Abstract
In micro-abrasive water jet polishing (MAWJP) technology, where abrasive particles serve as polishing tools, particles tend to form large clusters, leading to increased nozzle wear and diminished material polishing quality. Achieving a polishing solution with good dispersion stability is crucial for enhancing polishing accuracy and minimizing nozzle wear. Therefore, this study employed three dispersants with distinct dispersion mechanisms to examine the impact of each dispersant’s concentration on the dispersion stability of the polishing solution across various abrasive concentrations. Through experimentation, the optimal dispersant type and concentration ratio of abrasive to dispersant were determined, and the effect of the selected dispersant on jet polishing performance was validated. The results of the dispersion stability experiment indicated that, in comparison to Na(PO3)6 and polyethylene glycol (PEG), the polishing solution containing 1.0–2.0 wt% phosphoric ester compounds exhibited a more stable dispersion effect (zeta potential < −50 mV) and superior dispersibility, characterized by a smaller average particle size. Furthermore, K9 optical glass was subjected to fixed-point and local polishing using phosphoric ester compounds as the dispersant. The fixed-point polishing experiment revealed that, at a dispersant concentration of 1.0 wt% and an abrasive concentration of 20 wt%, a smooth and symmetrical material removal profile could be achieved. In the local polishing experiment, the reduction rate of the root mean square of the surface roughness (RMS) increased from 54.33% to 82.24%, and the reduction rate of peak-to-valley height difference in surface (PV) increased from 38.84% to 68.97%. In conclusion, the incorporation of a dispersant proves effective in enhancing the dispersion stability of the polishing solution and dispersibility of the abrasive particles, thereby improving the surface quality of the materials in MAWJP.
1. Introduction
Optical components featuring aspheric and free-form surfaces are progressively employed in aerospace, astronomy, national defense technology, and various other fields [1,2,3]. Consequently, there is an escalating demand for enhanced optical performance and surface accuracy in these components. Conventional mechanical polishing techniques, including cutting, grinding, and milling, necessitate direct contact between the tool and the part surface throughout the process. The polishing procedure generates heat and induces tool wear, leading to potential damage or sub-damage to the surfaces of the optical components. In pursuit of high-precision optical surfaces, and to mitigate surface damage, researchers have consistently investigated ultra-precision polishing technologies, including ion beam polishing, airbag polishing, magnetorheological polishing, abrasive water jet polishing, and vibration-assisted polishing [4]. Micro-abrasive water jet polishing (MAWJP) is an advanced polishing technology derived from abrasive water jet technology. Functioning as a non-contact polishing process, MAWJP avoids tool wear and thermal stress on the workpiece surface during polishing, rendering it apt for processing and polishing micro, complex-shaped workpiece surfaces [5]. This technology has found broad applications and has been extensively employed in the finishing of optical components and microgrooves [6].
Fähnle et al. [7] were pioneers in suggesting the application of abrasive jet technology for polishing BK7 glass. Their experimental outcomes demonstrated a decrease in the root mean square of the surface roughness (RMS) value after polishing, from 350 nm to 24 nm, while preserving a surface roughness of 1.6 nm. This substantiates the viability of employing abrasive water jet technology for optical surface polishing and shape modification. Subsequently, abrasive water jet polishing technology has garnered significant attention from a diverse group of scholars. Li et al. [8] optimized the removal profile derived from experiments and utilized it for polishing BK7 glass. The experimental outcomes demonstrated a decrease in the peak-to-valley height difference in the surface (PV) from 1.492λ to 0.294λ (λ = 632.8 nm), and in the RMS from 0.166λ to 0.029λ. Sun et al. [9] conducted a study involving fluid jet polishing on fused silica glass with a diameter of 120 mm and a thickness of 20 mm. The study explored the effects of abrasive slurry and incidence distance on polishing accuracy. The experimental outcomes indicated a reduction in the PV of the polished workpieces from 0.178λ to 0.046λ, and the RMS decreased from 1/25λ to 0.007λ. This suggests a substantial enhancement in the surface accuracy of the workpieces’ materials due to fluid jet polishing. Zhu et al. [10] observed a substantial enhancement in the surface accuracy of workpiece materials through the use of abrasive water jet technology for polishing brittle and hard materials. Liu et al. [11] conducted jet polishing experiments on hard and brittle materials, achieving a successful reduction in the surface roughness of silicate glass from 2.32 μm to 0.35 μm and silicon nitride material from 2.63 μm to 0.34 μm. Anbarasu et al. [12] introduced the concept of multi-stage polishing and investigated the impact of various parameter combinations on the surface roughness of BK7 optical glass through experiments. They concluded that employing large abrasive particles for initial-stage polishing and smaller abrasive particles for subsequent stages could more efficiently reduce the surface roughness of the workpiece.
The stability of the liquid jet significantly impacts the polishing accuracy of MAWJP. Upon exiting the nozzle, the disruptive influence of air on the jet beam induces a gradual loss of coherence, leading to unstable and unpredictable polishing points. This phenomenon hinders the assurance of surface accuracy during the polishing process for the workpiece [13]. Perec et al. [14] found that, when corundum is used as an abrasive, it can cause severe wear on the focusing tube. This is because, before abrasive particles enter the jet, they reflect multiple times from the outer surface of the jet and the inner surface of the focusing tube, causing various forms of wear of the focusing tube. Moreover, the long-time use of focusing tubes exposed to wear will lead to a loss of precision [15]. Furthermore, the dispersion stability of the polishing solution is influenced by the agglomeration and sedimentation of particles in the liquid, consequently impacting the polishing efficiency and quality [16,17]. Numerous scholars have undertaken significant studies on the dispersion stability of the polishing solution. Luo et al. [18] investigated the characteristics of the jet before and after the addition of polymers to the abrasive suspension, studying its effect on polishing optical glass. Their experiment revealed a reduced jet dispersion angle after the addition of the polymers, while the surface roughness of the jet-polished area remained consistent. Wang et al. [19] performed process parameter experiments on K9 optical glass, discovering that employing mixed additives resulted in a more uniform surface roughness distribution for the polished workpiece. Additionally, the boundary between the polished and non-polished areas could be reduced. Kowsari [20] introduced PEO polymers with various molecular weights and concentrations into the polishing solution in order to explore their impact on the width, shape, and centerline roughness of the microchannels. The study indicated that the addition of eight million molecular weight PEO polymers to the polishing solution effectively decreased the width of the microchannels and reduced the centerline roughness, resulting in a V-shape in the cross-section of the processed microchannels. However, an excessive concentration of PEO polymers induced transverse oscillations in the jet.
