Optimization of Deposition Parameters of SnO2 Particles on Tubular Alumina Substrate for H2 Gas Sensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Characterizations
2.2. Gas Sensor Fabrication and Sensing Measurement
3. Results and Discussion
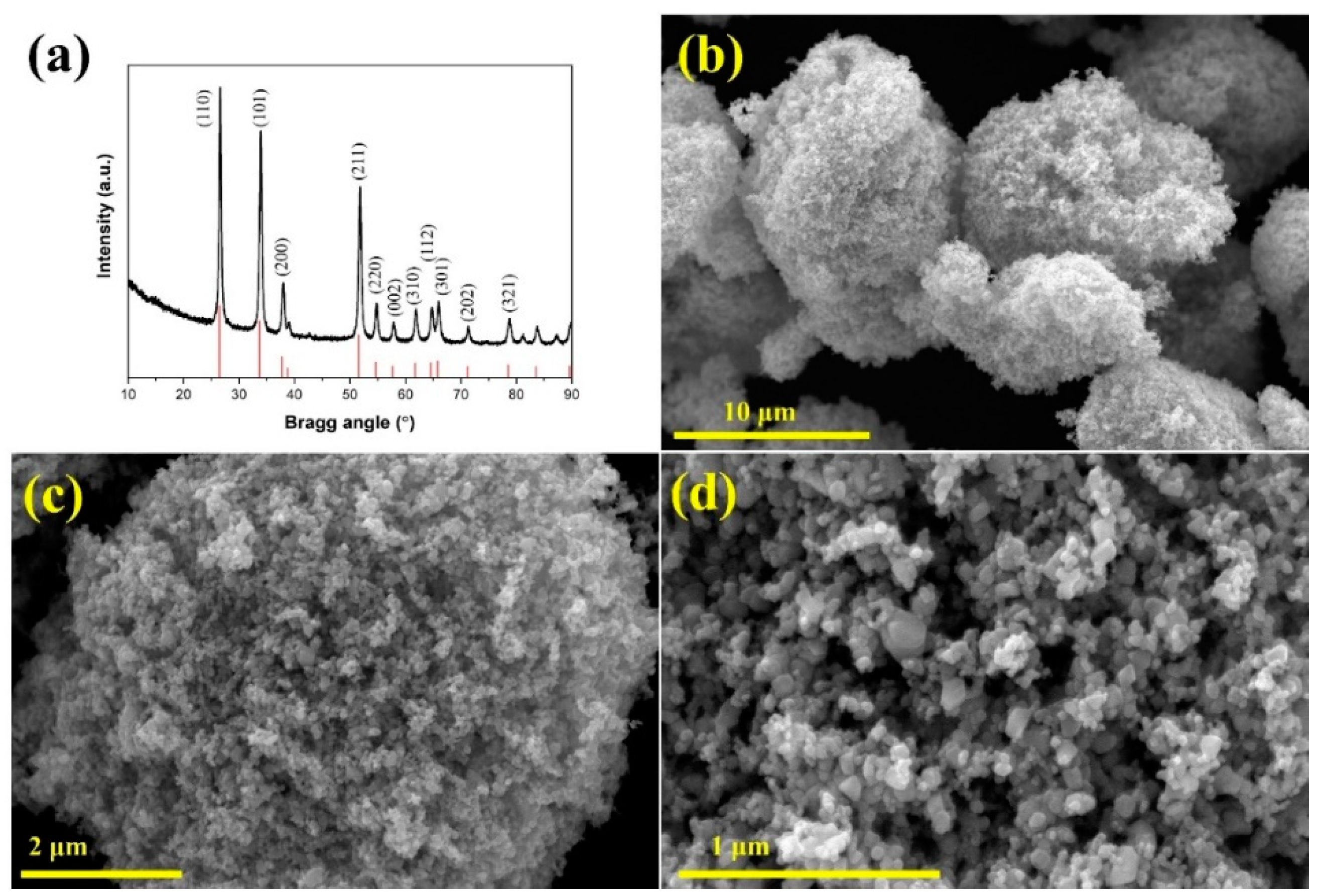
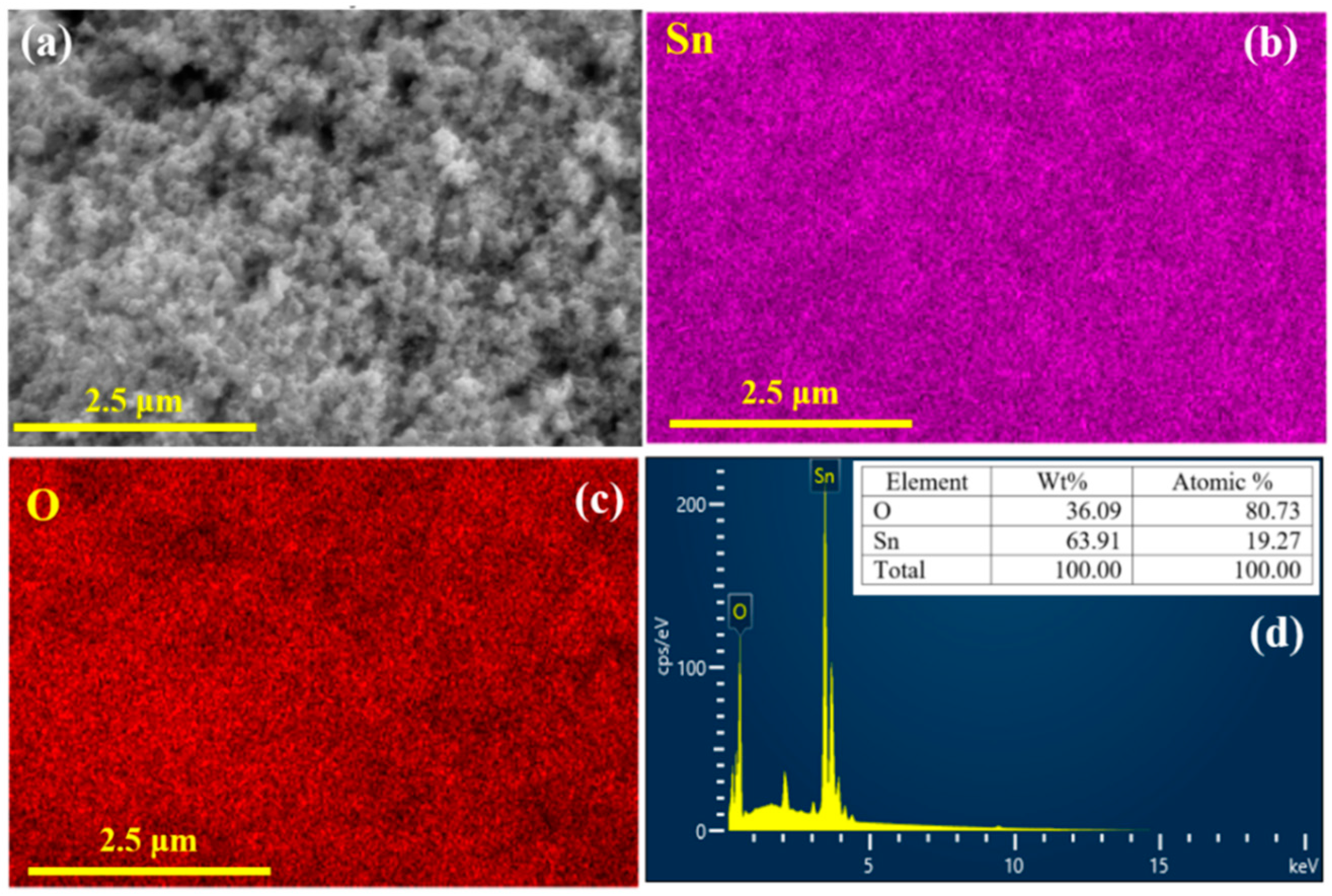
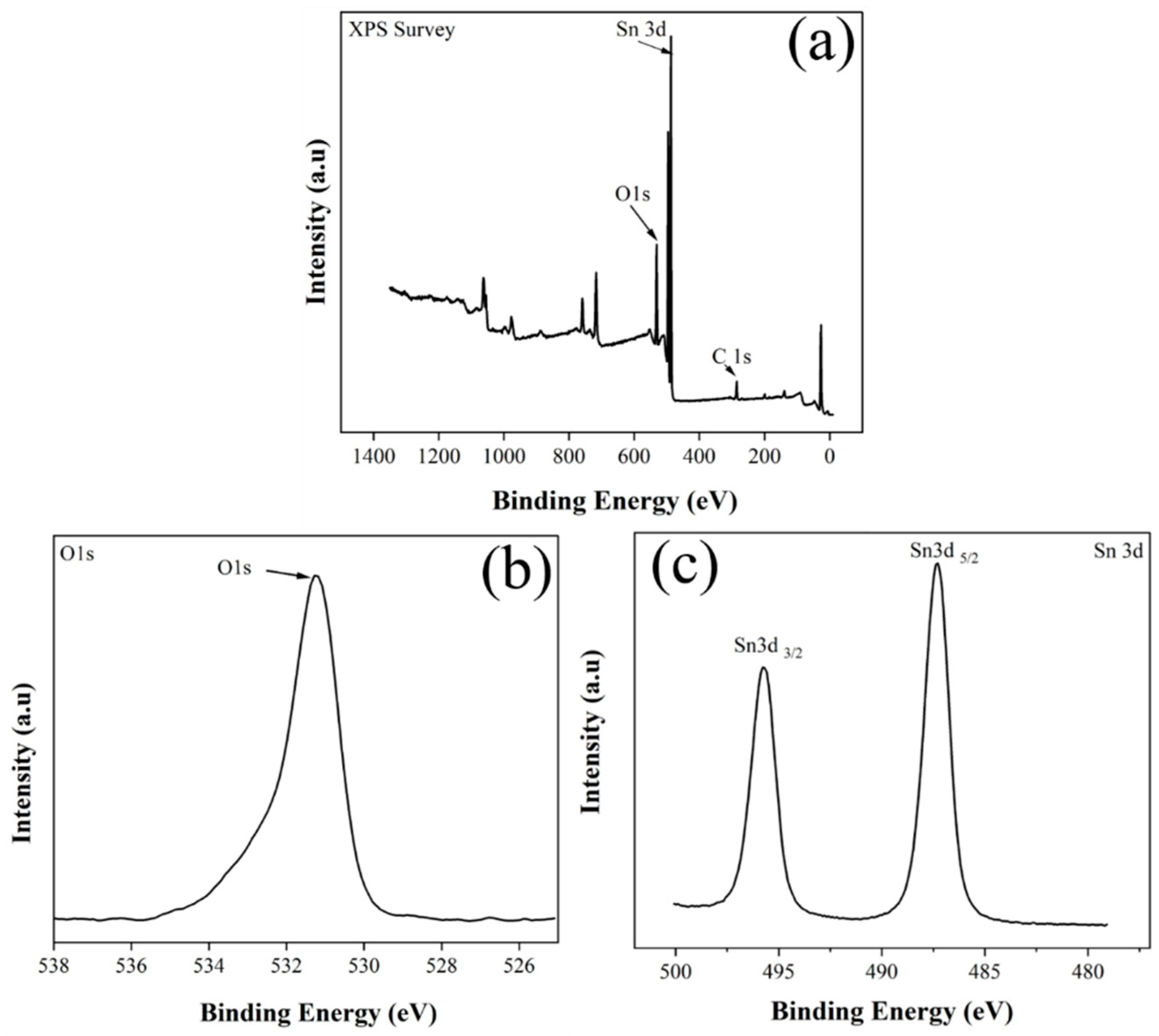
3.1. Characterization Studies
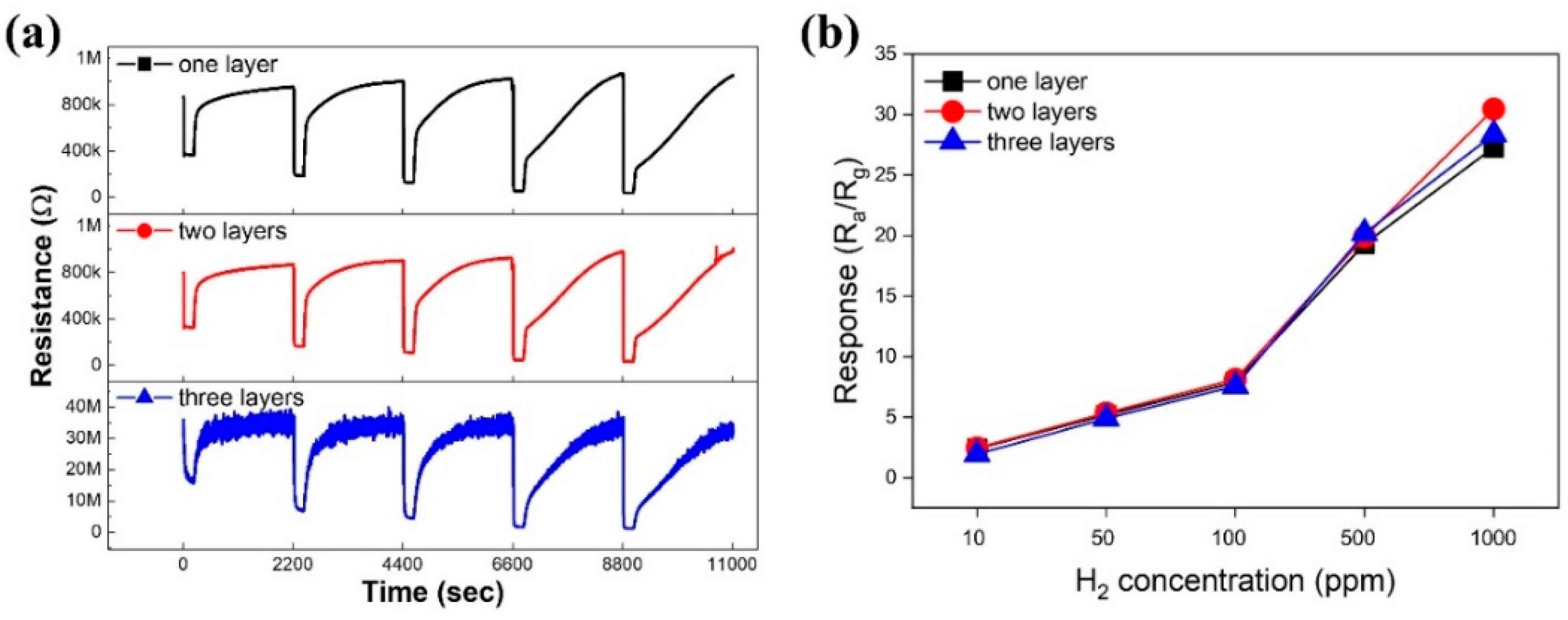
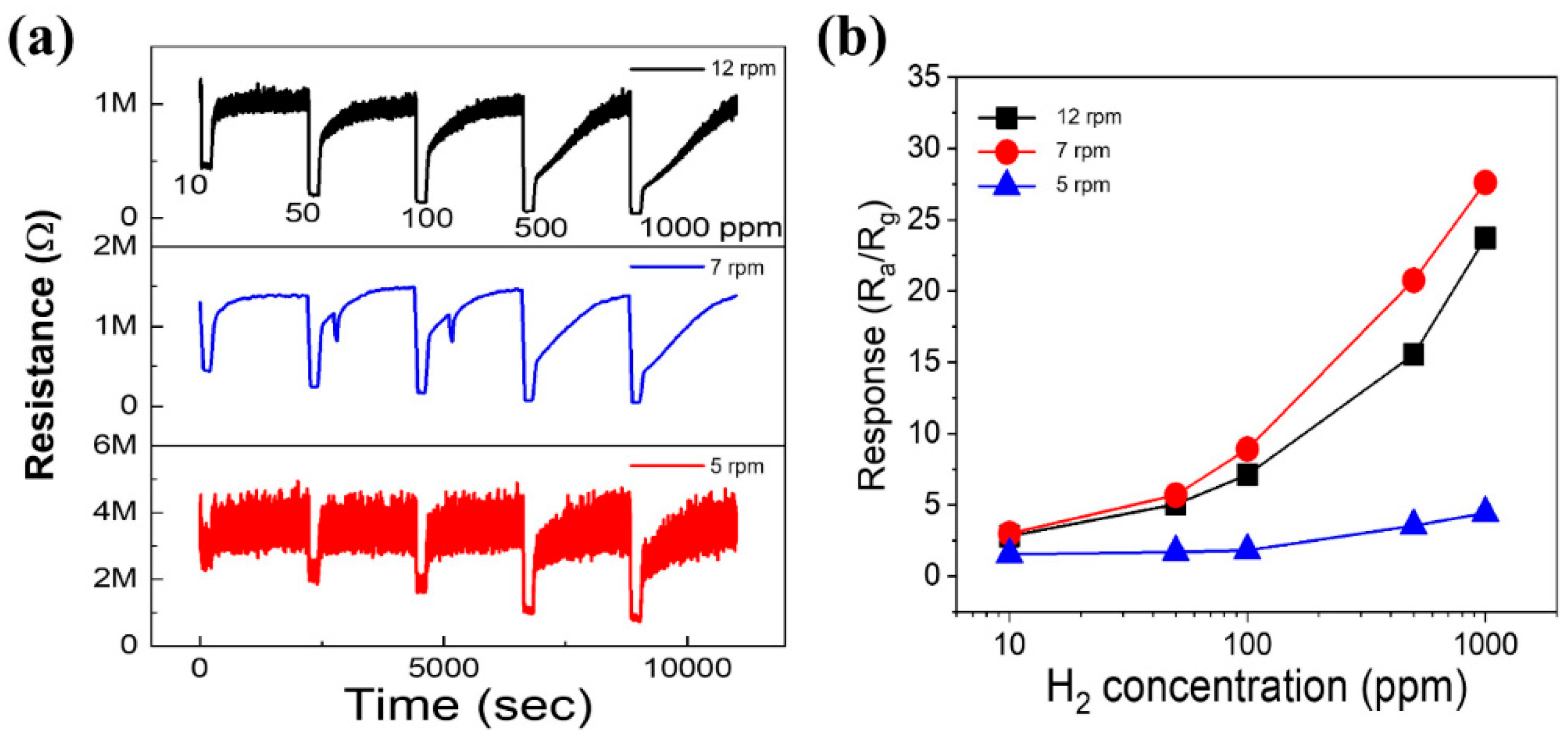
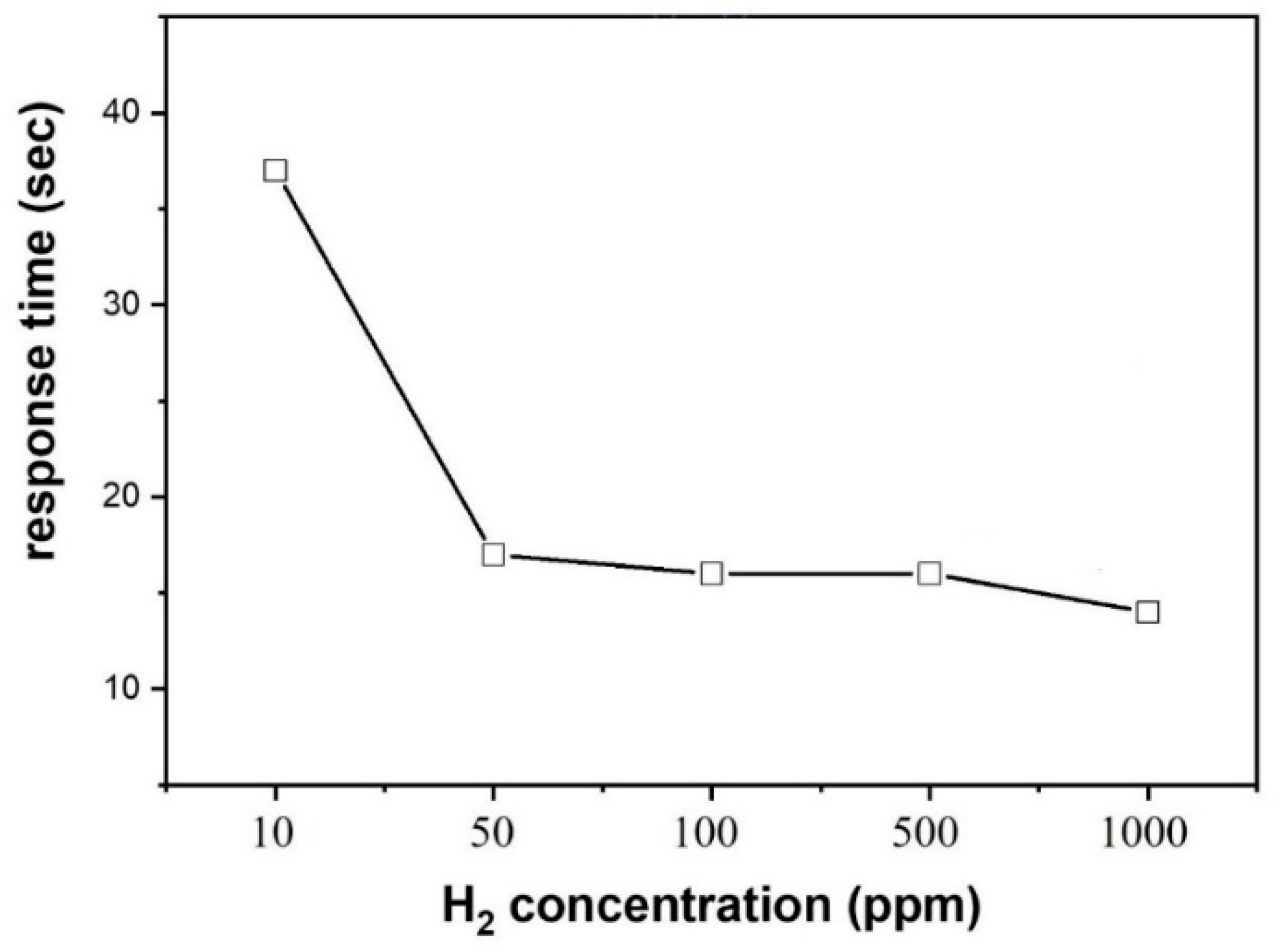
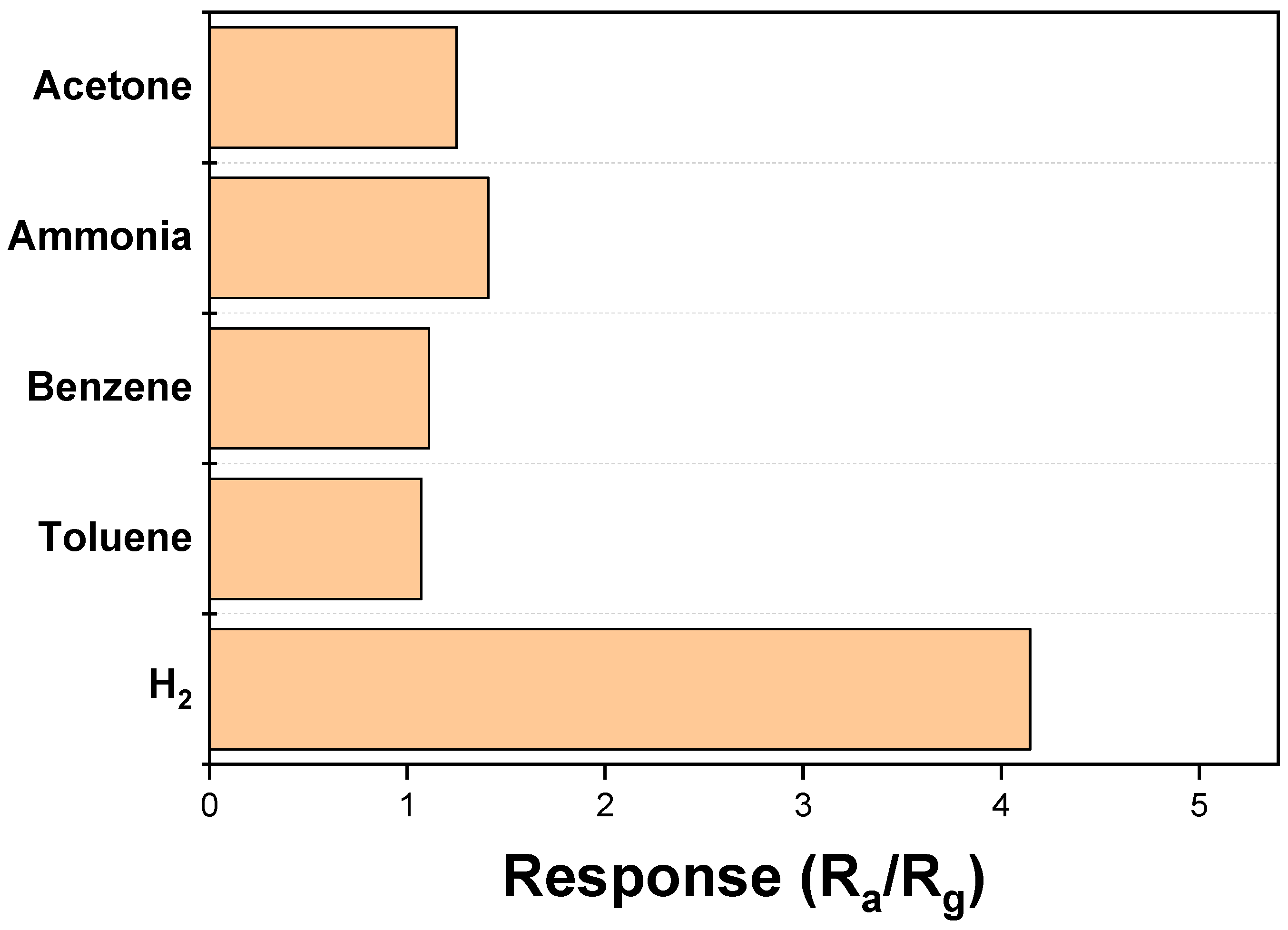
3.2. Gas Sensing Investigations
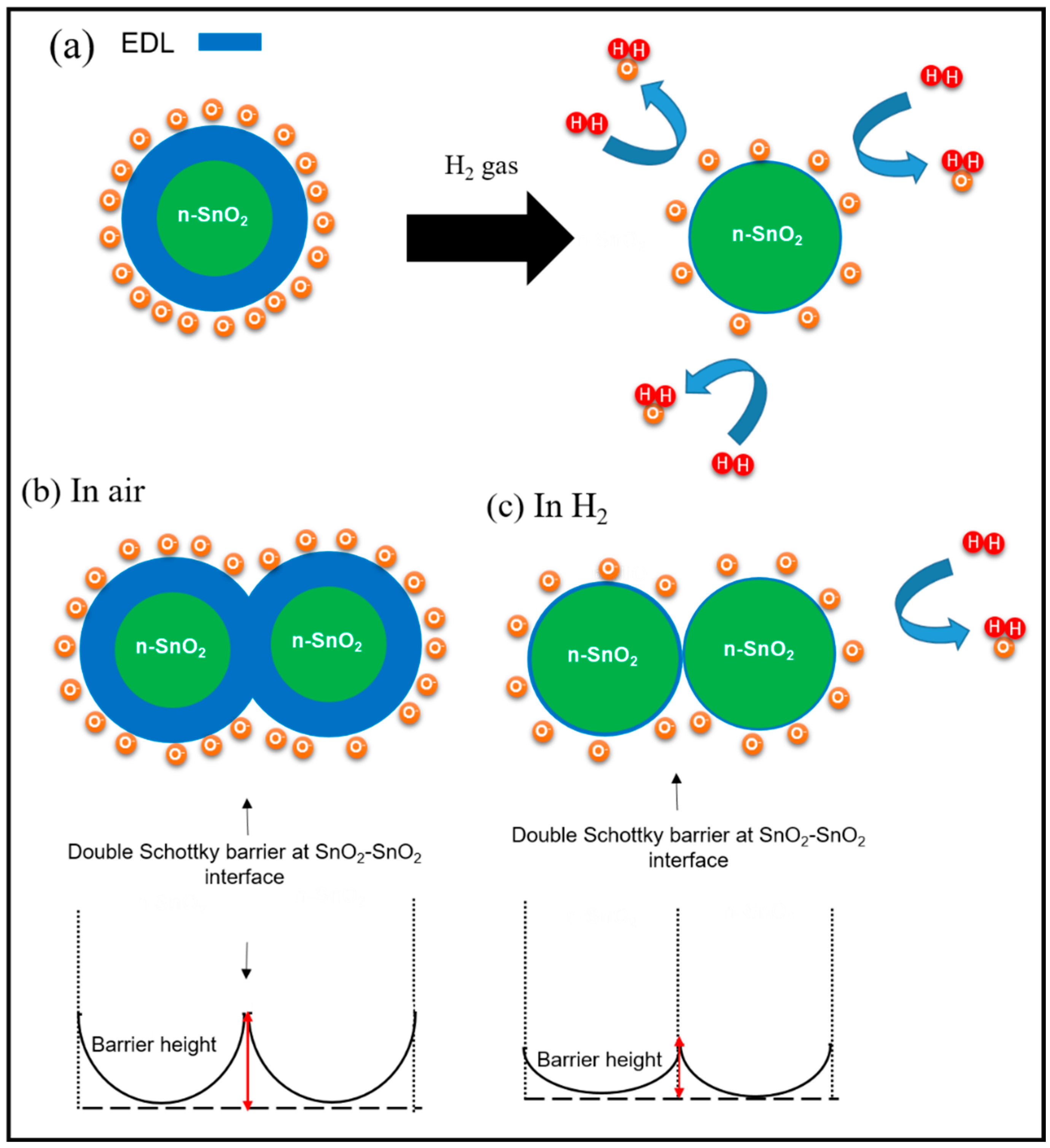
3.3. Proposed Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, B.; Sharma, A.; Kim, J.S. Recent advances on H2 sensor technologies based on MOX and FET devices: A review. Sens. Actuators B Chem. 2018, 262, 758–770. [Google Scholar] [CrossRef]
- Li, Z.; Yao, Z.; Haidry, A.A.; Plecenik, T.; Xie, L.; Sun, L.; Fatima, Q. Resistive-type hydrogen gas sensor based on TiO2: A review. Int. J. Hydrogen Energy 2018, 43, 21114–21132. [Google Scholar] [CrossRef]
- Mobtakeri, S.; Habashyani, S.; Çoban, Ö.; Budak, H.F.; Kasapoğlu, A.E.; Gür, E. Effect of growth pressure on sulfur content of RF-magnetron sputtered WS2 films and thermal oxidation properties of them toward using Pd decorated WO3 based H2 gas sensor. Sens. Actuators B Chem. 2023, 381, 133485. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Sharma, A.; Vikraman, D.; Lee, Y.