Adsorption of Coxsackievirus in Sediments: Influencing Factors, Kinetics, and Isotherm Modeling
Abstract
1. Introduction
2. Material and Methods
2.1. Preparation of Viruses
2.2. Virus Detection
2.3. Virus Adsorption Batch Experiment
2.3.1. Sediment Particle Size
2.3.2. Temperature
2.3.3. pH Values
2.3.4. Humic Acid
2.3.5. Initial Concentrations
2.4. Adsorption Kinetics
2.5. Adsorption Isotherm
3. Results and Discussion
3.1. Effect of Different Factors on Virus Adsorption
3.1.1. Sediment Particle Size
3.1.2. Temperature
3.1.3. pH Values
3.1.4. Humic Acid
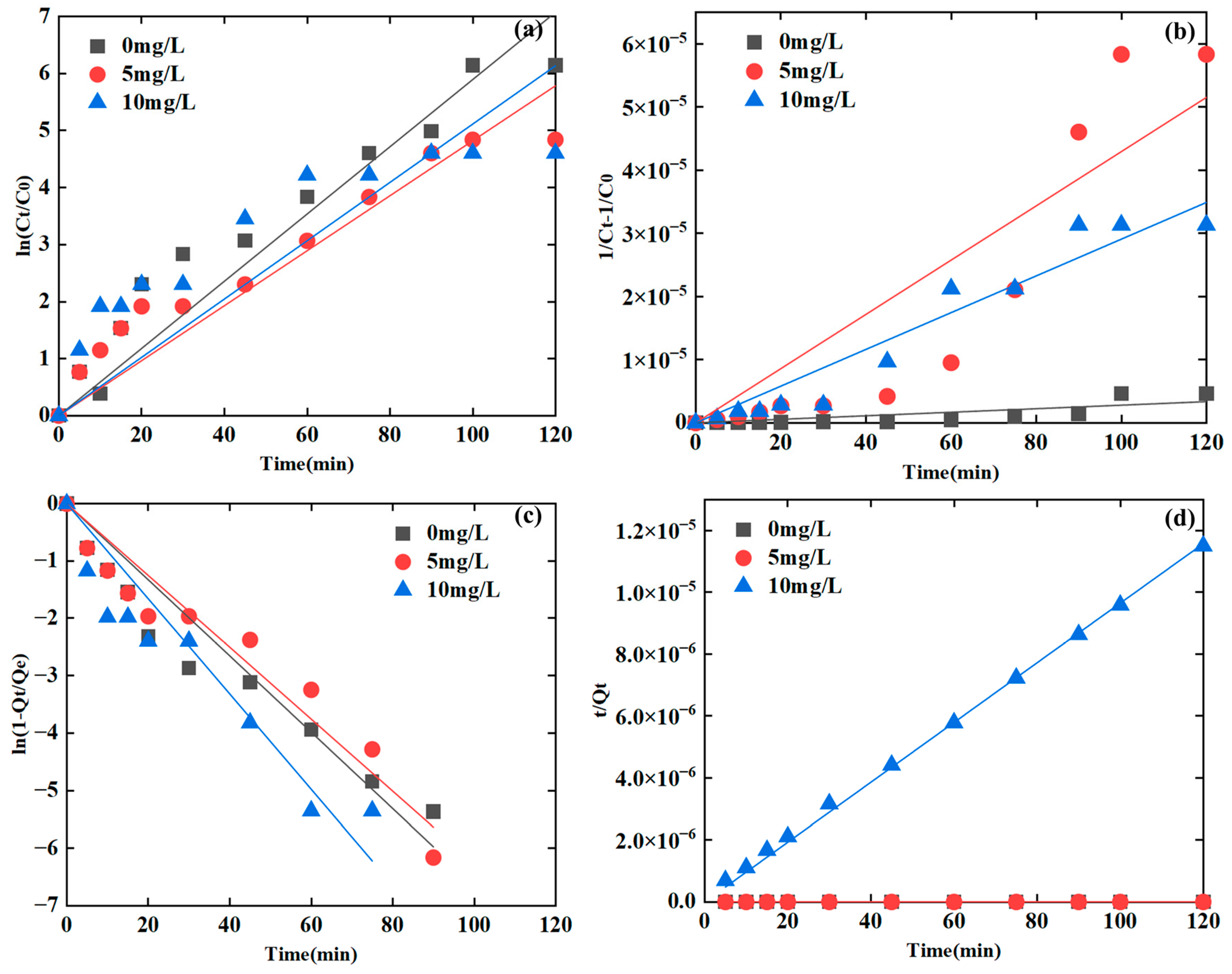
3.1.5. Initial Concentrations
3.2. Kinetic Modelling
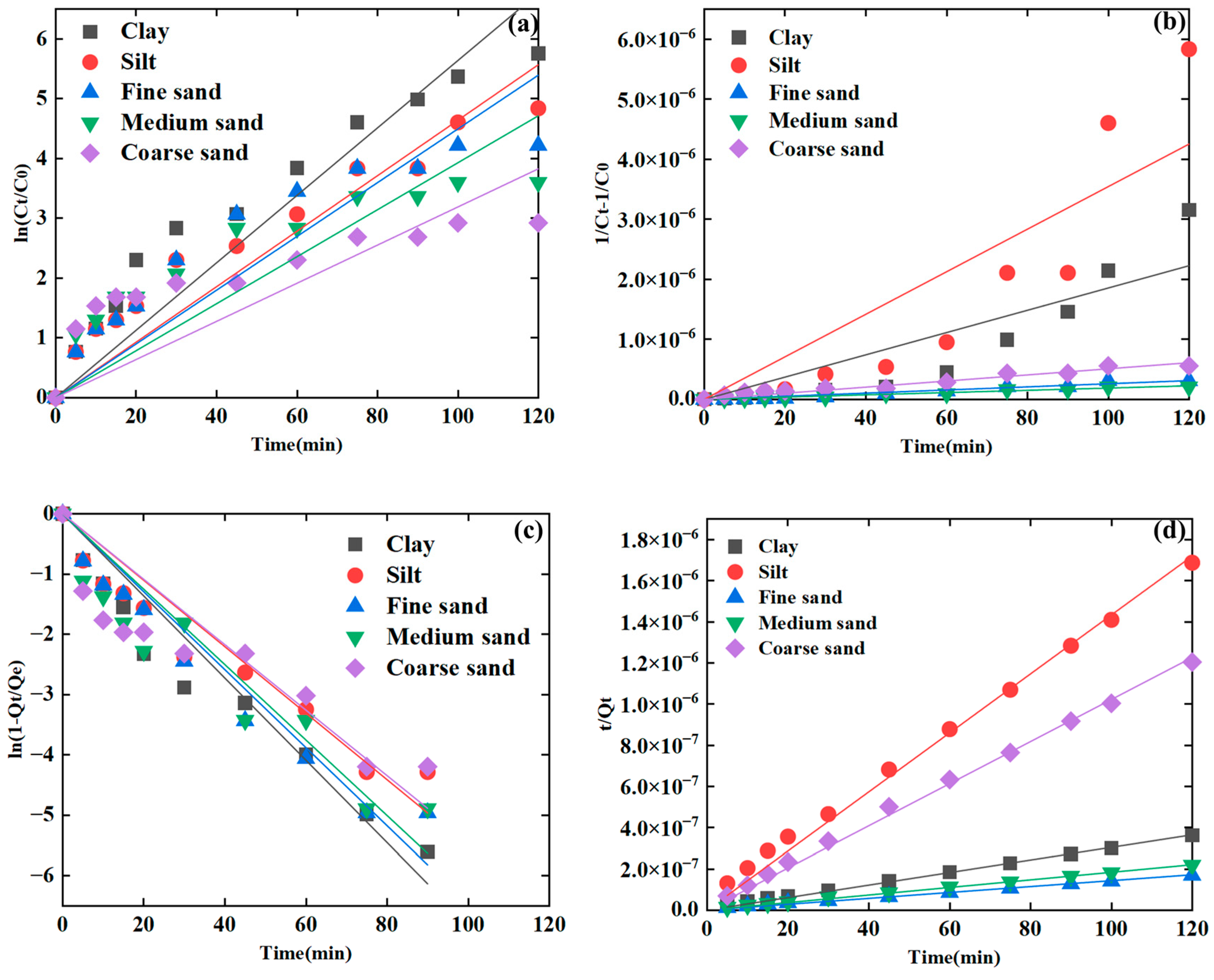
3.2.1. Fitting of the Kinetic Models with Varied Sediment Particle Sizes
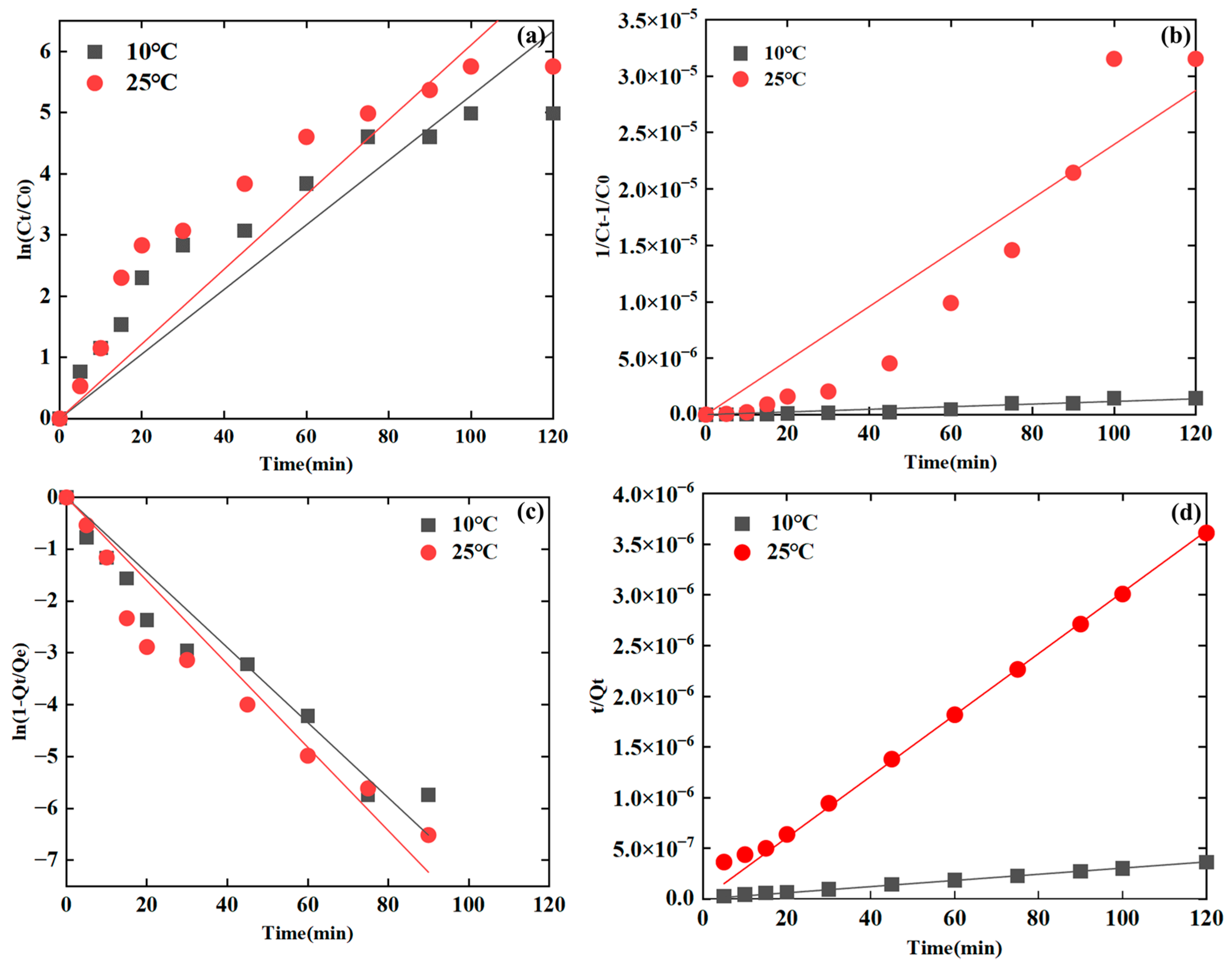
3.2.2. Fitting of the Kinetic Models with Varied Temperatures
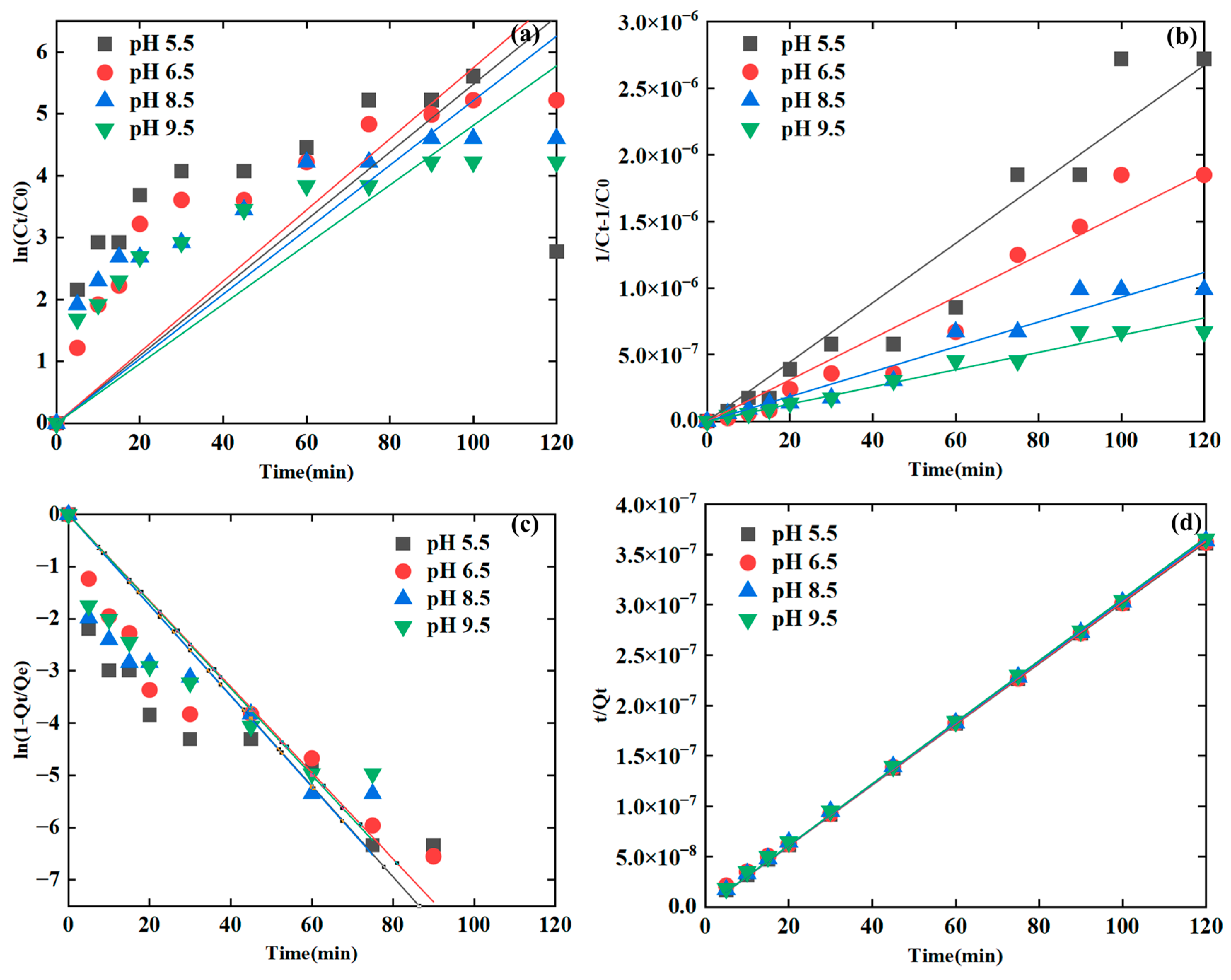
3.2.3. Fitting of the Kinetic Models with Varied pH Values
3.2.4. Fitting of the Kinetic Models with Varied Humic Acid Concentrations
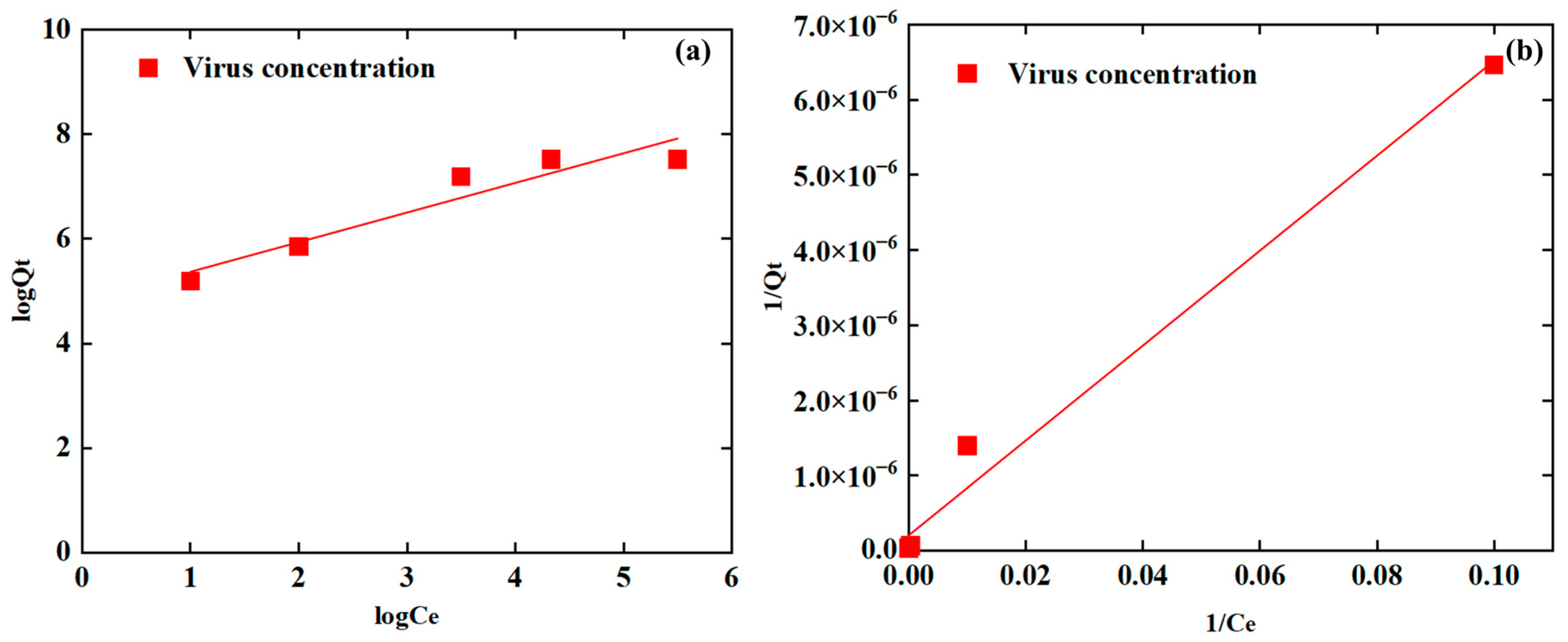
3.3. Isotherm Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rimstad, E. Examples of emerging virus diseases in salmonid aquaculture. Aquac. Res. 2011, 42, 86–89. [Google Scholar] [CrossRef]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.-F.; et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef]
- Yazeed, V.W.; Eunice, U.-J.; Dippenaar, M.A. Potential SARS-CoV-2 contamination of groundwater as a result of mass burial: A mini-review. Sci. Total Environ. 2022, 835, 155473. [Google Scholar] [CrossRef]