The characteristics of the polishing solution play a crucial role in the jet stability and polishing performance of MAWJP. Therefore, it is essential to formulate a polishing solution with good dispersibility and material removal performance to achieve high surface quality for optical glass. This study initially analyzes the influence of different types and concentrations of dispersants on the dispersibility stability of the polishing solution through static sedimentation experiments. It further investigates the zeta potential and average particle size in the samples after the addition of dispersants, exploring the impact of dispersants on the dispersibility of abrasive particles. The study then utilizes SEM technology to visually discuss the effects of the dispersants at different concentrations. Finally, polishing experiments on K9 optical glass are conducted to verify the influence of dispersants on polishing quality.
In contrast to previous studies focusing on a single dispersant, this study employs various methods to compare the effects of dispersants with different dispersing mechanisms. It identifies the optimal type of dispersant and the best combination of abrasive and dispersant concentrations, significantly enhancing the polishing quality. Moreover, this study goes beyond dispersibility analysis, confirming the practical application of the selected dispersants in jet polishing. It demonstrates their positive impact on K9 optical glass polishing, revealing their potential to improve the manufacturing process of optical components.
2. Materials and Methods
2.1. Experimental Materials
In this study, the abrasive particles utilized in the polishing solution were alumina particles with an average diameter of 1 μm (purity: 99.9%, model: XBH-300), procured from Shanghai Xinbiao Inspection Instrument Manufacturing Co., Ltd. (Shanghai, China). The following three dispersants were employed: sodium hexametaphosphate (Na(PO3)6, AR grade) with anionic properties, polyethylene glycol (abbreviated as PEG, MW ≈ 4000, AR grade) with nonionic properties, and phosphoric ester compounds with anionic properties (model: ZW-S06). Among these, Na(PO3)6 was acquired from Zhiyuan Chemical Reagent Co., Ltd. (Tianjin, China), PEG from Yatai United Chemical Co., Ltd. (Wuxi, China), and phosphoric ester compounds from Zhongwei Grinding Technology Co. The solvent used for the polishing solution was ultrapure water. The polishing sample for the micro-abrasive water jet experiments was K9 optical glass, with a diameter of 70 mm and a thickness of 4 mm, purchased from Oupute Technology Co., Ltd. (Beijing, China).
2.2. Preparation of Polishing Solution
The abrasive concentration is defined as the mass of abrasive contained in each liter of ultrapure water, and the dispersant concentration is defined as the ratio of the mass of the dispersant to the mass of ultrapure water. To investigate the impact of different dispersant types and concentrations on the dispersion stability of the polishing solution, a specific quantity of dispersant was added to 100 cm3 of ultrapure water. The dispersant was thoroughly dissolved in the ultrapure water through continuous stirring with a magnetic stirrer for 60 min. Subsequently, a designated amount of alumina particles was introduced to the solution while maintaining continuous stirring. The final polishing solution samples were prepared by thorough stirring for two hours with the magnetic stirrer. The combinations of abrasive concentration and dispersant concentration of all polishing solution samples involved in this study are shown in Table 1.

Table 1.
The combinations of abrasive concentration and dispersant concentration of all polishing solution samples.
2.3. Characterization of Dispersion Stability and Abrasive Particle Dispersibility
To characterize the dispersion stability of the polishing solution and the dispersibility of the abrasive particles, a series of static sedimentation experiments were conducted on each formulated polishing solution sample. The effects of the dispersant type and concentration on the dispersion stability and abrasive particle dispersibility were analyzed by measuring the zeta potential and average particle size of the samples. Finally, SEM analyses were performed on the dispersants with the best dispersing effect to explore the effect of different concentrations of dispersants from a visual perspective.
Initially, the prepared polishing solution samples underwent sequential static sedimentation experiments in reagent bottles. The dispersion stability was preliminarily assessed by observing whether the sample in the reagent bottle exhibited noticeable delamination after 30 min.
A zeta potential/particle size analyzer (model: ZETASIZER NANO ZSP, produced by Malvern Instruments, Malvern, UK, capable of measuring sample concentrations ranging from 1 ppm to 40% w/v) was employed to measure the zeta potential and average particle size of the samples. The 1-cm3 sample of thoroughly stirred polishing solution was dropped into the sample chamber, and its zeta potential was measured using the electrophoretic light scattering (ELS) method, while its particle size was measured using the dynamic light scattering (DLS) method at 25 °C. Each sample was measured three times, and the average value was taken as the final result. The dispersion stability of the polishing solution was categorized into the following five assessments based on the zeta potential values [21]: rapid coagulation or flocculation (0 to ±5 mV), incipient instability (±10 mV to ±30 mV), moderate stability (±30 mV to ±40 mV), good stability (±40 mV to ±60 mV), and excellent stability (≥±60 mV). Therefore, the impact of the dispersants on the stability of the polishing solution samples can be determined by the zeta potential. A larger average particle size indicates poorer dispersibility, while a smaller average particle size suggests better dispersibility.
The scanning electron microscope (model: EVO 10, produced by Zeiss, Oberkochen, Germany) was used to observe the microscopic morphology. The carrier stage was covered with aluminum foil fixed with conductive adhesive. A 1-cm3 sample of the polishing solution was dropped onto the foil and dried using an infrared lamp. The sample was then gold-sprayed and observed under SEM.