-A.; Sivakumar, P.; Korvink, J.G. Room-temperature response of MOF-derived Pd@PdO core shell/γ-Fe2O3 microcubes decorated graphitic carbon based ultrasensitive and highly selective H2 gas sensor. J. Colloid Interface Sci. 2023, 652, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Tian, Q.; Wu, G.; Bian, W.; Sun, N.; Wang, X. Highly sensitive and stable H2 gas sensor based on p-PdO-n-WO3-heterostructure-homogeneously-dispersing thin film. Int. J. Hydrogen Energy 2022, 47, 17821–17834. [Google Scholar] [CrossRef]
- Ai, T.; Zhang, J.; Li, J.; Zhang, Y.; Yin, Y.; Lu, J. Ultrafast response of Pt functionalized Fe2(MoO4)3 nanoflower gas sensors for ultra-low ppm level H2 gas detection. J. Alloys Comp. 2024, 970, 172567. [Google Scholar] [CrossRef]
- Yang, D.; Gopal, R.A.; Lkhagvaa, T.; Choi, D. Metal-oxide gas sensors for exhaled-breath analysis: A review. Meas. Sci. Technol. 2021, 32, 102004. [Google Scholar] [CrossRef]
- Urita, Y.; Watanabe, T.; Maeda, T.; Sasaki, Y.; Ishihara, S.; Hike, K.; Sanaka, M.; Nakajima, H.; Sugimoto, M. Breath hydrogen gas concentration linked to intestinal gas distribution and malabsorption in patients with small-bowel pseudo-obstruction. Biomark. Insights 2009, 4, BMI-S2139. [Google Scholar] [CrossRef]
- Breedon, M.; Miura, N. Augmenting, H2 sensing performance of YSZ-based electrochemical gas sensors via the application of Au mesh and YSZ coating. Sens. Actuators B Chem. 2013, 182, 40–44. [Google Scholar] [CrossRef]
- Fechete, A.; Wlodarski, W.; Kalantar-Zadeh, K.; Holland, A.; Antoszewski, J.; Kaciulis, S.; Pandolfi, L. SAW-based gas sensors with rf sputtered InOx and PECVD SiNx films: Response to H2 and O3 gases. Sens. Actuators B Chem. 2006, 118, 362–367. [Google Scholar] [CrossRef]
