Abstract
In recent years, the consumption of pastry and bakery products has grown considerably, and consumers are increasingly tempted to choose products from an organoleptic point of view. At the same time, consumers are also interested in having a healthy diet, respectively, products with special sensory properties, but with a low sucrose content. Substituting the sucrose in these products with apple puree represents an alternative to obtaining cakes with a lower sugar content and, in addition, in obtaining products with high nutritional value, with the bioactive compounds from apples having special properties on health. The purpose of this work was to analyze both the physicochemical properties, the total content of polyphenols and the antioxidant activity of the apple puree samples, as well as their variation during the storage period. The physicochemical properties analyzed were: moisture content, titratable acidity, ash content, pH, water activity, total soluble solids content and color. Regarding the content of the bioactive compounds, the total content of polyphenols and the antioxidant capacity were determined by the Folin–Ciocalteu method, respectively, the DPPH method. The results showed that apple puree is an important source of polyphenols, and these are the main factors influencing antioxidant activity. The analysis of the properties of the three products obtained from the apple will allow you to choose one of them or a combination of them in order to obtain the highest degree of sugar substitution and the highest nutritional value of the products. The degree of substitution will be correlated with technological parameters, baking temperature and time.
1. Introduction
In recent years, a very large number of cases of obesity (over 1 billion) have been registered. Also, the number of type II diabetes cases is increasing (over 300 million). The main cause of obesity is the energy imbalance, more precisely the imbalance between the calories consumed and those burned, and the main causes of type II diabetes are: obesity and excessive consumption of sucrose [1,2,3]. Lately, the consumption of sugar has experienced a significant increase. While our ancestors relied mainly on natural sugars from fruit and honey, today sugar is an added ingredient in most processed foods [4]. In addition to the natural sugar present in foods such as grains, fruits, vegetables and milk, manufacturers add sugar to baked goods, both to enhance the sweet taste and for the multiple roles it plays (in terms of texture, flavor, color and preservation) [5]. Considering the current food trends, it is necessary to implement some preventive measures that involve both educating the population in order to choose a healthy diet, as well as constraining producers in order to reduce and substitute the amount of sugar in food products [2,6,7,8,9]. These measures represent a real challenge, both in educating the consumer who, most of the time, chooses food products according to their organoleptic properties, as well as in the production of foods with low sugar content, but which present sensory characteristics accepted by the consumer [2,6,7,8,9]. Pastry products are foods that contain large amounts of sucrose, which makes them calorie dense and prone to overconsumption. An alternative in reducing the sugar in these products could be replacing sugar with substitutes, such as: apple puree or other fruits, Stevia, inulin, date syrup or powder, oligofructose, apple pomace, Nypa fructicans syrup, grape syrup or polyols [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. The apple (Malus domestica) is a widely consumed fruit throughout the world, being one of the most cultivated fruit species and being in the top five most consumed fruits [26,27]. They are preferred by consumers due to their flavor and high nutritional value, being rich in vitamins, minerals and phenolic compounds [28,29,30]. Properly stored, these can be available throughout the year both in its biological form and in the form of juices, purees, cider, frozen or dried slices [28,31,32]. The processing of apples implies the loss of 25% of their mass; thus, parts such as pulp, peel and seeds are thrown away [31,33]. Thus, the use of apple purees in the production of food products represents an alternative both to avoid waste and to improve the nutritional value of the finished products [24,25,31,34,35,36]. Apples attract the attention of the scientific community through their content in bioactive compounds, such as phenolic compounds (phenolic acids, flavonoids) [26]. The presence and concentration of phenolic compounds depends on the anatomical part of the apple, species, storage, environment, and degree of ripening. The main phenolic acids in apples are the caffeic acid and the p-coumaric acid, and the most important flavonoids are: quercetin, catechin, epicatechin [32,37]. Due to their high content of hydroxyl groups and high antioxidant capacity, phenolic compounds play a very important role in the human body, disrupting oxidation processes. By providing electrons separated from the phenol group or hydrogen atoms, polyphenols prevent the production of free radicals [29]. The high content of antioxidants in apples can prevent the occurrence of diseases caused by oxidative stress (cardiovascular diseases, asthma, diabetes, cancer) [26,27,29,38,39]. Also, apples are an important source of mineral elements, in the composition of which there are both macroelements (Na, Mg, Ca, K) and microelements (Cu, Mn, Zn, Fe) [40,41]. During the storage of apples, numerous physical, chemical and biochemical changes take place. Considering the variation in the concentration of bioactive compounds and the physicochemical properties of apples, the authors proposed to study the differences that appear between the anatomical parts of apples and those that appear in different stages of ripening/storage. In this study, three products were analyzed (raw puree, cooked puree and apple peel extract) in two stages of maturity/storage.
The three forms in which the apple can be used as a substitute were analyzed, and we will choose which is the most suitable version for industrial use. The compositions of the three variants and their stability were analyzed so that the apple puree can be used for a longer time without changing its composition, while preserving its nutritional value.
2. Materials and Methods
2.1. Chemicals and Reagents
The reagents used for the experimental determinations were: methanol (Honeywell, Steinheim, Germany), gallic acid (Sigma Aldrich, St. Louis, MO, USA), 2,2-di(4-tetr-octylphenyl)-1-picrylhydrazyl (Sigma Aldrich, Steinheim, Germany), Folin reagent–Ciocâlteu (Sigma Aldreich, Steinheim, Germany), sodium carbonate (Merck Milipore, Darmstadt, Germany), sodium hydroxide (Honeywell, Germany), glacial acetic acid (Chimreactiv, Bucharest, Romania), Trolox (6-hydroxy-2,5,7,8-tetramethychroman-2-carboxylic acid).