2.4. Polishing Experiments
To explore the impact of abrasive particle dispersibility on polishing quality, fixed-point polishing and local area (6 mm × 6 mm) polishing experiments were performed on K9 optical glass. These experiments were executed on a customized high-precision micro-abrasive water jet polishing platform, which consists of a three-axis working table, a control system, a hydraulic system, and a circulating system, as illustrated in Figure 1. The experimental parameters are detailed in Table 2.
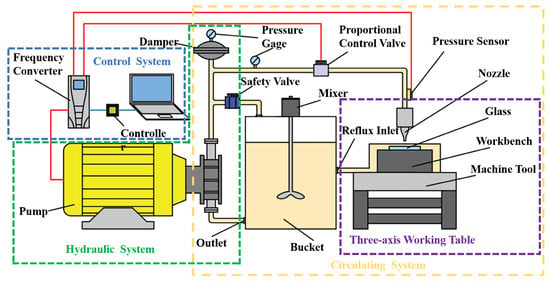
Figure 1.
Micro-abrasive water jet polishing system.

Table 2.
Experiment parameters.
For fixed-point polishing, experiments were conducted on different abrasive concentrations with the same dispersant concentration and on the same abrasive concentration with different dispersant concentrations. The removal profile of materials was analyzed to assess the impact of the dispersant on the polishing quality. In the case of local polishing, a polishing solution with an abrasive concentration of 20 g/dm3 and a phosphoric ester compounds dispersant concentration of 1.0 wt% was employed. The polishing path was set as the grid path, as depicted in Figure 2. The surface morphology of the workpieces was measured using a laser interferometer (model: VeriFire, produced by Zygo, Middlefield, CT, USA), and the measured RMS and PV values were utilized to evaluate the quality of the polishing.
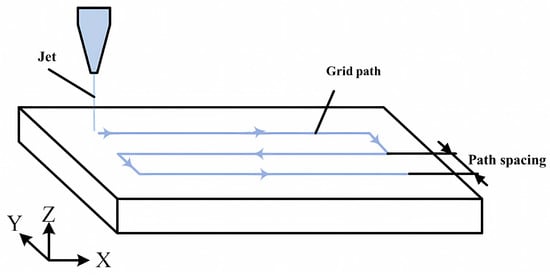
Figure 2.
Polishing path: grid path.
3. Results and Discussion
3.1. Dispersion Stability Analysis
In the polishing solutions with abrasive concentrations of 20 g/dm3, 60 g/dm3, and 100 g/dm3, various concentrations of Na(PO3)6 were added, and the sedimentation conditions after standing for 30 min are depicted in Figure 3. The corresponding photos are presented in Figure A1 in Appendix A, where reagent bottle numbers 1, 2, 3, 4, and 5, respectively, represent Na(PO3)6 concentrations of 0, 0.5 wt%, 1.0 wt%, 1.5 wt%, and 2.0 wt%. It can be observed from Figure A1 that, at the same Na(PO3)6 concentration, the sedimentation degree of the polishing solution increased, and delamination became more pronounced as the abrasive concentration rose. With different Na(PO3)6 concentrations at the same abrasive concentration, the sedimentation degree of the polishing solution varied. As shown in Figure 3, when the Na(PO3)6 concentration was 0.5 wt% and the abrasive concentration was 20 g/dm3, the abrasive particles hardly sedimented, indicating good dispersion stability in the polishing solution. However, at abrasive concentrations of 60 g/dm3 and 100 g/dm3, the abrasive particles showed slight sedimentation, and noticeable sedimentation occurred when the Na(PO3)6 concentration exceeded 1.0 wt%. At the same abrasive concentration, an increase in the Na(PO3)6 concentration initially improved and later decreased the dispersion stability of the polishing solution. Conversely, at the same Na(PO3)6 concentration, an increase in abrasive concentration intensified particle sedimentation, accentuated delamination, and deteriorated the dispersion stability.
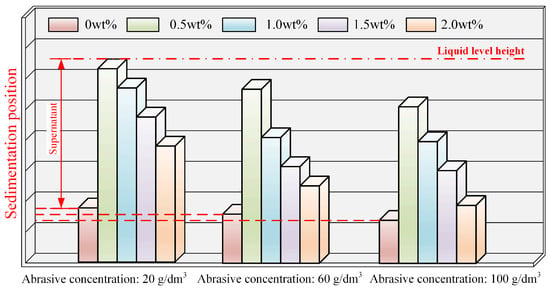
Figure 3.
Sedimentation conditions of polishing solution at different abrasive and Na(PO3)6 concentrations.
PEG, as a dispersant, was introduced into the polishing solution with varying abrasive concentrations, and the sedimentation patterns after 30 min are depicted in Figure 4. Actual photographs are presented in Figure A2 in Appendix A, with reagent bottle numbers 1, 2, 3, 4, 5, and 6 corresponding to PEG concentrations of 0, 0.5 wt%, 1.0 wt%, 1.5 wt%, 2.0 wt%, and 3.0 wt%, respectively. In comparison to samples with abrasive concentrations of 60 g/dm3 and 100 g/dm3, the dispersion stability of the polishing solution was slightly superior at an abrasive concentration of 20 g/dm3, with the delamination phenomenon becoming more pronounced as the abrasive concentration increased. At an abrasive concentration of 100 g/dm3, all of the samples approached a complete sedimentation state in approximately 15 min. Through analysis, it can be concluded that, at low abrasive concentrations, the content of abrasive particles in the polishing solution is lower, resulting in a reduced probability of particles coalescing into larger particles compared to high abrasive concentrations. Consequently, the sedimentation rate of particles at a low abrasive concentration is relatively slow. Under the same abrasive concentration, there was almost no significant difference between the samples with different PEG concentrations. Therefore, the static sedimentation experiments were analyzed, leading to the conclusion that changes in abrasive concentration influence the sedimentation rate of the polishing solution, but PEG has a limited impact on the dispersion stability of the alumina polishing solution.