- Meeran, M.N.; Saravanan, S.P.; Shkir, M.; Kannan, S.K. Fabrication of transition-metal (Zn, Mn, Cu)-based MOFs as efficient sensor materials for detection of H2 gas by clad modified fiber optic gas sensor technique. Opt. Fiber Technol. 2021, 65, 102614. [Google Scholar] [CrossRef]
- Cho, S.H.; Suh, J.M.; Jeong, B.; Lee, T.H.; Choi, K.S.; Eom, T.H.; Kim, T.; Jang, H.W. Fast responding and highly reversible gasochromic H2 sensor using Pd-decorated amorphous WO3 thin films. Chem. Eng. J. 2022, 446, 136862. [Google Scholar]
- Shi, Y.; Xu, H.; Liu, T.; Zeb, S.; Nie, Y.; Zhao, Y. Advanced development of metal oxide nanomaterials for H2 gas sensing applications. Mater. Adv. 2021, 2, 1530–1569. [Google Scholar] [CrossRef]
- Yang, B.; Myung, N.V.; Tran, T.T. 1D metal oxide semiconductor materials for chemiresistive gas sensors: A review. Adv. Electron. Mater. 2021, 7, 2100271. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Kong, Y.; Li, Y.; Cui, X.; Su, L.; Ma, D.; Lai, T. SnO2 nanostructured materials used as gas sensors for the detection of hazardous and flammable gases: A review. Nano Mater. Sci. 2022, 4, 339–350. [Google Scholar] [CrossRef]
- Li, G.; Du, K.; Wang, X.; Wang, X.; Chen, B.; Qiu, C.; Xu, J. Pd nanoparticles decorated SnO2 ultrathin nanosheets for highly sensitive H2 sensor: Experimental and theoretical studies. Int. J. Hydrogen Energy 2024, 50, 761–771. [Google Scholar] [CrossRef]
