Caries in Primary Molars: Is Silver Diamine Fluoride Effective in Prevention and Treatment? A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Processing
2.3. Eligibility Criteria
- −
- Population: children, both male and female, who had a primary molar treated with SDF;
- −
- Intervention: SDF on deciduous molar;
- −
- Comparison: untreated deciduous molars with SDF;
- −
- Outcome: effectiveness of SDF in arresting caries or preventing its development on deciduous molars.
2.4. Data Processing
2.5. Article Identification Procedure
2.6. Study Evaluation
2.7. Quality Assessment
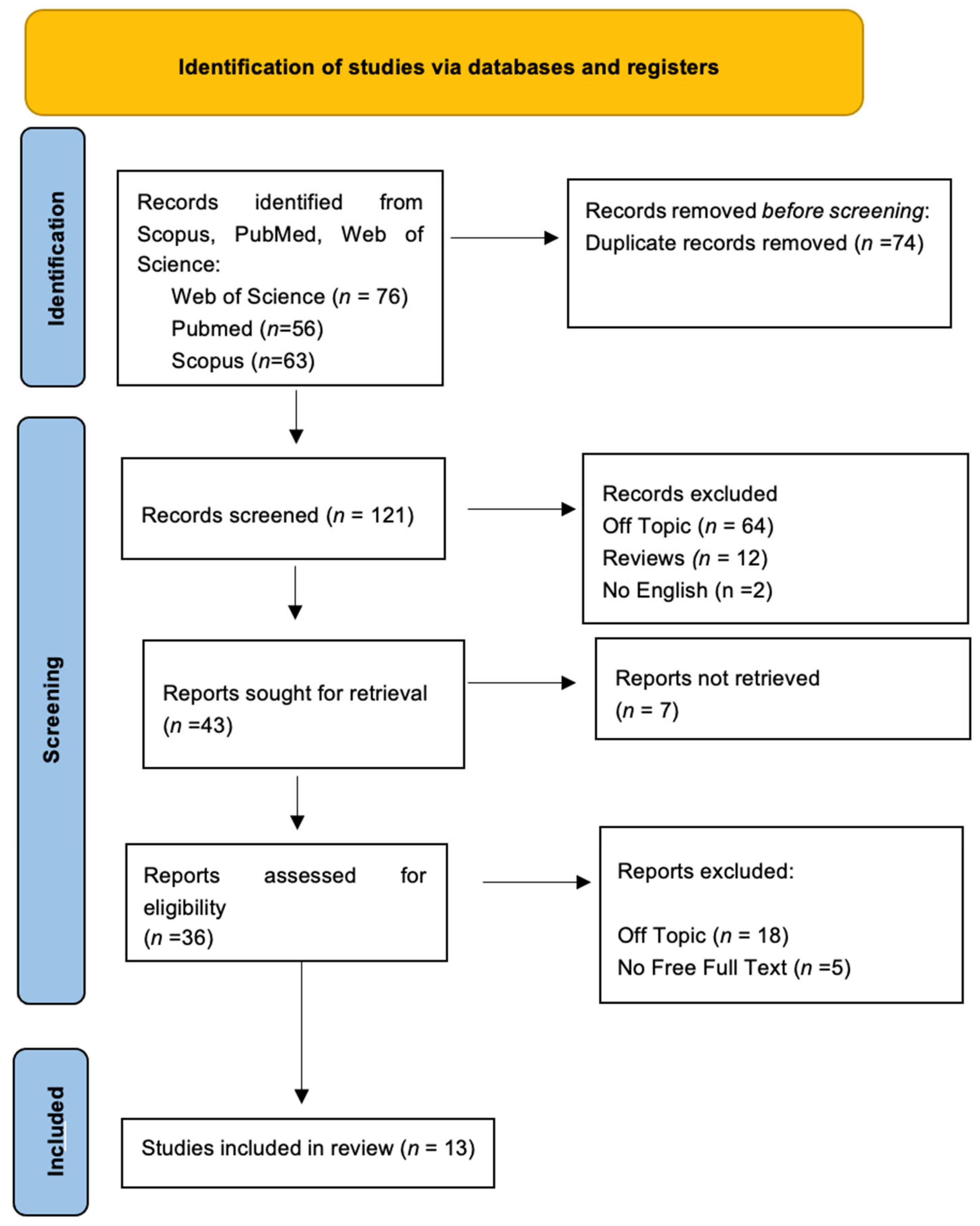
3. Results
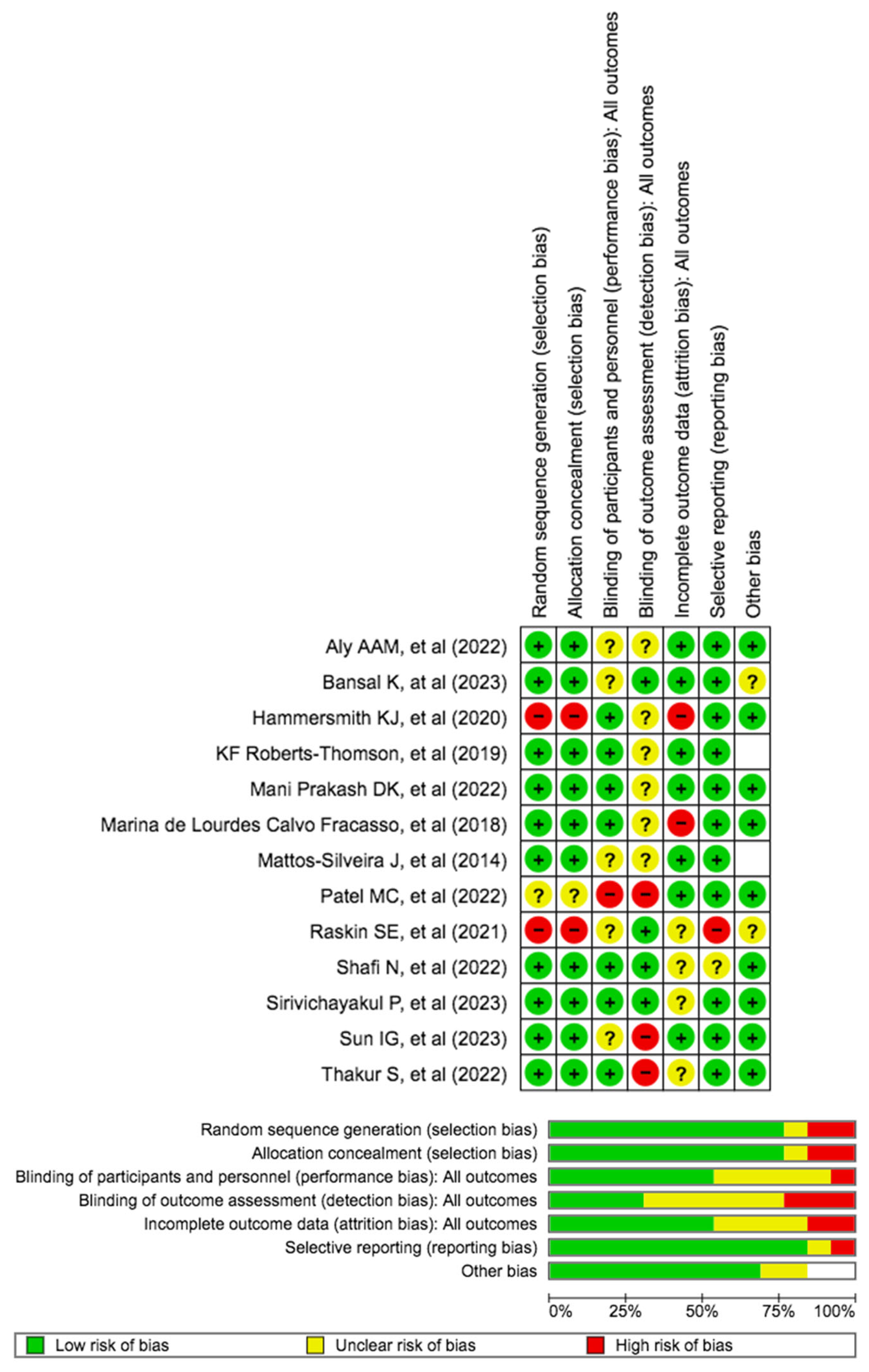
3.1. Quality Assessment and Risk of Bias
3.2. Relevant Findings of the Studies
4. Discussion
4.1. Comparative Efficacy of SDF in Various Treatment Modalities
4.2. Clinical Performance and Long-Term Outcomes of SDF
4.3. Optimizing SDF Application Protocols and Treatment Outcomes
4.4. Population-Level Impact and Public Health Considerations
4.5. Limitations
- Heterogeneous designs: Variability in methodologies and participant characteristics hampers direct comparisons, impacting generalizability.
