Abstract
Atherosclerosis, a disease that mainly affects human blood vessels, can cause various cerebral ischaemic diseases such as coronary heart disease and peripheral arterial disease. However, conventional drugs for the treatment of atherosclerosis have the disadvantages of low bioavailability and high toxicity. Bowl-shaped particles not only have the excellent properties of traditional spherical particles, such as improved drug distribution, increased drug absorption, reduced drug toxicity and side effects, but also are easier to circulate in the blood for a long time, have reduced immune rejection and have a larger specific surface area. Chitosan/polycaprolactone bowl-shaped particles were prepared via electrostatic spraying, and the effects of precursor solution concentration and polymer ratio on particle morphology were investigated. Chitosan/polycaprolactone composite bowl-shaped particles containing hirudin were prepared under optimal parameters for sustained anticoagulation. The anticoagulant molecules of hirudin could be continuously released from the composite scaffold as the bowl particles degraded. The biocompatibility and haemocompatibility of the composite particles were assessed using mouse glial cells and rabbit blood, and the results showed that the cell viability of the drug-loaded particles was overall above 90% and the haemolysis rate was below 2%. By controlling the release rate of hirudin, bowl-shaped particles can achieve a long-term anticoagulant drug delivery system and have wider application potential as a novel blood contact material.
1. Introduction
Atherosclerosis (AS) is a common cardiovascular disease that seriously threatens human health [1]. The deposition of oxidised lipoproteins in the arterial wall promotes macrophage aggregation [2] and foam cell formation [3], a process that leads to the accumulation of reactive oxygen species (ROS) [4]. ROS also exacerbate inflammation by inducing oxidative damage, DNA denaturation, and apoptosis [5]. This inflammation promotes the formation of vascular defects that can lead to bleeding, plaque, and thrombosis. When atherosclerotic plaques are present, these areas become slightly acidic [6], leading to macrophage apoptosis and aggregation, resulting in thrombus formation. Cardiovascular diseases caused by atherosclerosis are extremely dangerous to human health, and their incidence is increasing every year, making them the leading cause of death in China and even the world. Atherosclerosis is responsible for more than 75% of the disability and death from cardiovascular diseases in China, indicating that atherosclerosis has become one of the most serious factors endangering the health of Chinese citizens [7]. Therefore, early treatment of atherosclerosis is of great clinical importance.
Hirudin (rH), a peptide extracted from the peripheral glands of leeches, is a safe natural anticoagulant with beneficial effects in the treatment of atherosclerosis due to its pharmacological effects, such as anticoagulation, antithrombotic, anti-inflammatory, endothelial cell protection and antifibrotic effects, without causing thrombocytopenia [8]. A promising class of anticoagulants, hirudins can be used to treat various thrombotic diseases [9], especially venous thrombosis and diffuse vascular coagulation. In addition, unlike most peptides, hirudin is highly permeable and stable, is not destroyed by trypsin and chymotrypsin, and does not decompose with slight changes in pH or temperature, so it can be taken orally, and the decomposed fragments also have anticoagulant effects; however, some special conditions, such as the simultaneous presence of strong alkalinity and high temperature, can lead to the irreversible inactivation of hirudin [10]. Traditional extraction methods for hirudin include water extraction and organic solvent extraction. The water extraction method involves crude extraction with 0.9% saline at four times the volume of the original hirudin solution, followed by the removal of large amounts of heteroproteins with 10–15% trichloroacetic acid [11]. The organic solvent extraction method is to use alcohol, acetone, etc., as the extraction solvent [12]. In recent years, leeches and Chinese patent medicines or compounded preparations containing leeches have been widely used in the clinical treatment of cardiovascular and cerebrovascular diseases.
Nanotechnology can improve the bioavailability of drugs. By precisely controlling the size, drug loading, and composition of nanomaterial microparticles, it can provide a brand new idea for therapeutic development in AS. Artificially prepared drug-loaded nanoparticles have the advantages of drug solubilization, long half-life, fewer non-specific reactions, an active targeting effect, and multifunctionality as a whole, which suggest great potential in the direction of AS therapy [13,14,15,16,17,18]. However, engineered nanoparticles suffer from immune clearance and difficulty in delivering the drug to the lesion. Erythrocytes are the most abundant cells in the blood. As a result of long-term natural selection, their unique biconcave disc-like morphology and cellular composition have enabled them to acquire excellent biological properties. Researchers have produced a variety of nanoparticles that mimic erythrocytes, which have been widely used in biomedical fields such as drug delivery, bioimaging, and tissue engineering [19].
Bowl-shaped particles are a common type of erythrocyte-like particle with a morphology of unidirectional concave structures with distinct directionality. The radial depression creates unique cavities in the particles, enhancing deformability and providing specific space for drug loading. In addition, the sagging surface of the bowl-shaped particles can adhere tightly to the surface of an object, similar to a suction cup, after the air in the cavity has been squeezed out by an external force. Although they are promising, the number of bowl-shaped particles developed is small and the preparation methods are generally limited. The preparation methods currently available fall into two broad categories. One is based on templates combined with hierarchical self-assembly, but this method is very complex [20,21,22]. The other is the electrostatic spraying method combined with solvent evaporation or solvent diffusion. However, the second method also suffers from poor control of the morphology of the formed microparticles and inhomogeneous particle size [23,24].
Chitosan (CS) is a cationic, weakly basic polysaccharide of natural polysaccharides containing free amino groups that can be protonated by H+ to form polycationic polymers, which are naturally broad-spectrum antimicrobial, safe and non-toxic, biocompatible and degradable, and can be used as a drug carrier material to control drug release, prolong drug efficacy and reduce toxicity and side effects [25,26,27]. Polycaprolactone (PCL) is a polymer with good biodegradability, biocompatibility, and physico-mechanical properties, which is widely used in biomedical materials, drug-controlled release systems, and tissue engineering scaffolds [28,29]. Recently, we successfully prepared CS/PCL bowl-shaped particles (CS/PCL MPs) using the electrostatic spraying method, which had good morphological stability, drug-loading properties, and acid-triggered solubilisation properties. These microparticles can be used as drug carriers for drug loading, while their acid-triggered solubilisation property can enable the drug to achieve a local release effect at the lesion site and in the specific human microenvironment, which is promising for AS therapy and even tumour therapy.
