Performance Evaluation of L1-Norm-Based Blind Deconvolution after Noise Reduction with Non-Subsampled Contourlet Transform in Light Microscopy Images
Abstract
1. Introduction
2. Materials and Methods
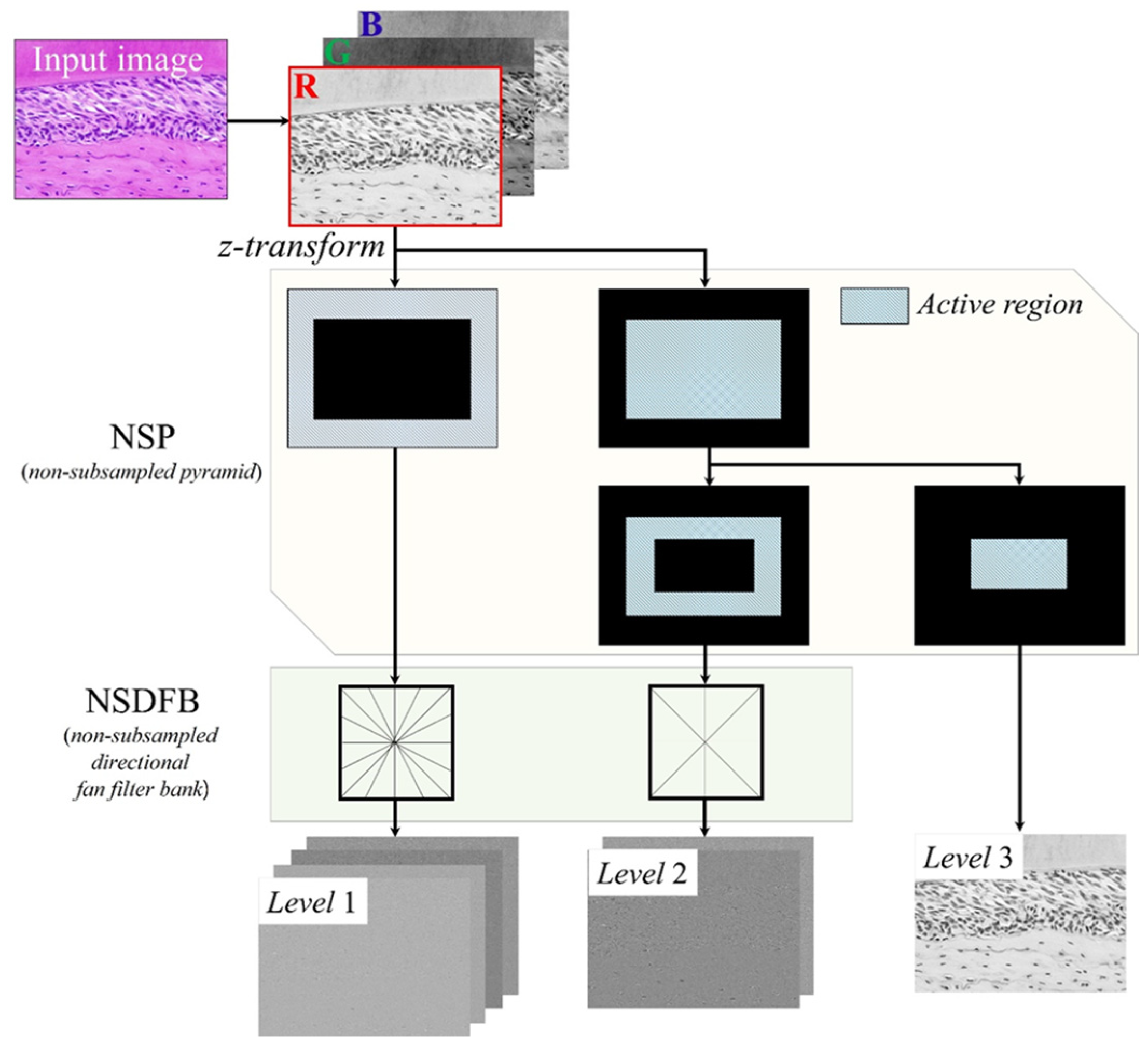
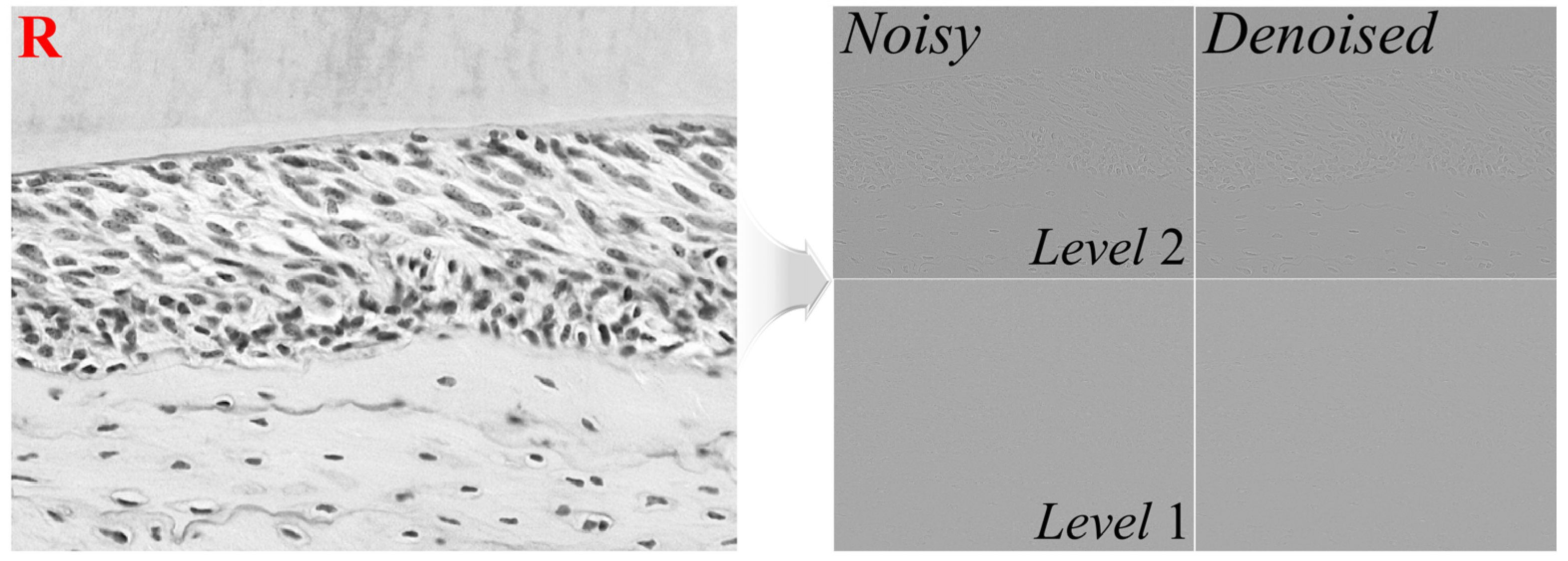
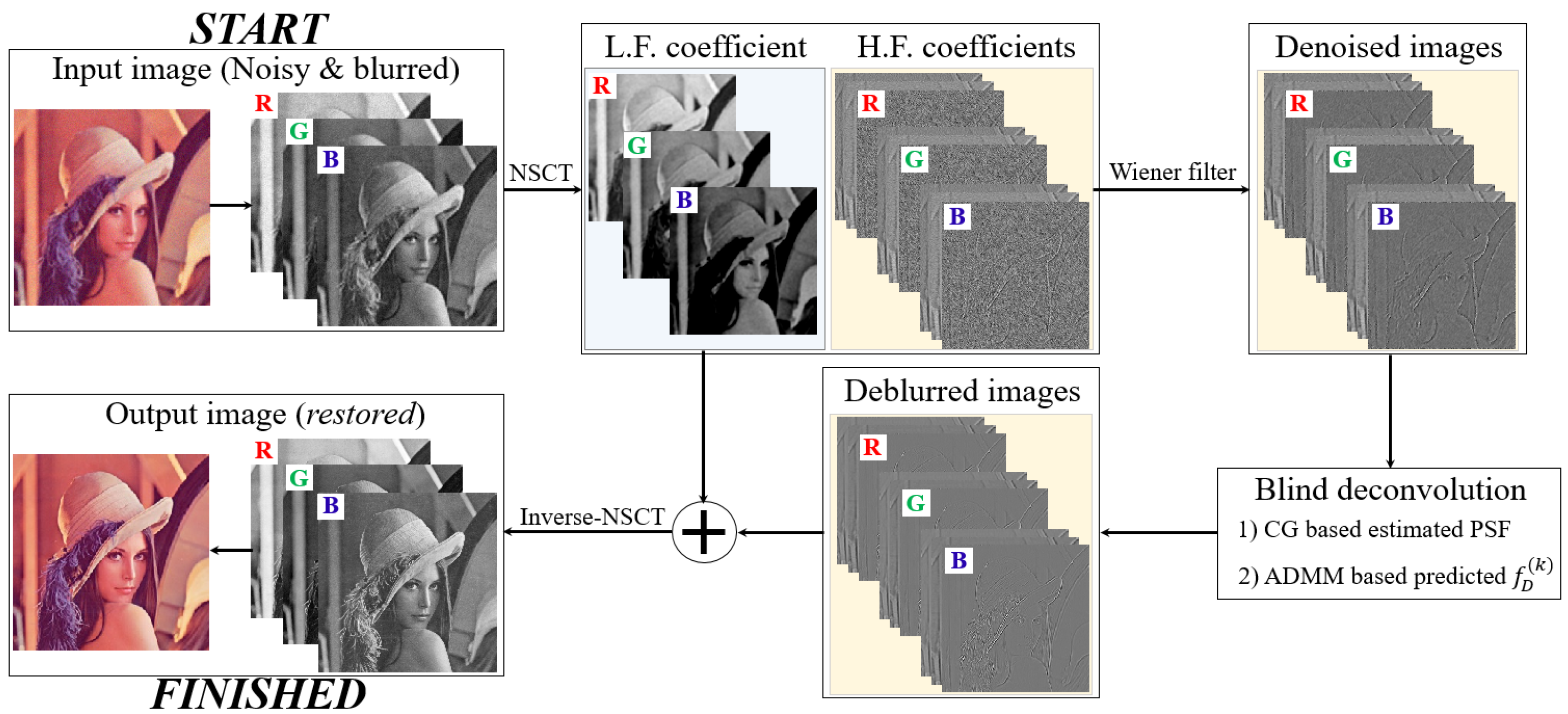
2.1. Proposed Restoration Scheme for Microscopic Images
2.2. Materials and Conditions for the Simulation and Experiment
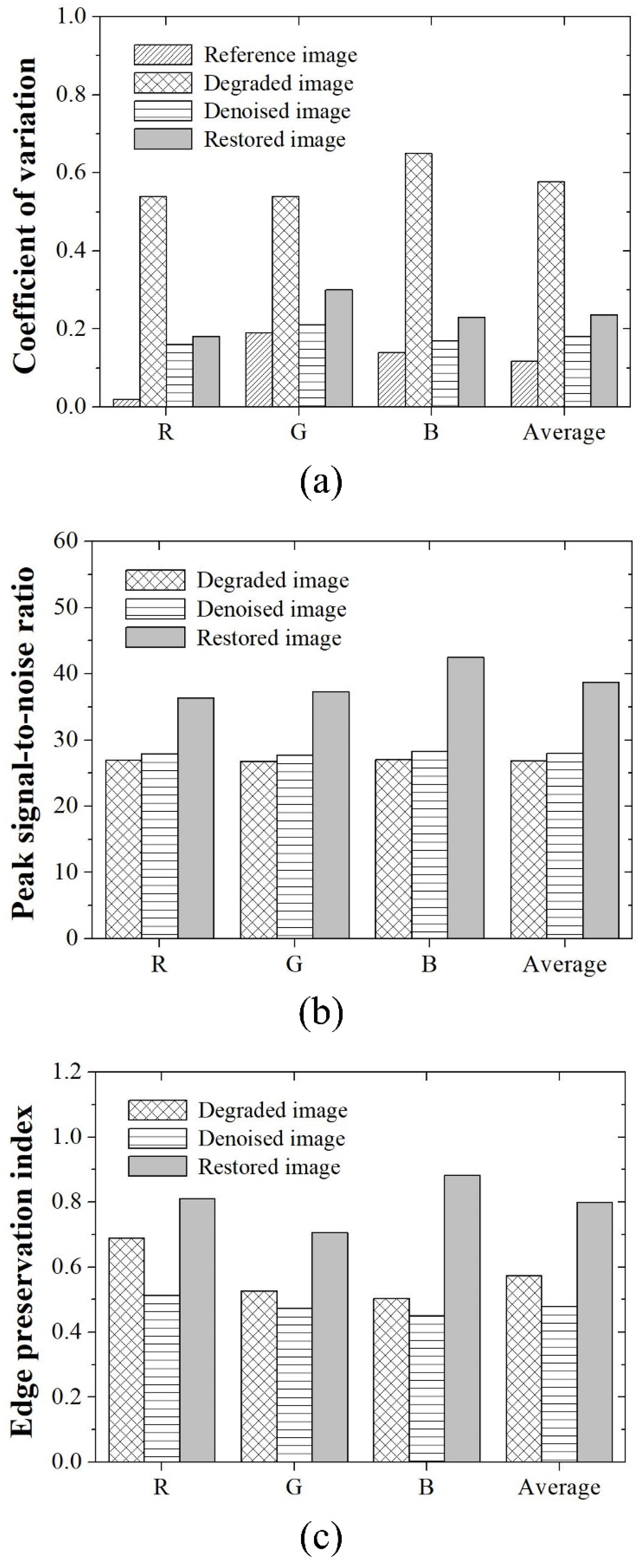
2.3. Quantitative Image Quality Evaluation
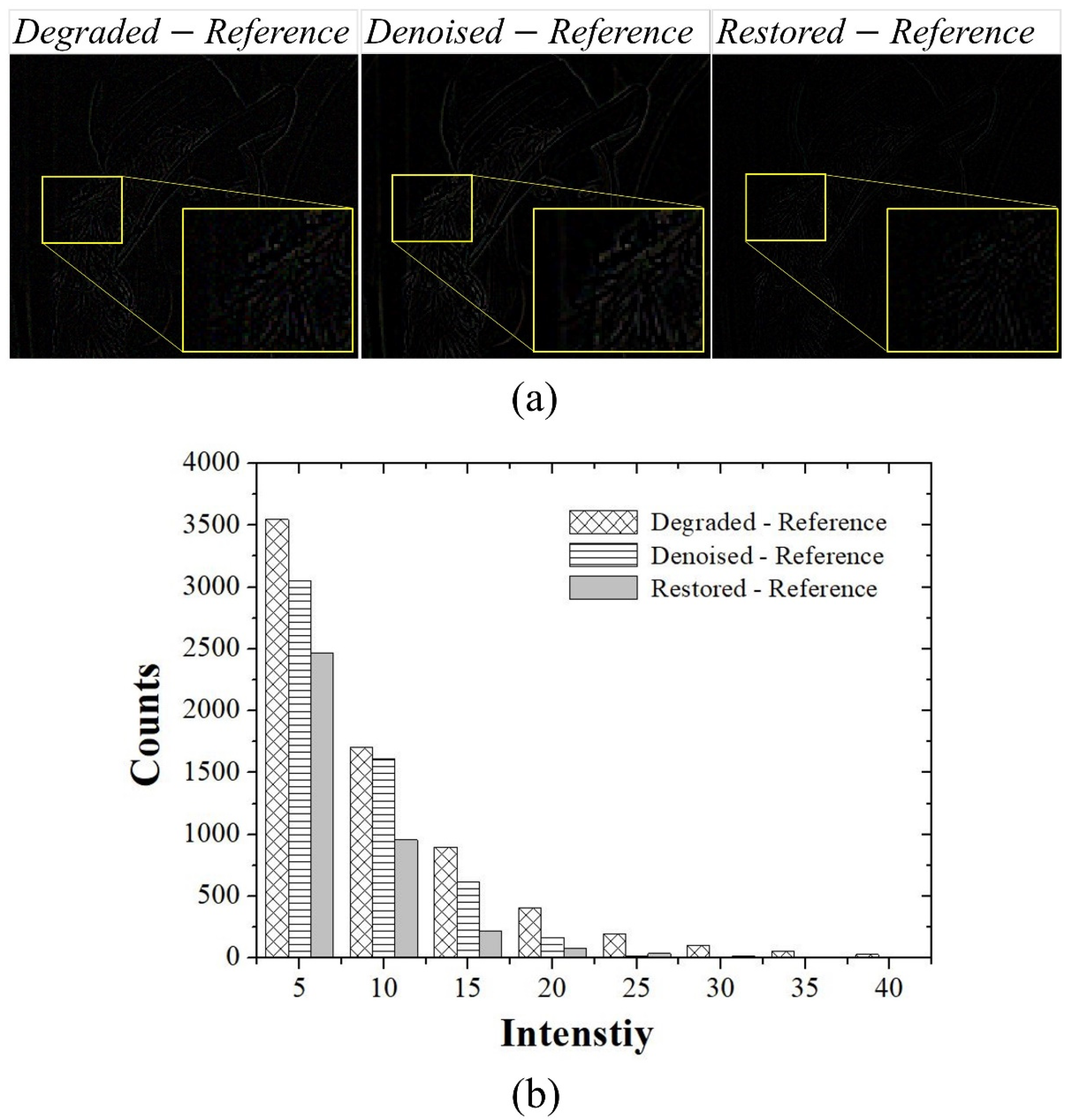
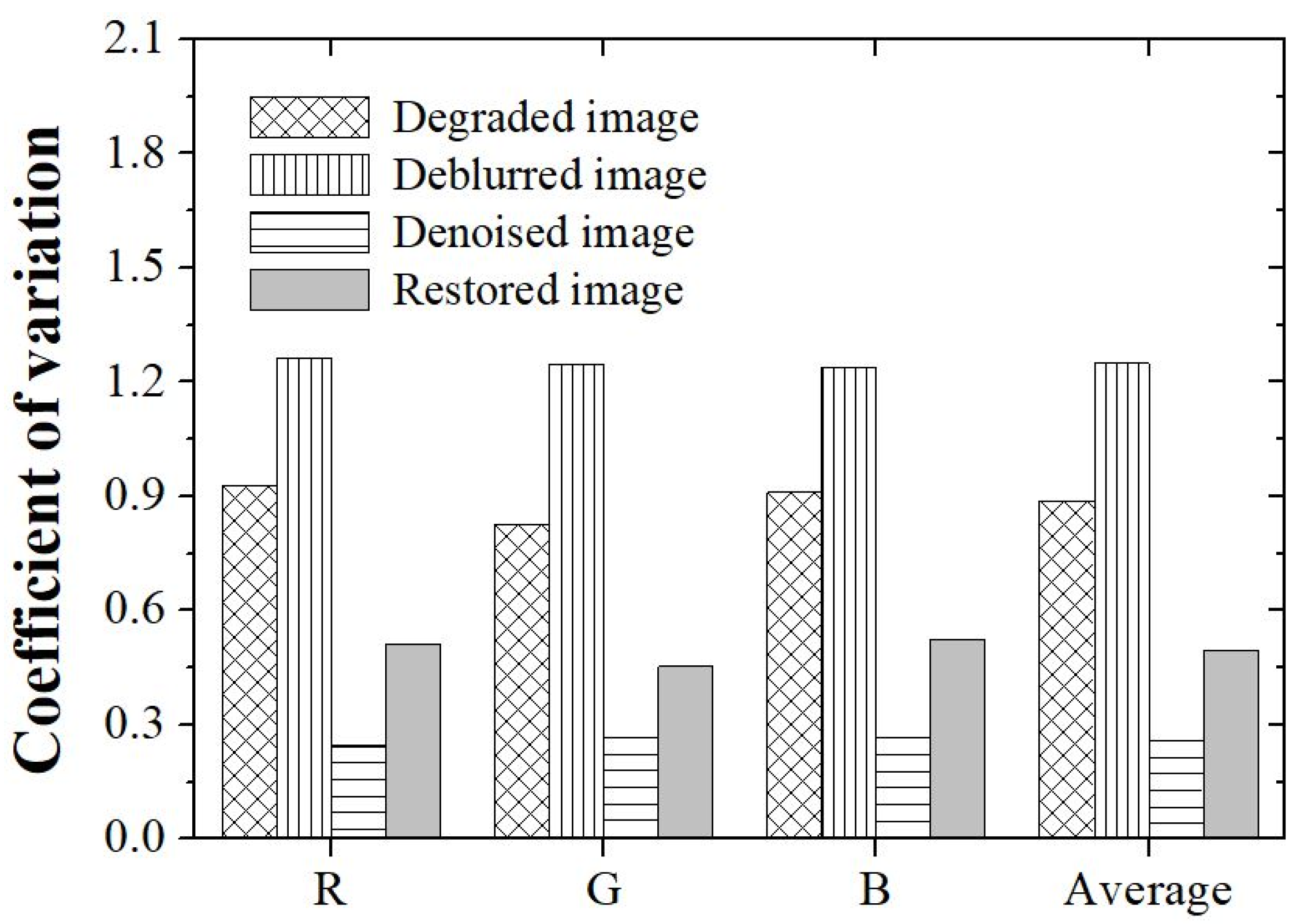
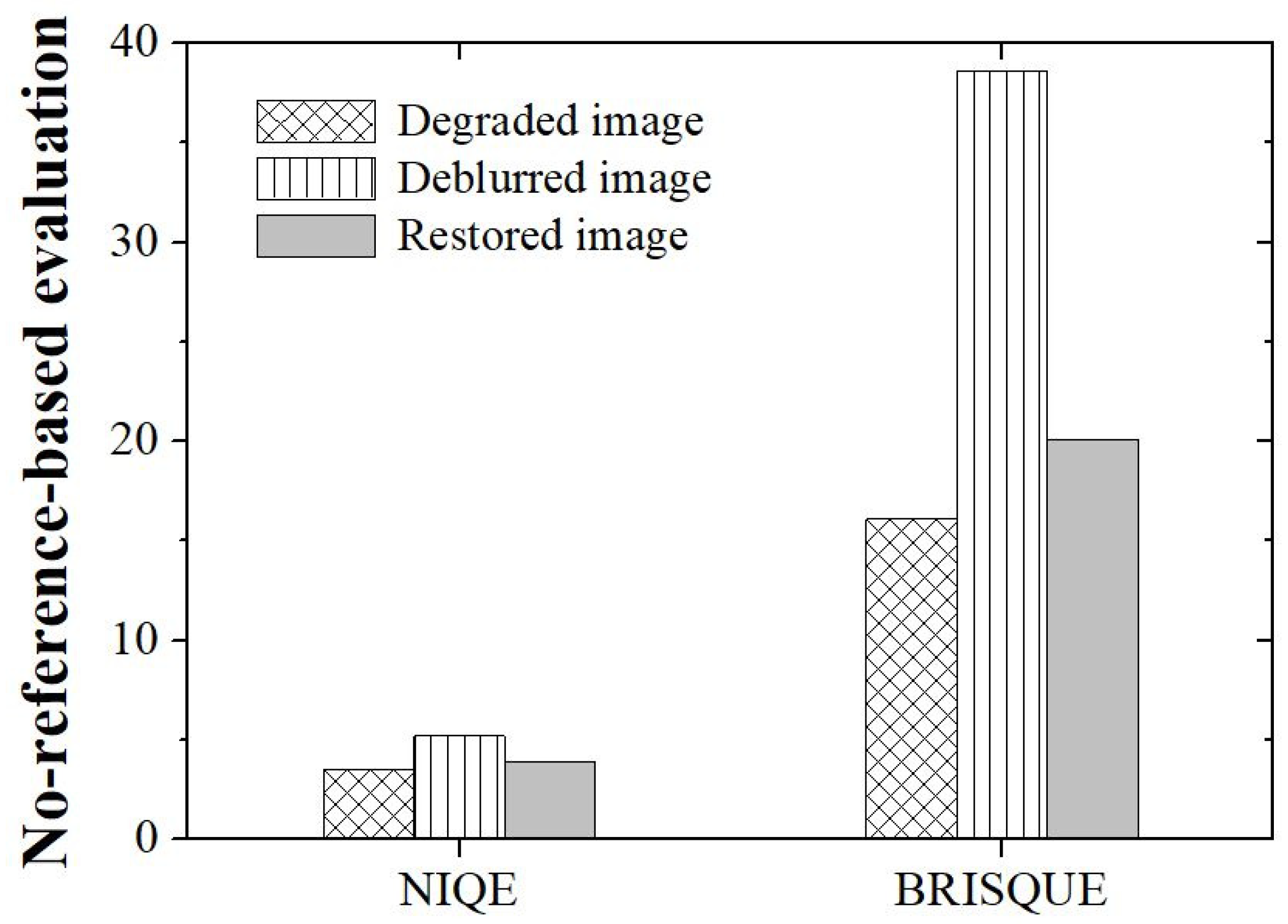
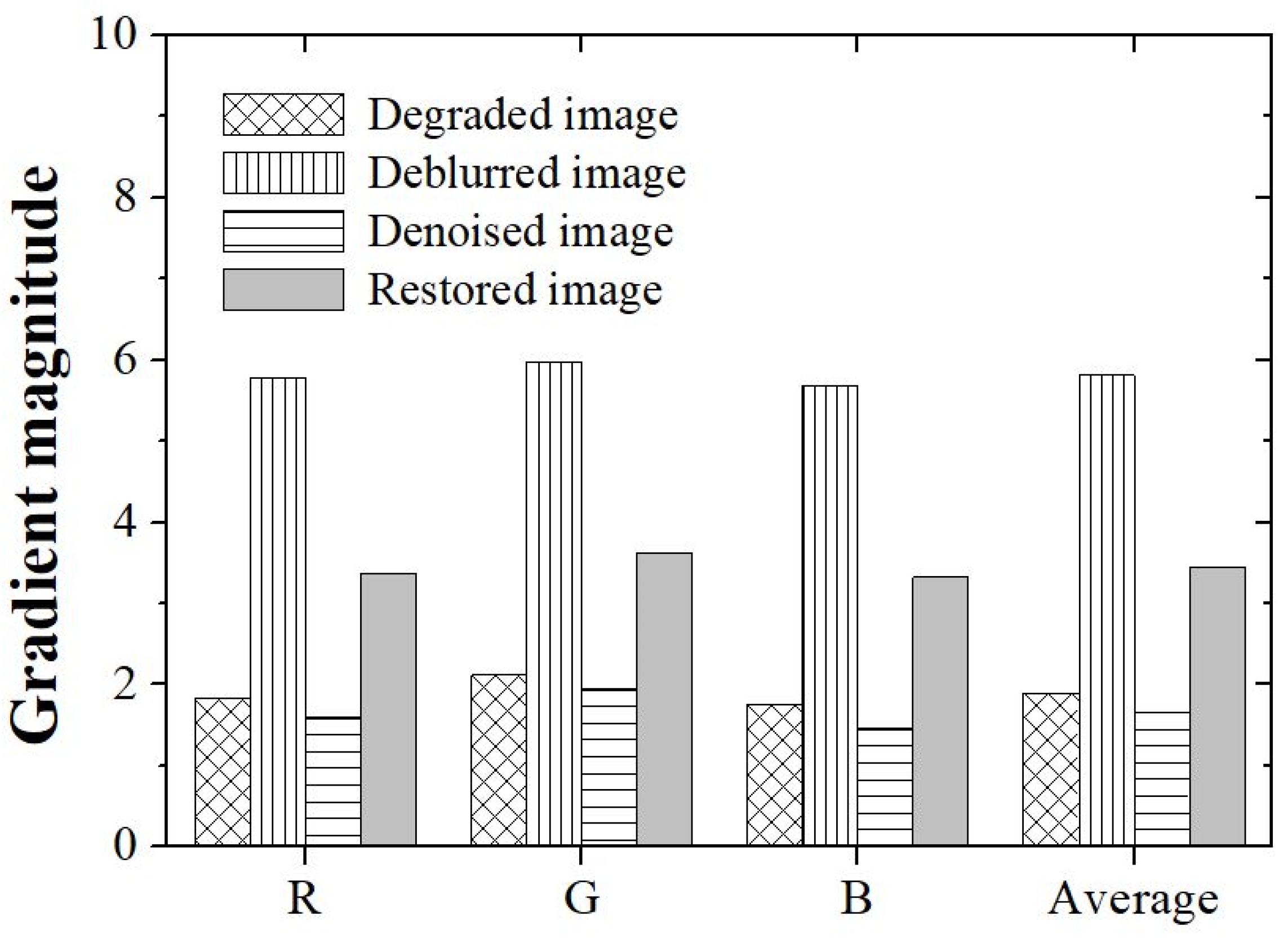
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Xue, F.; Qu, F.; Ho, Y.P.; Blu, T. On-the-fly estimation of a microscopy point spread function. Opt. Express 2018, 26, 26120–26133. [Google Scholar] [CrossRef]
- Cole, R.W.; Jinadasa, T.; Brown, C.M. Measuring and interpreting point spread functions to determine confocal microscope resolution and ensure quality control. Nat. Protoc. 2011, 6, 1929–1941. [Google Scholar] [CrossRef]
- Sarder, P.; Nehorai, A. Deconvolution methods for 3-D fluorescence microscopy images. IEEE Signal Process. Mag. 2006, 23, 32–45. [Google Scholar] [CrossRef]
- Born, M.; Wolf, E. Principles of Optics: 60th Anniversary Edition, 7th ed.; Cambridge University Press: Cambridge, UK, 2020; pp. 281–285. [Google Scholar]
- Kirshner, H.; Aguet, F.; Sage, D.; Unser, M. 3-D PSF fitting for fluorescence microscopy: Implementation and localization application. J. Microsc. 2013, 249, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Wiener, N. Extrapolation, Interpolation, and Smoothing of Stationary Time Series: With Engineering Applications; The MIT Press: Cambridge, MA, USA, 1964; pp. 1–163. [Google Scholar]