- Staining and acceptance concerns: aesthetic issues related to SDF staining raise acceptance challenges, introducing subjective biases in evaluation.
- Recruitment challenges and sample size variability: recruitment difficulties and varying sample sizes across studies affect representativeness and statistical reliability.
- Short to medium-term follow-up: many studies have relatively short follow-up periods (a week), limiting insights into the long-term (months) effectiveness and side effects of SDF.
- Retrospective nature: studies with retrospective designs, like Hammersmith et al., face challenges related to incomplete data and potential bias.
- Applicability to high-risk populations: generalizing SDF’s effectiveness to diverse pediatric populations with varying risk profiles remains a challenge.
- Inconsistent outcome measures: diverse outcome measures across studies complicate the synthesis of evidence and hinder standardization.
- Limited socioeconomic and cultural diversity: some studies lack diverse socioeconomic and cultural representation, impacting the broad applicability of findings.
4.6. Strengths of the Review
4.7. Implications for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AgF | Stannous fluoride |
| ART | Atraumatic restorative technique |
| ECC | Early childhood caries |
| NaF | Sodium-fluoride |
| SDF | Silver Diamine Fluoride |
| SMART | Silver-modified atraumatic restorative technique |
References
- Campanella, V.; Gallusi, G.; Nardi, R.; Mea, A.; Di Taranto, V.; Montemurro, E.; Marzo, G.; Libonati, A. Dentinal Substrate Variability and Bonding Effectiveness: Sem Investigation. J. Biol. Regul. Homeost. Agents 2020, 34, 49–54. [Google Scholar]
- Marchetti, E.; Tecco, S.; Caterini, E.; Casalena, F.; Quinzi, V.; Mattei, A.; Marzo, G. Alcohol-Free Essential Oils Containing Mouthrinse Efficacy on Three-Day Supragingival Plaque Regrowth: A Randomized Crossover Clinical Trial. Trials 2017, 18, 154. [Google Scholar] [CrossRef]
- Mummolo, S.; Tieri, M.; Tecco, S.; Mattei, A.; Albani, F.; Giuca, M.R.; Marzo, G. Clinical Evaluation of Salivary Indices and Levels of Streptococcus Mutans and Lactobacillus in Patients Treated with Occlus-o-Guide. Eur. J. Paediatr. Dent. Off. J. Eur. Acad. Paediatr. Dent. 2014, 15, 367–370. [Google Scholar]
- Rosa, M.; Quinzi, V.; Marzo, G. Paediatric Orthodontics Part 1: Anterior Open Bite in the Mixed Dentition. Eur. J. Paediatr. Dent. 2019, 20, 80–82. [Google Scholar] [CrossRef]
- Crincoli, V.; Anelli, M.G.; Quercia, E.; Piancino, M.G.; Di Comite, M. Temporomandibular Disorders and Oral Features in Early Rheumatoid Arthritis Patients: An Observational Study. Int. J. Med. Sci. 2019, 16, 253–263. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Malcangi, G.; Inchingolo, A.M.; Piras, F.; Settanni, V.; Garofoli, G.; Palmieri, G.; Ceci, S.; Patano, A.; De Leonardis, N.; et al. Benefits and Implications of Resveratrol Supplementation on Microbiota Modulations: A Systematic Review of the Literature. Int. J. Mol. Sci. 2022, 23, 4027. [Google Scholar] [CrossRef]
- Di Stasio, D.; Lauritano, D.; Romano, A.; Salerno, C.; Minervini, G.; Minervini, G.; Gentile, E.; Serpico, R.; Lucchese, A. In Vivo Characterization of Oral Pemphigus Vulgaris by Optical Coherence Tomography. J. Biol. Regul. Homeost. Agents 2015, 29, 39–41. [Google Scholar]
- Inchingolo, A.M.; Inchingolo, A.D.; Latini, G.; Garofoli, G.; Sardano, R.; De Leonardis, N.; Dongiovanni, L.; Minetti, E.; Palermo, A.; Dipalma, G.; et al. Caries Prevention and Treatment in Early Childhood: Comparing Strategies. A Systematic Review. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 11082–11092. [Google Scholar] [CrossRef]
- Contaldo, M.; Della Vella, F.; Raimondo, E.; Minervini, G.; Buljubasic, M.; Ogodescu, A.; Sinescu, C.; Serpico, R. Early Childhood Oral Health Impact Scale (ECOHIS): Literature Review and Italian Validation. Int. J. Dent. Hyg. 2020, 18, 396–402. [Google Scholar] [CrossRef]
- Di Spirito, F.; Amato, A.; Di Palo, M.P.; Ferraro, G.A.; Baroni, A.; Serpico, R.; Contaldo, M. COVID-19 Related Information on Pediatric Dental Care Including the Use of Teledentistry: A Narrative Review. Children 2022, 9, 1942. [Google Scholar] [CrossRef]
- Malcangi, G.; Inchingolo, A.D.; Inchingolo, A.M.; Santacroce, L.; Marinelli, G.; Mancini, A.; Vimercati, L.; Maggiore, M.E.; D’oria, M.T.; Hazballa, D.; et al. COVID-19 Infection in Children, Infants and Pregnant Subjects: An Overview of Recent Insights and Therapies. Microorganisms 2021, 9, 1964. [Google Scholar] [CrossRef]
- Patano, A.; Cirulli, N.; Beretta, M.; Plantamura, P.; Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Malcangi, G.; Marinelli, G.; Scarano, A.; et al. Education Technology in Orthodontics and Paediatric Dentistry during the COVID-19 Pandemic: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 6056. [Google Scholar] [CrossRef]
- Dipalma, G.; Inchingolo, A.D.; Inchingolo, F.; Charitos, I.A.; Di Cosola, M.; Cazzolla, A.P. Focus on the Cariogenic Process: Microbial and Biochemical Interactions with Teeth and Oral Environment. J. Biol. Regul. Homeost. Agents 2021, 35, 429–440. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Palladino, A.; Inchingolo, A.M.; Dipalma, G. Oral Piercing and Oral Diseases: A Short Time Retrospective Study. Int. J. Med. Sci. 2011, 8, 649–652. [Google Scholar] [CrossRef]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Di Blasio, M.; Ronsivalle, V.; Cicciù, M. Children Oral Health and Parents Education Status: A Cross Sectional Study. BMC Oral Health 2023, 23, 787. [Google Scholar] [CrossRef]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Fiorillo, L.; Cervino, G.; Cicciù, M. The Association between Parent Education Level, Oral Health, and Oral-Related Sleep Disturbance. An Observational Crosssectional Study. Eur. J. Paediatr. Dent. 2023, 24, 218–223. [Google Scholar] [CrossRef]
- Rossi, F.; Tortora, C.; Paoletta, M.; Marrapodi, M.M.; Argenziano, M.; Di Paola, A.; Pota, E.; Di Pinto, D.; Di Martino, M.; Iolascon, G. Osteoporosis in Childhood Cancer Survivors: Physiopathology, Prevention, Therapy and Future Perspectives. Cancers 2022, 14, 4349. [Google Scholar] [CrossRef]