The equipment used was: Zhicheng ZRD-A5055 oven (Zhicheng Instrument, Shanghai, China) Shimadzu 300 UV—VIS—NIR spectrometer (Tokyo, Japan), Konica Minolta CR—400 colorimeter (Konica Minolta, Tokyo, Japan), Nabertherm L9 C6 calcination furnace (Nabertherm, Lilienthal, Germany), analytical balance Partner AS 220.RS (Radwag, Torunska, Poland), Hermle Z 216 MK centrifuge (Hermle Labortechnik, Wemingen, Germany), Hirschmann burette (Solarus, Eberstadt, Germany), Mettler Toledo pH meter (Seven Compact, Heusenstamm, Germany), tabletop Abbe refractometer, AquaLab 4TE water activity meter (Meter Group, Pullman WA, USA), the Mark 10 texturometer (Mark 10 ESM301 Corporation, Copiague, NY, USA)).
2.2. Raw Materials
The Champion apple cultivars were used to obtain the purees and peel extract. The apples were purchased from a local producer in Fălticeni, Suceava, Romania. Three types of products were analyzed: raw mash, cooked mash and peel extract. The samples were analyzed in different stages of apple ripening: stage I (January), stage II (February).
2.3. Sample Preparation
To obtain the raw apple puree, the apples were washed, peeled and then blended for 5 min at maximum speed. To obtain the cooked puree, proceed as in the case of the raw puree, followed by the evaporation of the water. The sample was boiled at 100 degrees Celsius until the mass was reduced by half.
The peel extract was obtained by boiling the peels in 100 mL of water and evaporating it to 50 mL. Figure 1 shows the scheme for obtaining the raw, cooked mash and the peel extract.
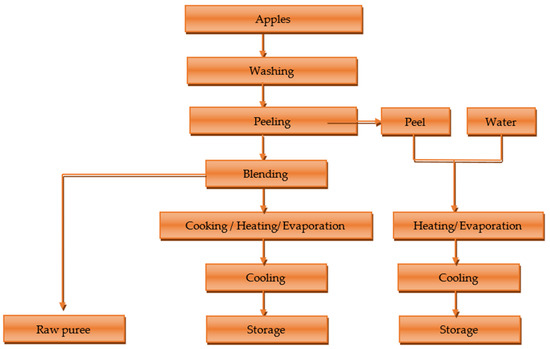
Figure 1.
Scheme for obtaining raw, cooked puree and peel extract.
2.4. Determination Methods of Physicochemical Characteristics
2.4.1. Moisture Content
Moisture content determination was performed according to AOAC Official Method 934.06. This procedure involves drying 5 g of sample at 103 ± 2 °C until a constant mass is obtained [28,42,43,44,45]. Three determinations were performed for each sample.
2.4.2. Ash Content
The ash content was determined by calcining the sample at 550–600 °C in the calcination furnace until a constant mass was obtained [28,42,43,44,45].
2.4.3. Titratable Acidity
The titratable acidity was determined by titrating with NaOH 0.1 N the sample obtained from 5 g of sample and 50 mL of water. The titration was carried out until pH = 8.1, and ml NaOH 0.1 N were converted into g malic acid equivalent (MAE)/kg [27,41,43,46,47,48,49,50,51,52].
2.4.4. pH
The pH was determined at room temperature using a Mettler Toledo pH meter (Seven Compact, Heusenstamm, Germany) [41]. Three measurements were performed for each of the three products in triplicate.
2.4.5. Total Soluble Solids Content
To determine the content of total soluble substances in the samples, measurements were carried out by placing a few drops of the sample on a tabletop Abbe refractometer. Three measurements were performed for each sample, and the results were expressed in °Brix/g/100 g [27,39].
2.4.6. Water Activity
The water activity measurements for the samples were obtained using an Aqua Lab 4TE water activity meter from Meter Group, located in Pullman, Washington, DC, USA. These measurements were performed in triplicate.
2.4.7. Total Polyphenol Content (TPC)
The total content of polyphenols was determined by the Folin–Ciocalteu method [53]. The extract for determining the total content of polyphenols was obtained by mixing 1 g of the sample with 10 mL of acidified methanol in a volumetric flask. After one hour, the extract was filtered, and 0.2 mL of the extract was introduced into a volumetric flask, over which 1.8 mL of deionized water, Folin–Ciocalteu reagents (10 mL of 1:10 reagent) and sodium carbonate were added (8 mL of 7.5% concentration). The color formation time was 2 h. The analyses were performed in triplicate for each product, at λ = 765 nm, using the Shimadzu 300 UV—VIS—NIR spectrophotometer (Tokyo, Japan), and the results were expressed in mg gallic acid equivalent (GAE)/kg fresh mass (FM), using its calibration curve [28,30,43].
2.4.8. Antioxidant Activity
The determination of the antioxidant activity was carried out according to the method described by Mateescu et al. (2022) and Thaipong et al. (2006) [43,54]. The sample was left to extract in methanol for 24 h at −20 °C. Then, the mixture obtained from 150 μL of extract and 2850 μL of 2,2-di(4-tetr-octylphenyl)-1-picrylhydrazyl (DPPH) solution was left for one hour in the dark. Each sample was analyzed in triplicate at λ = 515 nm using the Shimadzu 300 UV—VIS—NIR spectrophotometer (Tokyo, Japan), and the results were expressed in μM Trolox/g fresh mass, using its calibration curve [43,54]. One of the most used methods for evaluating the antioxidant capacity is represented by the capture of free radicals [42]. Percent radical scavenging activity of methanolic extracts was determined according to the method described by Sethi (2020) [30,55]. Thus, for the mixture consisting of 0.1 mL of extract and DPPH (3.9 mL), the absorbance was measured at λ = 515 nm using the Shimadzu 300 UV—VIS—NIR spectrophotometer (Tokyo, Japan). The radical scavenging percentage was calculated by the formula [30,51,55,56,57]:
2.4.9. Color
The color of the samples was determined with a Konica Minolta CM 700 Chromameter (Konica Minolta, Tokyo, Japan) by the CIEL lab method. Three measurements were taken for each sample of the following parameters: L*, a*, b*: L* (brightness) represents how dark the sample is, ranging from 0 (black) to 100 (white); a* represents the color variation from green (−) to red (+); b* represents the color variation from blue (−) to yellow (+).
