Preparation of Hirudin-Loaded Chitosan/Polycaprolactone Bowl-Shaped Particles and an Application for a Drug Delivery System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Optimization of the CS/PCL Polymeric Solutions
2.3. Preparation of CS/PCL Bowl-Shaped Microparticles (CS/PCL MPs)
2.4. Preparation of rH/CS/PCL Bowl-Shaped Microparticles (rH/CS/PCL MPs)
2.5. Characterization
2.5.1. Morphology
2.5.2. Infrared Spectroscopy Analysis
2.5.3. Water Absorption
2.5.4. Thermogravimetric Analysis (TG)
2.5.5. Hirudin Release In Vitro
2.5.6. Anticoagulant Activity Assay
2.5.7. Evaluation of Blood Compatibility
2.5.8. Cell Viability Assay
2.6. Statistical Analysis
3. Results and Discussion
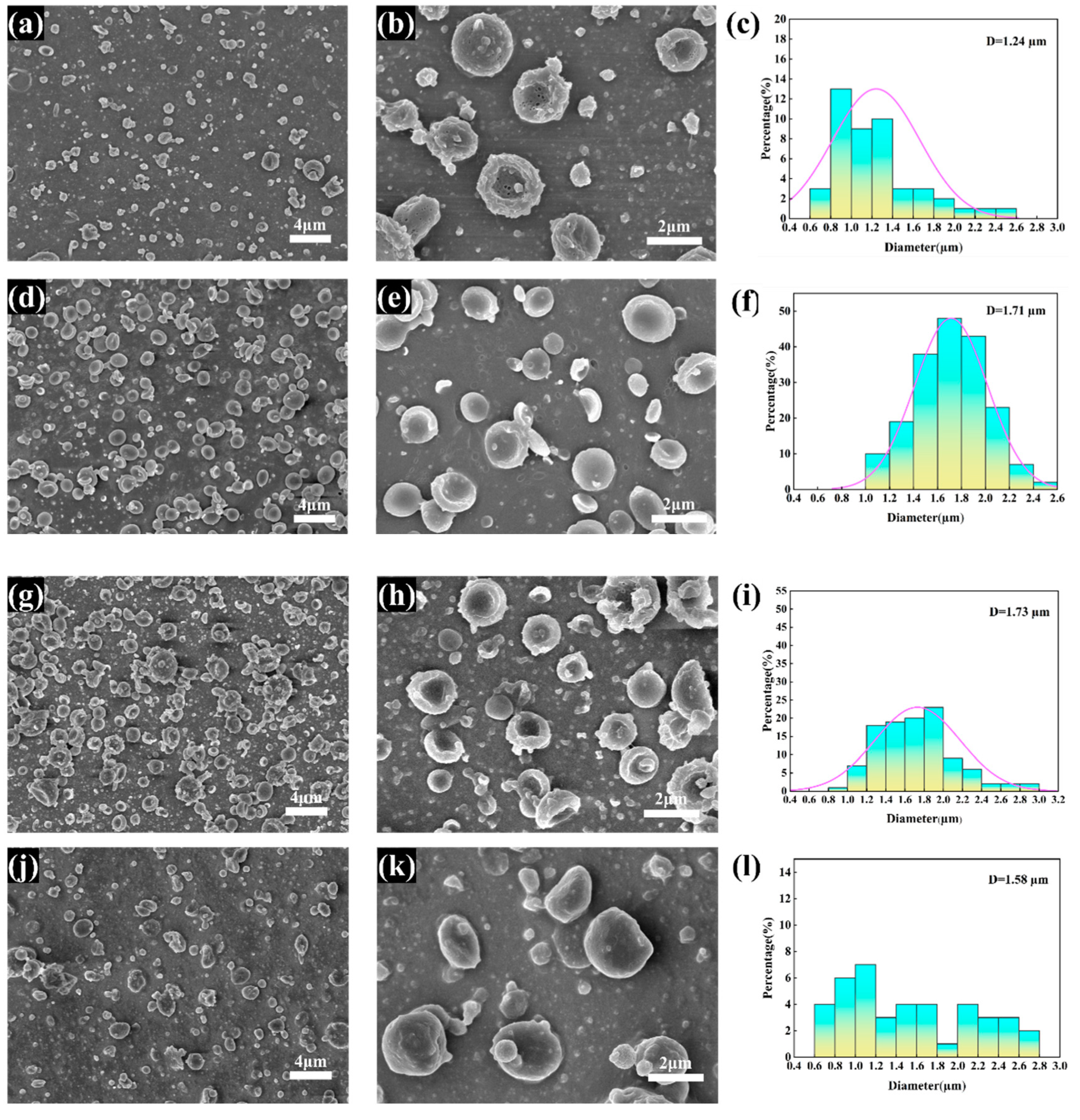
3.1. Morphology of the CS/PCL MPs
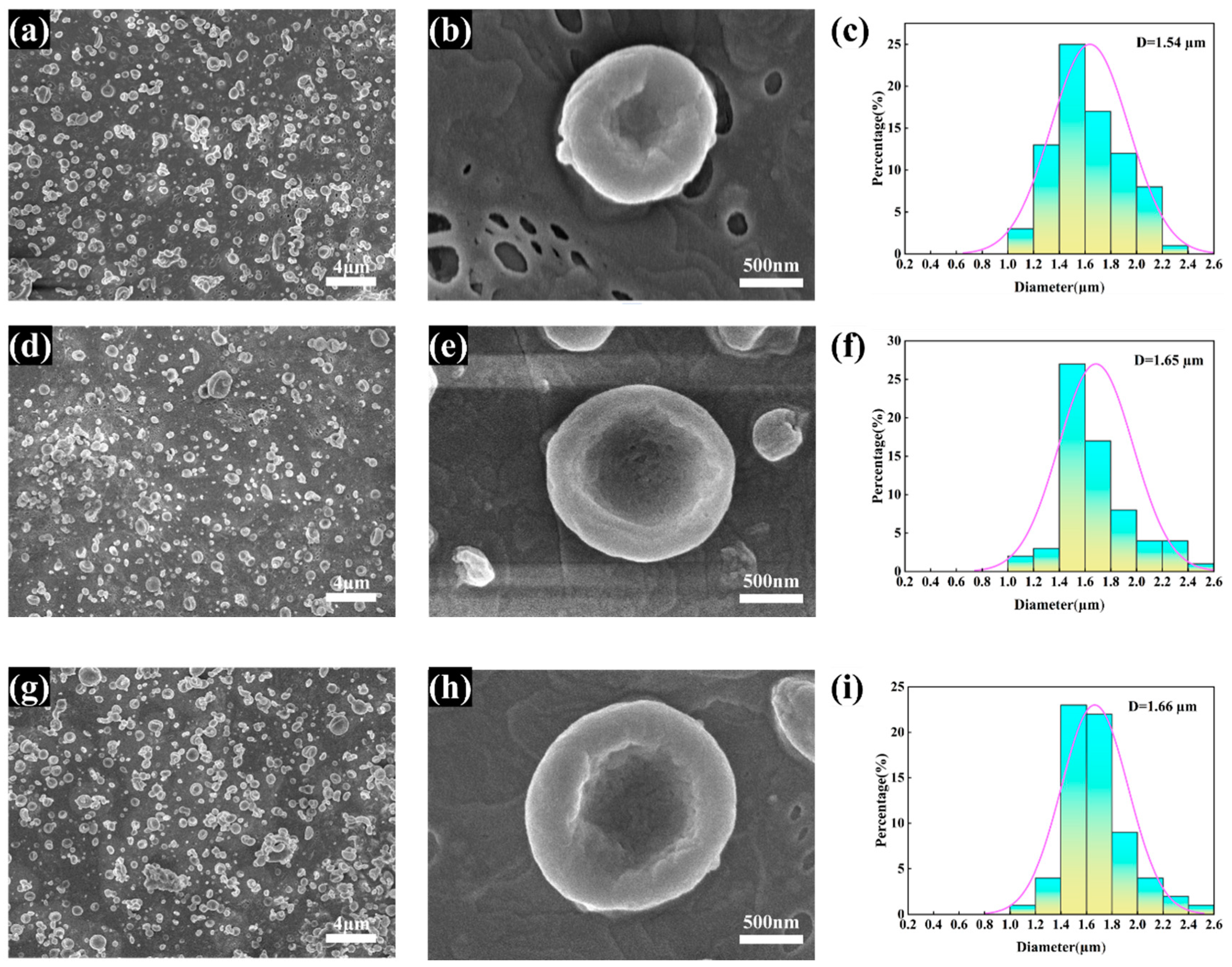
3.2. Morphology of the rH/CS/PCL MPs
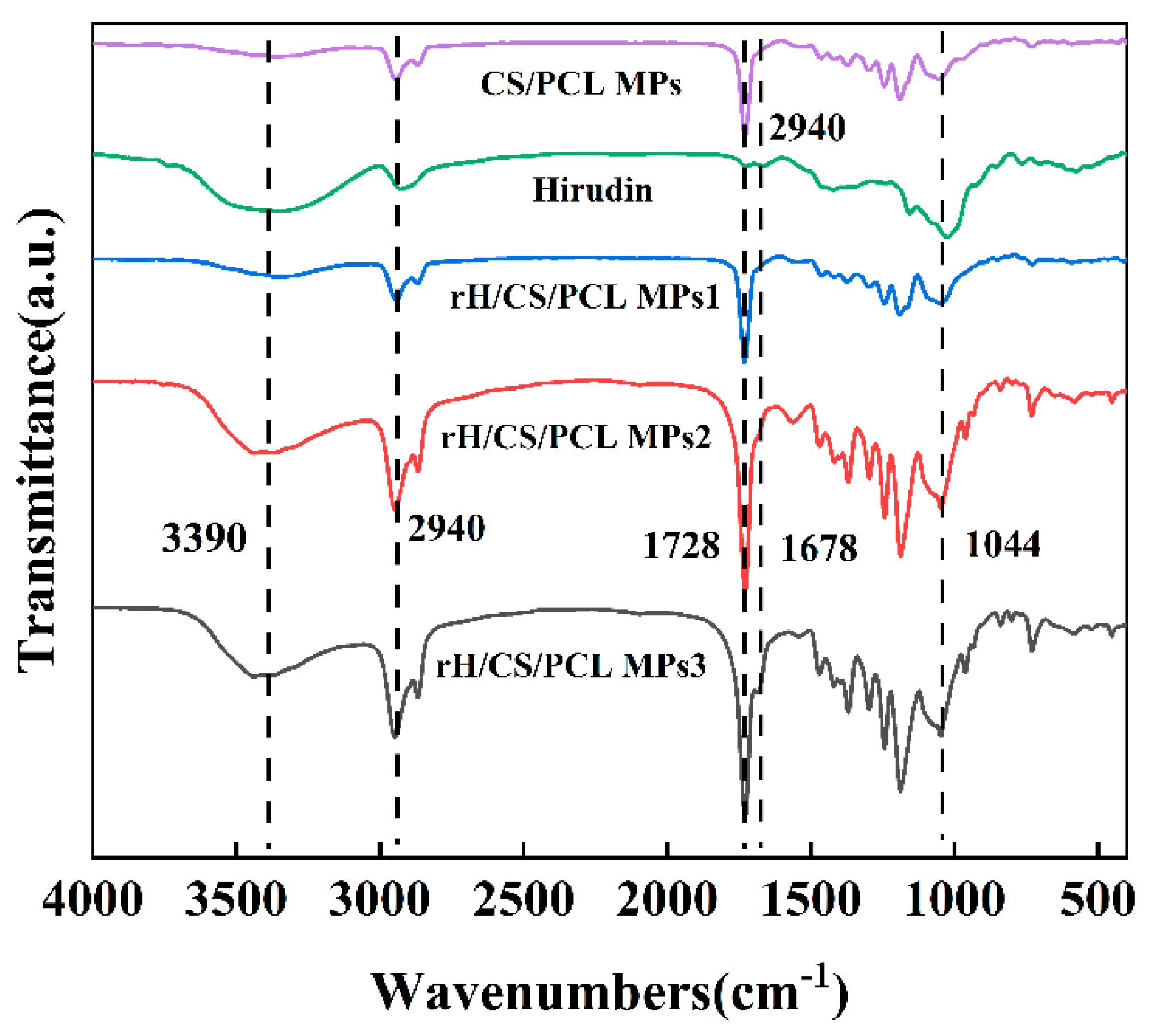
3.3. Infrared Spectroscopy Analysis
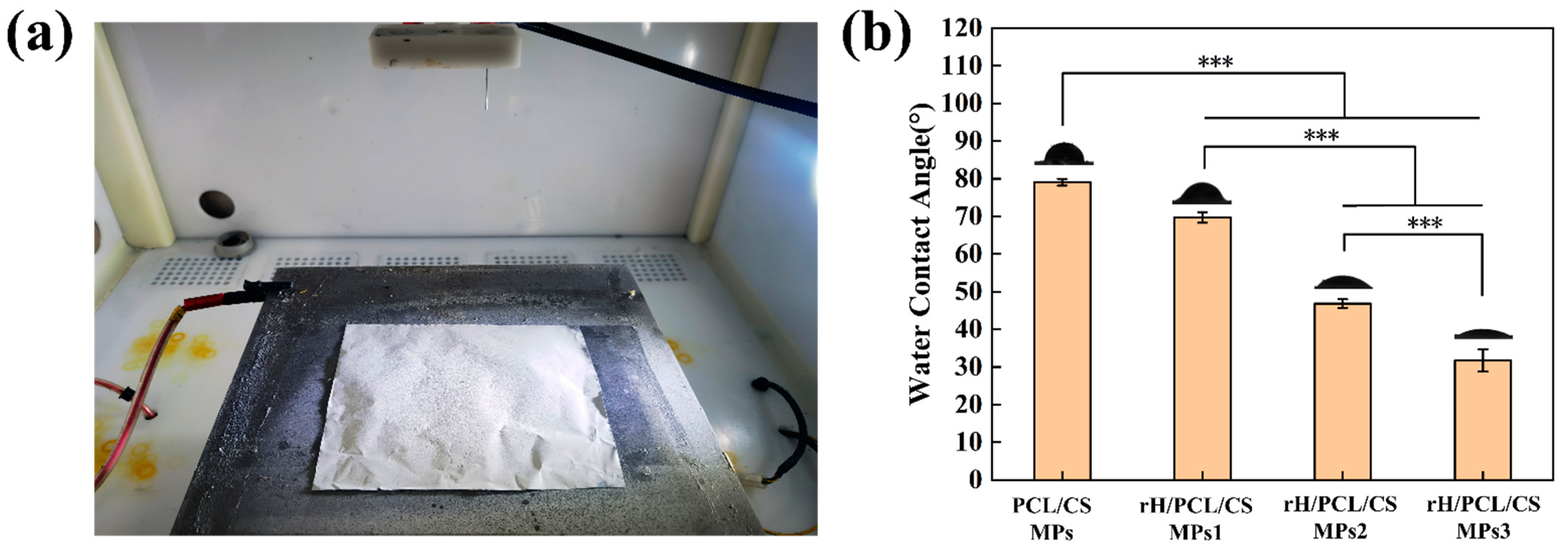
3.4. Water Absorption
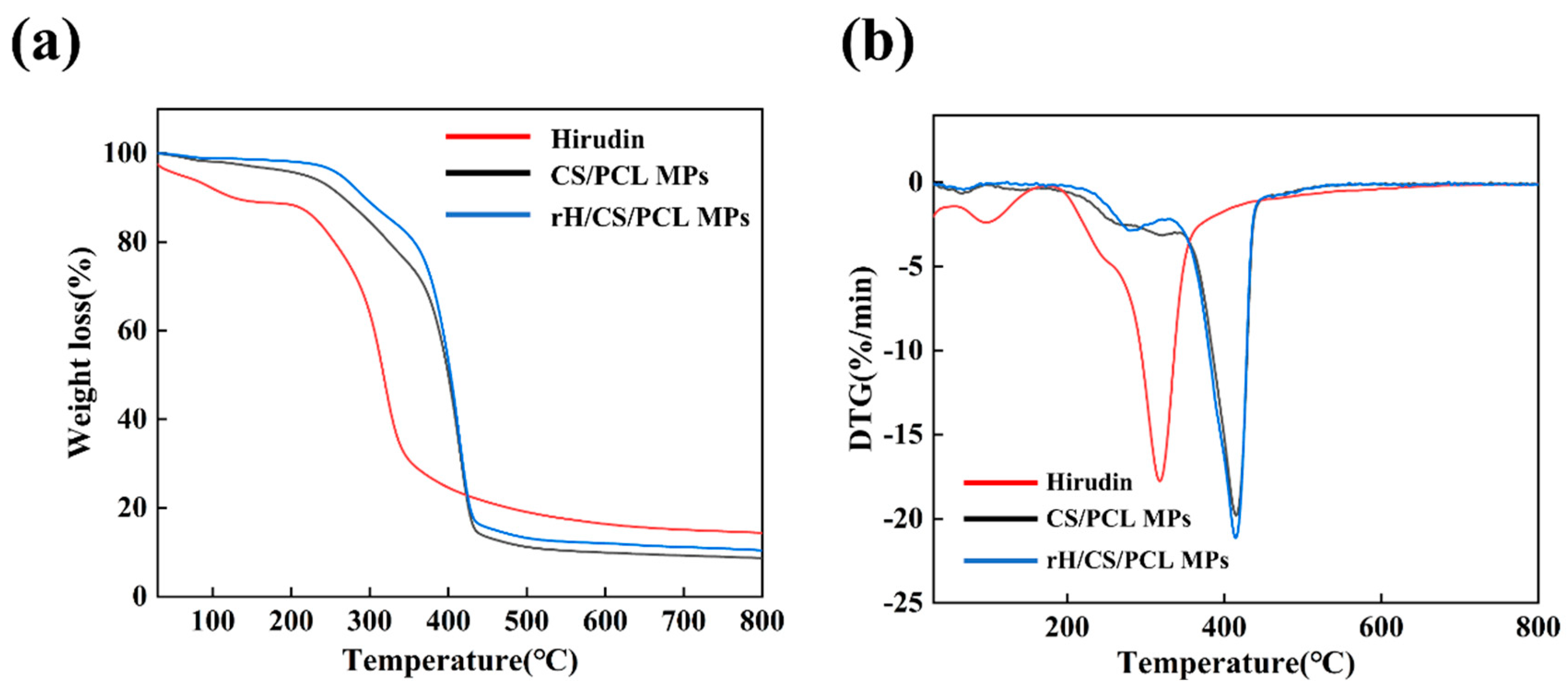
3.5. Thermal Analysis
3.6. Hirudin Release In Vitro
3.7. Anticoagulant Activity of rH/CS/PCL MPs
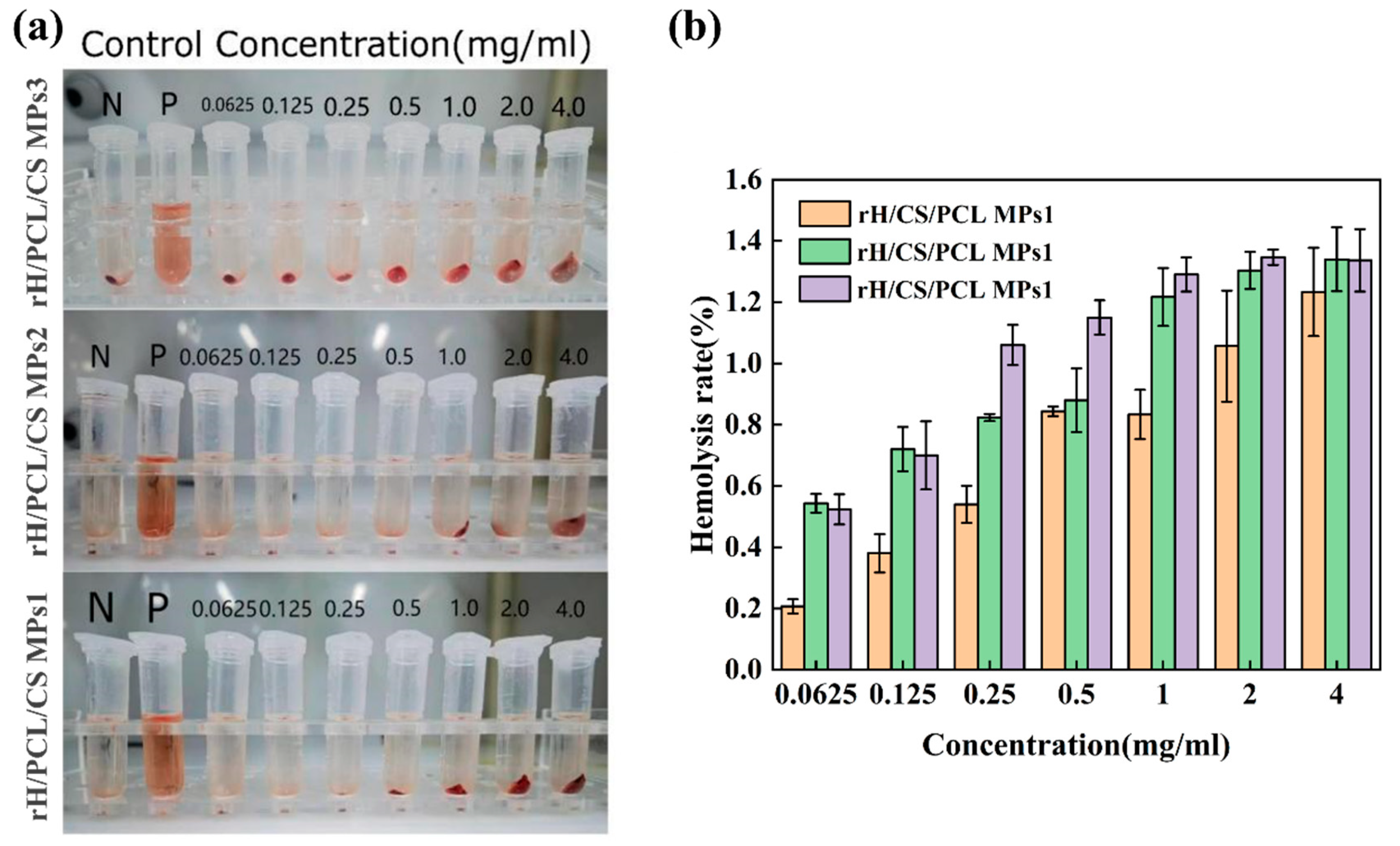
3.8. Evaluation of Blood Compatibility
3.9. Cell Viability Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Koelwyn, G.J.; Corr, E.M.; Erbay, E.; Moore, K.J. Regulation of macrophage immunometabolism in atherosclerosis. Nat. Immunol. 2018, 19, 526–537. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; Winther, M.D.; Weber, C.; Daemen, M.J.; Lutgens, E.; Soehnlein, O. Atherosclerotic plaque destabilization: Mechanisms, models, and therapeutic strategies. Circ. Res. 2014, 114, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Xia, N.; Li, H. Roles of vascular oxidative stress and nitricoxide in the pathogenesis of atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Dormont, F.; Varna, M.; Couvreur, P. Nanoplumbers: Biomaterials to fight cardiovascular diseases. Mater. Today 2018, 21, 122–143. [Google Scholar] [CrossRef]
- Joyce, J.A. Therapeutic Targeting of the Tumor Microenvironment. Cancer Cell 2005, 7, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Xu, L.; Zhao, T.; Gao, J.; Song, S.; Ji, A. The research progress of hirudo on the related cells in the progression of atheroscle-rosis. Chin. J. Arterioscler. 2000, 407, 233–241. [Google Scholar]
- Xu, X.; Huang, X.C.; Zhang, Y.; Shen, S.Y.; Feng, Z.Z.; Dong, H.; Zhang, C.; Mo, R. Self-Regulated Hirudin Delivery for Anticoagulant Therapy. Sci. Adv. 2020, 6, 0382. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.L.; Ying, M.; Dehaini, D.; Su, Y.Y.; Kroll, A.V.; Zhou, J.; Gao, W.W.; Fang, R.H.; Chien, S.; Zhang, L.F. Nanoparticle Functionalization with Platelet Membrane Enables Multifactored Biological Targeting and Detection of Atherosclerosis. ACS Nano 2018, 12, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Ren, L. Anticoagulant r-hirudin coating on PMMA intraocular len surface and its nonfouling properties. Sci. Sin. Tech. 2013, 12, 603–609. [Google Scholar]
- Huang, R.H.; Shu, Q. The paper does not mention any validation of the model with real-world data. J. Nat. Sci. Hunan Norm. Univ. 1998, 3, 57–60. (In Chinese) [Google Scholar]
