan, M.-D.; Bui Thi, B.-H.; Nguyen, M.-T.; Tran Minh, D.; Nguyen, A.-D.; Le, T.-M.; Nguyen, T.-H.; Nguyen, T.-D.; Tran, V.-C.; et al. The Effect of Drying Methods on Chlorophyll, Polyphenol, Flavonoids, Phenolic Compounds Contents, Color and Sensory Properties, and in Vitro Antioxidant and Anti-Diabetic Activities of Dried Wild Guava Leaves. Dry. Technol. 2023, 41, 1291–1302. [Google Scholar] [CrossRef]
- Shonte, T.T.; Duodu, K.G.; de Kock, H.L. Effect of Drying Methods on Chemical Composition and Antioxidant Activity of Underutilized Stinging Nettle Leaves. Heliyon 2020, 6, e03938. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, Y.; Zhou, W.; He, S.; Yang, M.; Xue, Q.; Wang, Y.; Zhao, T.; Cao, J.; Khan, A.; et al. Phenolic Composition, Antioxidant and Cytoprotective Effects of Aqueous-methanol Extract from Anneslea Fragrans Leaves as Affected by Drying Methods. Int. J. Food Sci. Technol. 2021, 56, 4807–4819. [Google Scholar] [CrossRef]
- Bai, J.-W.; Xiao, H.-W.; Ma, H.-L.; Zhou, C.-S. Artificial Neural Network Modeling of Drying Kinetics and Color Changes of Ginkgo biloba Seeds during Microwave Drying Process. J. Food Qual. 2018, 2018, 3278595. [Google Scholar] [CrossRef]
- Boateng, I.D.; Yang, X.-M. Thermal and Non-Thermal Processing Affect Maillard Reaction Products, Flavor, and Phytochemical Profiles of Ginkgo biloba Seed. Food Biosci. 2021, 41, 101044. [Google Scholar] [CrossRef]
- Boateng, I.D.; Yang, X.-M.; Tahany, A.A.A.; Li, Y.-Y. Yolandani Drying Methods Affect Organoleptic and Physicochemical Properties of Rehydrated Ginkgo Seed Slices. Ind. Crops Prod. 2021, 160, 113166. [Google Scholar] [CrossRef]
- Amoussa, A.M.O.; Zhang, L.; Lagnika, C.; Riaz, A.; Zhang, L.; Liu, X.; Beta, T. Effects of Preheating and Drying Methods on Pyridoxine, Phenolic Compounds, Ginkgolic Acids, and Antioxidant Capacity of Ginkgo biloba Nuts. J. Food Sci. 2021, 86, 4197–4208. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Wang, W.; Wang, T.; Yu, N. Colors, Bioactive Compounds, and Antioxidant Capacity of Ginkgo biloba Seeds Affected by Drying Conditions. J. Food Meas. Charact. 2021, 15, 3953–3961. [Google Scholar] [CrossRef]
- Lee, C.Y.; Jaworski, A.W. Phenolics and Browning Potential of White Grapes Grown in New York. Am. J. Enol. Vitic. 1988, 39, 337–340. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Končić, M.Z.; Kremer, D.; Gruz, J.; Strnad, M.; Biševac, G.; Kosalec, I.; Šamec, D.; Piljac-Žegarac, J.; Karlović, K. Antioxidant and Antimicrobial Properties of Moltkia petraea (Tratt.) Griseb. Flower, Leaf and Stem Infusions. Food Chem. Toxicol. 2010, 48, 1537–1542. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Chu, Q.; Li, L.; Duan, X.; Zhao, M.; Wang, Z.; Wang, Z.; Ren, X.; Li, C.; Ren, G. Effect Mechanism of Different Drying Methods on the Quality and Browning for Daylily. LWT 2023, 182, 114862. [Google Scholar] [CrossRef]
- Chung, H.-S.; Lee, J.H. Comparative Evaluation of Physicochemical Properties of Pine Needle Powders Prepared by Different Drying Methods. Prev. Nutr. Food Sci. 2015, 20, 143–147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yilmaz, A.; Alibas, I. The Impact of Drying Methods on Quality Parameters of Purple Basil Leaves. J. Food Process. Preserv. 2021, 45, e15638. [Google Scholar] [CrossRef]
- Chou, S.; Chen, B.; Chen, J.; Wang, M.; Wang, S.; Croft, H.; Shi, Q. Estimation of Leaf Photosynthetic Capacity from the Photochemical Reflectance Index and Leaf Pigments. Ecol. Indic. 2020, 110, 105867. [Google Scholar] [CrossRef]