- Masciopinto, C.; Osvalda, D.G.; Scrascia, M.; Fortunato, F.; La Rosa, G.; Suffredini, E.; Pazzani, C.; Prato, R.; Montagna, M.T. Human health risk assessment for the occurrence of enteric viruses in drinking water from wells: Role of flood runoff injections. Sci. Total Environ. 2019, 666, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Karunanidhi, D.; Subramani, T.; Srinivasamoorthy, K. Sources and consequences of groundwater contamination. Arch. Environ. Contam. Toxicol. 2021, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Chand, S.; Chand, S.; Rout, P.R.; Naik, S.K. Emerging groundwater contaminants: A comprehensive review on their health hazards and remediation technologies. Groundw. Sustain. Dev. 2023, 20, 100868. [Google Scholar] [CrossRef]
- Lee, H.; Kim, M.; Lee, J.E.; Lim, M.; Kim, M.; Kim, J.-M.; Jheong, W.-H.; Kim, J.; Ko, G. Investigation of norovirus occurrence in groundwater in metropolitan Seoul, Korea. Sci. Thetotal Environ. 2011, 409, 2078–2084. [Google Scholar] [CrossRef] [PubMed]
- Fout, G.S.; Martinson, B.C.; Moyer, M.W.N.; Dahling, D.R. A multiplex reverse transcription-PCR Method for detection of human enteric viruses in groundwater. Appl. Environ. Microbiol. 2003, 69, 3158–3164. [Google Scholar] [CrossRef]
- Opere, W.M.; John, M.; Ombori, O. Occurrence of enteric viruses in surface water and the relationship with changes in season and physical water quality dynamics. Adv. Virol. 2020, 2020, 9062041. [Google Scholar] [CrossRef]
- Lanrewaju, A.A.; Enitan-Folami, A.M.; Sabiu, S.; Edokpayi, J.N.; Swalaha, F.M. Global public health implications of human exposure to viral contaminated water. Front. Microbiol. 2022, 13, 981896. [Google Scholar] [CrossRef]
