Measurement of the Mechanical Impedance of Ear Cartilage and Development of a Coupler for Vibrator Evaluation of Cartilage Conduction Hearing Aids
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
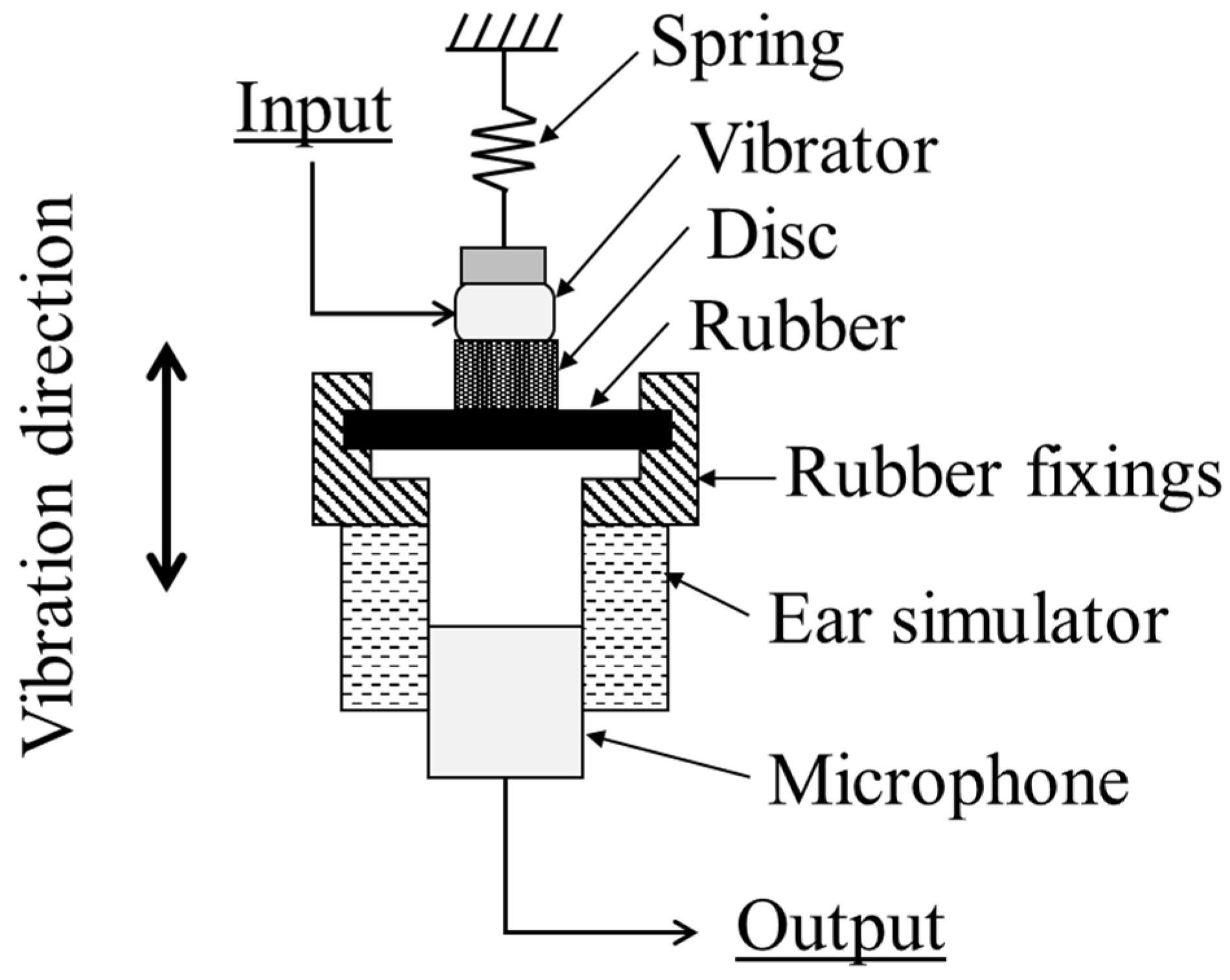
2.2. Coupler Simulating the Ear Cartilage
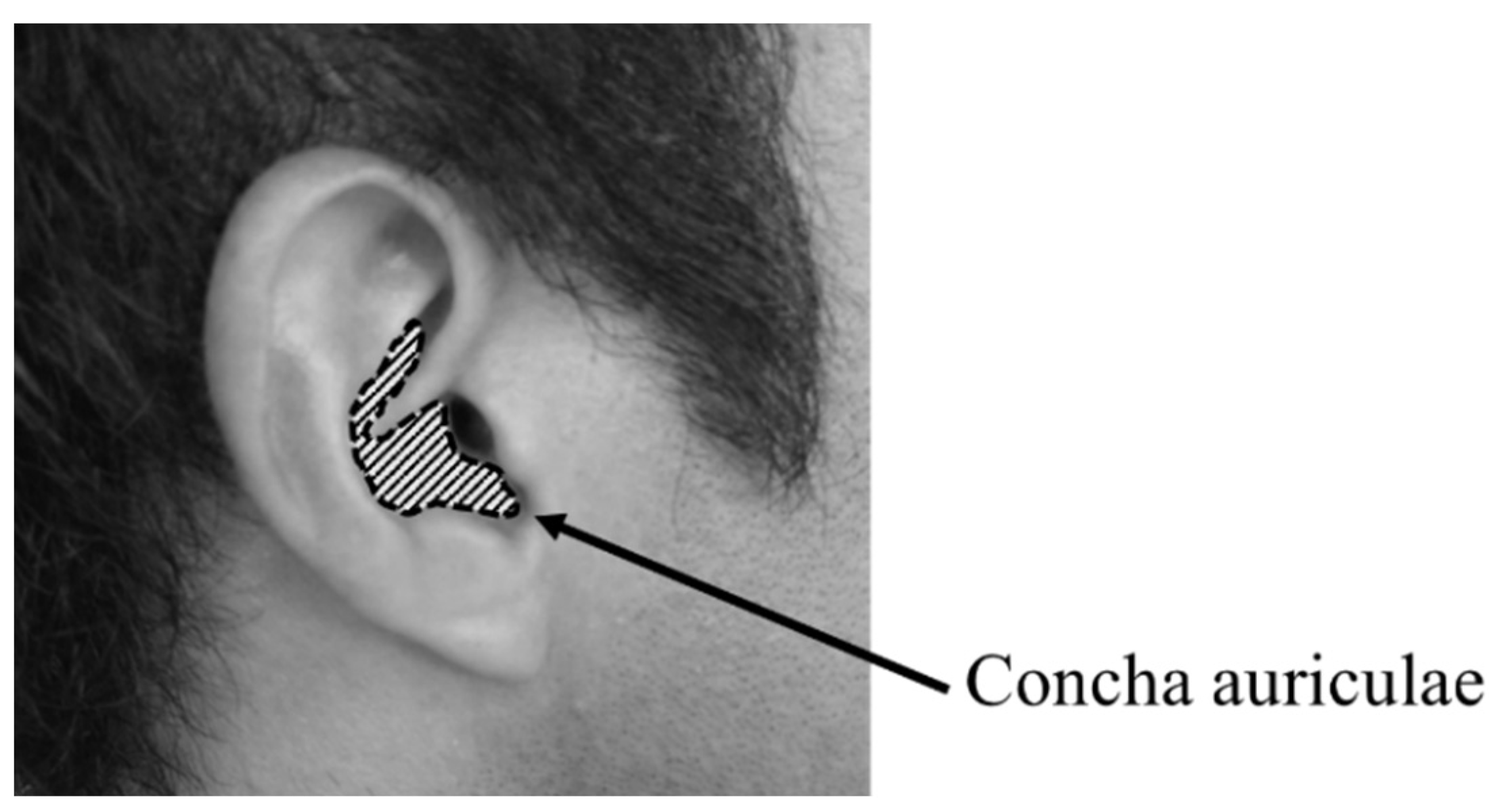
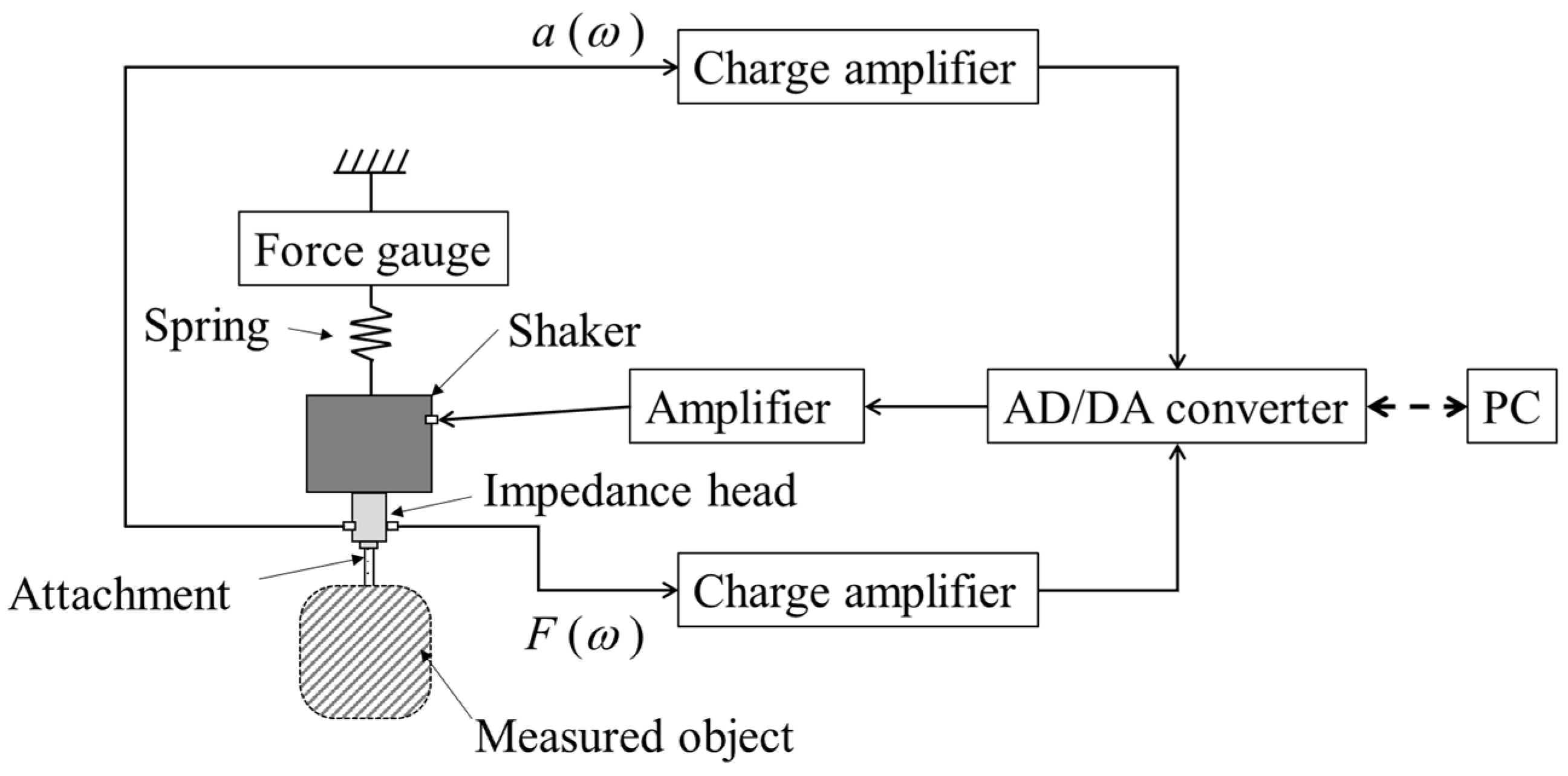
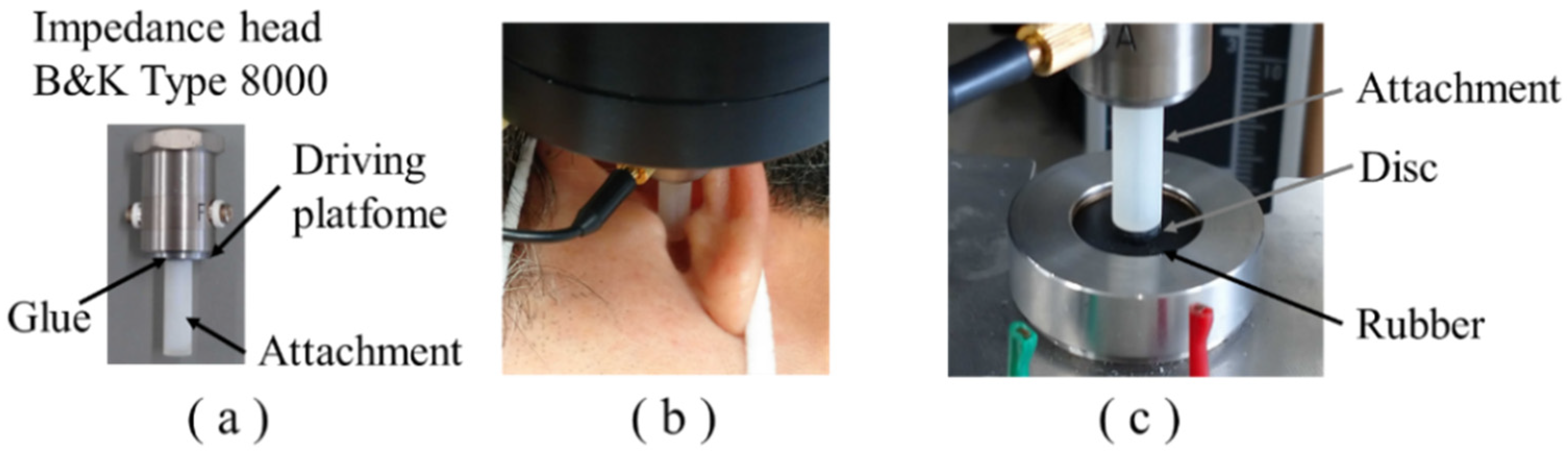
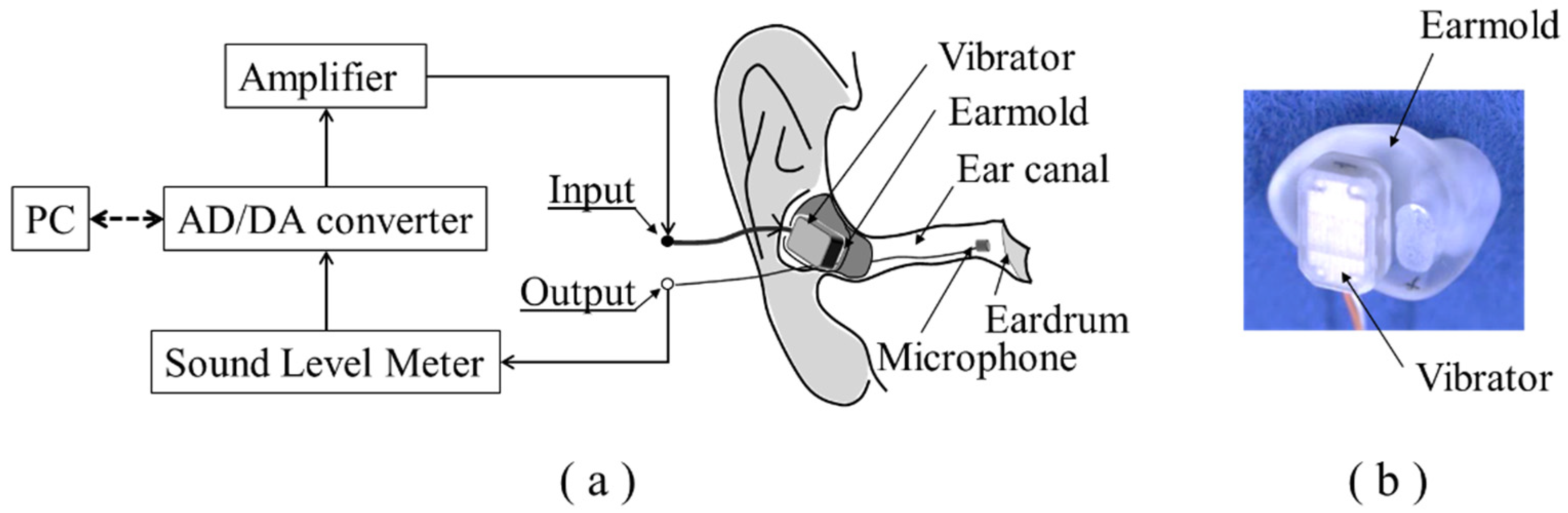
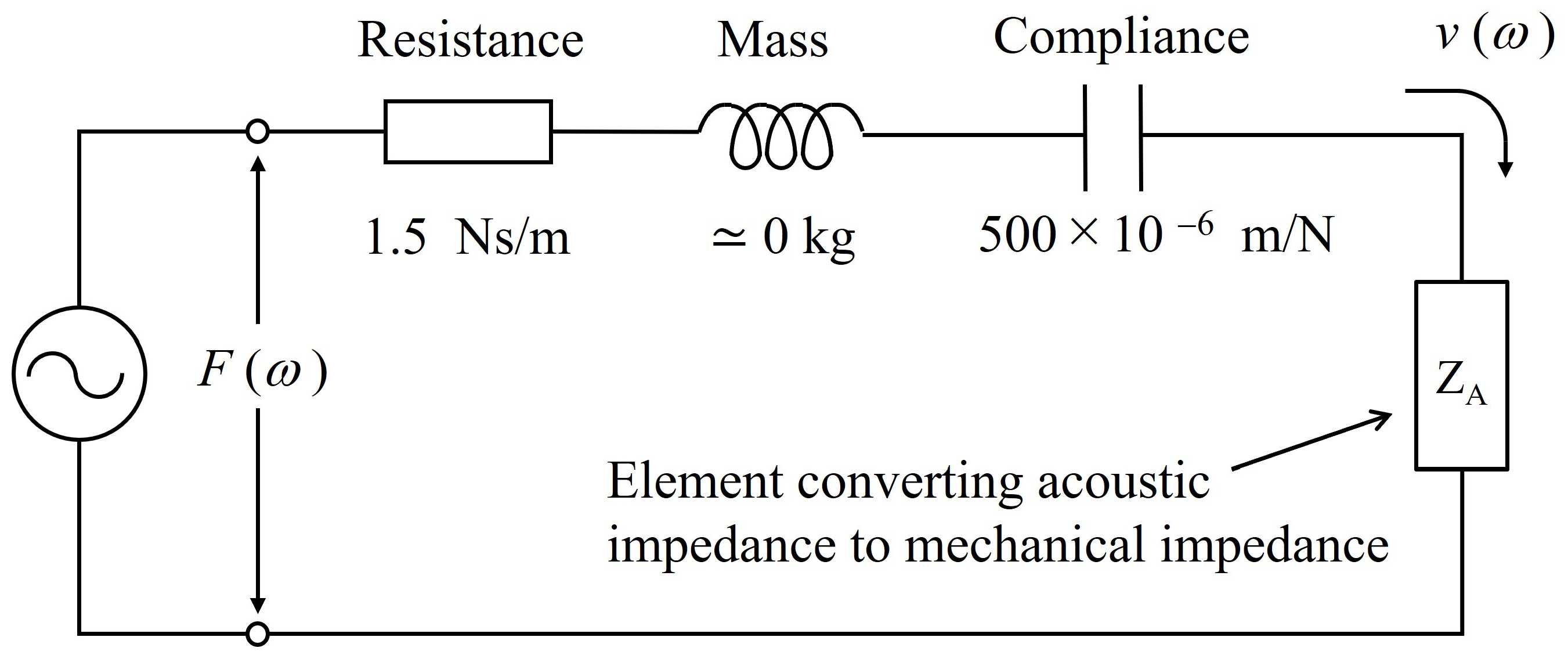
2.3. Mechanical Impedance Measurement Methods
2.4. Sound Pressure Measurement Methods
3. Results
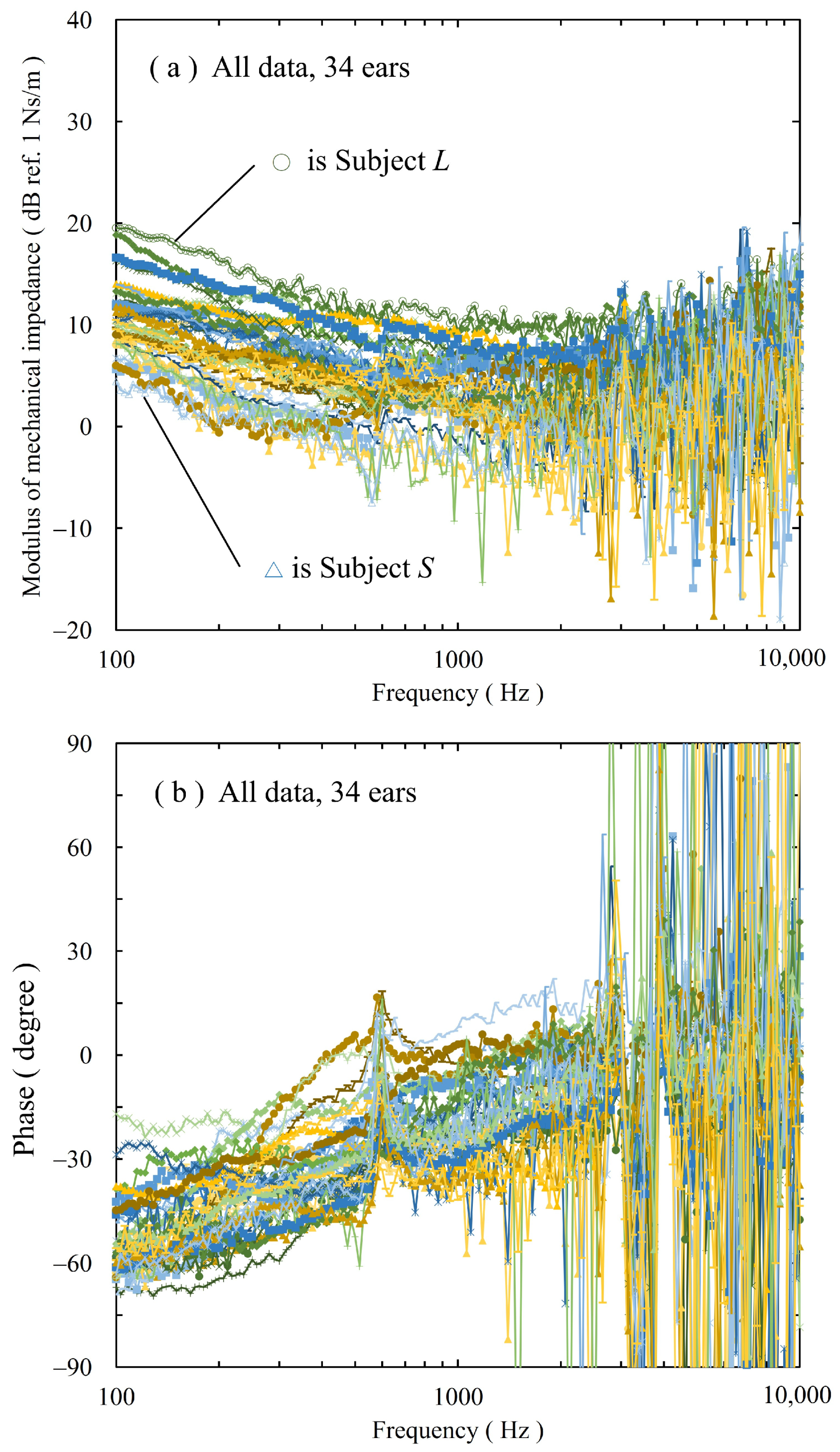
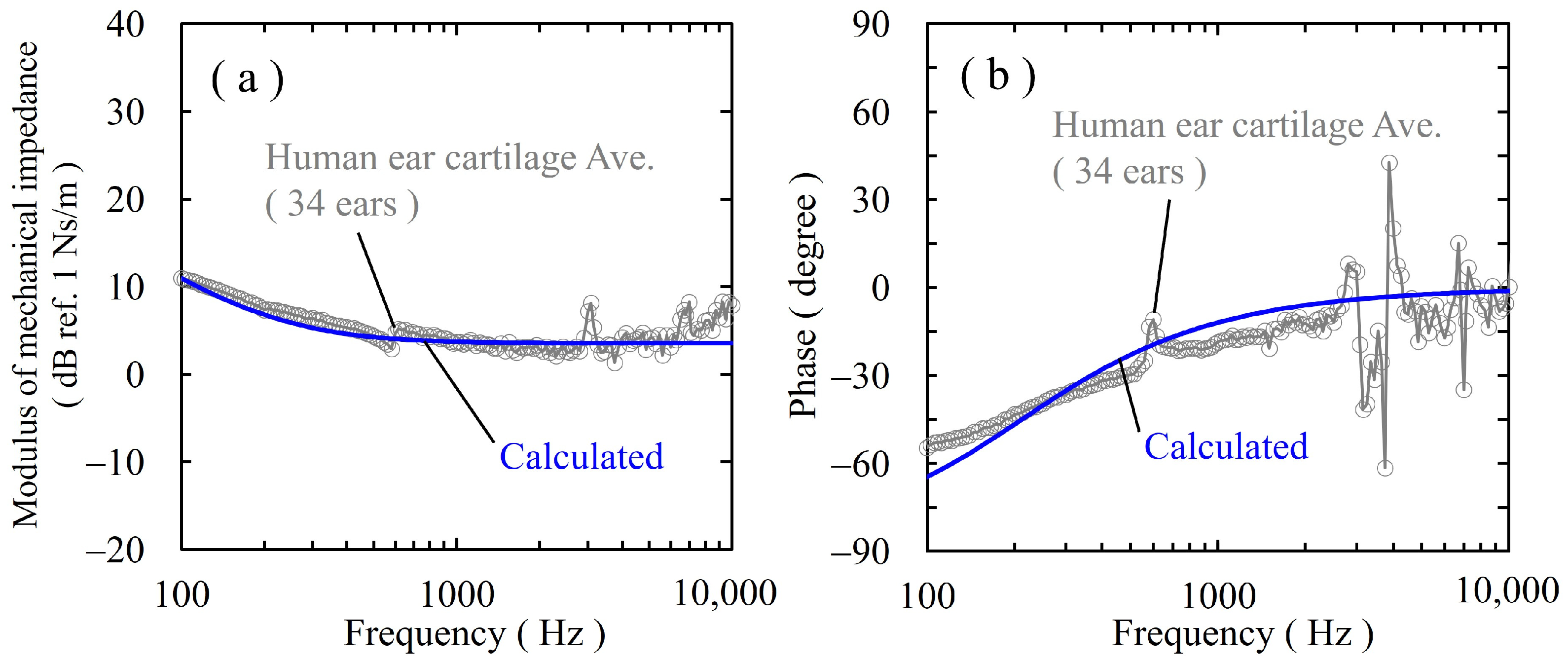
3.1. Mechanical Impedance of Ear Cartilage
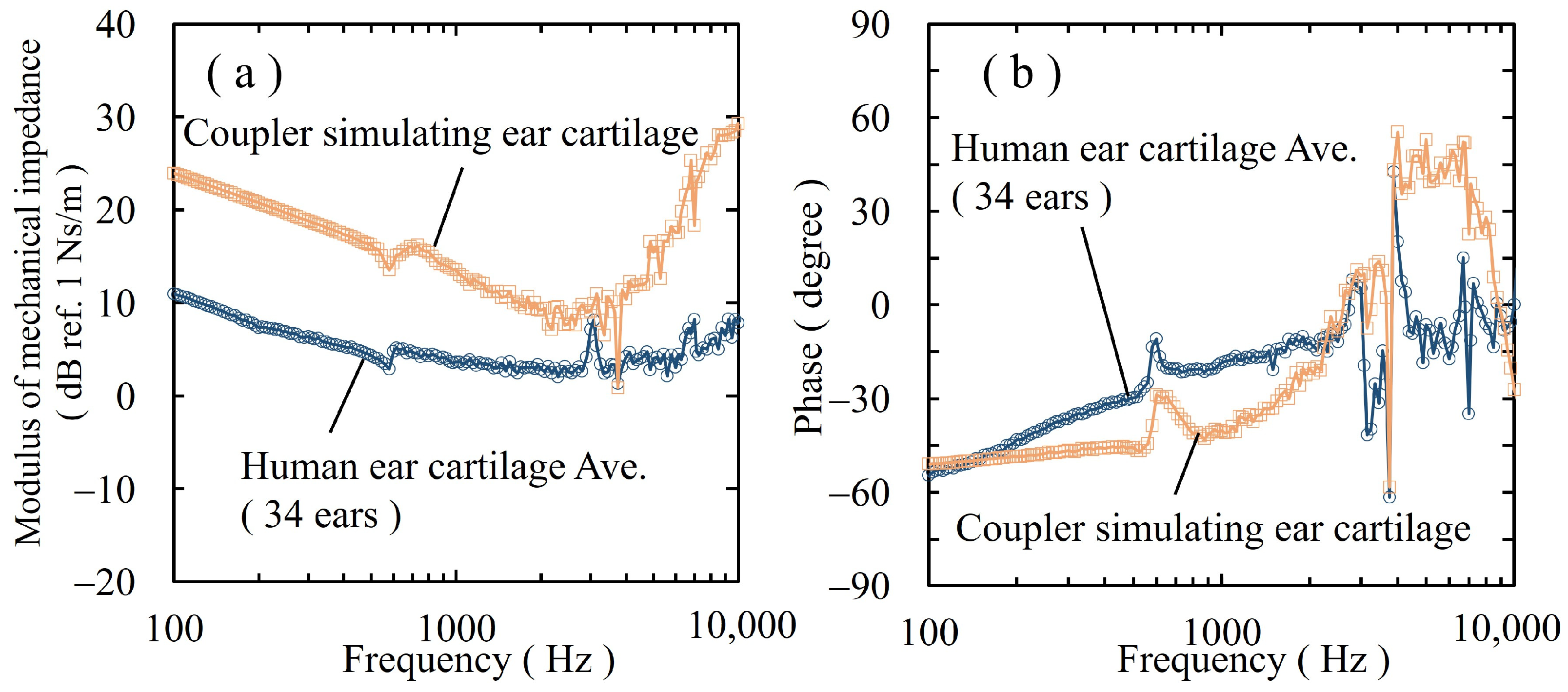
3.2. Mechanical Impedance of the Coupler Simulating the Ear Cartilage
3.3. Sound Pressure Characteristics in Human Ear Cartilage Conduction