The aim of this study was to prepare chitosan/polycaprolactone bowl-shaped microparticles with stable morphology and to realise their drug-carrying and drug-releasing functions for the natural anticoagulant drug hirudin (rH). The morphology of the microparticles was controlled by controlling the concentration of the precursor solution and the solute ratio in the electrostatic spraying process to obtain microparticles with uniform and stable size and morphology. The method of preparing CS/PCL bowl-shaped microparticles proposed in this paper is simple and effective, which can meet the requirements of drug-carrying and drug-releasing functions, and provides an efficient, low-toxicity, simple, and economical idea for the treatment of AS.
2. Materials and Methods
2.1. Materials
Polycaprolactone (PCL, MW = 2000, dried and used, Macklin, Shanghai, China), chitosan (CS, MW = 30,000 Da, Macklin, Shanghai, China), and hirudin (Macklin, Shanghai, China) were utilized. The solvents used were tetrahydrofuran (THF, analytically pure, Macklin, Shanghai, China), glacial acetic acid (HAC, analytically pure, Macklin, Shanghai, China), and deionised water (from BK-DI30 pure water preparation system, Shanghai Baibokang Instrument Co., Shanghai, China). PBS buffer (pH = 7.2 and pH = 5.8, Aladdin, Shanghai, China) was utilized. Activated partial thromboplastin time (APTT) reagent (Leagene, Beijing, China), Calcein/PI Cell Viability/Cytotoxicity Assay Kit (Beyotime, Shanghai, China) and cell counting kit-8 (CCK-8, Biosharp, Hefei, China) were utilized.
2.2. Preparation and Optimization of the CS/PCL Polymeric Solutions
The precursor solution was optimised by varying the solution concentration and solute ratio to obtain the best parameters for the preparation of bowl-shaped particles. Several bowl-shaped particles were prepared using 3%, 4%, and 5% (w/v) PCL/CS (3:1) in aqueous acetic acid/tetrahydrofuran (3:2). First, CS powder was slowly added to 10% v/v aqueous acetic acid solution and stirred at 50 °C for at least 2 h (until a clear solution was formed). Then, PCL particles were slowly added to THF and stirred at 35 °C for at least 30 min (until a clear liquid was formed). Finally, the CS and PCL solutions were mixed 3:2 and stirred well. Table 1 shows the detailed process parameters for the CS/PCL precursor solution.

Table 1.
Single-factor experiment design for CS/PCL bowl-shaped microparticles.
2.3. Preparation of CS/PCL Bowl-Shaped Microparticles (CS/PCL MPs)
CS/PCL microparticles were prepared using a uniaxial electrospray device (TL-BM300, Tongli, China) by applying the electrostatic spray method. The electrospray device consisted of a syringe pump, a plastic syringe, a 21-gauge stainless steel needle used as a capillary nozzle, and a high-voltage power supply. The configured solution was loaded into the syringe and the flow rate of the needle was precisely controlled by the syringe pump. The needle was scanned and sprayed above the electrospray chamber, which was connected to the positive pole of the high-voltage power supply, and the receiving plate, covered with aluminium foil, was connected to the negative pole of the high-voltage power supply to collect the electrostatic sprayed microparticles.
The electrostatic spraying experiment was carried out in an electrostatic spraying room. The ambient temperature was maintained at room temperature (25 °C) and the relative humidity was 15–20%. The applied voltage and high voltage were between 18 kV, the jet distance was 18 cm, and the micro-pump supplied the solution at a rate of 0.6 mL/h. The temperature of the solution in its spray configuration was the same as that of the spraying environment.
2.4. Preparation of rH/CS/PCL Bowl-Shaped Microparticles (rH/CS/PCL MPs)
To prepare hirudin-containing bead-shaped particles, hirudin and chitosan were first added to acetic acid solution and stirred at 50 °C for 2 h, and then the rH/CS solution was mixed with PCL solution and stirred well. Finally, the precursor solution was prepared into dish-shaped particles through electrostatic spraying. The process parameters were the optimal ones obtained in Section 2.3. In this way, composite particles of rH/CS/PCL MPs1, rH/CS/PCL MPs2, and rH/CS/PCL MPs3 were obtained. Hirudin has excellent stability, which means that acetic acid solution and tetrahydrofuran will not inactivate hirudin by destroying its structure [30,31]. The specific parameters are listed in Table 2.

Table 2.
Single-factor experiment design for rH/CS/PCL bowl-shaped microparticles.
2.5. Characterization
2.5.1. Morphology
The morphology of the dried CS/PCL microparticles was observed via scanning electron microscopy (SEM, JSM-7500F, JEOL, Tokyo, Japan) with an accelerated voltage of 5 kV. All samples used for SEM measurements were dried at room temperature. To increase the conductivity of the sample and improve the imaging clarity, gold was sprayed three times in a vacuum for 60 s each time. The average particle diameter was determined by measuring the diameters of at least 100 particles using ImageJ Launcher v 1.41 software.
2.5.2. Infrared Spectroscopy Analysis
FTIR spectra were recorded over the range of 4000–400 cm−1 with an FTIR spectrometer (FTIR, FITR-1500, JOSVOK, Tianjin, China), and the composition present in the microparticles was inferred from the obtained spectral pending frequency positions, intensity, and shape. All samples were grounded together with KBr and pressed into thin sheets for testing.
2.5.3. Water Absorption
The static water contact angle of the microparticles was measured using a DCAT21 contact angle goniometer (dataphysics, Filderstadt, German). The water droplet volume was 5 μL. Particle hydrophilicity was measured by placing 5 μL water droplets on 2 × 2 cm2 layers of CS/PCL MPs and rH/CS/PCL MPs. Each sample was prepared by electrostatic spraying for 10 h.
2.5.4. Thermogravimetric Analysis (TG)
Hirudin, CS/PCL MPs, and rH/CS/PCL MPs3 were thermogravimetrically analysed using a thermogravimetric analyser with a temperature range from 30 °C to 800 °C and a heating rate of 15 °C/min. Tests were performed under nitrogen at a flow rate of 50 mL/min. The mass of each test sample was approximately 5–10 mg.
2.5.5. Hirudin Release In Vitro
The OD value of hirudin was measured using a UV-visible spectrophotometer, and then the OD concentration standard curve was plotted according to the OD values of hirudin solutions at different concentrations, and the following regression equation was obtained: Y = 0.0014X − 0.00163, R2 = 0.99962, where “Y” is the absorbance and “X” is the concentration of hirudin. Three groups of rH/CS/PCL MPs were placed in centrifuge tubes, and then 2 mL of PBS buffer at different pH (pH = 5.8, pH = 7.2) was added to each group to assess the rate of drug release from rH/CS/PCL MPs in different environments. Studies have shown that lysosomes in macrophages play a role in the initiation and progression stages of atherosclerotic plaque [32]. The pH of lysosomes/endosomes ranges from 4.5 to 7.0 and pH = 5.8 is close to the middle of the pH range; therefore, PBS buffer at pH = 5.8 was chosen to test the rate of hirudin release from rH/CS/PCL MPs in an acidic environment [33]. The normal intracellular pH of tissues is between 7.0 and 7.2, which is why PBS with pH = 7.2 was chosen. rH/PBS OD values were measured to assess the rate of release of rH from the rH/CS/PCL MPs after incubation at 37 °C with continuous shaking for 2, 4, 6, 8, 12, 24, 48, 96, 192 and 384 h.