- Lampe, J.; Voss, H. A survey on variational characterizations for nonlinear eigenvalue problems. ETNA Electron. Trans. Numer. Anal. 2021, 55, 1–75. [Google Scholar] [CrossRef]
- Landweber, L. An Iteration Formula for Fredholm Integral Equations of the First Kind. Am. J. Math. 1951, 73, 615–624. [Google Scholar] [CrossRef]
- Beck, A.; Teboulle, M. A Fast Iterative Shrinkage-Thresholding Algorithm for Linear Inverse Problems. SIAM J. Imaging Sci. 2009, 2, 183–202. [Google Scholar] [CrossRef]
- Dey, N.; Blanc-Feraud, L.; Zimmer, C.; Roux, P.; Kam, Z.; Olivo-Marin, J.C.; Zerubia, J. Richardson-Lucy algorithm with total variation regularization for 3D confocal microscope deconvolution. Microsc. Res. Tech. 2006, 69, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Lucy, L.B. An iterative technique for the rectification of observed distributions. Astron. J. 1974, 79, 745. [Google Scholar] [CrossRef]
- Richardson, W.H. Bayesian-Based Iterative Method of Image Restoration*. J. Opt. Soc. Am. 1972, 62, 55–59. [Google Scholar] [CrossRef]
- Hom, E.F.; Marchis, F.; Lee, T.K.; Haase, S.; Agard, D.A.; Sedat, J.W. AIDA: An adaptive image deconvolution algorithm with application to multi-frame and three-dimensional data. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2007, 24, 1580–1600. [Google Scholar] [CrossRef] [PubMed]
- Sage, D.; Donati, L.; Soulez, F.; Fortun, D.; Schmit, G.; Seitz, A.; Guiet, R.; Vonesch, C.; Unser, M. DeconvolutionLab2: An open-source software for deconvolution microscopy. Methods 2017, 115, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Stockham, T.G.; Cannon, T.M.; Ingebretsen, R.B. Blind deconvolution through digital signal processing. Proc. IEEE 1975, 63, 678–692. [Google Scholar] [CrossRef]
- Cannon, M. Blind deconvolution of spatially invariant image blurs with phase. IEEE Trans. Acoust. Speech Signal Process. 1976, 24, 58–63. [Google Scholar] [CrossRef]