- Pandey, G.; Bhardwaj, M.; Kumar, S.; Lawaniya, S.D.; Kumar, M.; Dwivedi, P.K.; Awasthi, K. Synergistic effects of Pd-Ag decoration on SnO/SnO2 nanosheets for enhanced hydrogen sensing. Sens. Actuators B Chem. 2024, 402, 135062. [Google Scholar] [CrossRef]
- Kim, D.S.; Ahemad, M.J.; Le, T.D.; Lee, H.J.; Yu, Y.T. Synergistic effects of bimetallic Pd-Au (alloy)/SnO2 nanocomposites for low temperature and selective hydrogen gas sensors. Mater. Sci. Eng. B 2024, 299, 116939. [Google Scholar] [CrossRef]
- Kumar, V.; Gautam, Y.K.; Gautam, D.; Kumar, A.; Adalati, R.; Singh, B.P. highly sensitive and selective hydrogen gas sensor with humidity tolerance using pd-capped SnO2 thin films of various thicknesses. Fuels 2023, 4, 279–294. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, X.Y.; Chen, J.A.; Fang, J.B.; Zi, T.Q.; Zhu, L.; Liu, C.; Han, M.; Cao, Y.Q.; Li, A.D. Ultrasensitive and wide-range flexible hydrogen sensor based on Pd nanoparticles decorated ultrathin SnO2 Film. Adv. Electron. Mater. 2023, 9, 2201047. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M.; Leonardi, S.G.; Neri, G. A novel gas sensor based on Ag/Fe2O3 core-shell nanocomposites. Ceram. Int. 2016, 42, 18974–18982. [Google Scholar] [CrossRef]
- Gui, Y.H.; Tu, Y.S.; Guo, H.S.; Qin, X.Y.; Tian, K.; Qin, X.M.; Guo, D.J.; Guo, X. Microwave-assisted efficient synthesis of ZnO nanospheres for low temperature NO2 gas sensor. Mater. Sci. Eng. B 2024, 299, 117031. [Google Scholar] [CrossRef]