- Osvalda, D.G.; Caggiano, G.; Bagordo, F.; Barbuti, G.; Brigida, S.; Lugoli, F.; Grassi, T.; La Rosa, G.; Lucentini, L.; Uricchio, V.F.; et al. Enteric viruses and fecal bacteria indicators to assess groundwater quality and suitability for irrigation. Int. J. Environ. Res. Public Health 2017, 14, 558. [Google Scholar] [CrossRef]
- Upfold, N.S.; Luke, G.A.; Knox, C. Occurrence of human enteric viruses in water sources and shellfish: A focus on Africa. Food Environ. Virol. 2021, 13, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Quanrud, D.M.; Carroll, S.M.; Gerba, C.P.; Arnold, R.G. Virus removal during simulated soil-aquifer treatment. Water Res. 2003, 37, 753–762. [Google Scholar] [CrossRef]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E.J. Risk factors for human disease emergence. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Rehmann, L.L.C.; Welty, C.; Harvey, R.W. Stochastic Analysis of Virus Transport in Aquifers. Water Resour. Res. 1999, 35, 1987–2006. [Google Scholar] [CrossRef][Green Version]
- Bales, R.C.; Hinkle, S.R.; Kroeger, T.W.; Stocking, K.; Gerba, C.P. Bacteriophage adsorption during transport through porous media: Chemical perturbations and reversibility. Environ. Sci. Technol. 1991, 25, 2088–2095. [Google Scholar] [CrossRef]
- La Rosa, G.; Fratini, M.; Libera, S.d.; Iaconelli, M.; Muscillo, M. Emerging and potentially emerging viruses in water environments. Alcohol Alcohol. 2012, 48, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.K. Processes in pathogenic biocolloidal contaminants transport in saturated and unsaturated porous media: A Review. Water Air Soil Pollut. 2011, 216, 239–256. [Google Scholar] [CrossRef]