- Carasso, A.S. Linear and Nonlinear Image Deblurring: A Documented Study. Siam J. Numer. Anal. SIAM J. NUMER ANAL 1999, 36, 1659–1689. [Google Scholar] [CrossRef]
- Kundur, D.; Hatzinakos, D. Blind image deconvolution. IEEE Signal Process. Mag. 1996, 13, 43–64. [Google Scholar] [CrossRef]
- Holmes, T.J. Blind deconvolution of quantum-limited incoherent imagery: Maximum-likelihood approach. J. Opt. Soc. Am. A 1992, 9, 1052–1061. [Google Scholar] [CrossRef]
- Krishnamurthi, V.; Liu, Y.H.; Bhattacharyya, S.; Turner, J.N.; Holmes, T.J. Blind deconvolution of fluorescence micrographs by maximum-likelihood estimation. Appl. Opt. 1995, 34, 6633–6647. [Google Scholar] [CrossRef]
- Yang, Y.; Galatsanos, N.P.; Stark, H. Projection-based blind deconvolution. J. Opt. Soc. Am. A 1994, 11, 2401–2409. [Google Scholar] [CrossRef]
- Mika, S.; Schölkopf, B.; Smola, A.; Müller, K.-R.; Scholz, M.; Rätsch, G. Kernel PCA and De-Noising in Feature Spaces. Adv. Neural Inf. Process. Syst. 1999, 11, 536–542. [Google Scholar]
- Kenig, T.; Kam, Z.; Feuer, A. Blind image deconvolution using machine learning for three-dimensional microscopy. IEEE Trans. Pattern Anal. Mach. Intell. 2010, 32, 2191–2204. [Google Scholar] [CrossRef] [PubMed]
- Meiniel, W.; Olivo-Marin, J.C.; Angelini, E.D. Denoising of Microscopy Images: A Review of the State-of-the-Art, and a New Sparsity-Based Method. IEEE Trans. Image Process 2018, 27, 3842–3856. [Google Scholar] [CrossRef] [PubMed]
- Willis, B.; Roysam, B.; Turner, J.N.; Holmes, T.J. Iterative, constrained 3-D image reconstruction of transmitted light bright-field micrographs based on maximum likelihood estimation. J. Microsc. 1993, 169, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Tower, T.T.; Tranquillo, R.T. Alignment maps of tissues: I. Microscopic elliptical polarimetry. Biophys. J. 2001, 81, 2954–2963. [Google Scholar] [CrossRef]