3.4. Sound Pressure Characteristics of the Coupler Simulating the Ear Cartilage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Report on Hearing; World Health Organization: Geneva, Switzerland, 2021; ISBN 9789240020481. [Google Scholar]
- Reinfeldt, S.; Håkansson, B.; Taghavi, H.; Eeg-Olofsson, M. New developments in bone-conduction hearing implants: A review. Med. Devices 2015, 8, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, H.; Yanai, S.; Nishimura, T.; Sakaguchi, T.; Iwakura, T.; Yoshino, K. Development of cartilage conduction hearing aid. Arch. Mat. Sci. Eng. 2010, 42, 104–110. [Google Scholar]
- Shimokura, R.; Hosoi, H.; Iwakura, T.; Nishimura, T.; Matsui, T. Development of monaural and binaural behind-the-ear cartilage conduction hearing aids. Appl. Acoust. 2013, 74, 1234–1240. [Google Scholar] [CrossRef]
- Nishimura, T.; Hosoi, H.; Saito, O.; Shimokura, R.; Yamanaka, T.; Kitahara, T. Cartilage conduction hearing aids for severe conduction hearing loss. Otol. Neurotol. 2018, 39, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Suwento, R.; Widodo, D.W.; Airlangga, T.J.; Alviandi, W.; Watanuki, K.; Nakanowatari, N.; Hosoi, H.; Nishimura, T. Clinical Trial for Cartilage Conduction Hearing Aid in Indonesia. Audiol. Res. 2021, 11, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Nairn, E.M.; Chen, A.S.; Nishimura, T.; Berezovsky, A.; Stucken, E.Z. Hearing Outcomes of a New Cartilage Conduction Device vs. Bone Conduction Devices. Otolaryngol. Head Neck Surg. 2023, 168, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Shimada, A.; Nakano, S.; Kondo, E.; Takeyama, T.; Fukuda, J.; Udaka, J.; Okamoto, H.; Takeda, N. Effects of FM system fitted into the normal hearing ear or cartilage conduction hearing aid fitted into the affected ear on speech-in-noise recognition in Japanese children with unilateral congenital aural atresia. J. Med. Investig. 