- Li, S.-H.; Chu, Z.; Meng, F.-F.; Luo, T.; Hu, X.-Y.; Huang, S.-Z. Highly sensitive gas sensor based on SnO2 nanorings for detection of isopropanol. J. Alloys Comp. 2016, 688, 712–717. [Google Scholar] [CrossRef]
- Mohd Chachuli, S.A.; Hamidon, M.N.; Mamat, M.S.; Ertugrul, M.; Abdullah, N.H. A Hydrogen gas sensor based on TiO2 nanoparticles on alumina substrate. Sensors 2018, 18, 2483. [Google Scholar] [CrossRef]
- Moon, J.; Hedman, H.-P.; Kemell, M.; Tuominen, A.; Punkkinen, R. Hydrogen sensor of Pd-decorated tubular TiO2 layer prepared by anodization with patterned electrodes on SiO2/Si substrate. Sens. Actuators B. Chem. 2016, 222, 190–197. [Google Scholar] [CrossRef]
- Lee, J.H. Technological realization of semiconducting metal oxide–based gas sensors. In Gas Sensors Based on Conducting Metal Oxides; Elsevier: Amsterdam, The Netherlands, 2019; pp. 167–216. [Google Scholar]
- Weng, T.-F.; Ho, M.-S.; Sivakumar, C.; Balraj, B.; Chung, P.-F. VLS growth of pure and Au decorated β-Ga2O3 nanowires for room temperature CO gas sensor and resistive memory applications. Appl. Surf. Sci. 2020, 533, 147476. [Google Scholar] [CrossRef]
- Sertel, B.C.; Sonmez, N.A.; Kaya, M.D.; Ozcelik, S. Development of MgO: TiO2 thin films for gas sensor applications. Ceram. Int. 2019, 45, 2917–2921. [Google Scholar] [CrossRef]
- Chen, E.-X.; Fu, H.-R.; Lin, R.; Tan, Y.-X.; Zhang, J. Highly Selective and Sensitive Trimethylamine Gas Sensor Based on Cobalt Imidazolate Framework Material. ACS Appl. Mater. Interfaces 2014, 6, 22871–22875. [Google Scholar] [CrossRef]