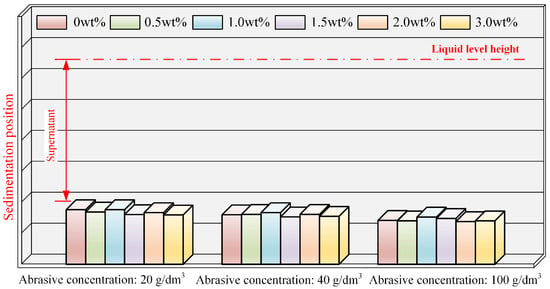
Figure 4.
Sedimentation conditions of polishing solution at different abrasive and PEG concentrations.
The results of the static sedimentation experiment for the polishing solution with added phosphoric ester compounds are depicted in Figure 5. Actual photographs are displayed in Figure A3 in Appendix A, with reagent bottle numbers 1, 2, 3, 4, 5, and 6 corresponding to phosphoric ester compounds concentrations of 0, 0.5 wt%, 1.0 wt%, 1.5 wt%, 2.0 wt%, and 3.0 wt%, respectively. A shown in Figure 5 and Figure A3, when the phosphoric ester compounds concentration was 0, the sedimentation level of the polishing solution increased with the rise in abrasive concentration. Under different abrasive concentrations, Sample 2 (0.5 wt%), Sample 3 (1.0 wt%), Sample 4 (1.5 wt%), and Sample 5 (2.0 wt%) all exhibited excellent dispersion stability without noticeable delamination; however, Sample 6 (3.0 wt%) showed slight delamination. Based on these experiments, it can be inferred that the phosphoric ester compounds dispersant displays superior dispersion stability compared to that of Na(PO3)6 and PEG.
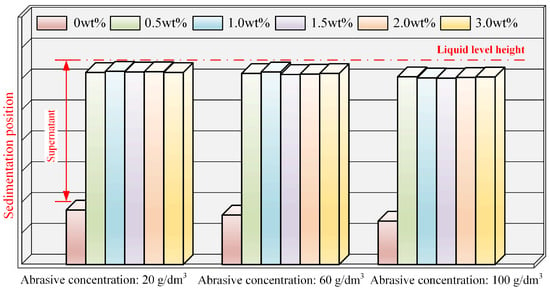
Figure 5.
Sedimentation conditions of polishing solution at different abrasive and phosphoric ester compounds concentrations.
3.2. Zeta Potential and Average Particle Size Analysis
The dispersion mechanism of Na(PO3)6 arises from the anions produced by hydrolysis attaching to the particle surfaces, forming an electric double layer and inducing repulsion between particles. As shown in Figure 6a, the absolute value of the zeta potential exhibited a negative correlation with an abrasive concentration at the same Na(PO3)6 concentration. This suggests a decrease in electrostatic repulsion between the particles as the abrasive concentration rises. Moreover, at a specific abrasive concentration, the absolute value of the zeta potential initially increased and then decreased with the rise in the Na(PO3)6 concentration. This phenomenon indicates that, when the Na(PO3)6 concentration reaches a certain threshold, hydrolyzed anions effectively cover the particle surface, enhancing the electrostatic repulsion between particles. However, surpassing this threshold may cause excessive anions to compress the electric double layer, diminishing electrostatic repulsion and hindering stable dispersion. It is noteworthy that, at an abrasive concentration of 100 g/dm3, the zeta potential fluctuated between −1.773 mV and −14.463 mV with varying Na(PO3)6 concentration, indicating an incipient instability state with poor dispersion stability and significant flocculation or agglomeration. When the Na(PO3)6 concentration was 0.5 wt% and the abrasive concentration was 20 g/dm3, the zeta potential reached −50.313 mV, ensuring good dispersion stability in the polishing solution. Therefore, maintaining a balance between the Na(PO3)6 concentration and the abrasive concentration is crucial for a stable dispersion state.
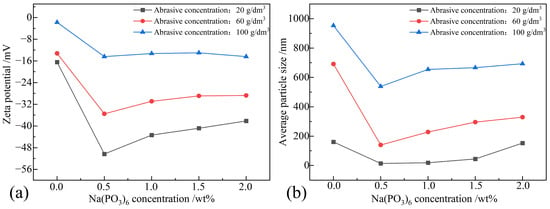
Figure 6.
Zeta potential and average particle size of polishing solution samples when Na(PO3)6 was used as a dispersant: (a) zeta potential; (b) average particle size.
Figure 6b illustrates that, with a Na(PO3)6 concentration of 0, the average particle size increased with the rise in the abrasive concentration. However, upon the addition of Na(PO3)6, there was a slight decrease in the average particle size. Under a constant abrasive concentration, when the Na(PO3)6 concentration was 0.5 wt%, the average particle size reached its minimum for that abrasive concentration. Nevertheless, with a further increase in the Na(PO3)6 concentration, the average particle size exhibited an upward trend. The average particle size was positively correlated with the abrasive concentration at any Na(PO3)6 concentration. This suggests that both a high abrasive concentration and a high Na(PO3)6 concentration may lead to the formation of large particle clusters, thereby reducing dispersibility.