2.5.6. Anticoagulant Activity Assay
The anticoagulant activity of rH/CS/PCL MPs was assessed by measuring the activated partial thromboplastin time (APTT) [34]. Fresh mouse blood was used as a blank blood sample for clotting time assessment and was placed in a test tube containing 3.8% (w/v) sodium citrate solution (1:9 sodium citrate solution and blood, v/v). 300 µL of blood was added and 30 µL of 50 μg/mL rH/CS/PCL MPs solution was added. The mixture was then incubated at 37 °C for 20 min and centrifuged at 2000 rpm for 10 min to collect the supernatant. The APTT of each sample was determined using commercially available APTT kits according to the kit instructions: equilibrate the APTT ellagic acid solution to room temperature, invert and mix, and take 0.1 mL each of the plasma to be tested (including normal control plasma) and the APTT ellagic acid solution and mix well. Then, place in a 37 °C water bath for 5 min, gently mixing several times. After this, 0.1 mL of CaCl2 (25 mM) warmed to 37 °C was added, immediately timed, placed under constant shaking, and removed after approximately 30 s to observe the time of appearance of fibrin filaments, and this was repeated twice to obtain the mean.
2.5.7. Evaluation of Blood Compatibility
A haemolytic activity assay was performed with 4% red blood cell suspension, which was purchased from Henan Yuechi Biotechnology Co., Ltd., Zhengzhou, China. First, rH/CS/PCL MPs were dispersed in PBS solution with concentrations of 0.0625, 0.125, 0.25, 0.5, 1, 2, and 4 mg mL−1. Subsequently, 100 μL 4% red blood cell suspension was added into 1 mL microparticle/PBS solution at each concentration. The positive and negative controls were the distilled water containing 4% red blood cell suspension and PBS, respectively. All samples were incubated at 37 °C for 1 h and then centrifuged at 2000 rpm min−1 for 3 min to obtain supernatants. The absorbance of the supernatants was measured at 545 nm (JK-UVS-752N, SHANGHAI JINGKE, China), and haemolysis percentages of bowl-shaped particles were calculated by using the following Formula (1) [35].
where Ax is the OD545 of the test sample, A− is the OD545 of the negative control group, and A+ is the OD545 of the positive control group.
2.5.8. Cell Viability Assay
BV2 cells were inoculated into 96-well culture plates for proliferation. When the number of cells in each well reached approximately 1 × 104, DMEM (modified Duchenne eagle medium) containing 10% fetal bovine serum was added and transferred to an incubator (5% CO2, 37 °C) for 24 h. CS/PCL MPs and rH/CS/PCL MPs were reconstituted to 20 mg/mL with PBS buffer and the particle solution was then diluted to 200 μg/mL with culture medium for use. At the end of incubation, the old medium in the wells was aspirated and then 100 μL of CS/PCL MPs, rH/CS/PCL MPs1, rH/CS/PCL MPs2, rH/CS/PCL MPs3 at a concentration of 200 μg/mL was added and incubation continued for 48 h. At the end of incubation, the medium containing the pellet was aspirated and the experimental group was washed three times with PBS. Then, 10 μL CCK-8 solution (5 mg mL−1) and 100 μL fresh medium were added and incubation continued for 4 h. Cells without CCK-8 were used as a blank correction. Finally, the cytotoxicity of MPs was determined by measuring the absorbance of the solution at 450 nm using an enzyme marker and comparing the survival of cells in the medium containing MPs with that of the reference group.
The viability of BV2 cells was characterised using Calcein-AM. The digested BV2 cells were diluted (20-fold) and evenly dispersed into confocal dishes, and the culture medium was replenished to 1 mL and incubated in a cell culture incubator for 24 h to make the cells completely adherent to the wall. CS/PCL MPs and rH/CS/PCL MPs were reconstituted to 20 mg/mL with PBS buffer and the particle solution was then diluted to 200 μg/mL with culture medium for use. After aspirating the medium from the dish, 1 mL of cell culture medium, 1 mL of CS/PCL MPs diluted in medium (200 μg/mL), 1 mL of rH/CS/PCL MPs1 diluted in medium (200 μg/mL), or 1 mL of rH/CS/PCL MPs2 diluted in medium (200 μg/mL) was added to the confocal dish. The confocal dish was incubated in a cell culture incubator for 48 h. An amount of 5 μL of Calcein-AM dye was dissolved in 5 mL of buffer solution, and the solution was mixed by repeated shaking. The culture medium containing the drug was aspirated, and 500 μL of diluted dye was added to each dish and incubated in the incubator for half an hour. The culture medium was removed and the cells were gently washed with PBS buffer along the periphery of the dish, avoiding directly blowing on the cells. After removing the PBS from the dish, 1 mL of fresh culture medium was added and used to take fluorescent images of the cell survival status. In the confocal microscope, the excitation wavelength of Calcein-AM (green fluorescence) was set at 488 nm, and the emission wavelength was collected at 500–550 nm.
2.6. Statistical Analysis
The results of statistical analyses are expressed as mean ± standard deviation (SD). Results between groups were analysed for statistical significance via one-way ANOVA at the 95% confidence level using SPSS 19.0 software. Probability values (p) of <0.05 were considered statistically significant, * p < 0.05, ** p < 0.01, *** p < 0.001.