2.4.10. Antimicrobial Analysis
Compact dry-type plates with lyophilized culture media were used for the microbiological analysis of the three samples. The samples were prepared by dilutions of 1:10 with saline solution, and 1 g of the prepared samples was added to the culture medium. The purees and peel extract were tested for Salmonella, Clostridium botulinum, Enterococcus, yeasts, molds, total germ count, Bacillus cereus, Escherichia coli and coliforms. The plates were kept for different times at different temperatures depending on the species, as follows: Salmonella—24 h at 42 °C [58], Clostridium botulinum—48 h at 37 °C, Enterococcus, Escherichia coli and coliforms, Bacillus cereus and the total number of germs—48 h at 35 °C [59], yeasts and molds—3 days at 30 °C [60].
2.4.11. Mineral Content
The mineral content of apple purees and apple peel extract was determined according to the method described by Laura Agripina Scripcă (2021), using ICP-MS [41,61,62].
2.4.12. Statistical Analysis
The experiments were performed in triplicate. Mean values and standard deviations for all investigated parameters were calculated using statistical software SPSS 25.0 (trial version) from IBM, New York, NY, USA. Data differences were assessed through analysis of variance (ANOVA) followed by Tukey’s HSD test. Additionally, Origin Pro 2019b 9.6.5.169 (Student Version) Copyright© 1991–2019 Origin Lab Corporation, Northampton, MA, USA, was employed for analysis. Values followed by different superscript letters (a, b, c) are statistically different at 95% confidence level.
3. Results
3.1. Moisture Content
The high water absorption capacity of starch in apple varieties is responsible for their increased moisture content, this being associated with weak molecular attraction forces between starch granules [43,63]. The sample with the highest moisture content is represented by the peel extract (89.76% ± 0.049%), this being obtained by boiling the peels in water. The raw mash had a higher moisture content (86.73% ± 1.10%) than the boiled one (77.09% ± 2.48%) because, by boiling the mash, the water in its composition evaporates (Figure 2). Also, a decrease in moisture content was observed with the increase in the degree of ripening of the apples.
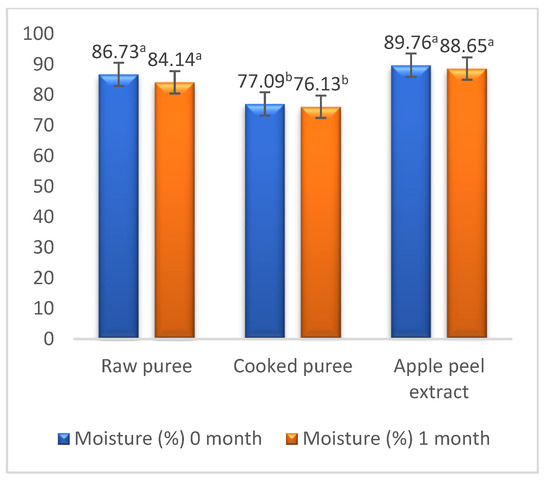
Figure 2.
Moisture content. Values followed by different superscript letters (a,b) are statistically different at 95% confidence level.
3.2. Ash Content
The percentage of ash in apples varies depending on the degree of ripening and the anatomical part of the apple. Thus, with the increase in the degree of ripening of the apples, they lost part of their water content, which led to an increase in the percentage of ash. In addition, the ash content also varied depending on the anatomical part of the apple; thus, a significantly (p < 0.05) higher content was determined in the apple peel compared to the apple purees, approximately double compared to the raw puree: 2.18% ± 0.01% and 1.05% ± 0.01% (Figure 3). A higher percentage of ash (1.05% ± 0.01%) was determined in the boiled apple puree because, by boiling, part of the contained water was evaporated (Figure 3).
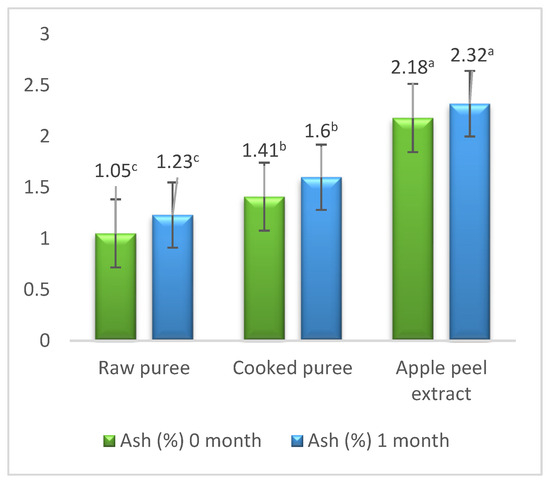
Figure 3.
Ash content. Values followed by different superscript letters (a,b,c) are statistically different at 95% confidence level.
3.3. Titrable Acidity
Previous studies have shown that, during fruit storage, the content of organic acids increases. In this study, we obtained similar results, so that, during the storage period, the content of organic acids increased for each of the three samples as follows: in the case of the raw mash, it increased from 2.98 g MAE/kg to 5.49 g MAE/kg; for the cooked puree, it increased from 2.49 g MAE/kg to 5.3 g MAE/kg; and in the case of the bark extract, it varied from 0.92 g MAE/kg to 2.57 g MAE/kg (Figure 4).
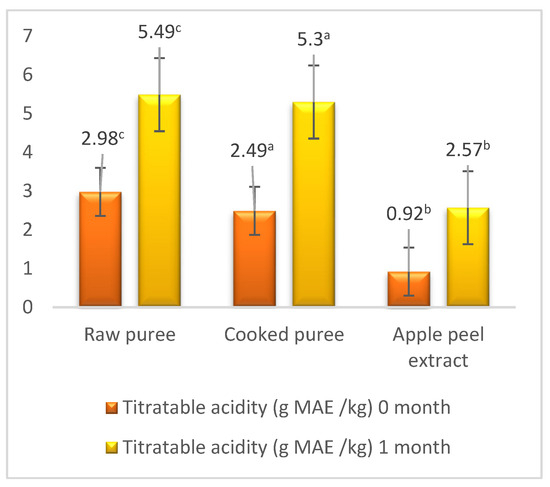
Figure 4.