- Rossi, F.; Tortora, C.; Di Martino, M.; Di Paola, A.; Di Pinto, D.; Marrapodi, M.M.; Argenziano, M.; Pota, E. Alteration of Osteoclast Activity in Childhood Cancer Survivors: Role of Iron and of CB2/TRPV1 Receptors. PLoS ONE 2022, 17, e0271730. [Google Scholar] [CrossRef]
- Geduk, N.; Ozdemir, M.; Erbas Unverdi, G.; Ballikaya, E.; Cehreli, Z.C. Clinical and Radiographic Performance of Preformed Zirconia Crowns and Stainless-Steel Crowns in Permanent First Molars: 18-Month Results of a Prospective, Randomized Trial. BMC Oral Health 2023, 23, 828. [Google Scholar] [CrossRef]
- Benjamin, R.M. Oral Health: The Silent Epidemic. Public Health Rep. 2010, 125, 158–159. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Corelli, R.; Mingrone, R.; Inchingolo, A.M.; Dipalma, G. Simple Technique for Augmentation of the Facial Soft Tissue. Sci. World J. 2012, 2012, 262989. [Google Scholar] [CrossRef][Green Version]
- Tecco, S.; Crincoli, V.; Di Bisceglie, B.; Saccucci, M.; Macrĺ, M.; Polimeni, A.; Festa, F. Signs and Symptoms of Temporomandibular Joint Disorders in Caucasian Children and Adolescents. Cranio J. Craniomandib. Pract. 2011, 29, 71–79. [Google Scholar] [CrossRef]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Almeida, L.E.; Ronsivalle, V.; Cicciù, M. Prevalence of Temporomandibular Disorders (TMD) in Obesity Patients: A Systematic Review and Meta-Analysis. J. Oral Rehabil. 2023, 50, 1544–1553. [Google Scholar] [CrossRef]
- Contaldo, M.; Luzzi, V.; Ierardo, G.; Raimondo, E.; Boccellino, M.; Ferati, K.; Bexheti-Ferati, A.; Inchingolo, F.; Di Domenico, M.; Serpico, R.; et al. Bisphosphonate-Related Osteonecrosis of the Jaws and Dental Surgery Procedures in Children and Young People with Osteogenesis Imperfecta: A Systematic Review. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 556–562. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Patano, A.; Coloccia, G.; Ceci, S.; Inchingolo, A.M.; Marinelli, G.; Malcangi, G.; Montenegro, V.; Laudadio, C.; Di Pede, C.; et al. The Efficacy of a New AMCOP® Elastodontic Protocol for Orthodontic Interceptive Treatment: A Case Series and Literature Overview. Int. J. Environ. Res. Public Health 2022, 19, 988. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Villabruna, B.; Inchingolo, A.M.; Dipalma, G. Severe Anisocoria after Oral Surgery under General Anesthesia. Int. J. Med. Sci. 2010, 7, 314–318. [Google Scholar] [CrossRef]
- Inchingolo, F.; Inchingolo, A.M.; Malcangi, G.; De Leonardis, N.; Sardano, R.; Pezzolla, C.; de Ruvo, E.; Di Venere, D.; Palermo, A.; Inchingolo, A.D.; et al. The Benefits of Probiotics on Oral Health: Systematic Review of the Literature. Pharmaceuticals 2023, 16, 1313. [Google Scholar] [CrossRef]
- Mungara, J.; Injeti, M.; Joseph, E.; Elangovan, A.; Sakthivel, R.; Selvaraju, G. Child’s Dental Fear: Cause Related Factors and the Influence of Audiovisual Modeling. J. Indian Soc. Pedod. Prev. Dent. 2013, 31, 215–220. [Google Scholar] [CrossRef]
- Marrelli, M.; Tatullo, M.; Dipalma, G.; Inchingolo, F. Oral Infection by Staphylococcus Aureus in Patients Affected by White Sponge Nevus: A Description of Two Cases Occurred in the Same Family. Int. J. Med. Sci. 2012, 9, 47–50. [Google Scholar] [CrossRef]
- Marinelli, G.; Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Limongelli, L.; Montenegro, V.; Coloccia, G.; Laudadio, C.; Patano, A.; Inchingolo, F.; et al. White Spot Lesions in Orthodontics: Prevention and Treatment. A Descriptive Review. J. Biol. Regul. Homeost. Agents 2021, 35, 227–240. [Google Scholar] [CrossRef]
- Patano, A.; Malcangi, G.; Sardano, R.; Mastrodonato, A.; Garofoli, G.; Mancini, A.; Inchingolo, A.D.; Di Venere, D.; Inchingolo, F.; Dipalma, G.; et al. White Spots: Prevention in Orthodontics-Systematic Review of the Literature. Int. J. Environ. Res. Public Health 2023, 20, 5608. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Ceci, S.; Patano, A.; Inchingolo, A.M.; Montenegro, V.; Di Pede, C.; Malcangi, G.; Marinelli, G.; Coloccia, G.; Garibaldi, M.; et al. Elastodontic Therapy of Hyperdivergent Class II Patients Using AMCOP® Devices: A Retrospective Study. Appl. Sci. 2022, 12, 3259. [Google Scholar] [CrossRef]
- Lanteri, V.; Cossellu, G.; Farronato, M.; Ugolini, A.; Leonardi, R.; Rusconi, F.; De Luca, S.; Biagi, R.; Maspero, C. Assessment of the Stability of the Palatal Rugae in a 3D-3D Superimposition Technique Following Slow Maxillary Expansion (SME). Sci. Rep. 2020, 10, 2676. [Google Scholar] [CrossRef]
- Maspero, C.; Fama, A.; Cavagnetto, D.; Abate, A.; Farronato, M. Treatment of Dental Dilacerations. J. Biol. Regul. Homeost. Agents 2019, 33, 1623–1628. [Google Scholar]
- Paolantoni, G.; Marenzi, G.; Blasi, A.; Mignogna, J.; Sammartino, G. Findings of a Four-Year Randomized Controlled Clinical Trial Comparing Two-Piece and One-Piece Zirconia Abutments Supporting Single Prosthetic Restorations in Maxillary Anterior Region. BioMed Res. Int. 2016, 2016, 8767845. [Google Scholar] [CrossRef][Green Version]
- Minervini, G.; Marrapodi, M.M.; Cicciù, M. Online Bruxism-related Information: Can People Understand What They Read? A Cross-Sectional Study. J. Oral Rehabil. 2023, 50, 1211–1216. [Google Scholar] [CrossRef]
- Crystal, Y.O.; Niederman, R. Silver Diamine Fluoride Treatment Considerations in Children’s Caries Management. Pediatr. Dent. 2016, 38, 466–471. [Google Scholar]
- Malcangi, G.; Patano, A.; Morolla, R.; De Santis, M.; Piras, F.; Settanni, V.; Mancini, A.; Di Venere, D.; Inchingolo, F.; Inchingolo, A.D.; et al. Analysis of Dental Enamel Remineralization: A Systematic Review of Technique Comparisons. Bioengineering 2023, 10, 472. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, R. Behavior Assessment of Children in Dental Settings: A Retrospective Study. Int. J. Clin. Pediatr. Dent. 2011, 4, 35–39. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4999635/ (accessed on 23 January 2024).
