Extracellular Matrix Tunes the Regenerative Potential of Fetal Stem Cells
Abstract
1. Introduction
2. Materials & Methods
2.1. Human fNPC and fSDSC Culture
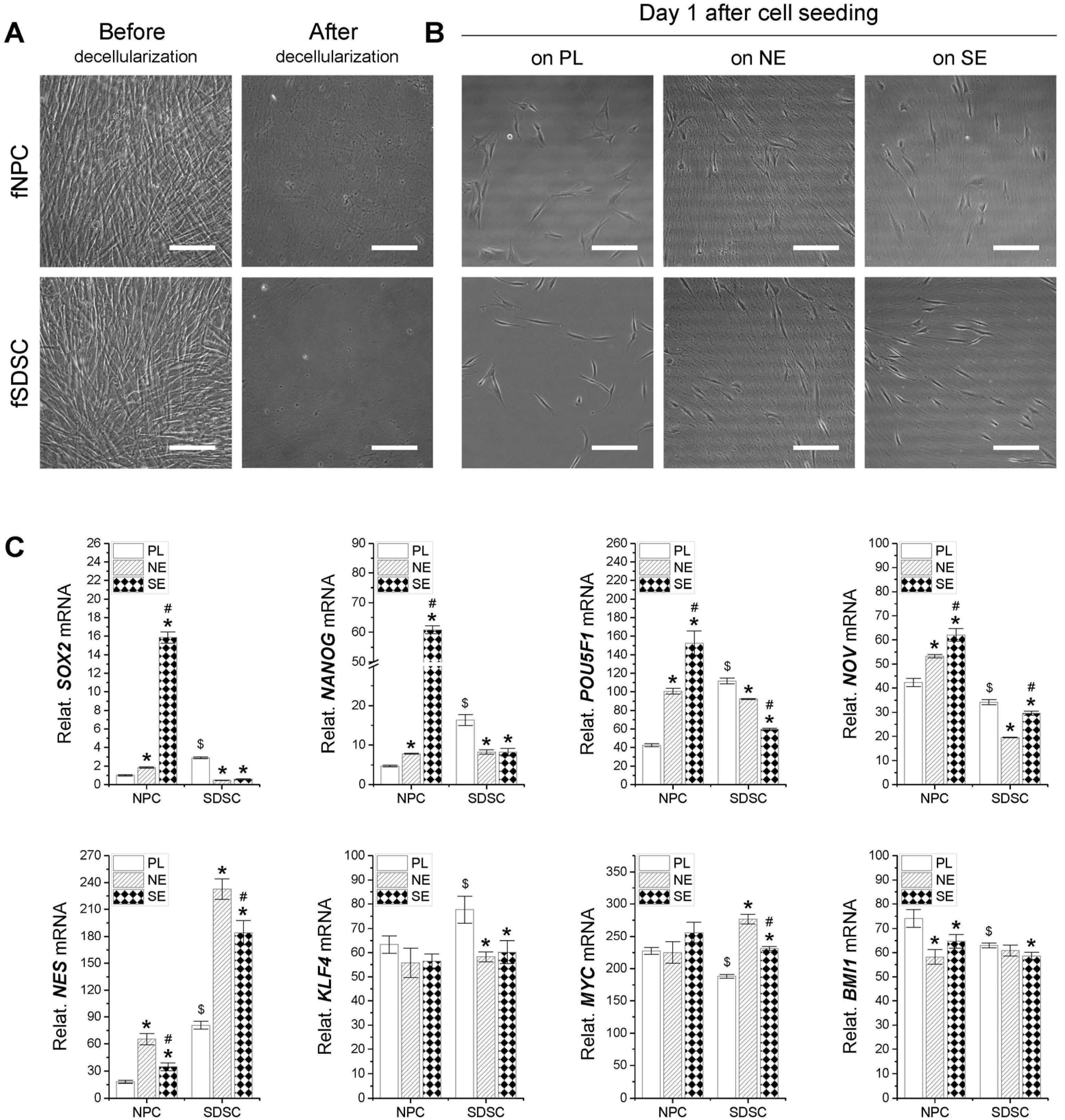
2.2. Preparation of dECMs
2.3. Evaluation of Proliferation, Surface Markers, and Stemness Genes of Expanded fSDSCs and fNPCs
2.4. Immunofluorescence Staining of dECMs
2.5. Induction and Assessment of Adipogenesis, Osteogenesis, and Chondrogenesis
2.6. Statistical Analysis
3. Results
3.1. Assessment of Stemness-Related Gene Expression in Fetal Stem Cells after Expansion on dECMs
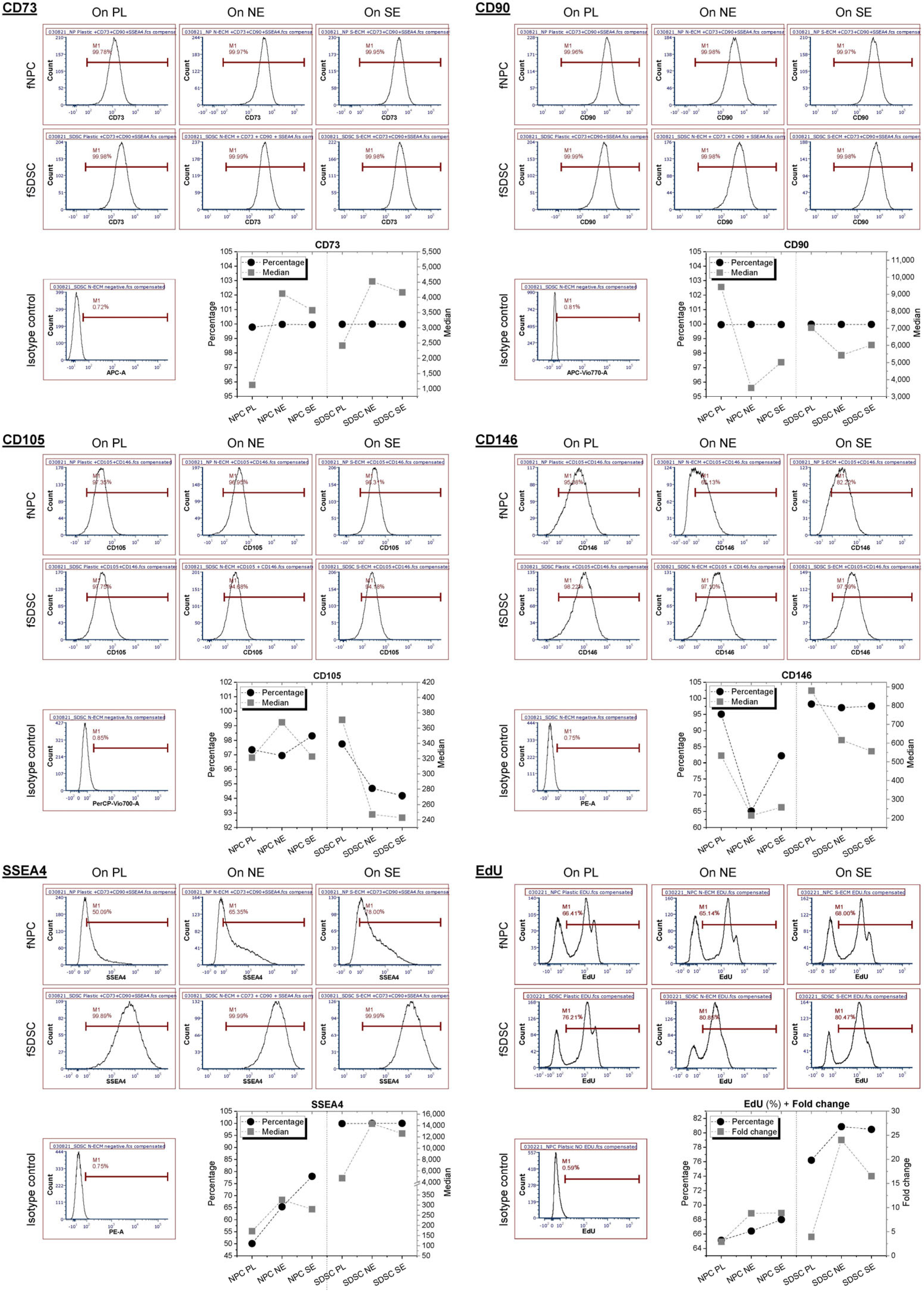
3.2. Assessment of Surface Marker Expression in Fetal Stem Cells after Expansion on dECMs
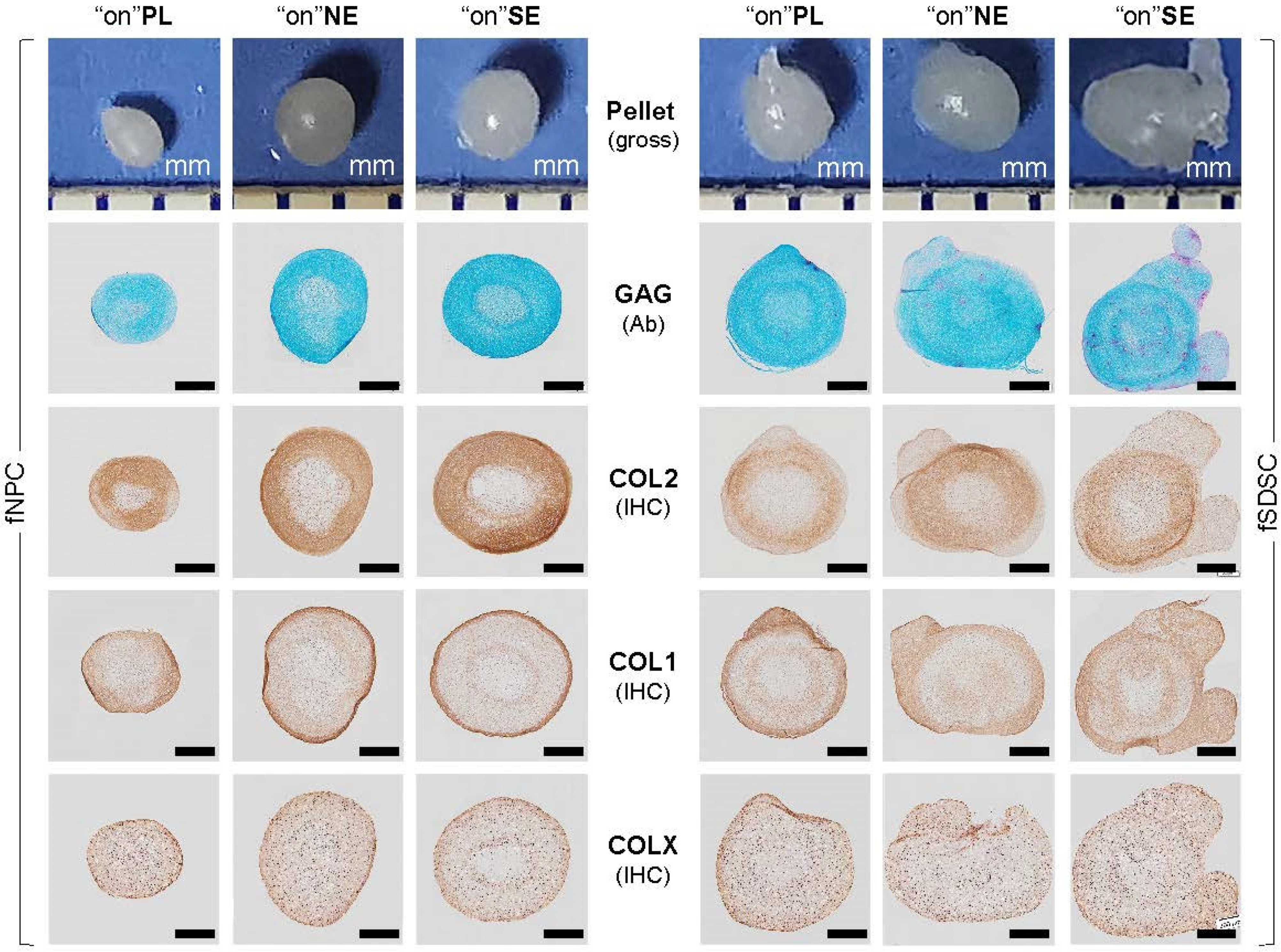
3.3. Chondrogenic Capacity of Fetal Stem Cells after Growth on dECMs

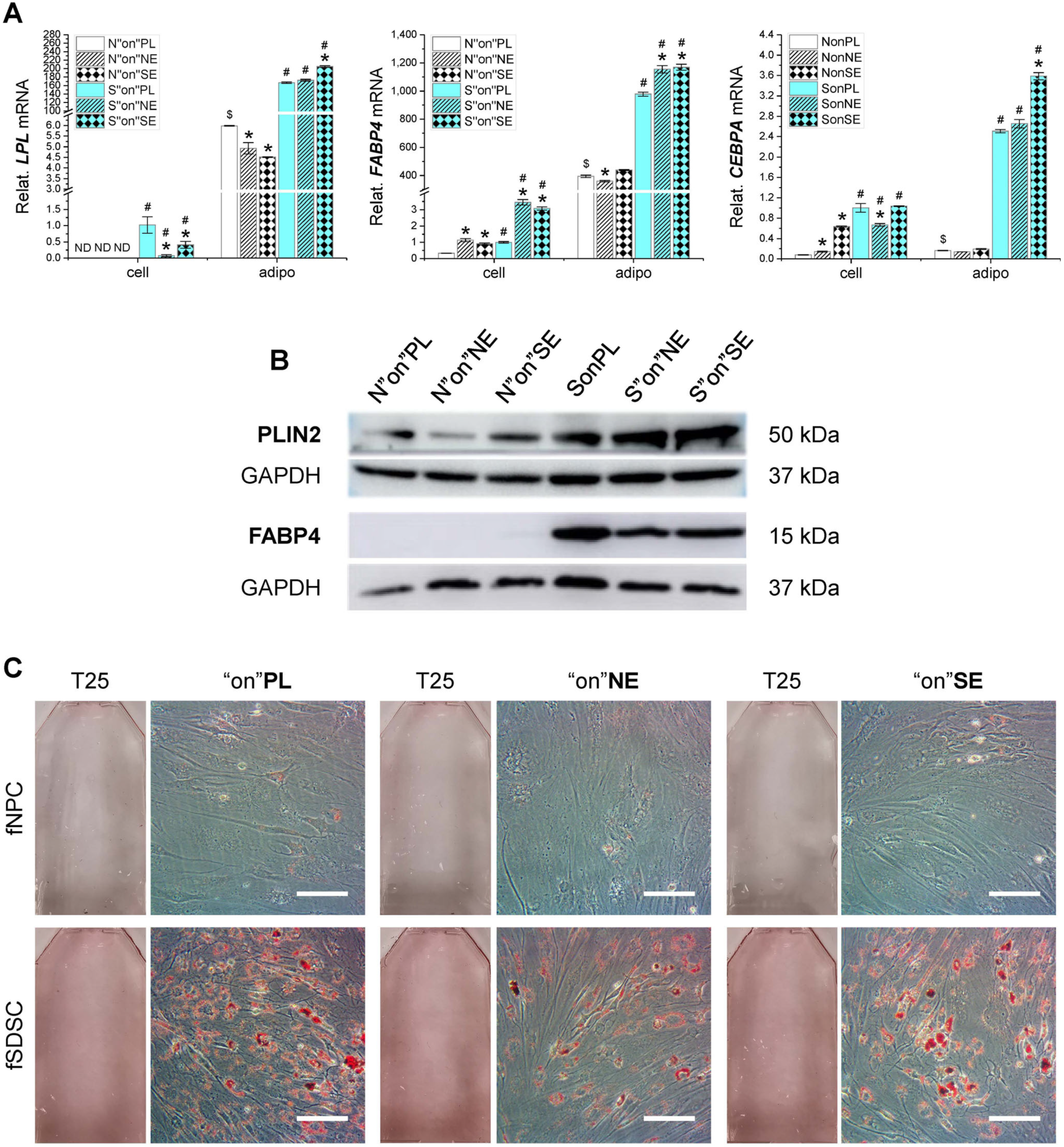
3.4. Adipogenic Capacity of Fetal Stem Cells after Growth on dECMs
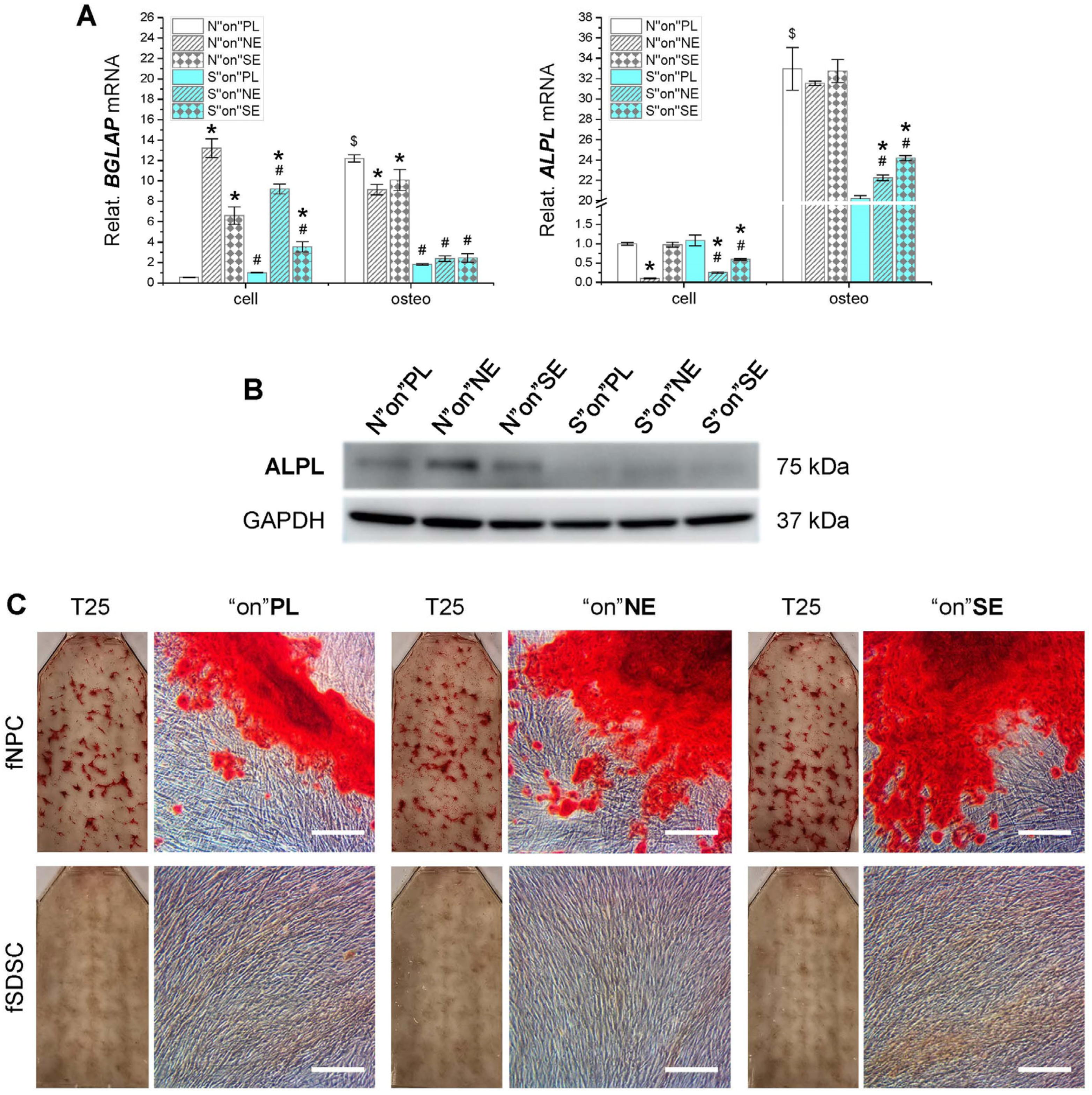
3.5. Osteogenic Capacity of Fetal Stem Cells after Growth on dECMs
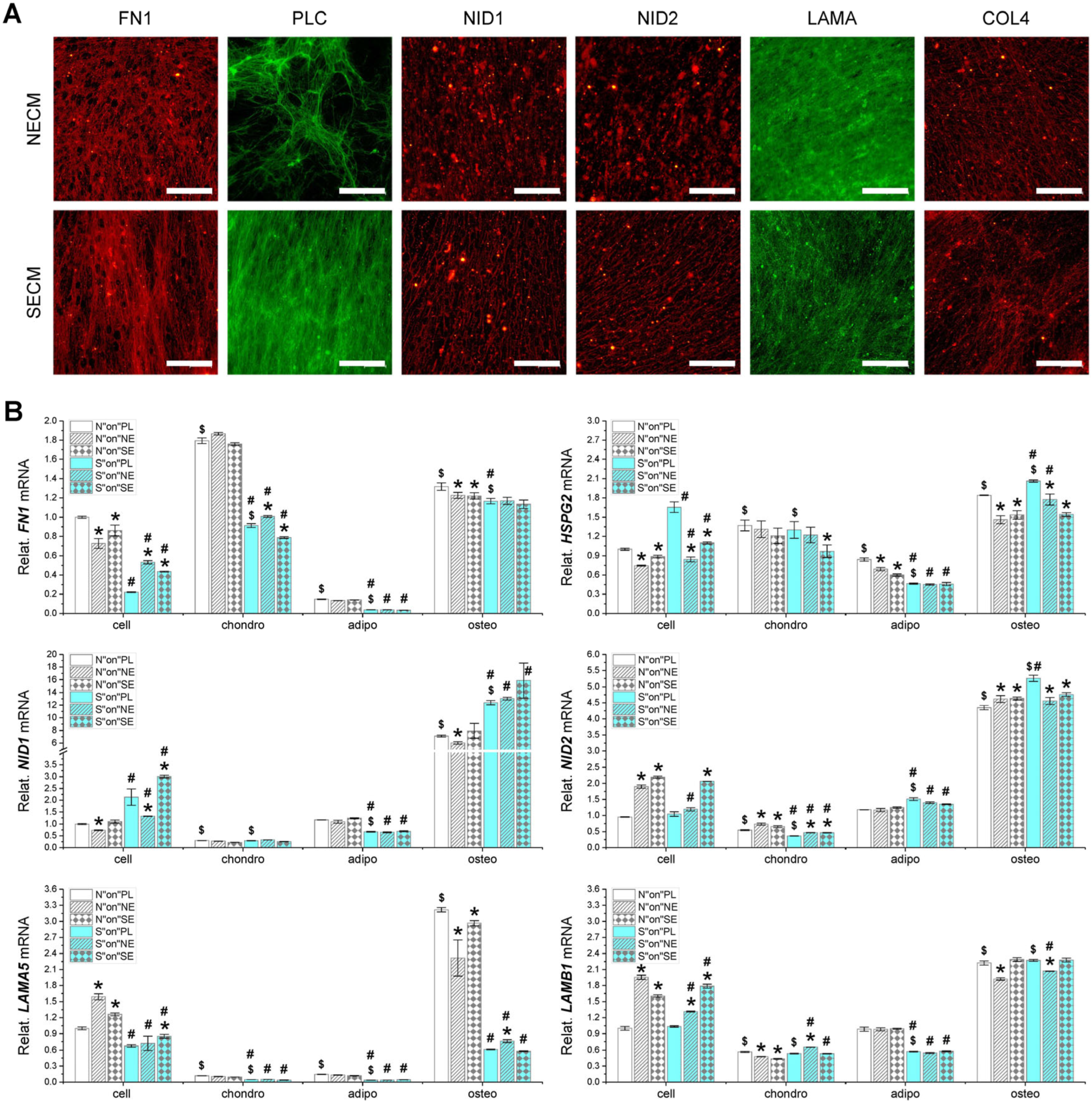
3.6. Major Matrix Protein Expression in Fetal Stem Cells, Their Matrix, and Three-Lineage Differentiated Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toh, W.S.; Foldager, C.B.; Pei, M.; Hui, J.H. Advances in mesenchymal stem cell-based strategies for cartilage repair and regeneration. Stem Cell Rev. Rep. 2014, 10, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pei, M. Cell senescence: A challenge in cartilage engineering and regeneration. Tissue Eng. Part B Rev. 2012, 18, 270–287. [Google Scholar] [CrossRef]