- Prigent, S.; Dutertre, S.; Bidaud-Meynard, A.; Bertolin, G.; Michaux, G.; Kervrann, C. Sparse denoising and adaptive estimation enhances the resolution and contrast of fluorescence emission difference microscopy based on an array detector. Opt. Lett. 2023, 48, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fang, C.; Xie, X.; Yang, Y.; Mei, W.; Jin, D.; Fei, P. High-throughput, high-resolution deep learning microscopy based on registration-free generative adversarial network. Biomed. Opt. Express 2019, 10, 1044–1063. [Google Scholar] [CrossRef] [PubMed]
- van Kempen, G.M.; van Vliet, L.J. Background estimation in nonlinear image restoration. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2000, 17, 425–433. [Google Scholar] [CrossRef]
- Chaux, C.; Blanc-Féraud, L.; Zerubia, J. Wavelet-Based Restoration Methods: Application to 3D Confocal Microscopy Images; SPIE: Bellingham, WA, USA, 2007; Volume 6701. [Google Scholar]
- Izeddin, I.; Boulanger, J.; Racine, V.; Specht, C.G.; Kechkar, A.; Nair, D.; Triller, A.; Choquet, D.; Dahan, M.; Sibarita, J.B. Wavelet analysis for single molecule localization microscopy. Opt. Express 2012, 20, 2081–2095. [Google Scholar] [CrossRef]
- Sun, D.; Shi, Y.; Feng, Y. Blind deblurring and denoising via a learning deep CNN denoiser prior and an adaptive L0-regularised gradient prior for passive millimetre-wave images. IET Image Process. 2020, 14, 4774–4784. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, Z.; Huang, Z.; Hua, X.; Hong, H.; Li, L. HINRDNet: A half instance normalization residual dense network for passive millimetre wave image restoration. Infrared Phys. Technol. 2023, 132, 104722. [Google Scholar] [CrossRef]
- Cunha, A.L.D.; Zhou, J.; Do, M.N. The Nonsubsampled Contourlet Transform: Theory, Design, and Applications. IEEE Trans. Image Process. 2006, 15, 3089–3101. [Google Scholar] [CrossRef]