- Farkas, K.; Varsani, A.; Pang, L. Adsorption of rotavirus, MS2 bacteriophage and surface-modified silica nanoparticles to hydrophobic matter. Food Environ. Virol. 2015, 7, 261–268. [Google Scholar] [CrossRef]
- Diamond, M.S.; Kanneganti, T.-D. Innate immunity: The first line of defense against SARS-CoV-2. Nat. Immunol. 2022, 23, 165–176. [Google Scholar] [CrossRef]
- Gassilloud, B.; Gantzer, C. Adhesion-aggregation and inactivation of poliovirus 1 in groundwater stored in a hydrophobic container. Appl. Environ. Microbiol. 2005, 71, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Schijven, J.F.; Hassanizadeh, S.M. Removal of viruses by soil passage: Overview of modeling, processes, and parameters. Crit. Rev. Environ. Sci. Technol. 2000, 30, 49–127. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Wagstaffe, M.; Creutzburg, M.; Ugolotti, A.; Kulkarni, S.; Jeromin, A.; Krekeler, T.; Feuerherd, M.; Herrmann, A.; Ebert, G.; et al. Adsorption and Inactivation of SARS-CoV-2 on the surface of anatase TiO2(101). ACS Appl. Mater. Interfaces 2023, 15, 8770–8782. [Google Scholar] [CrossRef]
- Zhang, L.; Xi, T.; Zhu, D.; Shen, H.; Su, W.; Yu, P.; Lyu, C. Adsorption-enhanced photocatalytic waterborne virus inactivation by graphite carbon nitride conjugated with covalent organic frameworks. Chem. Eng. J. 2023, 472, 144893. [Google Scholar] [CrossRef]
- Merhi, T.; Atasi, O.; Coetsier, C.; Lalanne, B.; Roger, K. Assessing suspension and infectivity times of virus-loaded aerosols involved in airborne transmission. Proc. Natl. Acad. Sci. USA 2022, 119, e2204593119. [Google Scholar] [CrossRef] [PubMed]