3. Results and Discussion
3.1. Morphology of the CS/PCL MPs
The variation in microscopic morphology in the obtained CS/PCL bowl-shaped particles with solution concentration and solute ratio is shown in Figure 1. When spherical particles begin to dry, because the droplet movement process of the outside of the contact with air flow velocity is faster, the outer layer of the droplet first dries and solidifies, and then forms a solid “shell”, so that the volume of the internal changes is not immediately reflected in the external shape. However, as the interior of the droplet also begins to dry, the solute left behind by the drying of the solution inside is not enough to fill the internal structure, resulting in the formation of a cavity inside the droplet. Eventually, the cavity collapses and the whole sphere collapses into a bowl shape [36,37]. At a solution concentration of 3% (Figure 1a–c), due to the low solute content, low solution viscosity, and small diameter of the atomised droplets, the drying rate inside and outside the droplets is similar, and no cavities are formed, resulting in fewer and smaller-diameter bowl-shaped particles. At a solute concentration of 4% (Figure 1d–f), a large number of regular bowl-shaped particles are produced. This is due to the moderate droplet diameter and the large difference in drying rates between the inside and outside; the particles are highly collapsed and flattened with one side concave. When the solute concentration is increased to 5% (Figure 1g–i), the mass of the solute in the atomised droplets increases with the increase in mass volume fraction, resulting in larger particle diameters and thicker walls.
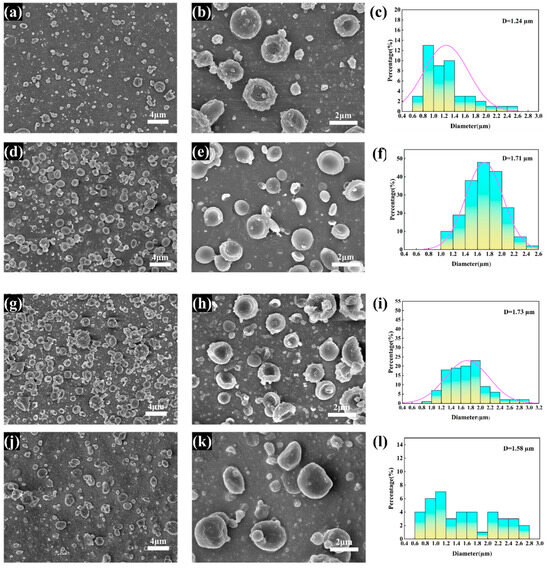
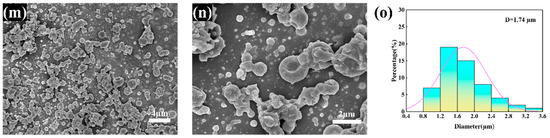
Figure 1.
SEM images and diameter distribution of MPs prepared at different solute concentrations (w/v): (a–c) 3%, (d–f) 4%, (g–i) 5%. SEM images and diameter distribution of MPs prepared with different PCL and CS ratios at the concentration of 4% w/v: (j–l) PCL and CS ratio of 7:1; (m–o) PCL and CS ratio of 1:1.
The mass ratio of PCL to CS in the solution is one of the most important factors in particle generation. When the mass ratio of PCL:CS was 7:1, the number of particles produced by the spraying process was very small, which can be inferred from the images (Figure 1j–l), due to the fact that PCL is easily soluble in the aqueous solution of THF and glacial acetic acid; a large amount of PCL in the large mass ratio of PCL is sprayed directly onto the receiver plate to solidify and does not bind with CS to form particles, and the small amount of particles formed by CS also forms irregular concave cake-like particles in the process. When the ratio of PCL:CS was reduced to 3:1 (Figure 1d–f), the state of the microparticles changed significantly and bowl-shaped MPs with regular morphology were formed, and when the mass ratio was reduced to 1:1 (Figure 1m–o), although a few of the formed microparticles had the morphology of bowl-shaped microparticles, adhesion of particles caused by a large amount of chitosan and the direct formation of particles by chitosan were very obvious.
The elemental distribution of CS/PCL MPs is shown in Figure 2, where the three elements C, N, and O are uniformly distributed; since only CS contains nitrogen in PCL ((C6H10O2)n) and CS ((C6H11NO4)n), it can be demonstrated that chitosan and polycaprolactone are uniformly distributed in the particles.
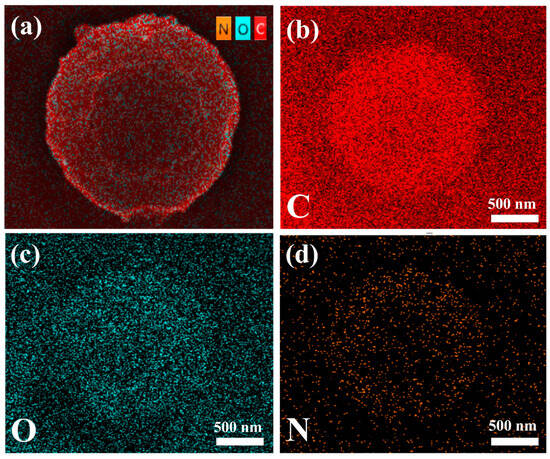
Figure 2.
Elemental mapping of a single CS/PCL MP (4% w/v, PCL:CS = 3:1): (a) the mixture of elements; (b) C element; (c) O element; (d) N element.
3.2. Morphology of the rH/CS/PCL MPs
After obtaining bowl-shaped MPs that could be stably prepared with a regular shape, the morphological stability and drug loading capacity of the microparticles were tested by adding hirudin to the precursor solution. Hirudin amounts of 20, 40, and 60 mg were added to the precursor solution and electrostatically sprayed. As can be seen from the SEM images (Figure 3), the morphology of the bowl-shaped microparticles did not change significantly after the addition of different amounts of hirudin, which proves that the structure of the CS/PCL bowl-shaped MPs investigated in the present experiments is quite stable.
Figure 3.
SEM image and diameter distribution of rH/CS/PCL MPs1 (a–c), rH/CS/PCL MPs2 (d–f), and rH/CS/PCL MPs3 (g–i).
3.3. Infrared Spectroscopy Analysis
The FTIR spectra of hirudin, CS/PCL MPs, and rH/CS/PCL MPs are shown in Figure 4. Among them, PCL is the most abundant and therefore its characteristic spectrum dominates, producing sharp peaks in the band region in the range of 1720–1740 cm−1 due to carbonyl stretching vibration; the C–H stretching vibration peak of saturated hydrocarbons appears near 2940 cm−1 [38]. The broad and absorption peaks at 3390 cm−1 and 1044 cm−1 are attributed to N–H and C–O in chitosan [39]. Comparing the IR spectra of hirudin in Figure 4, the two characteristic peaks of rH/CS/PCL MPs at 1678 cm−1 and 3123–3692 cm−1 were further enhanced due to the fact that hirudin is also rich in –NH and –C=C. This indicates that hirudin was successfully immobilised in CS/PCL MPs and that the amount of hirudin immobilised increased as the concentration of the hirudin solution increased [40].