Titratable acidity. Values followed by different superscript letters (a,b,c) are statistically different at 95% confidence level.
3.4. Total Soluble Solids Content
In the case of raw and cooked mash, the total content of soluble solids did not show significant differences during the storage period and varied from 12.06 °Brix to 12.8 °Brix in the case of raw mash, respectively from 18.56 °Brix to 18.66 °Brix for cooked mash (p < 0.05). In the case of the apple peel extract, the total content of soluble solids increased by a percentage of 64.35% (Table 1).

Table 1.
Total soluble solids, water activity and pH.
3.5. Water Activity and pH
Regarding the water activity, significant differences were not found (p > 0.05) (Table 1). Similar results were obtained by Balestra et al. (2011) [61]. The pH of the samples had an insignificant increase during the storage period of the apples. Increases in pH from 2.05% to 9.98% were obtained (Table 1); similar results were obtained by Yu et al. (2019), Rinaldi et al. (2021), Nistor et al. (2015) and Balestra et al. (2011) [32,41,61,64].
3.6. Total Phenolic Content
The variations in the total content of polyphenols during the storage period of the samples are presented in Figure 5. The total content of polyphenols of the analyzed samples varied from 600.66 mg GAE/kg to 972.33 mg GAE/kg (Figure 5). The highest content of polyphenols was determined in the peel extract (972.33 mg GAE/kg); similar results were also obtained by Loncaric et al. (2014) [27]. Also, in the case of the peel extract, there was determined an increase in the total polyphenol content during the storage period. In the case of the raw mash, respectively the cooked mash, a decrease in the content of polyphenols was determined during the storage period. In the cooked mash, a higher content of polyphenols was obtained by up to 51% compared to the raw mash, this fact being due to the change in the concentration of the sample during the heat treatment, with similar results being obtained by Park et al. (2015) and Kampuse et al. (2019) [43,48,62]. The significant decrease in the total content of polyphenols in the cooked puree, compared to that of the raw puree, is due to the catalysis of the oxidation of phenolic compounds by polyphenoloxidase.
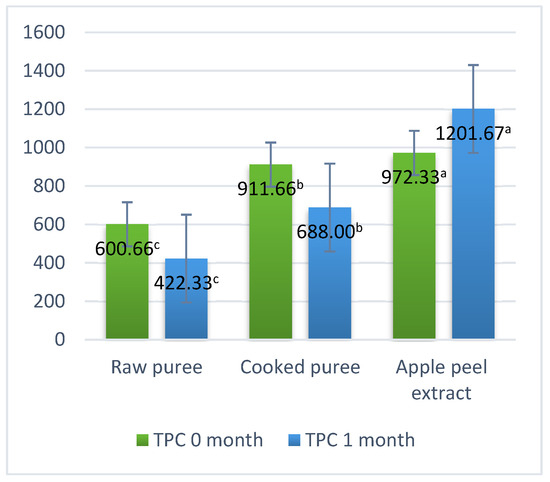
Figure 5.
Total polyphenol content. Values followed by different superscript letters (a,b,c) are statistically different at 95% confidence level.
3.7. Antioxidant Capacity
The antioxidant activity was determined by the DPPH method and varied between 0.77 μM of TE/g FM for the raw mash and 1.24 μM of TE/g FM for the cooked mash (Figure 6). This difference may be due to the thermal process to which the boiled puree was subjected, a process that led to a change in the concentration of the sample. During the storage period, a decrease in the antioxidant activity was recorded for all 3 samples (Figure 6). The main factors influencing antioxidant activity are phenolic compounds [29]. Based on the results obtained, it can be concluded that there is a correlation between the total content of polyphenols and the antioxidant activity, a conclusion also reached by Loncaric (2014), Park et al. (2015) and Kampuse et al. (2019) [27,48,62]. In the case of purees, with the decrease in the total content of polyphenols, the antioxidant activity also decreased; similar results were obtained by Loncaric et al. (2014) [27].
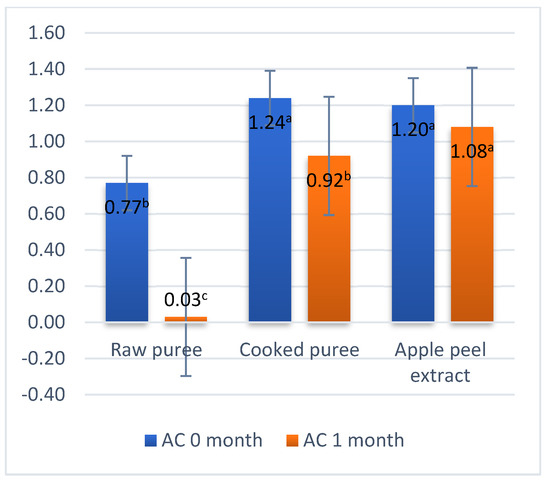
Figure 6.
Antioxidant capacity. Values followed by different superscript letters (a,b,c) are statistically different at 95% confidence level.
The peel extract had the highest antioxidant capacity of 94.01% inhibited DPPH, compared to the raw puree, which had the lowest antioxidant capacity of 63.93% inhibited DPPH. Based on the results obtained, a positive influence of the thermal treatment on the antioxidant activity of the samples can be observed, in the case of the puree not thermally treated, but a very large decrease in the antioxidant activity and percent radical scavenging activity can be observed (Figure 7). Mateescu (2022) also obtained increases in the antioxidant activity after heat treatment of the samples [43].