- Sheiham, A. Dental Caries Affects Body Weight, Growth and Quality of Life in Pre-School Children. Br. Dent. J. 2006, 201, 625–626. [Google Scholar] [CrossRef]
- Jackson, S.L.; Vann, W.F., Jr.; Kotch, J.B.; Pahel, B.T.; Lee, J.Y. Impact of Poor Oral Health on Children’s School Attendance and Performance. Am. J. Public Health 2011, 101, 1900–1906. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3222359/ (accessed on 20 January 2024). [CrossRef]
- De Grauwe, A.; Aps, J.K.; Martens, L.C. Early Childhood Caries (ECC): What’s in a Name? Eur. J. Paediatr. Dent. 2004, 5, 62–70. [Google Scholar]
- Feldens, C.A.; Giugliani, E.R.J.; Duncan, B.B.; de Drachler, M.L.; Vítolo, M.R. Long-Term Effectiveness of a Nutritional Program in Reducing Early Childhood Caries: A Randomized Trial. Community Dent. Oral Epidemiol. 2010, 38, 324–332. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Malcangi, G.; Semjonova, A.; Inchingolo, A.M.; Patano, A.; Coloccia, G.; Ceci, S.; Marinelli, G.; Di Pede, C.; Ciocia, A.M.; et al. Oralbiotica/Oralbiotics: The Impact of Oral Microbiota on Dental Health and Demineralization: A Systematic Review of the Literature. Children 2022, 9, 1014. [Google Scholar] [CrossRef]
- Finlayson, T.L.; Siefert, K.; Ismail, A.I.; Sohn, W. Psychosocial Factors and Early Childhood Caries among Low-Income African-American Children in Detroit. Community Dent. Oral Epidemiol. 2007, 35, 439–448. [Google Scholar] [CrossRef]
- Nunn, M.E.; Braunstein, N.S.; Krall Kaye, E.A.; Dietrich, T.; Garcia, R.I.; Henshaw, M.M. Healthy Eating Index Is a Predictor of Early Childhood Caries. J. Dent. Res. 2009, 88, 361–366. [Google Scholar] [CrossRef]
- Berkowitz, R.J. Causes, Treatment and Prevention of Early Childhood Caries: A Microbiologic Perspective. J. Can. Dent. Assoc. 2003, 69, 304–307. Available online: https://pubmed.ncbi.nlm.nih.gov/12734024/ (accessed on 20 January 2024).
- Palmer, C.A.; Kent, R., Jr.; Loo, C.Y.; Hughes, C.V.; Stutius, E.; Pradhan, N.; Dahlan, M.; Kanasi, E.; Arevalo Vasquez, S.S.; Tanner, A.C. Diet and Caries-Associated Bacteria in Severe Early Childhood Caries. Dent. Res. 2010, 89, 1224–1229. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2954266/ (accessed on 20 January 2024). [CrossRef]
- Crescente, G.; Minervini, G.; Spagnuolo, C.; Moccia, S. Cannabis Bioactive Compound-Based Formulations: New Perspectives for the Management of Orofacial Pain. Molecules 2023, 28, 106. [Google Scholar] [CrossRef]
- Minervini, G.; Del Mondo, D.; Russo, D.; Cervino, G.; D’Amico, C.; Fiorillo, L. Stem Cells in Temporomandibular Joint Engineering: State of Art and Future Persectives. J. Craniofac. Surg. 2022, 33, 2181–2187. [Google Scholar] [CrossRef]
- Minervini, G.; Lucchese, A.; Perillo, L.; Serpico, R.; Minervini, G. Unilateral Superior Condylar Neck Fracture with Dislocation in a Child Treated with an Acrylic Splint in the Upper Arch for Functional Repositioning of the Mandible. Cranio J. Craniomandib. Pract. 2017, 35, 337–341. [Google Scholar] [CrossRef]
- Ballini, A.; Cantore, S.; Signorini, L.; Saini, R.; Scacco, S.; Gnoni, A.; Inchingolo, A.D.; De Vito, D.; Santacroce, L.; Inchingolo, F.; et al. Efficacy of Sea Salt-Based Mouthwash and Xylitol in Improving Oral Hygiene among Adolescent Population: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 44. [Google Scholar] [CrossRef]
- Zaffarano, L.; Salerno, C.; Campus, G.; Cirio, S.; Balian, A.; Karanxha, L.; Cagetti, M.G. Silver Diamine Fluoride (SDF) Efficacy in Arresting Cavitated Caries Lesions in Primary Molars: A Systematic Review and Metanalysis. Int. J. Environ. Res. Public Health 2022, 19, 2917. [Google Scholar] [CrossRef]
- Shah, S.; Bhaskar, V.; Venkatraghavan, K.; Choudhary, P.; Trivedi, K. Silver Diamine Fluoride: A Review and Current Applications. J. Adv. Oral Res. 2014, 5, 15–35. Available online: https://www.academia.edu/89754115/Silver_Diamine_Fluoride_A_Review_and_Current_Applications (accessed on 21 January 2024). [CrossRef]
- Zheng, F.M.; Yan, I.G.; Duangthip, D.; Gao, S.S.; Lo, E.C.M.; Chu, C.H. Silver Diamine Fluoride Therapy for Dental Care. Jpn. Dent. Sci. Rev. 2022, 58, 249–257. [Google Scholar] [CrossRef]
- Daga, S.; Shetty, V.; Hegde, A. Silver Diamine Fluoride in Arresting Dentinal Caries in School Children. Indian J. Public Health Res. Dev. 2020, 11, 530–535. [Google Scholar] [CrossRef]
- Llodra, J.C.; Rodriguez, A.; Ferrer, B.; Menardia, V.; Ramos, T.; Morato, M. Efficacy of Silver Diamine Fluoride for Caries Reduction in Primary Teeth and First Permanent Molars of Schoolchildren: 36-Month Clinical Trial. J. Dent. Res. 2005, 84, 721–724. [Google Scholar] [CrossRef]
- Gao, S.S.; Chen, K.J.; Duangthip, D.; Wong, M.C.M.; Lo, E.C.M.; Chu, C.H. Arresting Early Childhood Caries Using Silver and Fluoride Products—A Randomised Trial. J. Dent. 2020, 103, 103522. [Google Scholar] [CrossRef]
- Chu, C.H.; Mei, L.; Seneviratne, C.J.; Lo, E.C.M. Effects of Silver Diamine Fluoride on Dentine Carious Lesions Induced by Streptococcus Mutans and Actinomyces Naeslundii Biofilms. Int. J. Paediatr. Dent. 2012, 22, 2–10. [Google Scholar] [CrossRef]
- Zhi, Q.H.; Lo, E.C.M.; Lin, H.C. Randomized Clinical Trial on Effectiveness of Silver Diamine Fluoride and Glass Ionomer in Arresting Dentine Caries in Preschool Children. J. Dent. 2012, 40, 962–967. [Google Scholar] [CrossRef]
- Holan, G.; Rahme, M.A.; Ram, D. Parents’ Attitude toward Their Children’s Appearance in the Case of Esthetic Defects of the Anterior Primary Teeth. J. Clin. Pediatr. Dent. 2009, 34, 141–145. [Google Scholar] [CrossRef]
- Holan, G.; Fuks, A.; Ketlz, N. Success Rate of Formocresol Pulpotomy in Primary Molars Restored with SSC vs. Amalgam. Pediatr. Dent. 2001, 24, 212–216. [Google Scholar]