- Alrammouz, R.; Podlecki, J.; Abboud, P.; Sorli, B.; Habchi, R. A review on flexible gas sensors: From materials to devices. Sens. Actuators A Phys. 2018, 284, 209–231. [Google Scholar] [CrossRef]
- Khan, S.; Ali, S.; Bermak, A. Recent developments in printing flexible and wearable sensing electronics for healthcare applications. Sensors 2019, 19, 1230. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Shi, N.; Jiang, X.; Chen, M.; Jiang, J.; Zheng, Y.; Wu, W.; Cui, D.; Haick, H.; Tang, N. Techniques for wearable gas sensors fabrication. Sens. Actuators B Chem. 2022, 353, 131133. [Google Scholar] [CrossRef]
- Bag, A.; Lee, N.E. Recent advancements in development of wearable gas sensors. Adv. Mater. Technol. 2021, 6, 2000883. [Google Scholar] [CrossRef]
- Wang, T.; Xu, H.; Wang, Y.; Zeng, Y.; Liu, B. Porous SnO2 triple-shelled hollow nanoboxes for high sensitive toluene detection. Mater. Lett. 2020, 264, 127320. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Guan, K.; Liu, Y.; Li, Y.; Sun, F.; Wang, X.; Zhang, C.; Feng, S.; Zhang, T. Influence of oxygen vacancies on the performance of SnO2 gas sensing by near-ambient pressure XPS studies. Sens. Actuators B Chem. 2023, 393, 134252. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, S.S.; Park, C. Pt nanoparticle decoration on femtosecond laser-irradiated SnO2 nanowires for enhancing C7H8 gas sensing. Sens. Actuators B Chem. 2023, 379, 133279. [Google Scholar] [CrossRef]
- Sun, K.M.; Song, X.Z.; Wang, X.F.; Li, X.; Tan, Z. Annealing temperature-dependent porous ZnFe2O4 olives derived from bimetallic organic frameworks for high-performance ethanol gas sensing. Mater. Chem. Phys. 2020, 241, 122379. [Google Scholar] [CrossRef]