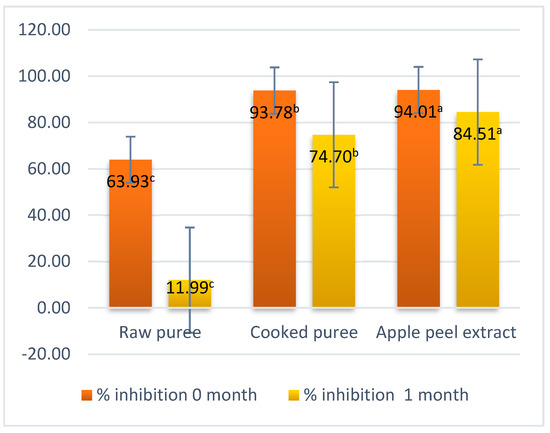
Figure 7.
Percent radical scavenging activity. Values followed by different superscript letters (a,b,c) are statistically different at 95% confidence level.
3.8. Color
The higher L* value of the raw mash compared to the cooked mash indicates that it is more susceptible to enzymatic browning than the thermally treated mash (Table 2) [63]. It can also be stated that thermal treatment leads to a decrease in the L* value, with similar results being obtained by Rinaldi (2021) [32].

Table 2.
Color parameters.
The obtained results agree with Rinaldi et al. [32], the decrease in the value of L* in the case of cooked puree due to caramelization, Maillard condensation, and the destruction of pigments due to the high temperatures used.
Obtaining negative values for a* and positive values for b* indicates that the samples have the color represented by shades of green and yellow. The lowest value of a* was obtained for the peel extract, respectively 0.78, and the tendency towards a positive value may be due to the red color of the peel.
3.9. Antimicrobial Analysis
Microbial contamination is indeed a major concern in the food industry and can significantly contribute to food spoilage and unacceptability. Microorganisms such as bacteria, molds and yeasts can affect food quality, taste, texture and pose health risks to consumers.
According to the obtained results, the samples presented a high microbiological stability. Regarding the apple peel extract, it showed the highest microbiological stability (Table 3); no species of microorganisms developed on the culture media due to the antimicrobial character of the antioxidants in the apple peel [49,50,51,52]. Regarding the microbiological stability of the cooked mash, it falls within the limit set by the Food Safety and Standard Regulations (FSSAI), European regulations and the Food and Drug Administration (FDA). The obtained results showed that the raw puree can be used 24 h after obtaining it.

Table 3.
Microbial analysis of apple purees and apple peel extract.
3.10. Mineral Content
Regarding the content of Ca and Mg in the cooked puree, similar data were obtained as those by Nistor et al. (2015), as well as in the case of the copper content in the raw apple puree [41]. The content of sodium, iron, zinc and manganese was lower than those obtained by Nistor et al. (2015) [41].
The highest mineral content was determined in apple peel extract, with the concentrations of microelements being significantly higher than the concentrations of macroelements in apple purees (p < 0.05) (Table 4).

Table 4.
Concentration values of mineral elements in apples puree and apple peel extract samples (mg/kg).
The element with the highest concentration in apple peel extract was sodium, followed by Mg, Ca, K. In raw and cooked puree, sodium was found in significantly lower concentrations than apple peel extract (p < 0.05).
In the case of raw puree, the highest concentration was in Mg, followed by Ca, K, and in the case of cooked puree, the concentration in Ca was highest, followed by K, and the concentration in Mg was significantly higher than in raw puree (p < 0.05).
In all samples, concentrations of microelements (Fe, Cu, Mn, Zn) were significantly lower (p < 0.05).
4. Conclusions
The results of this research confirmed the variation over time in the physicochemical properties of apple puree and apple peel extract. Thus, the total content of soluble solids, the ash content, the pH and the titratable acidity increased during the storage period. Instead, the total content of polyphenols and the antioxidant activity of the samples decreased during the storage period, with the exception of the polyphenol content of the peel extract. This fact leads to the confirmation of the existence of the link between the total content of polyphenols and the antioxidant activity, polyphenols being a factor influencing the antioxidant activity. Also, the results confirmed the variation in the physicochemical properties, polyphenol content and antioxidant activity depending on the anatomical part of the apple from which the samples were obtained. In addition, the results demonstrated an increase in polyphenol content and antioxidant activity in the case of thermally treated samples.
Knowing the physicochemical properties of apple purees allows the appropriate choice of technological parameters for obtaining pastry products in which they will totally or partially replace sugar. The three products obtained from apples can be used separately or in combination depending on the textural and organoleptic characteristics of the finished products. The microbiological analyses carried out allow for establishing the time interval within which the three products can be used safely. The use of puree and apple peel extract to replace sucrose in pastry and bakery products represents an alternative in obtaining healthier products both by reducing the energy value of the products obtained and by increasing the nutritional value due to the high content of polyphenols and mineral elements.
The proportions in which sugar can be replaced with apple puree will be followed, to what extent the liquid phase can be partially or totally formed from the apple peel extract, technological parameters (temperature and baking time), so that the resulting product is comparable to the samples of control in terms of texture, color, aroma and taste, and more valuable from a nutritional point of view.