- Rodríguez-Fuentes, D.E.; Fernández-Garza, L.E.; Samia-Meza, J.A.; Barrera-Barrera, S.A.; Caplan, A.I.; Barrera-Saldaña, H.A. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch. Med. Res. 2021, 52, 93–101. [Google Scholar] [CrossRef]
- Götherström, C.; Ringdén, O.; Westgren, M.; Tammik, C.; Le Blanc, K. Immunomodulatory effects of human foetal liver-derived mesenchymal stem cells. Bone Marrow Transplant. 2003, 32, 265–272. [Google Scholar] [CrossRef]
- Le Blanc, K.; Tammik, L.; Sundberg, B.; Haynesworth, S.E.; Ringdén, O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand. J. Immunol. 2003, 57, 11–20. [Google Scholar] [CrossRef]
- Montjovent, M.O.; Bocelli-Tyndall, C.; Scaletta, C.; Scherberich, A.; Mark, S.; Martin, I.; Applegate, L.A.; Pioletti, D.P. In vitro characterization of immune-related properties of human fetal bone cells for potential tissue engineering applications. Tissue Eng. Part A. 2009, 15, 1523–1532. [Google Scholar] [CrossRef]
- Allan, D.S. Using umbilical cord blood for regenerative therapy: Proof or promise? Stem Cells 2020, 38, 590–595. [Google Scholar] [CrossRef]
- Guillot, P.V.; Gotherstrom, C.; Chan, J.; Kurata, H.; Fisk, N.M. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells 2007, 25, 646–654. [Google Scholar] [CrossRef]
- Li, J.; He, F.; Pei, M. Creation of an in vitro microenvironment to enhance human fetal synovium-derived stem cell chondrogenesis. Cell Tissue Res. 2011, 345, 357–365. [Google Scholar] [CrossRef]