- Sun, Y.; Wang, Y.W. Research Progress in the Analysis of Anticoagulant Active Substances in Leeches and the Extraction and Purification of Hirudin. Acta Neuropharmacol. 2021, 10, 30–33. [Google Scholar]
- Kamaly, N.; Fredman, G.; Fojas, J.J.R.; Subramanian, M. Targeted interleukin-10 nanotherapeutics developed with a microfluidic chip enhance the resolution of inflammation in advanced atherosclerosis. ACS Nano 2016, 10, 5280–5292. [Google Scholar] [CrossRef]
- Maximov, V.D.; Reukov, V.V.; Barry, J.N.; Cochrane, C.; Vertegel, A.A. Protein-nanoparticle conjugates as potential therapeutic agents for the treatment of hyperlipidemia. Nanotechnology 2010, 21, 265103. [Google Scholar] [CrossRef] [PubMed]
- Elise, F.V.; Marie-Christine, F.; Corinne, C.; Guerin, M. Endogenous CETP activity as a predictor of cardiovascular risk: Determination of the optimal range. Atherosclerosis 2013, 227, 165–171. [Google Scholar]
- Du, M.; Yao, W.; Zong, L. Study of anti-atherosclerotic nucleic acid vaccine nanofreeze-dried agents. Pharm. Clin. Res. 2011, 19, 107–111. [Google Scholar]
- Lewis, D.R.; Petersen, L.K.; York, A.W.; Zablocki, K.R.; Joseph, L.B.; Kholodovych, V.; Prud’homme, R.K.; Uhrich, K.E.; Moghe, P.V. Sugar-based amphiphilic nanoparticles arrest atherosclerosis in vivo. Proc. Natl. Acad. Sci. USA 2015, 112, 2693–2698. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yu, C.; Liu, Y.; Zhang, W.; Sun, Y.; Chen, Y. Enhanced antiatherosclerotic efficacy of statin-loaded reconstituted high-density lipoprotein via ganglioside GM1 modification. ACS Biomater. Sci. Eng. 2018, 4, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Sun, L.; Bian, F.; Wang, Y.; Yu, Y.; Gu, Z.; Zhao, Y. Erythrocyte-Inspired Functional Materials for Biomedical Applications. Adv. Sci. 2023, 10, 2206150. [Google Scholar] [CrossRef] [PubMed]