Author Contributions
H.D. and A.S. contributed equally to the collection of data and preparation of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
This work was funded by the Ministry of Research, Innovation and Digitalization within Program 1—Development of national research and development system, Subprogram 1.2—Institutional Performance—RDI excellence funding projects, under contract no. 10PFE/2021.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rippe, J.M.; Angelopoulos, T.J. Relationship between Added Sugars Consumption and Chronic Disease Risk Factors: Current Understanding. Nutrients 2016, 8, 697. [Google Scholar] [CrossRef] [PubMed]
- Faruque, S.; Tong, J.; Lacmanovic, V.; Agbonghae, C.; Minaya, D.M.; Czaja, K. The Dose Makes the Poison: Sugar and Obesity in the United States—A Review. Polish J. Food Nutr. Sci. 2019, 69, 219–233. [Google Scholar] [CrossRef]
- Mastellos, N.; Gunn, L.H.; Felix, L.M.; Car, J.; Majeed, A. Transtheoretical Model Stages of Change for Dietary and Physical Exercise Modification in Weight Loss Management for Overweight and Obese Adults. Cochrane Database Syst. Rev. 2014, 2014, CD008066. [Google Scholar] [CrossRef]
- Belkova, J.; Rozkot, M.; Danek, P.; Klein, P.; Matonohova, J.; Podhorna, I. Sugar and Nutritional Extremism. Crit. Rev. Food Sci. Nutr. 2017, 57, 933–936. [Google Scholar] [CrossRef]
- Zaitoun, M.; Ghanem, M.; Harphoush, S. Sugars: Types and Their Functional Properties in Food and Human Health. Int. J. Public Health Res. 2018, 6, 93–99. [Google Scholar]
- Dadali, C. Sugar Replacement Applications in Cakes: Effect on Cake Texture and Sweetness. Int. J. Sci. Technol. Res. 2019, 5, 25–34. [Google Scholar] [CrossRef]
- Amies-Cull, B.; Briggs, A.D.M.; Scarborough, P. Estimating the Potential Impact of the UK Government’s Sugar Reduction Programme on Child and Adult Health: Modelling Study. BMJ 2019, 365, l1417. [Google Scholar] [CrossRef]
- Azaïs-Braesco, V.; Sluik, D.; Maillot, M.; Kok, F.; Moreno, L.A. A Review of Total & Added Sugar Intakes and Dietary Sources in Europe. Nutr. J. 2017, 16, 6. [Google Scholar] [CrossRef]
- Javanmardi, F.; Mousavi, M.M.; Ghazani, A.T.; Mahmoudpour, M.; Taram, F.; Pilevar, Z. Study on the effect of xylitol and maltitol as alternative sweeteners in sponge cakes. Curr. Nutr. Food Sci. 2020, 16, 403–409. [Google Scholar] [CrossRef]
- Stavale, M.D.O.; Assunção Botelho, R.B.; Zandonadi, R.P. Apple as Sugar Substitute in Cake. J. Culin. Sci. Technol. 2019, 17, 224–231. [Google Scholar] [CrossRef]
- Rodríguez-García, J.; Salvador, A.; Hernando, I. Replacing Fat and Sugar with Inulin in Cakes: Bubble Size Distribution, Physical and Sensory Properties. Food Bioprocess Technol. 2014, 7, 964–974. [Google Scholar] [CrossRef]
- Mehrabi, S.; Koushki, M.; Azizi, M.H. Effect of Grape Syrup as a Replacement for Sugar on the Chemical and Sensory Properties of Sponge Cake. Curr. Res. Nutr. Food Sci. 2017, 5, 126–136. [Google Scholar] [CrossRef]
- Alsirrag, M.A.; Hussein, A.A.; Awahd, H.A.; Awda, J.M.; Al-Masoudi, Z.M.; Almosawy, M.M. Phisco-Chemical Analysis and Sensory Evaluation of Iraqi Cake Incorporated with Grape and Date (Zahidi) Syrup. IOP Conf. Ser. Earth Environ. Sci. 2019, 388, 012053. [Google Scholar] [CrossRef]
- Mousavivand, H.; Hojati, M.; Jooyandeh, H.; Barzgar, H.; Zaki, H. Effect of Replacement of Sugar with Grape Syrup Powder on Characteristics of Cup Cake. J. Food Res. 2020, 30, 175–188. [Google Scholar]
- Alsenaien, W.A.; Alamer, R.A.; Tang, Z.X.; Albahrani, S.A.; Al-Ghannam, M.A.; Aleid, S.M. Substitution of Sugar with Dates Powder and Dates Syrup in Cookies Making. Adv. J. Food Sci. Technol. 2015, 8, 8–13. [Google Scholar] [CrossRef]
- Huțu, D.; Amariei, S. The Effects of Sugar and Fat Substitution on the Textural Properties of the Pie Dough. Food Environ. Saf. J. 2021, 20, 149–159. [Google Scholar] [CrossRef]
- Huțu, D.; Amariei, S. Effects of the Sugar and Fat Substitution on the Rheological Properties of the Pie Dough. Ukr. Food J. 2021, 10, 592–604. [Google Scholar] [CrossRef]
- Gao, J.; Brennan, M.A.; Mason, S.L.; Brennan, C.S. Effects of Sugar Substitution with “Stevianna” on the Sensory Characteristics of Muffins. J. Food Qual. 2017, 2017, 8636043. [Google Scholar] [CrossRef]
- Tsatsaragkou, K.; Methven, L.; Chatzifragkou, A.; Rodriguez-Garcia, J. The Functionality of Inulin as a Sugar Replacer in Cakes and Biscuits; Highlighting the Influence of Differences in Degree of Polymerisation on the Properties of Cake Batter and Product. Foods 2021, 10, 951. [Google Scholar] [CrossRef]
- Majzoobi, M.; Mohammadi, M.; Farahnaky, A. Simultaneous Reduction of Fat and Sugar in Cake Production; Effects of Changing Sucrose, Oil, Water, Inulin, and Rebaudioside A on Cake Batter Properties. J. Food Process. Preserv. 2020, 44, e14733. [Google Scholar] [CrossRef]
- Shahidi, B.; Kalantari, M.; Boostani, S. Preparation and Characterization of Sponge Cake Made with Grape Juice. Iran. Food Sci. Technol. Res. J. 2017, 13, 415–425. [Google Scholar]