- Okamura, L.H.; Cordero, P.; Palomino, J.; Parraguez, V.H.; Torres, C.G.; Peralta, O.A. Myogenic Differentiation Potential of Mesenchymal Stem Cells Derived from Fetal Bovine Bone Marrow. Anim. Biotechnol. 2018, 29, 1–11. [Google Scholar] [CrossRef]
- Pei, M.; Li, J.T.; Shoukry, M.; Zhang, Y. A review of decellularized stem cell matrix: A novel cell expansion system for cartilage tissue engineering. Eur. Cell Mater. 2011, 22, 333–343; discussion 343. [Google Scholar] [CrossRef]
- Pei, M. Environmental preconditioning rejuvenates adult stem cells’ proliferation and chondrogenic potential. Biomaterials 2017, 117, 10–23. [Google Scholar] [CrossRef]
- Assunção, M.; Dehghan-Baniani, D.; Yiu, C.H.K.; Später, T.; Beyer, S.; Blocki, A. Cell-Derived Extracellular Matrix for Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 602009. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, L.; Chen, X.; Liu, T.; Pan, G.; Cui, W.; Li, M.; Luo, Z.P.; Pei, M.; Yang, H.; et al. Culturing on decellularized extracellular matrix enhances antioxidant properties of human umbilical cord-derived mesenchymal stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 437–448. [Google Scholar] [CrossRef]
- Pei, M.; Zhang, Y.; Li, J.; Chen, D. Antioxidation of decellularized stem cell matrix promotes human synovium-derived stem cell-based chondrogenesis. Stem Cells Dev. 2013, 22, 889–900. [Google Scholar] [CrossRef]
- Sun, Y.; Yan, L.; Chen, S.; Pei, M. Functionality of decellularized matrix in cartilage regeneration: A comparison of tissue versus cell sources. Acta Biomater. 2018, 74, 56–73. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Yan, Z.; Pei, M. A prospect of cell immortalization combined with matrix microenvironmental optimization strategy for tissue engineering and regeneration. Cell Biosci. 