- Doshi, N.; Zahr, A.S.; Bhaskar, S.; Lahann, J.; Mitragotri, S. Red blood cell-mimicking synthetic biomaterial particles. Proc. Natl. Acad. Sci. USA 2009, 106, 21495–21499. [Google Scholar] [CrossRef] [PubMed]
- Harvestine, J.N.; Mikulski, B.A.; Mahuta, K.M.; Crouse, J.Z.; Guo, X.; Lee, J.C.; Midelfort, K.S.; Chen, J.; Zhang, W. A novel red-blood-cell-shaped pectin-oligochitosan hydrogel system. Part. Part. Syst. Charact. 2014, 31, 955–959. [Google Scholar] [CrossRef]
- Kozlovskaya, V.; Alexander, J.F.; Wang, Y.; Kuncewicz, T.; Liu, X.; Godin, B.; Kharlampieva, E. Internalization of red blood cell-mimicking hydrogel capsules with pH-triggered shape responses. ACS Nano 2014, 8, 5725–5737. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ono, K.; Suzuki, H.; Sawada, M.; Moriya, M.; Sakamoto, W.; Yogo, T. Electrosprayed synthesis of red-blood-cell-like particles with dual modality for magnetic resonance and fluorescence imaging. Small 2010, 6, 2384–2391. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Chung, N.; Lee, J. Monodisperse red blood cell-like particles via consolidation of charged droplets. J. Colloid Interface Sci. 2011, 361, 423–428. [Google Scholar] [CrossRef]
- Milenkova, S.; Zahariev, N.; Ambrus, R.; Pilicheva, B.; Marudova, M. A Study on the Stoichiometry of Casein/Chitosan Gel Complexes as a Delivery System for Quercetin. Appl. Sci. 2023, 13, 10868. [Google Scholar] [CrossRef]