- Krupa-Kozak, U.; Drabińska, N.; Rosell, C.M.; Piłat, B.; Starowicz, M.; Jeliński, T.; Szmatowicz, B. High-Quality Gluten-Free Sponge Cakes without Sucrose: Inulin-Type Fructans as Sugar Alternatives. Foods 2020, 9, 1735. [Google Scholar] [CrossRef]
- Wang, H.J.; Thomas, R.L. Direct Use of Apple Pomace in Bakery Products. J. Food Sci. 1989, 54, 618–620. [Google Scholar] [CrossRef]
- Jingrong, G.; Fezhong, H.; Xinbo, G.; Xian, Z.; Susan, L.M.; Margaret, A.B.; Charles, S.B. Image Analysis of the Sugar-Reduced Muffin Formulated with Stevianna® or Inulin as a Sugar Replacer. Grain Oil Sci. Technol. 2018, 1, 63–71. [Google Scholar] [CrossRef]
- Tai, Y.Y.; Alina, T.I.T.; Rosli, W.I.W. Improvement of Physico-Chemical Properties, Antioxidant Capacity and Acceptability of Carrot Cake by Partially Substituting Sugar with Concentrated Nypa Fruticans Sap. Pertanika J. Trop. Agric. Sci. 2019, 42, 883–902. [Google Scholar]
- Topuz, F.C.; Pazir, F. Characterization, Optimization, Physicochemical Properties, and Bioactive Components of Drum-Dried Apple Puree. J. Agric. Sci. Technol. 2019, 22, 109–119. [Google Scholar]
- Loncaric, A.; Dugalic, K.; Mihaljevic, I.; Jakobek, L.; Pilizota, V. Effects of Sugar Addition on Total Polyphenol Content and Antioxidant Activity of Frozen and Freeze-Dried Apple Purée. J. Agric. Food Chem. 2014, 62, 1674–1682. [Google Scholar] [CrossRef]
- Petkova, N.T.; Kuzmanova, S.; Bileva, T.; Valcheva, E.; Dobrevska, G.; Grozeva, N.; Popov, V. Influence of Conventional and Organic Agriculture Practices on the Total Phenols and Antioxidant Potential of Florina Apple Fruits. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1031, 012088. [Google Scholar] [CrossRef]
- Li, H.; Subbiah, V.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Phenolic Profiling of Five Different Australian Grown Apples. Appl. Sci. 2021, 11, 2421. [Google Scholar] [CrossRef]
- Sethi, S.; Joshi, A.; Arora, B.; Bhowmik, A.; Sharma, R.R.; Kumar, P. Significance of FRAP, DPPH, and CUPRAC Assays for Antioxidant Activity Determination in Apple Fruit Extracts. Eur. Food Res. Technol. 2020, 246, 591–598. [Google Scholar] [CrossRef]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.M.; Tou, J.C. A Comprehensive Analysis of the Composition, Health Benefits, and Safety of Apple Pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef]
- Rinaldi, M.; Langialonga, P.; Dhenge, R.; Aldini, A.; Chiavaro, E. Quality Traits of Apple Puree Treated with Conventional, Ohmic Heating and High-Pressure Processing. Eur. Food Res. Technol. 2021, 247, 1679–1688. [Google Scholar] [CrossRef]
- Rha, H.J.; Bae, I.Y.; Lee, S.; Yoo, S.H.; Chang, P.S.; Lee, H.G. Enhancement of Anti-Radical Activity of Pectin from Apple Pomace by Hydroxamation. Food Hydrocoll. 2011, 25, 545–548. [Google Scholar] [CrossRef]
- Reis, S.F.; Rai, D.K.; Abu-Ghannam, N. Apple Pomace as a Potential Ingredient for the Development of New Functional Foods. Int. J. Food Sci. Technol. 2014, 49, 1743–1750. [Google Scholar] [CrossRef]
- Macedo, I.B.; Romão de Lima, B.; Botelho, R.; Alencar, E.R.; Zandonadi, R. Dried Apples as Substitute for Refined Sugar in Pound Cakes. J. Acad. Nutr. Diet. 2019, 119, A126. [Google Scholar] [CrossRef]
- Sahni, P.; Shere, D.M. Utilization of Fruit and Vegetable Pomace as Functional Ingredient in Bakery Products: A Review. Asian J. Dairy Food Res. 2018, 37, 202–211. [Google Scholar] [CrossRef]
- Markowski, J.; Płocharski, W. Determination of Phenolic Compounds in Apples and Processed Apple Products. J. Fruit Ornam. Plant Res. 2006, 14, 133–142. [Google Scholar]
- Oszmiański, J.; Wolniak, M.; Wojdyło, A.; Wawer, I. Influence of Apple Purée Preparation and Storage on Polyphenol Contents and Antioxidant Activity. Food Chem. 2008, 107, 1473–1484. [Google Scholar] [CrossRef]
- Gerard, K.A.; Roberts, J.S. Microwave Heating of Apple Mash to Improve Juice Yield and Quality. Lwt 2004, 37, 551–557. [Google Scholar] [CrossRef]
- Scripcă, L.A.; Amariei, S. The Influence of Chemical Contaminants on the Physicochemical Properties of Unifloral and Multifloral Honey. Foods 2021, 10, 1039. [Google Scholar] [CrossRef]
- Nistor, O.V.; Stãnciuc, N.; Andronoiu, D.G.; Mocanu, G.D.; Botez, E. Ohmic Treatment of Apple Puree (Golden Delicious Variety) in Relation to Product Quality. Food Sci. Biotechnol. 2015, 24, 51–59. [Google Scholar] [CrossRef]
- Kozlowicz, K.; Kluza, F. Experimental characteristics of freezing of apple-pear puree with sweetening substances addition. Acta Agrophysica 2019, 7, 132. [Google Scholar]
- Mateescu, A.M.; Mureșan, A.E.; Pușcaș, A.; Mureșan, V.; Sestras, R.E.; Muste, S. Baby Food Purees Obtained from Ten Different Apple Cultivars and Vegetable Mixtures: Product Development and Quality Control. Appl. Sci. 2022, 12, 12462. [Google Scholar] [CrossRef]
- Lončarić, A.; Kopjar, M.; Piližota, V. Improving the Quality of Apple Purée. J. Food Sci. Technol. 2017, 54, 3201–3207. [Google Scholar] [CrossRef]