2019, 9, 7. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, X.; Liu, T.; Zhu, C.; Si, M.; Jargstorf, J.; Li, M.; Pan, G.; Gong, Y.; Luo, Z.P.; et al. SIRT1-dependent anti-senescence effects of cell-deposited matrix on human umbilical cord mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2018, 12, e1008–e1021. [Google Scholar] [CrossRef]
- Li, J.; Hansen, K.C.; Zhang, Y.; Dong, C.; Dinu, C.Z.; Dzieciatkowska, M.; Pei, M. Rejuvenation of chondrogenic potential in a young stem cell microenvironment. Biomaterials 2014, 35, 642–653. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, G.; Hill, R.C.; Dzieciatkowska, M.; Hansen, K.C.; Zhang, X.B.; Yan, Z.; Pei, M. Matrix reverses immortalization-mediated stem cell fate determination. Biomaterials 2021, 265, 120387. [Google Scholar] [CrossRef]
- Jones, B.A.; Pei, M. Synovium-derived stem cells: A tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng. Part B Rev. 2012, 18, 301–311. [Google Scholar] [CrossRef]
- Chen, S.; Fu, P.; Wu, H.; Pei, M. Meniscus, articular cartilage and nucleus pulposus: A comparative review of cartilage-like tissues in anatomy, development and function. Cell Tissue Res. 2017, 370, 53–70. [Google Scholar] [CrossRef]
- Pei, Y.A.; Pei, M. Hypoxia Modulates Regenerative Potential of Fetal Stem Cells. Appl. Sci. 2022, 12, 363. [Google Scholar] [CrossRef]