- Syngouna, V.I.; Chrysikopoulos, C.V. Interaction between viruses and clays in static and dynamic batch systems. Environ. Sci. Technol. 2010, 44, 4539–4544. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Zhao, B.; Zhang, C. Removal of bacteriophages MS2 and phiX174 from aqueous solutions using a red soil. J. Hazard. Mater. 2010, 180, 640–647. [Google Scholar] [CrossRef]
- Chen, F.; Yuan, X.; Song, Z.; Xu, S.; Yang, Y.; Yang, X. Gram-negative Escherichia coli promotes deposition of polymer-capped silver nanoparticles in saturated porous media. Environ. Sci. Nano 2018, 5, 1495–1505. [Google Scholar] [CrossRef]
- Loveland, J.P.; Ryan, J.N.; Amy, G.L.; Harvey, R.W. The reversibility of virus attachment to mineral surfaces. Colloids Surfaces. A Physicochem. Eng. Asp. 1996, 107, 205–221. [Google Scholar] [CrossRef]
- Erkurt, H.A.; Özyurt, M.; Özer, A. Adsorption by active and inactive aspergillus oryzae: Batch studies. Adsorpt. Sci. Technol. 2012, 30, 317–330. [Google Scholar] [CrossRef]
- Das, S.; Behera, B.C.; Mohapatra, R.K.; Pradhan, B.; Sudarshan, M.; Chakraborty, A.; Thatoi, H. Kinetic modeling and isotherm approach for biosorptive removal of hexavalent chromium using heat inactivated fungal biomass. Int. J. Chem. Kinet. 2023, 55, 365–380. [Google Scholar] [CrossRef]
- Cao, H.; Tsai, F.T.C.; Rusch, K.A. Salinity and soluble organic matter on virus sorption in sand and soil columns. Ground Water 2010, 48, 42–52. [Google Scholar] [CrossRef]
- Hornstra, L.M.; Schijven, J.F.; Waade, A.; Prat, G.S.; Smits, F.J.C.; Cirkel, G.; Stuyfzand, P.J.; Medema, G.J. Transport of bacteriophage MS2 and PRD1 in saturated dune sand under suboxic conditions. Water Res. 2018, 139, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Farkas, K.; Lin, S.; Hewitt, J.; Premaratne, A.; Close, M. Attenuation and transport of human enteric viruses and bacteriophage MS2 in alluvial sand and gravel aquifer media—Laboratory studies. Water Res. 2021, 196, 117051. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, L.; Li, W.; Hu, B.X.; Dai, Z. Bacterial community variations with salinity in the saltwater-intruded estuarine aquifer. Sci. Total Environ. 2021, 755 Pt 1, 142423. [Google Scholar] [CrossRef] [PubMed]
- Laine, R.F.; Goodfellow, G.; Young, L.J.; Travers, J.; Carroll, D.; Dibben, O.; Bright, H.; Kaminski, C.F. Structured illumination microscopy combined with machine learning enables the high throughput analysis and classification of virus structure. eLife 2018, 7, e40183. [Google Scholar] [CrossRef]
- Grabow, W.O.K. Bacteriophages: Update on application as models for viruses in water. Water SA 2001, 27, 251–268. [Google Scholar] [CrossRef]
- Leclerc, H.; Edberg, S.; Pierzo, V.; Delattre, J.M. Bacteriophages as indicators of enteric viruses and public health risk in groundwaters. J. Appl. Microbiol. 2000, 88, 5–21. [Google Scholar] [CrossRef]
- Fout, G.S.; Borchardt, M.A.; Kieke, B.A.; Karim, M.R. Human virus and microbial indicator occurrence in public-supply groundwater systems; meta-analysis of 12 international studies. Hydrogeol. J. 2017, 25, 903–919. [Google Scholar] [CrossRef]
- Petricevich, V.L.; Palomares, L.A.; González, M.; Ramírez, O.T. Parameters that determine virus adsorption kinetics: Toward the design of better infection strategies for the insect cell—Baculovirus expression system. Enzym. Microb. Technol. 2001, 29, 52–61. [Google Scholar] [CrossRef]