- Wilczyński, K.; Kobus, Z.; Nadulski, R.; Szmigielski, M. Assessment of the Usefulness of the Twin-Screw Press in Terms of the Pressing Efficiency and Antioxidant Properties of Apple Juice. Processes 2020, 8, 101. [Google Scholar] [CrossRef]
- Henríquez, C.; Almonacid, S.; Chiffelle, I.; Valenzuela, T.; Araya, M.; Cabezas, L.; Simpson, R.; Speisky, H. Determinación de La Capacidad Antioxidante, Contenido de Fenoles Totales y Composición Mineral de Diferentes Tejidos de Frutos de Cinco Variedades de Manzana Cultivadas En Chile. Chil. J. Agric. Res. 2010, 70, 523–536. [Google Scholar] [CrossRef]
- Landl, A.; Abadias, M.; Sárraga, C.; Viñas, I.; Picouet, P.A. Effect of High Pressure Processing on the Quality of Acidified Granny Smith Apple Purée Product. Innov. Food Sci. Emerg. Technol. 2010, 11, 557–564. [Google Scholar] [CrossRef]
- Kampuse, S.; Kruma, Z.; Klava, D.; Ozola, L.; Galoburda, R.; Straumite, E. The Evaluation of Organically Grown Apple Cultivars for Special Diet Puree Production. In Proceedings of the 13th Baltic Conference on Food Science and Technology “FOOD. NUTRITION. WELL-BEING”, Jelgava, Latvia, 2–3 May 2019; pp. 143–148. [Google Scholar] [CrossRef]
- Bakshi, R.A.; Aslam, A.; Khan, Z.S.; Fayaz, S.; Dar, B.N. Physiochemical, Sensorial, and Rheological Characteristics of Sauce Developed from Kashmiri Apples: Influence of Cultivars and Storage Conditions. Food Sci. Nutr. 2022, 10, 1685–1693. [Google Scholar] [CrossRef]
- Drake, S.R. Influence of Calcium Treatment on NMI Morphology. J. Food Sci. 2012, 48, 403–405. [Google Scholar] [CrossRef]
- Cheng, X.F.; Zhang, M.; Adhikari, B. Changes in Quality Attributes of Strawberry Purees Processed by Power Ultrasound or Thermal Treatments. Food Sci. Technol. Res. 2014, 20, 1033–1041. [Google Scholar] [CrossRef]
- Tosun, I.; Ustun, N.S.; Tekguler, B. Physical and Chemical Changes during Ripening of Blackberry Fruits. Sci. Agric. 2008, 65, 87–90. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, H.; Ren, S. Antioxidant Activity and HPLC Analysis of Polyphenol-Enriched Extracts from Industrial Apple Pomace. J. Sci. Food Agric. 2013, 93, 2502–2506. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Gomez, S.; Kuruvila, B.; Maneesha, P.K.; Joseph, M. Variation in Physico-Chemical, Organoleptic and Microbial Qualities of Intermediate Moisture Pineapple (Ananas Comosus (L.) Merr.) Slices during Storage. Food Prod. Process. Nutr. 2022, 4, 5. [Google Scholar] [CrossRef]
- Choo, W.S.; Yong, W.K. Antioxidant Properties of Two Species of Hylocereus Fruits. Adv. Appl. Sci. Res. 2011, 2, 418–425. [Google Scholar]
- Faramarzi, S.H.; Yadollahi, A.; Barzegar, M.; Sadraei, K.; Pacifico, S.; Jemric, T. Comparison of Phenolic Compounds’ Content and Antioxidant Activity between Some Native Iranian Apples and Standard Cultivar “Gala”. J. Agric. Sci. Technol. 2014, 16, 1601–1611. [Google Scholar]
- Nour, V.; Trandafir, I.; Ionica, M.E. Compositional Characteristics of Fruits of Several Apple (Malus Domestica Borkh.) Cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 228–233. [Google Scholar]
- Todea, D.; Cadar, O.; Simedru, D.; Roman, C.; Tanaselia, C.; Suatean, I.; Naghiu, A. Determination of Major-to-Trace Minerals and Polyphenols in Different Apple Cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 523–529. [Google Scholar] [CrossRef]
- Schmid, V.; Trabert, A.; Schäfer, J.; Bunzel, M.; Karbstein, H.P.; Emin, M.A. Modification of Apple Pomace by Extrusion Processing: Studies on the Composition, Polymer Structures, and Functional Properties. Foods 2020, 9, 1385. [Google Scholar] [CrossRef] [PubMed]
- Balestra, F.; Cocci, E.; Marsilio, G.; Rosa, M.D. Physico-Chemical and Rheological Changes of Fruit Purees during Storage. Procedia Food Sci. 2011, 1, 576–582. [Google Scholar] [CrossRef]
- Park, Y.S.; Im, M.H.; Ham, K.S.; Kang, S.G.; Park, Y.K.; Namiesnik, J.; Leontowicz, H.; Leontowicz, M.; Trakhtenberg, S.; Gorinstein, S. Quantitative Assessment of the Main Antioxidant Compounds, Antioxidant Activities and FTIR Spectra from Commonly Consumed Fruits, Compared to Standard Kiwi Fruit. Lwt 2015, 63, 346–352. [Google Scholar] [CrossRef]
- Dunarea de Jos Galati, U.; Botez, E.; Luca, E.; Danut Mocanu, G.; Viorela Nistor, O.; Dănuţ Mocanu, G.; Georgeta Andronoiu, D.; Timofti, M. Ohmic Heating Process Characterizations during Apple Puree Processing. J. Agroaliment. Process. Technol. 2013, 19, 228–236. [Google Scholar]
- Yu, P.; Yu, S.; Wang, J.; Guo, H.; Zhang, Y.; Liao, X.; Zhang, J.; Wu, S.; Gu, Q.; Xue, L.; et al. Bacillus CereusIsolated from Vegetables in China: Incidence, Genetic Diversity, Virulence Genes, and Antimicrobial Resistance. Front. Microbiol. 2019, 10, 948. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.-K.; Edith, A.A.; Thomas, A.D.; Mireille, D. Microbiological Quality of Raw Vegetables and Ready to Eat Products Sold in Abidjan (Cte DIvoire) Markets. Afr. J. Microbiol. Res. 2017, 11, 204–210. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).