- Katoch, A.; Sun, G.J.; Choi, S.W.; Byun, J.H.; Kim, S.S. Competitive influence of grain size and crystallinity on gas sensing performances of ZnO nanofibers. Sens. Actuators B Chem. 2013, 185, 411–416. [Google Scholar] [CrossRef]
- Katoch, A.; Abideen, Z.U.; Kim, J.H.; Kim, S.S. Crystallinity dependent gas-sensing abilities of ZnO hollow fibers. Met. Mater. Int. 2016, 22, 942–946. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Sakaguchi, I.; Hishita, S.; Ohsawa, T.; Suzuki, T.T. Decoration of Pt/Pd bimetallic nanoparticles on Ru-implanted WS2 nanosheets for acetone sensing studies. Appl. Surf. Sci. 2023, 641, 158478. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S.; Kim, H.W. How shell thickness can affect the gas sensing properties of nanostructured materials: Survey of literature. Sens. Actuators B Chem. 2018, 258, 270–294. [Google Scholar] [CrossRef]
- Cao, H.; Hu, Z.; Wei, X.; Wang, H.; Tian, X.; Ding, S. Conductometric ethanol gas sensor based on a bilayer film consisting of SnO2 film and SnO2/ZnSnO3 porous film prepared by magnetron sputtering. Sens. Actuators B Chem. 2023, 382, 133562. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceramics 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, W.; Li, Q. SnO2 as a gas sensor in detection of volatile organic compounds: A review. Sens. Actuators A Phys. 2022, 346, 113845. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, L.; Zhou, Q.; Zhang, Z.; Dong, Z.; Nie, L. In-situ synthesis of needle-like PdO-decorated NiO thin films on Al2O3 substrates for high-performance H2 sensors. Ceram. Int. 2022, 48, 31746–31754. [Google Scholar] [CrossRef]