Figure 7a illustrates the impact of the abrasive concentration and PEG concentration on the zeta potential. It can be observed from the graph that, at three different abrasive concentrations, there is no apparent linear relationship between the zeta potential of the polishing solution and the PEG concentration. This is because PEG, being a nonionic polymer, did not ionize in the solution, resulting in no correlation between the PEG concentration and the zeta potential at a certain abrasive concentration. When the abrasive concentration was 20 g/dm3, the zeta potential fluctuated between −14.817 mV and −16.950 mV, indicating that the polishing solution was in an incipient instability state. However, as the abrasive concentration increased to 100 g/dm3, the zeta potential fluctuated between −0.937 mV and −1.773 mV, indicating that the polishing solution was in the rapid coagulation or flocculation stage, suggesting poor dispersion stability. Additionally, it was observed that, under a constant PEG concentration, the absolute value of the zeta potential decreased with an increase in abrasive concentration. This is attributed to the reduced electrophoretic mobility of the solution with higher abrasive concentrations, leading to a decrease in the zeta potential. Overall, the maximum zeta potential in this experiment was −16.950 mV, indicating an unstable phase; therefore, PEG does not significantly enhance the dispersion stability of the alumina polishing solution.
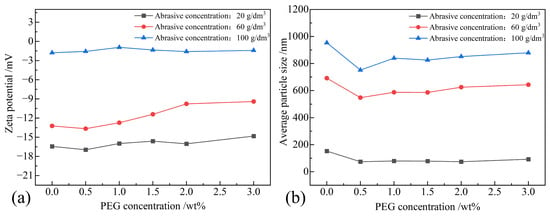
Figure 7.
Zeta potential and average particle size of polishing solution samples when PEG was used as a dispersant: (a) zeta potential; (b) average particle size.
The impact of different abrasive concentrations and PEG concentrations on the average particle size in the polishing solution is shown in Figure 7b. From the graph, it can be observed that, with an increase in abrasive concentration, the average particle size in the polishing solution also increased. As the PEG concentration increased, the average particle size initially decreased, then fluctuated within a small range. The analysis indicates that, when PEG serves as a dispersant, the average particle size distribution is narrower, implying a relatively weak enhancement in the dispersibility of the abrasive particles.
Figure 8a demonstrates the impact of phosphoric ester compounds and abrasive concentration on the zeta potential of the polishing solution. From the graph, it is evident that the absolute value of the zeta potential decreased with an increase in abrasive concentration and followed an initial increase and subsequent decrease trend with an increase in phosphoric ester compounds concentration. At an abrasive concentration of 20 g/dm3 and a phosphoric ester compounds concentration of 1.0 wt%, the zeta potential reached −61.117 mV; however, with an increase in phosphoric ester compounds concentration, the zeta potential decreased to −40.583 mV. Based on the zeta potential analysis, it can be inferred that, at an abrasive concentration of 20 g/dm3 and phosphoric ester compounds concentrations within the range of 0.5 wt% to 3.0 wt%, the polishing solution remained in a stable state, with the phosphoric ester compounds concentration of 1.0 wt% achieving excellent stability. At abrasive concentrations of 60 g/dm3 and 100 g/dm3, the zeta potential reached its maximum values of −45.133 mV and −40.404 mV, respectively, at phosphoric ester compounds concentrations of 1.0 wt% and 1.5 wt%, indicating a good stability. However, with an increase in phosphoric ester compounds concentration, the stability decreased, reaching a moderate stability at a concentration of 3.0 wt%. Overall, phosphoric ester compounds have a significant enhancing effect on the dispersion stability of the polishing solution. This is attributed to the multiple components in the mixture of phosphoric ester compounds, with their ionized ions adsorbing on the particle surface, strengthening the electrostatic repulsion between the particles. Additionally, the co-polymers formed after complete dissolution of phosphoric ester compounds adsorb onto the particle surface, providing strong steric hindrance, thus achieving good dispersion stability of the polishing solution.
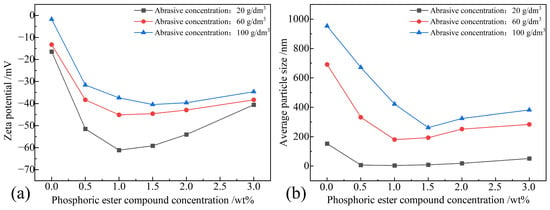
Figure 8.
Zeta potential and average particle size of polishing solution samples when phosphoric ester compounds were used as a dispersant: (a) zeta potential; (b) average particle size.
Figure 8b illustrates the relationship between the phosphoric ester compounds concentration, abrasive concentration, and average particle size. After adding phosphoric ester compounds, the minimum average particle size value, at 4.179 nm, was achieved at an abrasive concentration of 20 g/dm3 and a phosphoric ester compounds concentration of 1.0 wt%. As the abrasive concentration increased, the average particle size also increased, becoming nearly fifty times higher at an abrasive concentration of 100 g/dm3 compared to 20 g/dm3. Therefore, when phosphoric ester compounds are used as dispersants, the abrasive particles in the polishing solution exhibit better dispersibility at an abrasive concentration of 20 g/dm3 and a dispersant concentration of 1.0 wt%.
Through the above comparative analysis, it is evident that PEG is not suitable as a dispersant for an alumina polishing solution. Na(PO3)6 exhibits good dispersibility at an abrasive concentration of 20 g/dm3 and a dispersant concentration of 0.5 wt%. On the other hand, phosphoric ester compounds, when used as dispersants, demonstrate excellent dispersibility within the concentration range of 0.5 wt% to 2.0 wt%. Particularly, at an abrasive concentration of 20 g/dm3 and a dispersant concentration ratio of 1.0 wt%, phosphoric ester compounds show optimal dispersibility. By comparing the zeta potential, phosphoric ester compounds exhibit a superior dispersion stability compared to Na(PO3)6. Therefore, considering the effect of both dispersion stability on polishing solution and dispersibility on abrasive particles, the impact can be ranked as follows: phosphoric ester compounds > Na(PO3)6 > PEG.
3.3. SEM Analysis
Because phosphoric ester compounds exhibit the optimal dispersion stability and dispersibility in an alumina polishing solution, we conducted scanning electron microscope (SEM) analysis on polishing solution samples with different concentrations of phosphoric ester compounds at an abrasive concentration of 20 g/dm3 to intuitively analyze the dispersion characteristics of abrasive particles in the polishing solution, as shown in Figure 9. Figure 9a–d represent dispersant concentrations of 0, 0.5 wt%, 1.0 wt%, and 2.0 wt%, respectively. The regions marked with red circles in the figures indicate areas where abrasive particle aggregation is more pronounced.