- Do, M.N.; Vetterli, M. The contourlet transform: An efficient directional multiresolution image representation. IEEE Trans. Image Process. 2005, 14, 2091–2106. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, M.; Chen, L.; Yu, J. The Nonsubsampled Contourlet Transform Based Statistical Medical Image Fusion Using Generalized Gaussian Density. Comput. Math. Methods Med. 2015, 2015, 262819. [Google Scholar] [CrossRef] [PubMed]
- Bamberger, R.H.; Smith, M.J.T. A filter bank for the directional decomposition of images: Theory and design. IEEE Trans. Signal Process. 1992, 40, 882–893. [Google Scholar] [CrossRef]
- Diwakar, M.; Kumar, M. Edge preservation based CT image denoising using Wiener filtering and thresholding in wavelet domain. In Proceedings of the 2016 Fourth International Conference on Parallel, Distributed and Grid Computing (PDGC), Waknaghat, India, 22–24 December 2016; pp. 332–336. [Google Scholar]
- Cannistraci, C.V.; Abbas, A.; Gao, X. Median Modified Wiener Filter for nonlinear adaptive spatial denoising of protein NMR multidimensional spectra. Sci. Rep. 2015, 5, 8017. [Google Scholar] [CrossRef]
- Ju, S.; Kang, S.-H.; Lee, Y. Optimization of mask size for median-modified Wiener filter according to matrix size of computed tomography images. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2021, 1010, 165508. [Google Scholar] [CrossRef]
- Amer, A.; Mitiche, A.; Dubois, E. Reliable and fast structure-oriented video noise estimation. In Proceedings of the Proceedings. International Conference on Image Processing, Rochester, NY, USA, 22–25 September 2002; pp. 840–843. [Google Scholar]
- Bernas, T.; Barnes, D.; Asem, E.K.; Robinson, J.P.; Rajwa, B. Precision of light intensity measurement in biological optical microscopy. J. Microsc. 2007, 226, 163–174. [Google Scholar] [CrossRef]