- Minogue, B.M.; Richardson, S.M.; Zeef, L.A.; Freemont, A.J.; Hoyland, J.A. Characterization of the human nucleus pulposus cell phenotype and evaluation of novel marker gene expression to define adult stem cell differentiation. Arthritis Rheum. 2010, 62, 3695–3705. [Google Scholar] [CrossRef]
- Fauza, D.O.; Bani, M. Fetal Stem Cells in Regenerative Medicine: Principles and Translational Strategies, 1st ed.; Springer Nature: New York, NY, USA, 2016. [Google Scholar]
- Liu, C.; Pei, M.; Li, Q.; Zhang, Y. Decellularized extracellular matrix mediates tissue construction and regeneration. Front. Med. 2022, 16, 56–82. [Google Scholar] [CrossRef]
- Li, J.; He, F.; Pei, M. Chondrogenic priming of human fetal synovium-derived stem cells in an adult stem cell matrix microenvironment. Genes Dis. 2015, 2, 337–346. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Lu, Z.; Yan, L.; Pei, M. Commentary on ‘Surface markers associated with chondrogenic potential of human mesenchymal stromal/stem cells’. F1000Research 2020, 9, F1000 Faculty Rev-37. [Google Scholar] [CrossRef]
- He, F.; Chen, X.D.; Pei, M. Reconstruction of an in vitro tissue-specific microenvironment to rejuvenate synovium-derived stem cells for cartilage tissue engineering. Tissue Eng. Part A. 2009, 15, 3809–3821. [Google Scholar] [CrossRef]
- Pei, Y.A.; Mikaeiliagah, E.; Wang, B.; Zhang, X.; Pei, M. The matrix microenvironment influences but does not dominate tissue-specific stem cell lineage differentiation. Mater. Today Bio 2023, 23, 100805. [Google Scholar] [CrossRef]