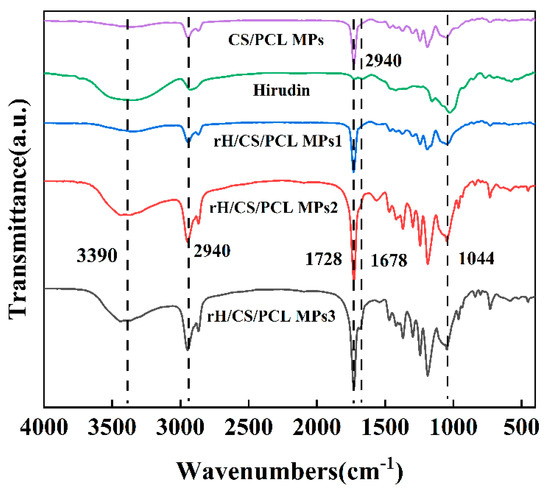
Figure 4.
Infrared spectra of hirudin, CS/PCL MPs, and rH/CS/PCL MPs.
3.4. Water Absorption
After a long deposition time, the particles will build up to form a layer. Figure 5a shows the macroscopic morphology of the rH/CS/PCL MPs3 layer formed after stacking rH/CS/PCL MPs3 for 10 h. The weight in grams per square metre (GSM) of the resulting mats was determined according to Formula (2).
where M1 is the mass of the bowl-shaped particles and their substrate; M2 is the mass of the substrate; S is the area of the layer. The calculated GSMs for the layers of CS/PCL MPs, rH/CS/PCL MPs1, rH/CS/PCL MPs2, and rH/CS/PCL MPs3 are all in the region of 6.9 g/m2. All layers in this experiment were obtained from single-channel work. In the actual production process, bowl-shaped particles can be mass-produced by increasing the number of working channels, and the productivity of bowl-shaped particles will increase several times or even a dozen times with an increase in the number of working channels.
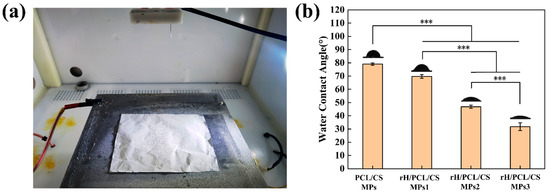
Figure 5.
Macroscopic morphology of the rH/CS/PCL MPs3 layer: (a). Hydrophobic angle test plot of CS/PCL MPs, rH/CS/PCL MPs1, rH/CS/PCL MPs2 and rH/CS/PCL MPs3: (b), n = 3. *** p < 0.001.
The hydrophobic angle testing of the prepared microparticles shows that when unloaded with hirudin, the prepared CS/PCL MPs formed a contact angle of 79°, which is less than ninety degrees (Figure 5b). It can be seen that the surface of the microparticles at this point has an affinity for water. As the concentration of hirudin in the particles increased, the contact angle gradually decreased to 69°, 47°, and 30°. It can be seen that the contact angles formed by the water droplets on the surface of the microparticles were all smaller than the contact angles formed when they were not loaded and showed a gradual decrease. It can be found that the prepared bowl-shaped microparticles have hydrophilic properties and show better hydrophilicity with the loading of drugs.
3.5. Thermal Analysis
Figure 6 shows the TGA and DTG plots of hirudin, CS/PCL MPs, and rH/CS/PCL MPs3, which were used to analyze the thermal stability of the bowl-shaped particulate scaffolds. The TGA curves in Figure 5a show that the thermal degradation behavior of hirudin, CS/PCL MPs, and rH/CS/PCL MPs3 increased from room temperature to 200 °C, and the weight loss at this stage was mainly due to the evaporation of free and bound water in the scaffolds, as well as dehydration of the macromolecular groups; then, the thermogravimetric curves reached a relatively flat state, and it can be seen that there was no significant weight loss in the three groups of samples in this temperature interval from the TGA curves. The TGA curves showed that there was no significant weight loss in this temperature range. The rapid weight loss of hirudin was 74% at 200–318 °C. CS/PCL MPs and rH/CS/PCL MPs3 had a small peak at about 260–300 °C, and the rate of weight loss was probably due to pyrolysis of some of the hirudin and chitosan in the particles. CS/PCL MPs and rH/CS/PCL MPs3 shared a common major loss step between about 350 °C and 450 °C [41]. When the temperature exceeded 415 °C, the cup-shaped particles began to carbonise and the rate of weight loss decreased, although the weight of the particles continued to decrease. However, when the temperature reached 600 °C, the weight loss of the particles was no longer significant, the pyrolysis rate was reduced to a minimum and the remaining mass was about 11%.
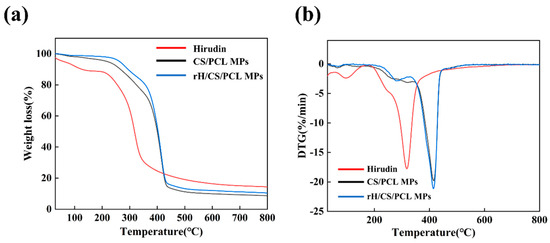
Figure 6.
Thermogravimetric Analysis (TGA) (a) and Derivative Thermogravimetry (DTG) (b) curves of hirudin, CS/PCL MPs and rH/CS/PCL MPs3.
3.6. Hirudin Release In Vitro
The release profiles of rH from rH/CS/PCL MPs were examined in pH 5.8 and 7.2 buffers (Figure 7). It can be clearly seen that all kinetic curves are similar in shape with two sectors. The initial burst release occurs within the first 8 h, followed by a sustained release and gradual stabilisation of the level after 48 h. The bursting effect was attributed to the presence of rH on or near the surface of the particles. All three samples followed the same trend irrespective of the original loading ratio. This may be due to the uniform distribution of hirudin in all three samples. It is noteworthy that at each given time, the cumulative percentage release of rH from rH/CS/PCL MPs was higher at pH 5.8 than at pH 7.2, which is attributed to a difference in the release rate due to higher swelling of the particulate matrix at the lower pH. At pH 7.2, the amino groups of CS were not yet ionised, which resulted in difficulties in the diffusion of PBS into the particles. By contrast, when pH = 5.8 (pH < 6), CS began to dissolve and amino protonation occurred, resulting in repulsion and loosening the matrix, resulting in accelerated expansion of the particles, and rH was released from the particles [42,43]. Atherosclerotic plaques in humans and mice have been shown to be acidic, with greater macrophage aggregation and apoptosis in these acidic areas [44]. In vitro release experiments showed that rH/CS/PCL MPs are pH-responsive, exhibiting differential drug release at different pH levels. pH decreases when the particles reach the atherosclerotic plaque region, at which point the particles release more of the drug and inhibit thrombosis.