- Kedziora, K.M.; Prehn, J.H.; Dobrucki, J.; Bernas, T. Method of calibration of a fluorescence microscope for quantitative studies. J. Microsc. 2011, 244, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Sutour, C.; Deledalle, C.-A.; Aujol, J.-F. Estimation of the Noise Level Function Based on a Nonparametric Detection of Homogeneous Image Regions. SIAM J. Imaging Sci. 2015, 8, 2622–2661. [Google Scholar] [CrossRef]
- Kendall, M.G. The Treatment of Ties in Ranking Problems. Biometrika 1945, 33, 239–251. [Google Scholar] [CrossRef]
- Kendall, M.G. A New Measure of Rank Correlation. Biometrika 1938, 30, 81–93. [Google Scholar] [CrossRef]
- Kim, K.; Kim, W.; Kang, S.; Park, C.; Lee, D.; Cho, H.; Seo, C.; Lim, H.; Lee, H.; Kim, G.; et al. A blind-deblurring method based on a compressed-sensing scheme in digital breast tomosynthesis. Opt. Lasers Eng. 2018, 110, 228–235. [Google Scholar] [CrossRef]
- Chan, S.H.; Khoshabeh, R.; Gibson, K.B.; Gill, P.E.; Nguyen, T.Q. An augmented Lagrangian method for video restoration. In Proceedings of the 2011 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Prague, Czech Republic, 22–27 May 2011; pp. 941–944. [Google Scholar]
- Kim, J.Y.; Kim, K.; Lee, Y. Application of Blind Deconvolution Based on the New Weighted L(1)-norm Regularization with Alternating Direction Method of Multipliers in Light Microscopy Images. Microsc. Microanal. 2020, 26, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Sattar, F.; Floreby, L.; Salomonsson, G.; Lovstrom, B. Image enhancement based on a nonlinear multiscale method. IEEE Trans. Image Process. 1997, 6, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Moorthy, A.K.; Bovik, A.C. No-reference image quality assessment in the spatial domain. IEEE Trans. Image Process 2012, 21, 4695–4708. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Soundararajan, R.; Bovik, A.C. Making a “Completely Blind” Image Quality Analyzer. IEEE Signal Process. Lett. 2013, 20, 209–212. [Google Scholar] [CrossRef]