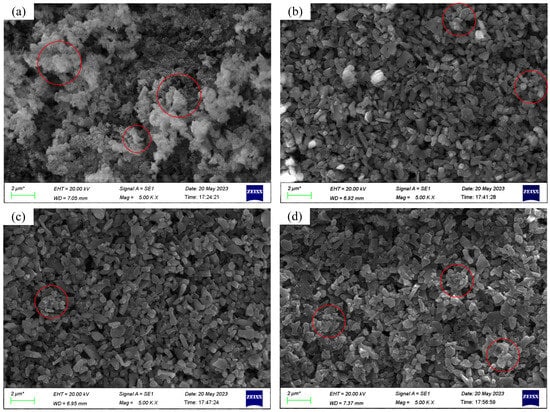
Figure 9.
SEM micrographs of polishing solution samples at an abrasive concentration of 20 g/dm3 with different phosphoric ester compounds concentrations: (a) phosphoric ester compounds concentration: 0; (b) phosphoric ester compounds concentration: 0.5 wt%; (c) phosphoric ester compounds concentration: 1.0 wt%; (d) phosphoric ester compounds concentration: 2.0 wt%. * Measuring scale: 2 μm.
From Figure 9a, it can be observed that, without the addition of a dispersant, a severe agglomeration of abrasive particles into large clusters occurred, and the abrasive particles in the polishing solution were almost present in block or flocculent forms. After adding a dispersant, as shown in Figure 9b,c, the dispersibility of abrasive particles was significant, and the overall distribution of abrasive particles was relatively uniform, with noticeable gaps between the particles. However, at a dispersant concentration of 0.5 wt%, some particles still exhibited cohesion, indicating that the amount of dispersant added was insufficient to fully cover the particle surfaces, leading to particle agglomeration. At a dispersant concentration of 1.0 wt%, the dispersibility of abrasive particles was highly significant, and there was no apparent agglomeration between the particles. However, at a dispersant concentration of 2.0 wt%, particle agglomeration reoccurred, and the overall dispersibility of abrasive particles decreased compared to the polishing solution containing 0.5 wt% and 1.0 wt% of dispersant. The analysis suggests that an excessively high concentration of phosphoric ester compounds causes co-polymer long chains to bind together, forming an adsorption layer around the particles and resulting in the particles being enveloped to form large clusters. Therefore, an excess of dispersant inhibits the dispersibility of abrasive particles.
From the SEM images, when phosphoric ester compounds are used as dispersants in an alumina polishing solution, concentrations ranging from 0.5 wt% to 2.0 wt% can achieve good dispersibility. The concentration of 1.0 wt% yields the best dispersibility, with abrasive particles exhibiting a uniform distribution. This observation further validates the conclusions drawn earlier through zeta potential measurements.
3.4. Polishing Performance Analysis
Figure 10 illustrates the impact of different abrasive concentrations on the fixed-point material removal profiles when phosphoric ester compounds were used as dispersants with a concentration of 1.0 wt%. At an abrasive concentration of 20 g/dm3, the material removal profile exhibited a relatively smooth and regular “W” shape. As the abrasive concentration increased, the material removal depth also increased, indicating an improvement in the removal rate. However, the material removal profile became irregular, especially at an abrasive concentration of 100 g/dm3, where the profile was highly irregular, with sharp transitions at the bottom. This is attributed to the decrease in abrasive dispersion stability because of the increase in abrasive concentration, leading to increased particle aggregation. This may cause temporary local blockages at the nozzle outlet, affecting the stability of the jet and consequently reducing the polishing quality.
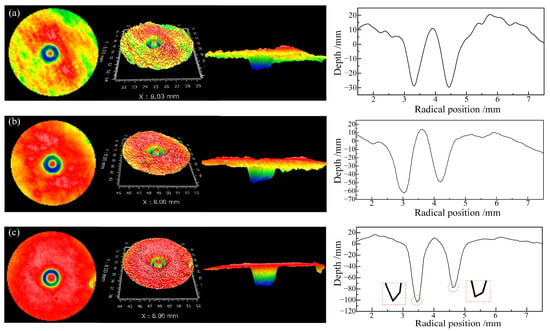
Figure 10.
Removal morphology and removal profile at a phosphoric ester compounds concentration of 1.0 wt% with different abrasive concentrations: (a) abrasive concentration: 20 g/dm3; (b) abrasive concentration: 60 g/dm3; (c) abrasive concentration: 100 g/dm3.
Figure 11 shows the influence of different phosphoric ester compounds concentrations on the fixed-point material removal profiles with an abrasive concentration of 20 g/dm3. When the phosphoric ester compounds concentration was 1.0 wt% and 1.5 wt%, the material removal profiles were similar, presenting a relatively smooth and regular shape. However, when the phosphoric ester compounds concentration was 0.5 wt% and 3.0 wt%, the removal profiles were less smooth at the bottom transition and were asymmetrical compared to the former concentrations. This indicates that insufficient or excessive dispersant concentration leads to inadequate dispersibility, causing locally unstable pressure on the target surface and uneven material removal profiles.
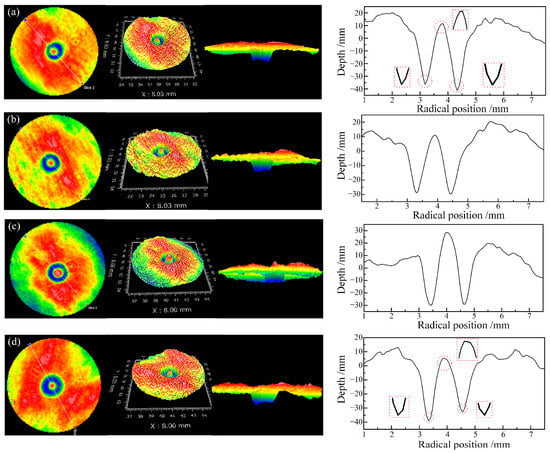
Figure 11.