- Gao, G.; Chen, S.; Pei, Y.A.; Pei, M. Impact of perlecan, a core component of basement membrane, on regeneration of cartilaginous tissues. Acta Biomater. 2021, 135, 13–26. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, T.L.; Toh, W.S.; Pei, M. The role of laminins in cartilaginous tissues: From development to regeneration. Eur. Cell Mater. 2017, 34, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, Y.; Yan, Z.; Zhang, X.B.; Pei, M. Impact of Fibronectin Knockout on Proliferation and Differentiation of Human Infrapatellar Fat Pad-Derived Stem Cells. Front. Bioeng. Biotechnol. 2019, 7, 321. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, S.; Pei, Y.A.; Pei, M. Nidogen: A matrix protein with potential roles in musculoskeletal tissue regeneration. Genes Dis. 2021, 9, 598–609. [Google Scholar] [CrossRef]
- Singh, P.; Schwarzbauer, J.E. Fibronectin and stem cell differentiation—Lessons from chondrogenesis. J. Cell Sci. 2012, 125 Pt 16, 3703–3712. [Google Scholar] [CrossRef]
- Taleb, S.; Cancello, R.; Clément, K.; Lacasa, D. Cathepsin s promotes human preadipocyte differentiation: Possible involvement of fibronectin degradation. Endocrinology 2006, 147, 4950–4959. [Google Scholar] [CrossRef] [PubMed]
- Uetaki, M.; Onishi, N.; Oki, Y.; Shimizu, T.; Sugihara, E.; Sampetrean, O.; Watanabe, T.; Yanagi, H.; Suda, K.; Fujii, H.; et al. Regulatory roles of fibronectin and integrin α5 in reorganization of the actin cytoskeleton and completion of adipogenesis. Mol. Biol. Cell 2022, 33, ar78. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, L.; Smas, C.; Sul, H.S. Pref-1 interacts with fibronectin to inhibit adipocyte differentiation. Mol. Cell Biol. 2010, 30, 3480–3492. [Google Scholar] [CrossRef]
| Antibody | Company Information | Concentration | Catalog No. |