- Contreras, V.; Toro, M.J.; Elías-Boneta, A.R.; Encarnación-Burgos, A. Effectiveness of Silver Diamine Fluoride in Caries Prevention and Arrest: A Systematic Literature Review. Gen. Dent. 2017, 65, 22–29. [Google Scholar]
- Nishino, M.; Yoshida, S.; Sobue, S.; Kato, J.; Nishida, M. Effect of Topically Applied Ammoniacal Silver Fluoride on Dental Caries in Children. J. Osaka Univ. Dent. Sch. 1969, 9, 149–155. [Google Scholar]
- Lo, E.C.; Chu, C.H.; Lin, H.C. A Community-Based Caries Control Program for Pre-School Children Using Topical Fluorides: 18-Month Results. J. Dent. Res. 2001, 80, 2071–2074. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Pacifici, A.; Gargari, M.; Inchingolo, A.D.; Inchingolo, A.M.; Dipalma, G.; Marrelli, M.; Abenavoli, F.M.; Pacifici, L. Use of Dermal-Fat Grafts in the Post-Oncological Reconstructive Surgery of Atrophies in the Zygomatic Region: Clinical Evaluations in the Patients Undergone to Previous Radiation Therapy. Head Face Med. 2012, 8, 33. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3527323/ (accessed on 21 January 2024). [CrossRef]
- Bijella, M.F.T.B.; Bijella, V.T.; Silva, M.S.M.B.D.; Lopes, E.S. Avaliação clínica da aplicação de diamino-fluoreto de prata a 12. Rev. Paul. Odontol. 1991, 13, 28–35. [Google Scholar]
- Mauro, S.; García Robles, E.; Cinque, C.; Squassi, A.F.; Bordoni, N.E. Eficiencia de tres fluoruros concentrados para la estabilización de caries de esmalte. Bol. Asoc. Argent. Odontol. Niños 2004, 33, 4–11. [Google Scholar]
- Mei, M.L.; Lo, E.C.-M.; Chu, C.-H. Clinical Use of Silver Diamine Fluoride in Dental Treatment. Compend. Contin. Educ. Dent. 2016, 37, 93–98. [Google Scholar]
- Boccellino, M.; Di Stasio, D.; Dipalma, G.; Cantore, S.; Ambrosio, P.; Coppola, M.; Quagliuolo, L.; Scarano, A.; Malcangi, G.; Borsani, E.; et al. Steroids and Growth Factors in Oral Squamous Cell Carcinoma: Useful Source of Dental-Derived Stem Cells to Develop a Steroidogenic Model in New Clinical Strategies. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8730–8740. [Google Scholar] [CrossRef]
- Vimercati, L.; De Maria, L.; Quarato, M.; Caputi, A.; Gesualdo, L.; Migliore, G.; Cavone, D.; Sponselli, S.; Pipoli, A.; Inchingolo, F.; et al. Association between Long COVID and Overweight/Obesity. J. Clin. Med. 2021, 10, 4143. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Gentile, M.; Inchingolo, A.M.; Dipalma, G. Non-Syndromic Multiple Supernumerary Teeth in a Family Unit with a Normal Karyotype: Case Report. Int. J. Med. Sci. 2010, 7, 378–384. [Google Scholar] [CrossRef]
- Inchingolo, F.; Ballini, A.; Cagiano, R.; Inchingolo, A.D.; Serafini, M.; De Benedittis, M.; Cortelazzi, R.; Tatullo, M.; Marrelli, M.; Inchingolo, A.M.; et al. Immediately Loaded Dental Implants Bioactivated with Platelet-Rich Plasma (PRP) Placed in Maxillary and Mandibular Region. Clin. Ter. 2015, 166, e146–e152. [Google Scholar] [CrossRef]
- Franco, R.; Gianfreda, F.; Miranda, M.; Barlattani, A.; Bollero, P. The Hemostatic Properties of Chitosan in Oral Surgery. Biomed. Biotechnol. Res. J. 2020, 4, 186. [Google Scholar] [CrossRef]
- Bollero, P.; Di Renzo, L.; Franco, R.; Rampello, T.; Pujia, A.; Merra, G.; De Lorenzo, A.; Docimo, R. Effects of New Probiotic Mouthwash in Patients with Diabetes Mellitus and Cardiovascular Diseases. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5827–5836. [Google Scholar] [CrossRef]
- Arrigoni, R.; Ballini, A.; Santacroce, L.; Cantore, S.; Inchingolo, A.; Inchingolo, F.; Di Domenico, M.; Quagliuolo, L.; Boccellino, M. Another Look at Dietary Polyphenols: Challenges in Cancer Preventionand Treatment. Curr. Med. Chem. 2022, 29, 1061–1082. [Google Scholar] [CrossRef]
- Santacroce, L.; Di Cosola, M.; Bottalico, L.; Topi, S.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Cazzolla, A.P.; Dipalma, G. Focus on HPV Infection and the Molecular Mechanisms of Oral Carcinogenesis. Viruses 2021, 13, 559. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Xhajanka, E.; Romeo, D.M.; Romeo, M.; Zappone, C.M.F.; Malcangi, G.; Scarano, A.; Lorusso, F.; et al. The Effectiveness of Osseodensification Drilling Protocol for Implant Site Osteotomy: A Systematic Review of the Literature and Meta-Analysis. Materials 2021, 14, 1147. [Google Scholar] [CrossRef]
- Crincoli, V.; Scivetti, M.; Di Bisceglie, M.B.; Pilolli, G.P.; Favia, G. Unusual Case of Adverse Reaction in the Use of Sodium Hypochlorite during Endodontic Treatment: A Case Report. Quintessence Int. Berl. Ger. 2008, 39, e70–e73. [Google Scholar]
- Di Domenico, M.; Pinto, F.; Quagliuolo, L.; Contaldo, M.; Settembre, G.; Romano, A.; Coppola, M.; Ferati, K.; Bexheti-Ferati, A.; Sciarra, A.; et al. The Role of Oxidative Stress and Hormones in Controlling Obesity. Front. Endocrinol. 2019, 10, 540. [Google Scholar] [CrossRef]
- Pisano, M.; Romano, A.; Di Palo, M.P.; Baroni, A.; Serpico, R.; Contaldo, M. Oral Candidiasis in Adult and Pediatric Patients with COVID-19. Biomedicines 2023, 11, 846. [Google Scholar] [CrossRef]
- Favia, G.; Tempesta, A.; Limongelli, L.; Crincoli, V.; Piattelli, A.; Maiorano, E. Metastatic Breast Cancer in Medication-Related Osteonecrosis Around Mandibular Implants. Am. J. Case Rep. 2015, 16, 621–626. [Google Scholar] [CrossRef]
- Camerota, L.; Ritelli, M.; Wischmeijer, A.; Majore, S.; Cinquina, V.; Fortugno, P.; Monetta, R.; Gigante, L.; Marfan Syndrome Study Group Tor Vergata University Hospital; Sangiuolo, F.C.; et al. Genotypic Categorization of Loeys-Dietz Syndrome Based on 24 Novel Families and Literature Data. Genes 2019, 10, 764. [Google Scholar] [CrossRef]
- Sammartino, G.; Gasparro, R.; Marenzi, G.; Trosino, O.; Mariniello, M.; Riccitiello, F. Extraction of Mandibular Third Molars: Proposal of a New Scale of Difficulty. Br. J. Oral Maxillofac. Surg. 2017, 55, 952–957. [Google Scholar] [CrossRef]