- Xue, W.; Zhang, L.; Mou, X.; Bovik, A.C. Gradient Magnitude Similarity Deviation: A Highly Efficient Perceptual Image Quality Index. IEEE Trans. Image Process. 2014, 23, 684–695. [Google Scholar] [CrossRef]
- Krishnan, D.; Tay, T.; Fergus, R. Blind deconvolution using a normalized sparsity measure. In Proceedings of the CVPR 2011, Colorado Springs, CO, USA, 20–25 June 2011; pp. 233–240. [Google Scholar]
- Xu, L.; Zheng, S.; Jia, J. Unnatural L0 Sparse Representation for Natural Image Deblurring. In Proceedings of the 2013 IEEE Conference on Computer Vision and Pattern Recognition, Portland, OR, USA, 23–28 June 2013; pp. 1107–1114. [Google Scholar]
- Krishnan, D.; Bruna, J.; Fergus, R. Blind Deconvolution with Non-local Sparsity Reweighting. arXiv 2013, arXiv:1311.4029. [Google Scholar]
- Kim, K.; Lee, M.-H.; Lee, Y. Investigation of a blind-deconvolution framework after noise reduction using a gamma camera in nuclear medicine imaging. Nucl. Eng. Technol. 2020, 52, 2594–2600. [Google Scholar] [CrossRef]
- Hajiaboli, M.R. An Anisotropic Fourth-Order Diffusion Filter for Image Noise Removal. Int. J. Comput. Vision. 2011, 92, 177–191. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.Y. Blind Deconvolution Based on Compressed Sensing with bi-I0-I2-norm Regularization in Light Microscopy Image. Int. J. Environ. Res. Public. Health 2021, 18, 1789. [Google Scholar] [CrossRef] [PubMed]
- Nasonov, A.; Krylov, A. An Improvement of BM3D Image Denoising and Deblurring Algorithm by Generalized Total Variation. In Proceedings of the 2018 7th European Workshop on Visual Information Processing (EUVIP), Tampere, Finland, 26–28 November 2018; pp. 1–4. [Google Scholar]