|---|---|---|---|
| CD73 Monoclonal Antibody (AD2), APC, human | eBioscienceTM, Fisher Scientific, Waltham, MA, USA | 0.125 μg/test | 17-0739-42 |
| Anti-CD146 Monoclonal Antibody (P1H12) PE, human | eBioscienceTM, Fisher Scientific | 0.125 μg/test | 12-1469-42 |
| PE anti-human SSEA-4 | BioLegend, Dedham, MA, USA | 0.125 μg/test | 330406 |
| CD90-APC-Vio® 770, human | Miltenyi Biotec, San Diego, CA, USA | 2 μL/test | 130-114-863 |
| CD105-PerCp-Vio® 700, human | eBioscienceTM | 2 μL/test | 130-112-170 |
| Gene Name | Full Name | TaqMan® Assay ID |
|---|---|---|
| Stemness-related genes | ||
| MYC | MYC proto-oncogene | Hs00153408_m1 |
| KLF4 | Kruppel-like factor 4 | Hs00358836_m1 |
| BMI1 | B lymphoma Mo-MLV insertion region 1 homolog | Hs00180411_m1 |
| POU5F1 | POU class 5 homeobox 1 | Hs04260367_gH |
| NES | Nestin | Hs04187831_g1 |
| NOV | Nephroblastoma overexpressed | Hs00159631_m1 |
| NANOG | Nanog homeobox | Hs02387400_g1 |
| SOX2 | SRY-box2 | Hs01053049_s1 |
| Adipogenesis-related genes | ||
| LPL | Lipoprotein lipase | Hs00173425_m1 |
| FABP4 | Fatty acid-binding protein 4 | Hs01086177_m1 |
| CEBPA | CCAAT/enhancer-binding protein alpha | Hs00269972_s1 |
| Osteogenesis-related genes | ||
| BGLAP | Bone gamma-carboxyglutamate protein | Hs01587814_g1 |
| ALPL | Alkaline phosphatase, liver | Hs01029144_m1 |
| COL1A1 | Type I collagen | Hs00164004_m1 |
| Chondrogenesis-related genes | ||
| SOX9 | SRY-Box 9 | Hs00165814_m1 |
| ACAN | Aggrecan | Hs00153936_m1 |
| Col2A1 | Type II collagen | Hs00156568_m1 |
| PRG4 | Proteoglycan 4 | Hs00981633_m1 |