- Saccomanno, S.; Berretin-Felix, G.; Paskay, L.C.; Manenti, R.J.; Quinzi, V. Myofunctional Therapy Part 4: Prevention and Treatment of Dentofacial and Oronasal Disorders. Eur. J. Paediatr. Dent. 2021, 22, 332–334. [Google Scholar] [CrossRef]
- Görürgöz, C.; Yangıncı, Y.; Akçam, M.O.; Orhan, K. Is It Possible to Reveal a Typical Swallowing Pattern for Specific Skeletal Malocclusion Types Using M-Mode Sonographic Imaging of Tongue Movements? J. Orofac. Orthop. 2023, 84, 392–404. [Google Scholar] [CrossRef]
- Van der Plas, P.P.J.M.; Streppel, M.; Pullens, B.; Koudstaal, M.J.; Mathijssen, I.M.J.; van Heesch, G.G.M.; Wolvius, E.B.; Joosten, K.F.M. Feeding and Swallowing Outcomes Following Mandibular Distraction Osteogenesis: An Analysis of 22 Non-Isolated Paediatric Cases. Int. J. Oral Maxillofac. Surg. 2022, 51, 892–899. [Google Scholar] [CrossRef]
- Grechi, T.H.; Itikawa, C.E.; Gallarreta, F.W.M.; Anselmo-Lima, W.T.; Valera, F.C.P.; Trawitzki, L.V.V. Effect of Rapid Maxillary Expansion on Masticatory and Swallowing Functions in Children with Posterior Crossbite. Braz. J. Otorhinolaryngol. 2023, 89, 101304. [Google Scholar] [CrossRef]
- Zhang, C.; Kimura, Y.; Matsumoto, K. The Effects of Pulsed Nd:YAG Laser Irradiation with Fluoride on Root Surface. J. Clin. Laser Med. Surg. 1996, 14, 399–403. [Google Scholar] [CrossRef]
- Miranda, M.; Martinez, L.S.; Franco, R.; Forte, V.; Barlattani, A.; Bollero, P. Differences between Warfarin and New Oral Anticoagulants in Dental Clinical Practice. Oral Implantol. 2016, 9, 151–156. [Google Scholar]
- Horst, J.A.; Ellenikiotis, H.; Milgrom, P.L. UCSF Protocol for Caries Arrest Using Silver Diamine Fluoride: Rationale, Indications and Consent. J. Calif. Dent. Assoc. 2016, 44, 16–28. [Google Scholar] [CrossRef]
- Oliveira, B.H.; Rajendra, A.; Veitz-Keenan, A.; Niederman, R. The Effect of Silver Diamine Fluoride in Preventing Caries in the Primary Dentition: A Systematic Review and Meta-Analysis. Caries Res. 2019, 53, 24–32. [Google Scholar] [CrossRef]
- Patel, M.; McTigue, D.J.; Thikkurissy, S.; Fields, H.W. Parental Attitudes Toward Advanced Behavior Guidance Techniques Used in Pediatric Dentistry. Pediatr. Dent. 2016, 38, 30–36. [Google Scholar]
- Food and Drug Administration, HHS. Food Labeling: Health Claims; Dietary Noncariogenic Carbohydrate Sweeteners and Dental Caries. Final Rule. Fed. Regist. 2006, 71, 15559–15564. [Google Scholar]
- Yamaga, R.; Nishino, M.; Yoshida, S.; Yokomizo, I. Diammine Silver Fluoride and Its Clinical Application. J. Osaka Univ. Dent. Sch. 1972, 12, 1–20. [Google Scholar]
- Ballikaya, E.; Ünverdi, G.E.; Cehreli, Z.C. Management of Initial Carious Lesions of Hypomineralized Molars (MIH) with Silver Diamine Fluoride or Silver-Modified Atraumatic Restorative Treatment (SMART): 1-Year Results of a Prospective, Randomized Clinical Trial. Clin. Oral Investig. 2022, 26, 2197–2205. [Google Scholar] [CrossRef]
- Mulder, R.; Potgieter, N.; Noordien, N. Penetration of SDF and AgF from the Infected Dentine towards the Unaffected Tooth Structure. Front. Oral Health 2023, 4, 1298211. [Google Scholar] [CrossRef]
- Sayed, M.; Nikaido, T.; Abdou, A.; Burrow, M.F.; Tagami, J. Potential Use of Silver Diammine Fluoride in Detection of Carious Dentin. Dent. Mater. J. 2021, 40, 820–826. [Google Scholar] [CrossRef]
- Ainoosah, S.E.; Levon, J.; Eckert, G.J.; Hara, A.T.; Lippert, F. Effect of Silver Diamine Fluoride on the Prevention of Erosive Tooth Wear in Vitro. J. Dent. 2020, 103, 100015. [Google Scholar] [CrossRef]
- Campanella, V.; Syed, J.; Santacroce, L.; Saini, R.; Ballini, A.; Inchingolo, F. Oral Probiotics Influence Oral and Respiratory Tract Infections in Pediatric Population: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8034–8041. [Google Scholar] [CrossRef]
- Fanali, S.; Tumedei, M.; Pignatelli, P.; Inchingolo, F.; Pennacchietti, P.; Pace, G.; Piattelli, A. Implant Primary Stability with an Osteocondensation Drilling Protocol in Different Density Polyurethane Blocks. Comput. Methods Biomech. Biomed. Engin. 2021, 24, 14–20. [Google Scholar] [CrossRef]
- Mei, M.L.; Ito, L.; Zhang, C.F.; Lo, E.C.M.; Chu, C.H. Effect of Laser Irradiation on the Fluoride Uptake of Silver Diamine Fluoride Treated Dentine. Lasers Med. Sci. 2015, 30, 985–991. [Google Scholar] [CrossRef]
- Crystal, Y.O.; Janal, M.N.; Hamilton, D.S.; Niederman, R. Parental Perceptions and Acceptance of Silver Diamine Fluoride Staining. J. Am. Dent. Assoc. 2017, 148, 510–518.e4. [Google Scholar] [CrossRef]
- Yee, R.; Holmgren, C.; Mulder, J.; Lama, D.; Walker, D.; van Palenstein Helderman, W. Efficacy of Silver Diamine Fluoride for Arresting Caries Treatment. J. Dent. Res. 2009, 88, 644–647. [Google Scholar] [CrossRef]
- Peng, J.J.-Y.; Botelho, M.G.; Matinlinna, J.P. Silver Compounds Used in Dentistry for Caries Management: A Review. J. Dent. 2012, 40, 531–541. [Google Scholar] [CrossRef]
- Rosenblatt, A.; Stamford, T.C.M.; Niederman, R. Silver Diamine Fluoride: A Caries “Silver-Fluoride Bullet”. J. Dent. Res. 2009, 88, 116–125. [Google Scholar] [CrossRef]
- Mei, M.-L.; Chu, C.-H.; Low, K.-H.; Che, C.-M.; Lo, E.-C.-M. Caries Arresting Effect of Silver Diamine Fluoride on Dentine Carious Lesion with S. Mutans and L. Acidophilus Dual-Species Cariogenic Biofilm. Med. Oral Patol. Oral Cirugia Bucal 2013, 18, e824–e831. [Google Scholar] [CrossRef]
- Sharma, G.; Puranik, M.P.; Sowmya, K.R. Approaches to Arresting Dental Caries: An Update. J. Clin. Diagn. Res. 2015, 9, ZE08–ZE11. [Google Scholar] [CrossRef]