Removal morphology and removal profile at an abrasive concentration of 20 g/dm3 with different phosphoric ester compounds concentrations: (a) phosphoric ester compounds concentration: 0.5 wt%; (b) phosphoric ester compounds concentration: 1.0 wt%; (c) phosphoric ester compounds concentration: 1.5 wt%; (d) phosphoric ester compounds concentration: 3.0 wt%.
Finally, local polishing experiments were conducted using grid path, and the surface morphology before and after polishing is shown in Figure 12. As a control group without the addition of a dispersant, the RMS of the polished K9 glass decreased from 32.291 nm to 14.748 nm, a reduction of 54.33%, and PV decreased from 135.832 nm to 83.071 nm, a reduction of 38.84%. With the addition of 1.0 wt% phosphoric ester compounds, the RMS of the polished K9 glass decreased from 25.067 nm to 4.451 nm, a reduction of 82.24%, and PV decreased from 113.053 nm to 35.078 nm, a reduction of 68.97%. Clearly, phosphoric ester compounds as dispersants significantly improved the polishing quality of MAWJP.
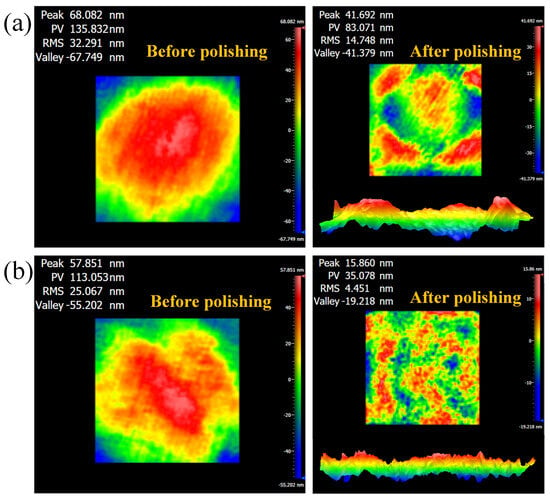
Figure 12.
Comparison of polished surface morphology of local area before and after the addition of dispersant: (a) without dispersant; (b) 1.0 wt% phosphoric ester compounds.
Overall, the experimental results indicate that phosphoric ester compounds can enhance the polishing quality of the polishing solution within a certain concentration range. Under the conditions of an abrasive concentration of 20 g/dm3 and a phosphoric ester compounds concentration of 1.0 wt%, the polishing effect is optimal, resulting in regular, smooth removal profiles and high-quality surface morphology.
4. Conclusions
In this study, the impact of different types of dispersants (Na(PO3)6, PEG, and phosphoric ester compounds), dispersant concentrations, and abrasive concentrations on the dispersion stability of a polishing solution and the dispersibility of abrasive particles were investigated through sedimentation experiments, zeta potential measurements, and particle size analysis. Additionally, the influence of phosphoric ester compounds as dispersants on jet polishing performance was explored through micro-abrasive water jet polishing experiments. The following conclusions were drawn:
- The dispersion stability of the polishing solution is negatively correlated with the abrasive concentration, showing an initial increase and subsequent decrease with an increasing dispersant concentration. The impact of different dispersants on the dispersion stability of the polishing solution and dispersibility of abrasive particles is ranked as follows: phosphoric ester compounds > Na(PO3)6 > PEG. Particularly, at a phosphoric ester compounds concentration of 1.0 wt% and an abrasive concentration of 20 g/L, the polishing solution exhibits the most stable dispersion effect. Na(PO3)6, at a concentration of 0.5 wt% and abrasive concentration of 20 g/dm3, performs great as well;
- For point polishing, the optimal concentration ratio of the polishing solution is a phosphoric ester compounds concentration of 1.0 wt% and an abrasive concentration of 20 g/dm3. At this concentration ratio, a smooth, regular, and symmetrical material removal profile is achieved;
- The polishing solution with the optimal concentration ratio significantly enhances the polishing quality, leading to a substantial reduction in RMS and PV. In local polishing experiments, compared to the polishing solution without a dispersant, the reduction rate of RMS increased from 54.33% to 82.24%, and the reduction rate of PV increased from 38.84% to 68.97%.
In summary, this study concentrated on the impact of dispersants on polishing quality but did not examine nozzle wear, which will be explored in future research.
Author Contributions
Conceptualization, L.L. and Y.Z.; methodology, D.J.; software, D.J.; validation, D.J., Y.Z. and L.L.; formal analysis, D.J.; investigation, Y.Z.; resources, H.Y.; data curation, Y.Z.; writing—original draft preparation, Y.Z.; writing—review and editing, D.J.; visualization, L.L.; supervision, L.L.; project administration, L.L.; funding acquisition, H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Guangxi Bagui Scholars Project (NO. 2019A02) and Guangxi Key Laboratory of Manufacturing System and Advanced Manufacturing Technology (Grant No. 15-140-305005).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Acknowledgments
Thanks to all who contributed to this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Figure A1.
Actual photos of sedimentation conditions of Na(PO3)6 as a dispersant: (a) abrasive concentration: 20 g/dm3; (b) abrasive concentration: 60 g/dm3; (c) abrasive concentration: 100 g/dm3.

Figure A2.
Actual photos of sedimentation conditions of PEG as a dispersant: (a) abrasive concentration: 20 g/dm3; (b) abrasive concentration: 60 g/dm3; (c) abrasive concentration: 100 g/dm3.

Figure A3.