| FBLN1 | Fibulin 1 | Hs00972609_m1 |
| FOXF1 | Forkhead box F1 | Hs00230962_m1 |
| Housekeeping internal gene | ||
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | Hs02758991_g1 |
| Antibody | Catalog No. | Company | Species | Working Conc. |
|---|---|---|---|---|
| Type II collagen | II6B3-c | Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA, USA | Mouse | 3 μg/mL |
| Type IV collagen | M3F7 | Mouse | 3 μg/mL | |
| Fibronectin | HFN 7.1 | Mouse | 3 μg/mL | |
| Laminin | PA1-16730 | Invitrogen, Waltham, MA, USA | Rabbit | 20 μg/mL |
| Col1A1 (3G3) | sc-293182 | Santa Cruz Biotechnology, Inc., Dallas, TX, USA | Mouse | 1 μg/mL |
| Perlecan (A7L6) | sc-33707 | Rat | 4 μg/mL | |
| Nidogen 1 (C-7) | sc-133175 | Mouse | 2 μg/mL | |
| Nidogen-2 (F-2) | sc-377424 | Mouse | 2 μg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, Y.A.; Patel, J.; Pei, M. Extracellular Matrix Tunes the Regenerative Potential of Fetal Stem Cells. Appl. Sci. 2024, 14, 1932. https://doi.org/10.3390/app14051932
Pei YA, Patel J, Pei M. Extracellular Matrix Tunes the Regenerative Potential of Fetal Stem Cells. Applied Sciences. 2024; 14(5):1932. https://doi.org/10.3390/app14051932
Chicago/Turabian StylePei, Yixuan Amy, Jhanvee Patel, and Ming Pei. 2024. "Extracellular Matrix Tunes the Regenerative Potential of Fetal Stem Cells" Applied Sciences 14, no. 5: 1932. https://doi.org/10.3390/app14051932
APA StylePei, Y. A., Patel, J., & Pei, M. (2024). Extracellular Matrix Tunes the Regenerative Potential of Fetal Stem Cells. Applied Sciences, 14(5), 1932. https://doi.org/10.3390/app14051932