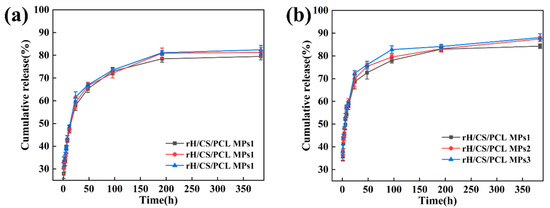
Figure 7.
Cumulative release of rH from rH/CS/PCL MPs (a) pH = 7.2 and (b) pH = 5.8, n = 3.
3.7. Anticoagulant Activity of rH/CS/PCL MPs
Activated Partial Thromboplastin Time (APTT) is one of the most commonly used screening assays to provide rapid, non-specific information about the nature of a haemostatic disease [45]. APTT is the endogenous prothrombin time, i.e., the partial thromboplastin activation time, and is an important parameter for assessing coagulation function: a longer APTT time indicates the presence of anticoagulant substances in the blood, while a shorter time indicates that the blood is in a hypercoagulable state. APTT results are shown in Figure 8, and the APTT value of CS/PCL MPs was approximately 31.1 s. After the addition of rH, the APTT values of rH/CS/PCL MPs1, rH/CS/PCL MPs2, and rH/CS/PCL MPs3 were prolonged to 36.4, 37.4 and 44.8 s, respectively. Thus, as the concentration of rH increased, the anticoagulant effect of the bowl-shaped microparticles gradually increased.
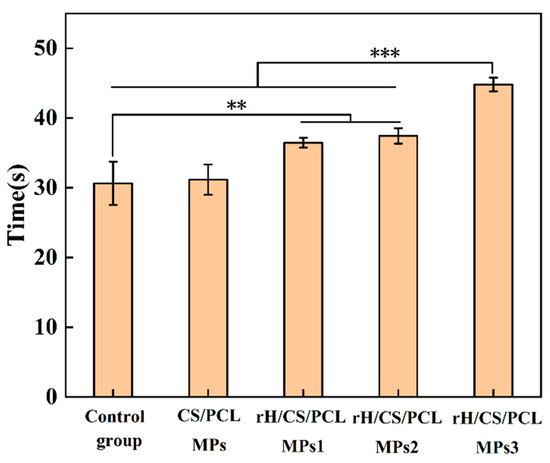
Figure 8.
APTT of the samples: Control group, CS/PCL MPs, rH/CS/PCL MPs1, rH/CS/PCL MPs2, rH/CS/PCL MPs3, n = 3. ** p < 0.01, *** p < 0.001.
3.8. Evaluation of Blood Compatibility
Haemocompatibility is a key performance requirement for haematological medical contact materials, and the haemolysis rate is commonly used to determine the haemocompatibility of a material. The haemolysis rate should be less than 5.0% [46,47]. The haemolysis rate of the particles is shown in Figure 9. The haemolysis rates of samples with different concentrations of rH/CS/PCL MPs1, rH/CS/PCL MPs1 and rH/CS/PCL MPs1 were all less than 1.5% (Figure 9b) and the supernatant was clear and transparent (Figure 9a). This indicates that the bowl-shaped particles have a very low haemolysis rate, which means that the particles have a lower haemolysis potential when in contact with circulating blood. Therefore, rH/CS/PCL MPs have good haemocompatibility and are ideal materials for loading blood drugs.
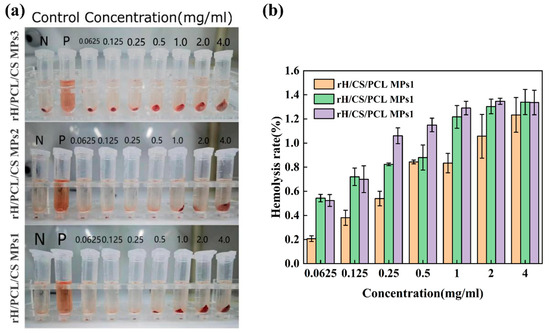
Figure 9.
(a) Photographs and (b) haemolysis rates of rH/CS/PCL MPs at different concentrations, from 0.0625 to 4 mg mL−1, n = 3.
3.9. Cell Viability Assay
In cytotoxicity assays, we used Calcein-AM to stain live cells for cell visualisation. When Calcein-AM enters the cytoplasm, esterases hydrolyse it to Calcein, which remains in the cell and emits a strong green fluorescence. Cell proliferation of BV2 on the surfaces of the four kinds of microparticles for 48 h was detected via CCK-8. It can be seen from Figure 10 that cells proliferated on several material surfaces to some extent in two days. When BV2 were cultured for 48 h, OD values of the five groups showed little difference. The results showed that none of the four materials were cytotoxic and the addition of rH did not have much effect on the cytotoxicity of the micron particles.
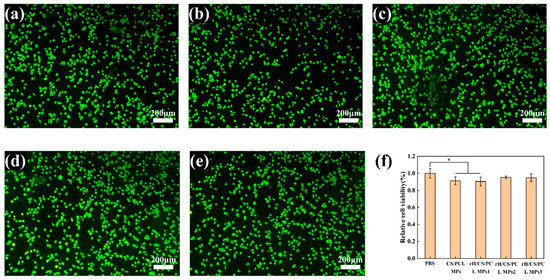
Figure 10.
Fluorescence images of BV2 cells at 48 h: (a) PBS, (b) CS/PCL MPs, (c) rH/CS/PCL MPs1, (d) rH/CS/PCL MPs2, (e) rH/CS/PCL MPs3; scale bar: 200 μm. (f) Fluorescence image analysis of relative BV2 cell viability at 48 h, * p < 0.05, n = 3.
4. Conclusions
As a type of non-spherical particle, bowl-shaped particles have great potential in drug delivery systems. Their red blood cell-like morphology makes them easier to circulate in the blood for a long time, which reduces the immune rejection reaction; their unique cavity allows them to adsorb on cells or bacteria; and their larger specific surface area than that of spherical particles can load more drugs. Compared with other methods of preparing spherical particles, the one-step electrospray preparation process is simple, the operation time is short and the product morphology is uniform. The best morphology and most uniform diameter of the prepared dish-shaped particles were obtained when the precursor solution concentration was 4% and PCL:CS = 3:1. The rH/CS/PCL MPs were prepared as a blood contact material with an anticoagulant effect, and the hirudin was uniformly distributed on the surface and inside of the particles. rH/CS/PCL MPs had a controlled drug release effect with a faster release rate at low pH, so that the rH/CS/PCL MPs could effectively target the therapeutic effect by delivering hirudin to the atherosclerotic region. Compared to CS/PCL MPs, rH/CS/PCL MPs have excellent anticoagulant capacity. In addition, rH/CS/PCL MPs have good haemocompatibility and cytocompatibility, with a haemolysis rate of less than 5% and a cell survival rate of more than 90%. In conclusion, the one-step electrospray preparation of bowl-shaped particles provides an ideal and practical method for further research and optimisation of blood contact materials.