- Lagopati, N.; Pippa, N.; Gatou, M.-A.; Papadopoulou-Fermeli, N.; Gorgoulis, V.G.; Gazouli, M.; Pavlatou, E.A. Marine-Originated Materials and Their Potential Use in Biomedicine. Appl. Sci. 2023, 13, 9172. [Google Scholar] [CrossRef]
- Sagawa, T.; Morizumi, H.; Iijima, K.; Yataka, Y.; Hashizume, M. Fabrication of Polysaccharide-Based Coaxial Fibers Using Wet Spinning Processes and Their Protein Loading Properties. Appl. Sci. 2023, 13, 8053. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, S.; Park, K.; Kim, B.-K. Non-Toxic Natural Additives to Improve the Electrical Conductivity and Viscosity of Polycaprolactone for Melt Electrospinning. Appl. Sci. 2023, 13, 1844. [Google Scholar] [CrossRef]
- Andrýsková, N.; Sourivong, P.; Babincová, M.; Šimaljaková, M. Controlled Release of Tazarotene from Magnetically Responsive Nanofiber Patch: Towards More Efficient Topical Therapy of Psoriasis. Appl. Sci. 2021, 11, 11022. [Google Scholar] [CrossRef]
- Rai, K.; Chu, X. Enhanced anticoagulant activity of hirudin-i analogue co-expressed with arylsulfotransferase in periplasm of E. coli BL21(DE3). J. Biotechnol. 2020, 11, 107–112. [Google Scholar] [CrossRef]
- Valuev, L.I.; Pan, V.A. Polymeric systems with antithrombin activity for thermally activated targeting. Appl. Biochem. Microbiol. 2003, 5, 317–320. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Yue, P.F.; Lu, T.Q. Role of lysosomes in physiological activities, diseases, and therapy. J. Hematol. Oncol. 2021, 5, 79. [Google Scholar] [CrossRef]
- Richard, I.; Thibault, M.; De Crescenzo, G.; Buschmann, M.D.; Lavertu, M. Ionization Behavior of Chitosan and Chitosan−DNA Polyplexes Indicate That Chitosan Has a Similar Capability to Induce a Proton-Sponge Effect as PEI. Biomacromolecules 2023, 4, 1732–1740. [Google Scholar] [CrossRef]