- Mei, M.L.; Lo, E.C.M.; Chu, C.H. Arresting Dentine Caries with Silver Diamine Fluoride: What’s Behind It? J. Dent. Res. 2018, 97, 751–758. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Roberts-Thomson, K.F.; Ha, D.H.; Wooley, S.; Meihubers, S.; Do, L.G. Community Trial of Silver Fluoride Treatment for Deciduous Dentition Caries in Remote Indigenous Communities. Aust. Dent. J. 2019, 64, 175–180. [Google Scholar] [CrossRef]
- Bansal, K.; Shamoo, A.; Mani, K.; Verma, A.; Mathur, V.P.; Tewari, N. Silver Diamine Fluoride Modified Atraumatic Restorative Treatment Compared to Conventional Restorative Technique on Carious Primary Molars-A Randomized Controlled Trial. J. Dent. 2023, 138, 104698. [Google Scholar] [CrossRef]
- Hammersmith, K.J.; DePalo, J.R.; Casamassimo, P.S.; MacLean, J.K.; Peng, J. Silver Diamine Fluoride and Fluoride Varnish May Halt Interproximal Caries Progression in the Primary Dentition. J. Clin. Pediatr. Dent. 2020, 44, 79–83. [Google Scholar] [CrossRef]
- Sirivichayakul, P.; Jirarattanasopha, V.; Phonghanyudh, A.; Tunlayadechanont, P.; Khumsub, P.; Duangthip, D. The Effectiveness of Topical Fluoride Agents on Preventing Development of Approximal Caries in Primary Teeth: A Randomized Clinical Trial. BMC Oral Health 2023, 23, 349. [Google Scholar] [CrossRef]
- De Fracasso, M.L.C.; Venante, H.S.; Santin, G.C.; Salles, C.L.F.; Provenzano, M.G.A.; Maciel, S.M. Performance of Preventive Methods Applied to the Occlusal Surface of Primary Teeth: A Randomized Clinical Study. Pesqui. Bras. Odontopediatria Clin. Integr. 2018, 18, 3675. [Google Scholar] [CrossRef]
- Aly, A.A.M.; Aziz, A.M.A.; Elghazawy, R.K.; El Fadl, R.K.A. Survival Analysis and Cost Effectiveness of Silver Modified Atraumatic Restorative Treatment (SMART) and ART Occlusal Restorations in Primary Molars: A Randomized Controlled Trial. J. Dent. 2023, 128, 104379. [Google Scholar] [CrossRef]
- Sun, I.G.; Duangthip, D.; Lo, E.C.M.; Chu, C.H. The Caries-Arrest Effectiveness of Silver Diamine Fluoride Treatment with Different Post-Treatment Instructions in Preschool Children: A Study Protocol for a Randomized Controlled Trial. Dent. J. 2023, 11, 145. [Google Scholar] [CrossRef]
- Mattos-Silveira, J.; Floriano, I.; Ferreira, F.R.; Viganó, M.E.F.; Frizzo, M.A.; Reyes, A.; Novaes, T.F.; Moriyama, C.M.; Raggio, D.P.; Imparato, J.C.P.; et al. New Proposal of Silver Diamine Fluoride Use in Arresting Approximal Caries: Study Protocol for a Randomized Controlled Trial. Trials 2014, 15, 448. [Google Scholar] [CrossRef]
- Raskin, S.E.; Tranby, E.P.; Ludwig, S.; Okunev, I.; Frantsve-Hawley, J.; Boynes, S. Survival of Silver Diamine Fluoride among Patients Treated in Community Dental Clinics: A Naturalistic Study. BMC Oral Health 2021, 21, 35. [Google Scholar] [CrossRef]
- Patel, M.C.; Makwani, D.A.; Bhatt, R.K.; Raj, V.; Patel, C.; Patel, F. Evaluation of Silver-Modified Atraumatic Restorative Technique versus Conventional Pulp Therapy in Asymptomatic Deep Carious Lesion of Primary Molars—A Comparative Prospective Clinical Study. J. Indian Soc. Pedod. Prev. Dent. 2022, 40, 383–390. [Google Scholar]
- Prakash, D.K.M.; Vinay, C.; Uloopi, K.S.; RojaRamya, K.S.; Penmatsa, C.; Chandana, N. Evaluation of Caries Arresting Potential of Silver Diamine Fluoride and Sodium Fluoride Varnish in Primary Molars: A Randomized Controlled Trial. J. Indian Soc. Pedod. Prev. Dent. 2022, 40, 377. [Google Scholar]
- Thakur, S.; Sojan, M.; Singhal, P.; Chauhan, D. A Comparative Study to Evaluate the Effectiveness of Silver Diamine Fluoride at Different Time Durations of Application in Treating Carious Primary Teeth: A Randomized Trial. Int. J. Clin. Pediatr. Dent. 2022, 15, S147–S150. [Google Scholar] [CrossRef]
- Shafi, N.; Kaur, H.; Choudhary, R.; Yeluri, R. Dilute Silver Diamine Fluoride (1:10) Versus Light Cure Calcium Hydroxide as Indirect Pulp Capping Agents in Primary Molars—A Randomized Clinical Trial. J. Clin. Pediatr. Dent. 2022, 46, 273–279. [Google Scholar] [CrossRef]


| Article screening Strategy | Database: Scopus, Web of Science and Pubmed |
| Keywords: “silver diamine fluoride” AND (“primary molar” OR “primary molars”) | |
| Boolean variable: “AND“ and “OR” | |
| Timespan: 2013–2023 | |
| Language: English |
| Authors | Type of Study | Age and Number of Participants | Aim of the study | Materials and Clinical Data | Results and Percentages |
|---|---|---|---|---|---|
| KF Roberts-Thomson et al. (2019) [77] | Randomized clinical trial | 210 children between 4 and 8 years with 384 eligible teeth | Effectiveness of silver fluoride (AgF) and stannous fluoride compared with atraumatic restorative technique (ART) in the treatment of posterior primary teeth | Two randomized groups of children with caries on primary molars: AgF and control group (ART technique) | AgF is effective as an ART technique in the treatment of posterior primary teeth. The study’s main unfavorable outcomes included dental discomfort, extractions, antibiotic usage, and more involved restorative procedures. Findings: neither of the treatment groups experienced many unfavorable effects. When comparing children treated with AgF to those treated with ART, the prevalence ratio of negative sequelae was lower at 0.18 |
| Bansal K, at al (2023) [78] | Randomized clinical trial | 226 children between 4 and 8 years old | Comparison of glass ionomer cement SMART technique (silver-modified atraumatic restorative technique) with conventional restoration | Children with asymptomatic caries in primary molars were randomly assessed in SMART and conventional groups | No significant difference between SMART and conventional techniques. The restoration success rates for the SMART and conventional groups were 38.4% and 45.8%, respectively (p = 0.105). In the SMART group, the acceptability of treatment was 79%, while in the conventional group, it was 56% (p < 0.001) |