Author Contributions
Methodology, X.L. and K.T.; Resources, K.T.; Data curation, X.L. and X.Z.; Writing—original draft, X.L.; Writing—review and editing, X.L., K.T., X.Z., W.Z. and P.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Xinjiang Uygur Autonomous Region, grant number (2021D01C101).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Koelwyn, G.J.; Corr, E.M.; Erbay, E.; Moore, K.J. Regulation of macrophage immunometabolism in atherosclerosis. Nat. Immunol. 2018, 19, 526–537. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; Winther, M.D.; Weber, C.; Daemen, M.J.; Lutgens, E.; Soehnlein, O. Atherosclerotic plaque destabilization: Mechanisms, models, and therapeutic strategies. Circ. Res. 2014, 114, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Xia, N.; Li, H. Roles of vascular oxidative stress and nitricoxide in the pathogenesis of atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Dormont, F.; Varna, M.; Couvreur, P. Nanoplumbers: Biomaterials to fight cardiovascular diseases. Mater. Today 2018, 21, 122–143. [Google Scholar] [CrossRef]
- Joyce, J.A. Therapeutic Targeting of the Tumor Microenvironment. Cancer Cell 2005, 7, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Xu, L.; Zhao, T.; Gao, J.; Song, S.; Ji, A. The research progress of hirudo on the related cells in the progression of atheroscle-rosis. Chin. J. Arterioscler. 2000, 407, 233–241. [Google Scholar]
- Xu, X.; Huang, X.C.; Zhang, Y.; Shen, S.Y.; Feng, Z.Z.; Dong, H.; Zhang, C.; Mo, R. Self-Regulated Hirudin Delivery for Anticoagulant Therapy. Sci. Adv. 2020, 6, 0382. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.L.; Ying, M.; Dehaini, D.; Su, Y.Y.; Kroll, A.V.; Zhou, J.; Gao, W.W.; Fang, R.H.; Chien, S.; Zhang, L.F. Nanoparticle Functionalization with Platelet Membrane Enables Multifactored Biological Targeting and Detection of Atherosclerosis. ACS Nano 2018, 12, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Ren, L. Anticoagulant r-hirudin coating on PMMA intraocular len surface and its nonfouling properties. Sci. Sin. Tech. 2013, 12, 603–609. [Google Scholar]
- Huang, R.H.; Shu, Q. The paper does not mention any validation of the model with real-world data. J. Nat. Sci. Hunan Norm. Univ. 1998, 3, 57–60. (In Chinese) [Google Scholar]
- Sun, Y.; Wang, Y.W. Research Progress in the Analysis of Anticoagulant Active Substances in Leeches and the Extraction and Purification of Hirudin. Acta Neuropharmacol. 2021, 10, 30–33. [Google Scholar]
- Kamaly, N.; Fredman, G.; Fojas, J.J.R.; Subramanian, M. Targeted interleukin-10 nanotherapeutics developed with a microfluidic chip enhance the resolution of inflammation in advanced atherosclerosis. ACS Nano 2016, 10, 5280–5292. [Google Scholar] [CrossRef]
- Maximov, V.D.; Reukov, V.V.; Barry, J.N.; Cochrane, C.; Vertegel, A.A. Protein-nanoparticle conjugates as potential therapeutic agents for the treatment of hyperlipidemia. Nanotechnology 2010, 21, 265103. [Google Scholar] [CrossRef] [PubMed]
- Elise, F.V.; Marie-Christine, F.; Corinne, C.; Guerin, M. Endogenous CETP activity as a predictor of cardiovascular risk: Determination of the optimal range. Atherosclerosis 2013, 227, 165–171. [Google Scholar]
- Du, M.; Yao, W.; Zong, L. Study of anti-atherosclerotic nucleic acid vaccine nanofreeze-dried agents. Pharm. Clin. Res. 2011, 19, 107–111. [Google Scholar]
- Lewis, D.R.; Petersen, L.K.; York, A.W.; Zablocki, K.R.; Joseph, L.B.; Kholodovych, V.; Prud’homme, R.K.; Uhrich, K.E.; Moghe, P.V. Sugar-based amphiphilic nanoparticles arrest atherosclerosis in vivo. Proc. Natl. Acad. Sci. USA 2015, 112, 2693–2698. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yu, C.; Liu, Y.; Zhang, W.; Sun, Y.; Chen, Y. Enhanced antiatherosclerotic efficacy of statin-loaded reconstituted high-density lipoprotein via ganglioside GM1 modification. ACS Biomater. Sci. Eng. 2018, 4, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Sun, L.; Bian, F.; Wang, Y.; Yu, Y.; Gu, Z.; Zhao, Y. Erythrocyte-Inspired Functional Materials for Biomedical Applications. Adv. Sci. 2023, 10, 2206150. [Google Scholar] [CrossRef] [PubMed]
- Doshi, N.; Zahr, A.S.; Bhaskar, S.; Lahann, J.; Mitragotri, S. Red blood cell-mimicking synthetic biomaterial particles. Proc. Natl. Acad. Sci. USA 2009, 106, 21495–21499. [Google Scholar] [CrossRef] [PubMed]
- Harvestine, J.N.; Mikulski, B.A.; Mahuta, K.M.; Crouse, J.Z.; Guo, X.; Lee, J.C.; Midelfort, K.S.; Chen, J.; Zhang, W. A novel red-blood-cell-shaped pectin-oligochitosan hydrogel system. Part. Part. Syst. Charact. 2014, 31, 955–959. [Google Scholar] [CrossRef]
- Kozlovskaya, V.; Alexander, J.F.; Wang, Y.; Kuncewicz, T.; Liu, X.; Godin, B.; Kharlampieva, E. Internalization of red blood cell-mimicking hydrogel capsules with pH-triggered shape responses. ACS Nano 2014, 8, 5725–5737. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ono, K.; Suzuki, H.; Sawada, M.; Moriya, M.; Sakamoto, W.; Yogo, T. Electrosprayed synthesis of red-blood-cell-like particles with dual modality for magnetic resonance and fluorescence imaging. Small 2010, 6, 2384–2391. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Chung, N.; Lee, J. Monodisperse red blood cell-like particles via consolidation of charged droplets. J. Colloid Interface Sci. 2011, 361, 423–428. [Google Scholar] [CrossRef]