2020, 67, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, T.; Oishi, N.; Ogawa, K. Who are good adult candidates for cartilage conduction hearing aids? Eur. Arch. Otorhinolaryngol. 2020; in press. [Google Scholar] [CrossRef]
- Nishimura, T.; Hosoi, H.; Saito, O.; Shimokura, R.; Yamanaka, T.; Kitahara, T. Sound localisation ability using cartilage conduction hearing aids in bilateral aural atresia. Int. J. Audiol. 2020, 59, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, T.; Oishi, N.; Ogawa, K. Efficacy of cartilage conduction hearing aids in children. Int. J. Pediatr. Otorhinolaryngol. 2021; in press. [Google Scholar] [CrossRef]
- Nishimura, T.; Hosoi, H.; Sugiuchi, T.; Matsumoto, N.; Nishiyama, T.; Takano, K.; Sugimoto, S.; Yazama, H.; Sato, T.; Komori, M. Cartilage conduction hearing aid fitting in clinical practice. J. Am. Acad. Audiol. 2021; in press. [Google Scholar]
- Nishimura, T.; Hosoi, H.; Morimoto, C.; Okayasu, T.; Shimokura, R.; Kitahara, T. Comparison of Cartilage Conduction Hearing Devices Designed by Ear Impression and Computed Tomography. Appl. Sci. 2023, 13, 6152. [Google Scholar] [CrossRef]
- Kitama, T.; Nishiyama, T.; Iwabu, K.; Wakabayashi, T.; Shimanuki, M.N.; Hosoya, M.; Oishi, N.; Ozawa, H. Comparison of Cartilage Conduction Hearing Aid, Bone Anchored Hearing Aid, and ADHEAR: Case Series of 6 Patients with Conductive and Mixed Hearing Loss. Appl. Sci. 2022, 12, 12099. [Google Scholar] [CrossRef]
- Flottorp, G.; Solberg, S. Mechanical impedance of human headbones (forehead and mastoid portion of the temporal bone) measured under ISO/IEC conditions. J. Acoust. Soc. Am. 1976, 59, 899. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Nim, N.; Stenfelt, S. The development of a whole-head human finite-element model for simulation of the transmission of bone-conducted sound. J. Acoust. Soc. Am. 2016, 140, 1635–1651. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, B.; Woelflin, F.; Tjellström, A.; Hodgetts, W. The mechanical impedance of the human skull via direct bone conduction implants. Med. Devices 2020, 13, 293–313. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, H.; Nishimura, T.; Shimokura, R.; Kitahara, T. Cartilage conduction as the third pathway for sound transmission. Auris Nasus Larynx 2019, 46, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, C.; Nishimura, T.; Hosoi, H.; Saito, O.; Fukuda, F.; Shimokura, R.; Yamanaka, T. Sound transmission by cartilage conduction in ear with fibrotic aural atresia. J. Rehabil. Res. Dev. 2014, 51, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, A.; Mashak, A. Review on rubbers in medicine: Natural, silicone and polyurethane rubbers. Review on rubbers in medicine: Natural, silicone and polyurethane rubbers. Plast. Rubber Compos. 2013, 42, 223–230. [Google Scholar] [CrossRef]
- Sheiko, S.S.; Dobrynin, A.V. Architectural Code for Rubber Elasticity: From Supersoft to Superfirm Materials. Macromolecules 2019, 52, 7531–7546. [Google Scholar] [CrossRef]
- Shimokura, R.; Nishimura, T.; Hosoi, H. Vibrational and Acoustical Characteristics of Ear Pinna Simulators That Differ in Hardness. Audiol. Res. 2021, 11, 327–334. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishikawa, S.-i.; Watanuki, K.; Iwakura, T. Measurement of the Mechanical Impedance of Ear Cartilage and Development of a Coupler for Vibrator Evaluation of Cartilage Conduction Hearing Aids. Appl. Sci. 2024, 14, 1536. https://doi.org/10.3390/app14041536
Ishikawa S-i, Watanuki K, Iwakura T. Measurement of the Mechanical Impedance of Ear Cartilage and Development of a Coupler for Vibrator Evaluation of Cartilage Conduction Hearing Aids. Applied Sciences. 2024; 14(4):1536. https://doi.org/10.3390/app14041536
Chicago/Turabian StyleIshikawa, Shin-ichi, Keisuke Watanuki, and Takashi Iwakura. 2024. "Measurement of the Mechanical Impedance of Ear Cartilage and Development of a Coupler for Vibrator Evaluation of Cartilage Conduction Hearing Aids" Applied Sciences 14, no. 4: 1536. https://doi.org/10.3390/app14041536
APA StyleIshikawa, S.-i., Watanuki, K., & Iwakura, T. (2024). Measurement of the Mechanical Impedance of Ear Cartilage and Development of a Coupler for Vibrator Evaluation of Cartilage Conduction Hearing Aids. Applied Sciences, 14(4), 1536. https://doi.org/10.3390/app14041536