Actual photos of sedimentation conditions of phosphoric ester compounds as a dispersant: (a) abrasive concentration: 20 g/dm3; (b) abrasive concentration: 60 g/dm3; (c) abrasive concentration: 100 g/dm3.
References
- Xie, M.; Pan, Y.; An, Z.; Huang, S.; Dong, M. Review on Surface Polishing Methods of Optical Parts. Adv. Mater. Sci. Eng. 2022, 2022, 8723269. [Google Scholar] [CrossRef]
- Wu, Z.; Shen, J.; Peng, Y.; Wu, X. Review on ultra-precision bonnet polishing technology. Int. J. Adv. Manuf. Technol. 2022, 121, 2901–2921. [Google Scholar] [CrossRef]
- Liu, F.; Wu, Y.; Chen, Q.; Liu, H.; Yan, F.; Zhang, S.; Wan, Y.; Wu, F. Overview of advanced manufacturing technology of large-aperture aspheric mirror. Opto-Electron. Eng. 2020, 47, 200203-1. [Google Scholar]
- Wang, Z.; Shi, C.; Zhang, P.; Yang, Z.; Chen, Y.; Guo, J. Recent Progress of Advanced Optical Manufacturing Technology. J. Mech. Eng. 2021, 57, 23–56. [Google Scholar]
- Wang, C.; Zhang, Z.; Cheung, C.F.; Luo, W.; Loh, Y.M.; Lu, Y.; Kong, L.; Wang, S. Maskless fluid jet polishing of optical structured surfaces. Precis. Eng. J. Int. Soc. Precis. Eng. Nanotechnol. 2022, 73, 270–283. [Google Scholar] [CrossRef]
- Ge, J.; Ren, Y.; Li, C.; Li, Z.; Yan, S.; Tang, P.; Xu, X.; Wang, Q. Ultrasonic coupled abrasive jet polishing (UC-AJP) of glass-based micro-channel for micro-fluidic chip. Int. J. Mech. Sci. 2023, 244, 108055. [Google Scholar] [CrossRef]
- Fahnle, O.W.; van Brug, H.; Frankena, H.J. Fluid jet polishing of optical surfaces. Appl. Opt. 1998, 37, 6771–6773. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, S.; Dai, Y.; Peng, X. Optimization and application of influence function in abrasive jet polishing. Appl. Opt. 2010, 49, 2947–2953. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Sun, L.; Jin, Y.; Wang, F.; Sun, D. Research on the fluid jet polishing in advanced optical fabrication. In Proceedings of the 7th International Symposium on Advanced Optical Manufacturing and Testing Technologies (AOMATT)—Advanced Optical Manufacturing Technologies, Harbin, China, 21–23 October 2014. [Google Scholar]
- Zhu, H.; Huang, C.; Wang, J.; Li, Q.; Che, C. Experimental study on abrasive waterjet polishing for hard–brittle materials. Int. J. Mach. Tools Manuf. 2009, 49, 569–578. [Google Scholar] [CrossRef]
- Liu, Z.W.; Liu, R. Study on Polishing Technology for Hard-Brittle Materials by a Micro Abrasive Water Jet. Adv. Mater. Res. 2014, 1027, 52–57. [Google Scholar] [CrossRef]
- Anbarasu, K.G.; Vijayaraghavan, L.; Arunachalam, N. Effect of multi stage abrasive slurry jet polishing on surface generation in glass. J. Am. Acad. Dermatol. 2019, 267, 384–392. [Google Scholar]
- Tricard, M.; Kordonski, W.; Shorey, A.; Evans, C. Magnetorheological Jet Finishing of Conformal, Freeform and Steep Concave Optics. CIRP Ann. Manuf. Technol. 2006, 55, 309–312. [Google Scholar] [CrossRef]
- Perec, A. Research into the Disintegration of Abrasive Materials in the Abrasive Water Jet Machining Process. Materials 2021, 14, 3940. [Google Scholar] [CrossRef] [PubMed]
- Perec, A.; Pude, F.; Grigoryev, A.; Kaufeld, M.; Wegener, K. A study of wear on focusing tubes exposed to corundum-based abrasives in the waterjet cutting process. Int. J. Adv. Manuf. Technol. 2019, 104, 2415–2427. [Google Scholar] [CrossRef]
- Liao, Y.P.; Wang, C.Y.; Hu, Y.N.; Song, Y.X. The Slurry for Glass Polishing by Micro Abrasive Suspension Jets. In Proceedings of the 8th China-Japan International Conference on Ultra-Precision Machining, Changsha, China, 21–23 August 2008; p. 322. [Google Scholar]
- Nguyen, T.; Shanmugam, D.; Wang, J. Effect of liquid properties on the stability of an abrasive waterjet. Int. J. Mach. Tools Manuf. 2008, 48, 1138–1147. [Google Scholar] [CrossRef]
- Luo, W.; Wang, C.; Song, Y.; Liao, Y. Characteristics and polishing effect of abrasive jet beam with polymer abrasive suspension additives. In Proceedings of the 13th International Symposium on Advances in Abrasive Technology, 1st Cross-Strait Conference on Precision Machining, Taipei, Taiwan, 19–22 September 2010; p. 9. [Google Scholar]
- Wang, R.J.; Wang, C.Y.; Wen, W.; Wang, J. Experimental study on a micro-abrasive slurry jet for glass polishing. Int. J. Adv. Manuf. Technol. 2016, 89, 451–462. [Google Scholar] [CrossRef]
- Kowsari, K.; James, D.; Papini, M.; Spelt, J. The effects of dilute polymer solution elasticity and viscosity on abrasive slurry jet micro-machining of glass. Wear 2014, 309, 112–119. [Google Scholar] [CrossRef]
- Prakash, S.; Mishra, R.; Malviya, R.; Sharma, P.K. Measurement Techniques and Pharmaceutical Applications of Zeta Potential: A Review. J. Chronother. Drug Deliv. 2014, 5, 33–40. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).