| Hammersmith KJ et al. (2020) [79] | Retrospective study | 131 patients with 185 interproximal carious lesions | Use of SDF and fluoride varnish in caries progress | Radiographic evidence of SDF at 12-month follow-up | No statistical difference in caries arrest between techniques. At a 12-month follow-up, radiographic evidence of non-progression was present in the majority of carious lesions (n = 155, 84.0%) |
| Sirivichayakul P et al. (2023) [80] | Randomized clinical trial | 190 preschoolers with 2685 cavities | Comparison of sodium fluoride (NaF) varnish, SDF, and control group | Semiannual application of agent and bitewing control | No statistical difference in caries progression between NaF, SDF, or the control group (p > 0.05) |
| Marina de Lourdes Calvo Fracasso et al. (2018) [81] | Randomized clinical trial | 32 children aged 36 to 60 months attending a pediatric clinic | Effectiveness of glass ionomer cement (Vitremer), resin sealant (Alpha Seal Light), and SDF (Cariostatic)-like preventive materials | Use of glass ionomer cement (Vitremer), resin sealant (Alpha Seal Light), and SDF (Cariostatic) with a control group | No statistical difference in caries progression (p = 0.154) and material retention (p = 0.214) |
| Aly AAM et al. (2022) [82] | Randomized clinical trial | 67 children aged 5–9 years old, with at least one asymptomatic primary molar with active caries | Comparison between SMART and ART restorations | Asymptomatic primary molar caries randomly assigned to SMART and ART restoration group after 12 months follow-up | No statistical difference between the SMART and ART restoration groups (p = 0.416) |
| Sun IG et al. (2023) [83] | Randomized clinical trial | 254 kindergarten children with active dentine caries | Effectiveness of SDF in caries’ arrest | Different use of SDF, with and without rinse, in two randomly assessed group | Arrest of caries lesions after 12-month follow-up |
| Mattos-Silveira J et al. (2014) [84] | Randomized clinical trial | Children/adolescents presenting at least one approximal initial caries lesion in primary molars/permanent premolars and molars | Study protocol of SDF in caries’ injury progression | Study of SDF, resin infiltration, and control group caries lesions in primary molars, permanent premolars, and molars | SDF is the most effective protocol |
| Raskin SE et al. (2021) [85] | Naturalistic study | patient n = 2269; teeth n = 7787 | Use of SDF in caries lesion arrest | Comparison of effectiveness of SDF alone, SDF with sedative filling, and SDF with same-day restoration | SDF is effective when applied alone and with filling in the lesion arrest |
| Patel MC et al. (2022) [86] | Prospective clinical study | Children aged 4–8 years; 60 asymptomatic primary molar teeth with caries | Assessment of SMART as a minimum intervention approach | Assessment between SMART and conventional approach | The conventional group showed 100%, and SMART observed 96.15% clinical success at 12 months follow-up (p > 0.05). SMART can be recommended as a potential biologic approach to managing asymptomatic deep dentinal lesions |
| Mani Prakash DK et al. (2022) [87] | Randomized controlled trial | 34 children aged 6–9 years with caries in both right and left primary molars without pulpal involvement | Effectiveness of SDF and sodium fluoride (NaF) varnish in stopping the carious process in primary molars | Evaluation of right and left primary molar caries in the SDF and NaF group after 6 and 12 months follow-up | SDF is more effective (p = 0.002) than NaF (p = 0.004)in stopping carious processes |
| Thakur S et al. (2022) [88] | Prospective clinical study | 49 children and 176 deciduous molars | Effectiveness of SDF in primary tooth treatment | Use of SDF in primary teeth lesions at different time durations of application in the treatment of three groups | SDF is successful in stopping the evolution of caries in cavitated and non-cavitated injury |
| Shafi N et al. (2022) [89] | Randomized clinical trial | 56 primary molars requiring indirect pulp treatment | Evaluation of SDF and light cure calcium hydroxide as treatment in primary molars | Evaluation of primary molar treatment with dilute SDF and light cure calcium hydroxide | SDF shows 96% of success in primary tooth treatment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inchingolo, F.; Inchingolo, A.D.; Latini, G.; Sardano, R.; Riccaldo, L.; Mancini, A.; Palermo, A.; Inchingolo, A.M.; Dipalma, G. Caries in Primary Molars: Is Silver Diamine Fluoride Effective in Prevention and Treatment? A Systematic Review. Appl. Sci. 2024, 14, 2055. https://doi.org/10.3390/app14052055
Inchingolo F, Inchingolo AD, Latini G, Sardano R, Riccaldo L, Mancini A, Palermo A, Inchingolo AM, Dipalma G. Caries in Primary Molars: Is Silver Diamine Fluoride Effective in Prevention and Treatment? A Systematic Review. Applied Sciences. 2024; 14(5):2055. https://doi.org/10.3390/app14052055
Chicago/Turabian StyleInchingolo, Francesco, Alessio Danilo Inchingolo, Giulia Latini, Roberta Sardano, Lilla Riccaldo, Antonio Mancini, Andrea Palermo, Angelo Michele Inchingolo, and Gianna Dipalma. 2024. "Caries in Primary Molars: Is Silver Diamine Fluoride Effective in Prevention and Treatment? A Systematic Review" Applied Sciences 14, no. 5: 2055. https://doi.org/10.3390/app14052055
APA StyleInchingolo, F., Inchingolo, A. D., Latini, G., Sardano, R., Riccaldo, L., Mancini, A., Palermo, A., Inchingolo, A. M., & Dipalma, G. (2024). Caries in Primary Molars: Is Silver Diamine Fluoride Effective in Prevention and Treatment? A Systematic Review. Applied Sciences, 14(5), 2055. https://doi.org/10.3390/app14052055