- Suzuki, T.; Sackmann, A.; Oprea, A.; Weimar, U.; Bârsan, N. Chemoresistive CO2 gas sensors based on La2O2CO3: Sensing mechanism insights provided by operando characterization. ACS Sens. 2020, 5, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Kumar, S.; Agrawal, H.; Moinuddin, M.G.; Kumar, M.; Sharma, S.K. An efficient hydrogen gas sensor based on hierarchical Ag/ZnO hollow microstructures. Sens. Actuators B Chem. 2021, 346, 130510. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Optimization of Deposition Parameters of SnO2 Particles on Tubular Alumina Substrate for H2 Gas Sensing. Appl. Sci. 2024, 14, 1567. https://doi.org/10.3390/app14041567
Lee MH, Mirzaei A, Kim HW, Kim SS. Optimization of Deposition Parameters of SnO2 Particles on Tubular Alumina Substrate for H2 Gas Sensing. Applied Sciences. 2024; 14(4):1567. https://doi.org/10.3390/app14041567
Chicago/Turabian StyleLee, Myoung Hoon, Ali Mirzaei, Hyoun Woo Kim, and Sang Sub Kim. 2024. "Optimization of Deposition Parameters of SnO2 Particles on Tubular Alumina Substrate for H2 Gas Sensing" Applied Sciences 14, no. 4: 1567. https://doi.org/10.3390/app14041567
APA StyleLee, M. H., Mirzaei, A., Kim, H. W., & Kim, S. S. (2024). Optimization of Deposition Parameters of SnO2 Particles on Tubular Alumina Substrate for H2 Gas Sensing. Applied Sciences, 14(4), 1567. https://doi.org/10.3390/app14041567