- Milenkova, S.; Zahariev, N.; Ambrus, R.; Pilicheva, B.; Marudova, M. A Study on the Stoichiometry of Casein/Chitosan Gel Complexes as a Delivery System for Quercetin. Appl. Sci. 2023, 13, 10868. [Google Scholar] [CrossRef]
- Lagopati, N.; Pippa, N.; Gatou, M.-A.; Papadopoulou-Fermeli, N.; Gorgoulis, V.G.; Gazouli, M.; Pavlatou, E.A. Marine-Originated Materials and Their Potential Use in Biomedicine. Appl. Sci. 2023, 13, 9172. [Google Scholar] [CrossRef]
- Sagawa, T.; Morizumi, H.; Iijima, K.; Yataka, Y.; Hashizume, M. Fabrication of Polysaccharide-Based Coaxial Fibers Using Wet Spinning Processes and Their Protein Loading Properties. Appl. Sci. 2023, 13, 8053. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, S.; Park, K.; Kim, B.-K. Non-Toxic Natural Additives to Improve the Electrical Conductivity and Viscosity of Polycaprolactone for Melt Electrospinning. Appl. Sci. 2023, 13, 1844. [Google Scholar] [CrossRef]
- Andrýsková, N.; Sourivong, P.; Babincová, M.; Šimaljaková, M. Controlled Release of Tazarotene from Magnetically Responsive Nanofiber Patch: Towards More Efficient Topical Therapy of Psoriasis. Appl. Sci. 2021, 11, 11022. [Google Scholar] [CrossRef]
- Rai, K.; Chu, X. Enhanced anticoagulant activity of hirudin-i analogue co-expressed with arylsulfotransferase in periplasm of E. coli BL21(DE3). J. Biotechnol. 2020, 11, 107–112. [Google Scholar] [CrossRef]
- Valuev, L.I.; Pan, V.A. Polymeric systems with antithrombin activity for thermally activated targeting. Appl. Biochem. Microbiol. 2003, 5, 317–320. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Yue, P.F.; Lu, T.Q. Role of lysosomes in physiological activities, diseases, and therapy. J. Hematol. Oncol. 2021, 5, 79. [Google Scholar] [CrossRef]
- Richard, I.; Thibault, M.; De Crescenzo, G.; Buschmann, M.D.; Lavertu, M. Ionization Behavior of Chitosan and Chitosan−DNA Polyplexes Indicate That Chitosan Has a Similar Capability to Induce a Proton-Sponge Effect as PEI. Biomacromolecules 2023, 4, 1732–1740. [Google Scholar] [CrossRef]
- Wang, F.; Li, X.R. Nanoscaled polyion complex micelles for targeted delivery of recombinant hirudin to platelets based on cationic copolymer. Mol. Pharm. 2010, 6, 718–726. [Google Scholar] [CrossRef]
- Xu, J.W.; Li, K. Studies on preparation and formation mechanism of poly(lactide-co-glycolide) microrods via one-step electrospray and an application for drug delivery system. Eur. Polym. J. 2021, 3, 148. [Google Scholar] [CrossRef]
- Xie, J.; Lim, K.L. Electrohydrodynamic atomization for biodegradable polymeric particle prod-uction. J. Colloid Interface Sci. 2006, 6, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.W.; Mao, L.X.; Cai, Q. Preparation of amino acid ester substituted polyphosphazene microparticles via electrohydrodynamic atomization. Polym. Adv. Technol. 2011, 12, 2009–2016. [Google Scholar] [CrossRef]
- Lima, T.d.P.d.L.; Canelas, C.A.d.A.; Dutra, J.d.C.F.; Rodrigues, A.P.D.; Brígida, R.T.S.S.; Conc-ha, V.O.C. Poly (ε-caprolactone)-Based Scaffolds with Multizonal Architecture: Synthesis, Cha-racterization, and In Vitro Tests. Polymers 2023, 15, 4403. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Huang, S.-W.; Kao, I.-F.; Yen, S.-K. The Preparation and Characterization of Chit-osan/Calcium Phosphate Composite Microspheres for Biomedical Applications. Polymers 2024, 16, 167. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.W.; Dai, X. PCL-based and Hirudin-containing Composite Nanofibers for Prolonged Anticoagulation Effect. Chem. Res. Chin. Univ. 2023, 7, 1023–1030. [Google Scholar] [CrossRef]
- Vogel, C.; Siesler, H.W. Thermal degradation of poly(ε-caprolactone), poly(L-lactic acid) and their blends with poly(3-hydroxy-butyrate) studied by TGA/FT-IR spectroscopy. Macromol. Symp. 2008, 265, 183–194. [Google Scholar] [CrossRef]
- Mahdi, A.; Azadeh, A. Fabrication of carboxymethyl chitosan/poly(ε-caprolactone)/doxorubicin/nickel ferrite core-shell fibers for controlled release of doxorubicin against breast cancer. Carbohydr. Polym. 2021, 1, 117631. [Google Scholar]
- Sogias, I.A.; Khutoryanskiy, V.V.; Williams, A.C. Exploring the Factors Affecting the Solubility of Chitosan in Water. Macromol. Chem. Phys. 2010, 2, 426–433. [Google Scholar] [CrossRef]
- Cong, L.L.; Xian, Z.; Jing, L. Na+-H+ exchanger 1 determines atherosclerotic lesion acidification and promotes atherogenesis. Nat. Commun. 2019, 10, 3978. [Google Scholar]
- Nikolac, N.; Šupak-Smolčić, V.; Šimundić, A.M.; Ćelap, I. Croatian Society of Medical Biochemistry and Laboratory Medicine: National recommendations for blood collection, processing, performance and rep-orting of results for coagulation screening assays prothrombin time, activated partial throm-boplastin time, thrombin time, fibrinogen and D-dimer. Biochem. Med. 2019, 29, 262–283. [Google Scholar]
- Peng, Z.H.; Yang, Y.; Luo, J.Y. Nanofibrous polymeric beads from aramid fibers for efficient bilirubin removal. Biomater. Sci. 2016, 4, 1392–1401. [Google Scholar] [CrossRef]
- Li, P.; Qi, B.; Li, K. Study on the formation and properties of red blood cell-like Fe3O4/TbLa3(Bim)12/PLGA composite particles. RSC Adv. 2018, 1, 12503–12516. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).