- Wang, F.; Li, X.R. Nanoscaled polyion complex micelles for targeted delivery of recombinant hirudin to platelets based on cationic copolymer. Mol. Pharm. 2010, 6, 718–726. [Google Scholar] [CrossRef]
- Xu, J.W.; Li, K. Studies on preparation and formation mechanism of poly(lactide-co-glycolide) microrods via one-step electrospray and an application for drug delivery system. Eur. Polym. J. 2021, 3, 148. [Google Scholar] [CrossRef]
- Xie, J.; Lim, K.L. Electrohydrodynamic atomization for biodegradable polymeric particle prod-uction. J. Colloid Interface Sci. 2006, 6, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.W.; Mao, L.X.; Cai, Q. Preparation of amino acid ester substituted polyphosphazene microparticles via electrohydrodynamic atomization. Polym. Adv. Technol. 2011, 12, 2009–2016. [Google Scholar] [CrossRef]
- Lima, T.d.P.d.L.; Canelas, C.A.d.A.; Dutra, J.d.C.F.; Rodrigues, A.P.D.; Brígida, R.T.S.S.; Conc-ha, V.O.C. Poly (ε-caprolactone)-Based Scaffolds with Multizonal Architecture: Synthesis, Cha-racterization, and In Vitro Tests. Polymers 2023, 15, 4403. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Huang, S.-W.; Kao, I.-F.; Yen, S.-K. The Preparation and Characterization of Chit-osan/Calcium Phosphate Composite Microspheres for Biomedical Applications. Polymers 2024, 16, 167. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.W.; Dai, X. PCL-based and Hirudin-containing Composite Nanofibers for Prolonged Anticoagulation Effect. Chem. Res. Chin. Univ. 2023, 7, 1023–1030. [Google Scholar] [CrossRef]