- Shittu, S.K.; Shehu, M.I.; Suleiman, J. Effect of the Drying Method on the Quality and Drying Characteristic of Mint Leaves (Mentha spicata L.). Fudma J. Sci. 2021, 5, 72–78. [Google Scholar] [CrossRef]
- Rubinskienė, M.; Viškelis, P.; Dambrauskienė, E.; Viškelis, J.; Karklelienė, R. Effect of Drying Methods on the Chemical Composition and Colour of Peppermint (Mentha × Piperita L.) Leaves. Zemdirb. Agric. 2015, 102, 223–228. [Google Scholar] [CrossRef]
- Nabi, F.; Arain, M.A.; Rajput, N.; Alagawany, M.; Soomro, J.; Umer, M.; Soomro, F.; Wang, Z.; Ye, R.; Liu, J. Health Benefits of Carotenoids and Potential Application in Poultry Industry: A Review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1809–1818. [Google Scholar] [CrossRef]
- Del Monte, D.; De Martino, L.; Marandino, A.; Fratianni, F.; Filomena Nazzaro, F.; De Feo, V. Phenolic content, antimicrobial and antioxidant activities of Hypericum perfoliatum L. Ind. Crops Prod. 2015, 74, 342–347. [Google Scholar] [CrossRef]
- Hazler Pilepić, K.; Maleš, Ž. Quantitative analysis of polyphenols in eighteen Hypericum taxa. Period. Biol. 2013, 115, 459–462. [Google Scholar]
- Xie, Z.; Liu, W.; Huang, H.; Slavin, M.; Zhao, Y.; Whent, M.; Blackford, J.; Lutterodt, H.; Zhou, H.; Chen, P.; et al. Chemical Composition of Five Commercial Gynostemma pentaphyllum Samples and Their Radical Scavenging, Antiproliferative, and Anti-inflammatory Properties. J. Agric. Food Chem. 2010, 58, 11243–11249. [Google Scholar] [CrossRef]
- Vladimir-Knežević, S.; Blažeković, B.; Bival Štefan, M.; Alegro, A.; Kőszegi, T.; Petrik, J. Antioxidant Activities and Polyphenolic Contents of Three Selected Micromeria Species from Croatia. Molecules 2011, 16, 1454–1470. [Google Scholar] [CrossRef]
- Casagrande, M.; Zanela, J.; Wagner, A.; Busso, C.; Wouk, J.; Iurckevicz, G.; Montanher, P.F.; Yamashita, F.; Malfatti, C.R.M. Influence of Time, Temperature and Solvent on the Extraction of Bioactive Compounds of Baccharis Dracunculifolia: In Vitro Antioxidant Activity, Antimicrobial Potential, and Phenolic Compound Quantification. Ind. Crops Prod. 2018, 125, 207–219. [Google Scholar] [CrossRef]
- Chen, X.; Zhong, W.; Shu, C.; Yang, H.; Li, E. Comparative Analysis of Chemical Constituents and Bioactivities of the Extracts from Leaves, Seed Coats and Embryoids of Ginkgo biloba L. Nat. Prod. Res. 2021, 35, 5498–5501. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Matlin, A.J.; Lowell, A.M.; Moore, M.J. The Biflavonoid Isoginkgetin Is a General Inhibitor of Pre-MRNA Splicing. J. Biol. Chem. 2008, 283, 33147–33154. [Google Scholar] [CrossRef] [PubMed]
- Goh, L.M.; Barlow, P.J.; Yong, C.S. Examination of Antioxidant Activity of Ginkgo biloba Leaf Infusions. Food Chem. 2003, 82, 275–282. [Google Scholar] [CrossRef]
- Suárez-González, E.; Sandoval-Ramírez, J.; Flores-Hernández, J.; Carrasco-Carballo, A. Ginkgo biloba: Antioxidant Activity and In Silico Central Nervous System Potential. Curr. Issues Mol. Biol. 2023, 45, 9674–9691. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Fini, A.; Gori, A.; Guidi, L.; Landi, M.; Sebastiani, F.; Tattini, M. Are Flavonoids Effective Antioxidants in Plants? Twenty Years of Our Investigation. Antioxidants 2020, 9, 1098. [Google Scholar] [CrossRef]
- Jurčević Šangut, I.; Šarkanj, B.; Karalija, E.; Šamec, D. A Comparative Analysis of Radical Scavenging, Antifungal and Enzyme Inhibition Activity of 3′-8″-Biflavones and Their Monomeric Subunits. Antioxidants 2023, 12, 1854. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).