- Brunet, D.; Vrscay, E.R.; Wang, Z. On the mathematical properties of the structural similarity index. IEEE Trans. Image Process 2012, 21, 1488–1499. [Google Scholar] [CrossRef] [PubMed]
- Patro, J.; Panda, S.; Mohanty, N.; Mishra, U.S. The Potential of Light Microscopic Features of the Oral Mucosa in Predicting Post-mortem Interval. Sultan Qaboos Univ. Med. J. 2021, 21, e34–e41. [Google Scholar] [CrossRef] [PubMed]
- Rakhshanfar, M.; Amer, M.A. Low-frequency image noise removal using white noise filter. In Proceedings of the 2018 25th IEEE International Conference on Image Processing (ICIP), Athens, Greece, 7–10 October 2018. [Google Scholar] [CrossRef]
- Gupta, S.K.; Pal, R.; Ahmad, A.; Melandsø, F.; Habib, A. Image denoising in acoustic microscopy using block-matching and 4D filter. Sci. Rep. 2023, 13, 13212. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Kim, J.-Y. Performance Evaluation of L1-Norm-Based Blind Deconvolution after Noise Reduction with Non-Subsampled Contourlet Transform in Light Microscopy Images. Appl. Sci. 2024, 14, 1913. https://doi.org/10.3390/app14051913
Kim K, Kim J-Y. Performance Evaluation of L1-Norm-Based Blind Deconvolution after Noise Reduction with Non-Subsampled Contourlet Transform in Light Microscopy Images. Applied Sciences. 2024; 14(5):1913. https://doi.org/10.3390/app14051913
Chicago/Turabian StyleKim, Kyuseok, and Ji-Youn Kim. 2024. "Performance Evaluation of L1-Norm-Based Blind Deconvolution after Noise Reduction with Non-Subsampled Contourlet Transform in Light Microscopy Images" Applied Sciences 14, no. 5: 1913. https://doi.org/10.3390/app14051913
APA StyleKim, K., & Kim, J.-Y. (2024). Performance Evaluation of L1-Norm-Based Blind Deconvolution after Noise Reduction with Non-Subsampled Contourlet Transform in Light Microscopy Images. Applied Sciences, 14(5), 1913. https://doi.org/10.3390/app14051913