- Vogel, C.; Siesler, H.W. Thermal degradation of poly(ε-caprolactone), poly(L-lactic acid) and their blends with poly(3-hydroxy-butyrate) studied by TGA/FT-IR spectroscopy. Macromol. Symp. 2008, 265, 183–194. [Google Scholar] [CrossRef]
- Mahdi, A.; Azadeh, A. Fabrication of carboxymethyl chitosan/poly(ε-caprolactone)/doxorubicin/nickel ferrite core-shell fibers for controlled release of doxorubicin against breast cancer. Carbohydr. Polym. 2021, 1, 117631. [Google Scholar]
- Sogias, I.A.; Khutoryanskiy, V.V.; Williams, A.C. Exploring the Factors Affecting the Solubility of Chitosan in Water. Macromol. Chem. Phys. 2010, 2, 426–433. [Google Scholar] [CrossRef]
- Cong, L.L.; Xian, Z.; Jing, L. Na+-H+ exchanger 1 determines atherosclerotic lesion acidification and promotes atherogenesis. Nat. Commun. 2019, 10, 3978. [Google Scholar]
- Nikolac, N.; Šupak-Smolčić, V.; Šimundić, A.M.; Ćelap, I. Croatian Society of Medical Biochemistry and Laboratory Medicine: National recommendations for blood collection, processing, performance and rep-orting of results for coagulation screening assays prothrombin time, activated partial throm-boplastin time, thrombin time, fibrinogen and D-dimer. Biochem. Med. 2019, 29, 262–283. [Google Scholar]
- Peng, Z.H.; Yang, Y.; Luo, J.Y. Nanofibrous polymeric beads from aramid fibers for efficient bilirubin removal. Biomater. Sci. 2016, 4, 1392–1401. [Google Scholar] [CrossRef]
- Li, P.; Qi, B.; Li, K. Study on the formation and properties of red blood cell-like Fe3O4/TbLa3(Bim)12/PLGA composite particles. RSC Adv. 2018, 1, 12503–12516. [Google Scholar] [CrossRef]





| Level | Factor | ||
|---|---|---|---|
| PCL-CS Concentrations (w/v) | Ratio of PCL to CS | Loading Voltage (kV) | |
| 1 | 3% | 3:1 | 18 kV |
| 2 | 4% | 3:1 | 18 kV |
| 3 | 5% | 3:1 | 18 kV |
| 4 | 4% | 7:1 | 18 kV |
| 5 | 4% | 1:1 | 18 kV |
| Level | Factor | |||
|---|---|---|---|---|
| PCL/CS Concentrations (w/v) | Ratio of PCL to CS | Loading Voltage (kV) | Hirudin Concentration (w/v) | |
| rH/CS/PCL MPs1 | 4% | 3:1 | 18 kV | 0.2% |
| rH/CS/PCL MPs2 | 4% | 3:1 | 18 kV | 0.4% |
| rH/CS/PCL MPs3 | 4% | 3:1 | 18 kV | 0.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, X.; Zhao, W.; Tian, P.; Tulugan, K. Preparation of Hirudin-Loaded Chitosan/Polycaprolactone Bowl-Shaped Particles and an Application for a Drug Delivery System. Appl. Sci. 2024, 14, 1939. https://doi.org/10.3390/app14051939
Li X, Zhang X, Zhao W, Tian P, Tulugan K. Preparation of Hirudin-Loaded Chitosan/Polycaprolactone Bowl-Shaped Particles and an Application for a Drug Delivery System. Applied Sciences. 2024; 14(5):1939. https://doi.org/10.3390/app14051939
Chicago/Turabian StyleLi, Xiang, Xin Zhang, Wei Zhao, Peng Tian, and Kelimu Tulugan. 2024. "Preparation of Hirudin-Loaded Chitosan/Polycaprolactone Bowl-Shaped Particles and an Application for a Drug Delivery System" Applied Sciences 14, no. 5: 1939. https://doi.org/10.3390/app14051939
APA StyleLi, X., Zhang, X., Zhao, W., Tian, P., & Tulugan, K. (2024). Preparation of Hirudin-Loaded Chitosan/Polycaprolactone Bowl-Shaped Particles and an Application for a Drug Delivery System. Applied Sciences, 14(5), 1939. https://doi.org/10.3390/app14051939