- Bruinsma, R.F.; Wuite, G.J.L.; Roos, W.H. Physics of viral dynamics. Nat. Rev. Phys. 2021, 3, 76–91. [Google Scholar] [CrossRef]
- Bartels, J.; Batista, A.G.; Kroll, S.; Maas, M.; Rezwan, K. Hydrophobic ceramic capillary membranes for versatile virus filtration. J. Membr. Sci. 2019, 570–571, 85–92. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.; Liu, L.; Wei, Z.; Ehrlich, E.S.; Liu, G.; Li, J.; Liu, X.; Wang, H.; Yu, X.-f. Coxsackievirus A16 infection induces neural cell and non-neural cell apoptosis in vitro. PLoS ONE 2014, 9, e111174. [Google Scholar] [CrossRef]
- Omo-Okoro, P.N.; Curtis, C.J.; Karásková, P.; Melymuk, L.; Oyewo, O.A.; Okonkwo, J.O. Kinetics, isotherm, and thermodynamic studies of the adsorption mechanism of PFOS and PFOA using inactivated and chemically activated maize tassel. Water Air Soil Pollut. 2020, 231, 485. [Google Scholar] [CrossRef]
- Mouri, M. Comparison between particle-size analyses of fine-grained soils by sieve-hydrometer and laser diffraction methods. J. Jpn. Soc. Civ. Eng. Ser. C Geosph. Eng. 2021, 77, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Badot, P.-M. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Venkata, M.S.; Ramanaiah, S.V.; Rajkumar, B.; Sarma, P.N. Removal of fluoride from aqueous phase by biosorption onto algal biosorbent Spirogyra sp.-IO2: Sorption mechanism elucidation. J. Hazard. Mater. 2007, 141, 465–474. [Google Scholar] [CrossRef]
- Xue, X.T.; Zhong, L.; Zhang, G.X.; Zhang, F.X. Adsorption kinetic study of reactive dyes on silk with octyl trimethyl ammonium bromide as accelerant. Adv. Text. Eng. 2011, 331, 291–297. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Z.; Gao, P.; Su, H.; Yu, Y.; Ren, N. Adsorption behavior of EE2 (17α-ethinylestradiol) onto the inactivated sewage sludge: Kinetics, thermodynamics and influence factors. J. Hazard. Mater. 2010, 175, 970–976. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, F.; Yin, S.; Wallace, C.D.; Soltanian, M.R.; Dai, Z.; Ritzi, R.W.; Ma, Z.; Zhan, C.; Lü, X. Application of upscaling methods for fluid flow and mass transport in multi-scale heterogeneous media: A critical review. Appl. Energy 2021, 303, 117603. [Google Scholar] [CrossRef]
- Kleber, M.; Bourg, I.C.; Coward, E.K.; Hansel, C.M.; Myneni, S.C.B.; Nunan, N. Dynamic interactions at the mineral–organic matter interface. Nat. Rev. Earth Environ. 2021, 2, 402–421. [Google Scholar] [CrossRef]
- Bellou, M.I.; Syngouna, V.I.; Tselepi, M.A.; Kokkinos, P.A.; Paparrodopoulos, S.C.; Vantarakis, A.; Chrysikopoulos, C.V. Interaction of human adenoviruses and coliphages with kaolinite and bentonite. Sci. Total Environ. 2015, 517, 86–95. [Google Scholar] [CrossRef]
- Chrysikopoulos, C.V.; Aravantinou, A.F. Virus attachment onto quartz sand: Role of grain size and temperature. J. Environ. Chem. Eng. 2014, 2, 796–801. [Google Scholar] [CrossRef]
- Ternes, T.A.; Andersen, H.; Gilberg, D.; Bonerz, M. Determination of estrogens in sludge and sediments by liquid extraction and GC/MS/MS. Anal. Chem. 2002, 74, 3498–3504. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Rashid, A.; Bhatti, H.N.; Iqbal, M.; Noreen, S. Fungal biomass composite with bentonite efficiency for nickel and zinc adsorption: A mechanistic study. Ecol. Eng. 2016, 91, 459–471. [Google Scholar] [CrossRef]
- Kumar, K.V.; Porkodi, K. Comments on “Removal of Congo red from aqueous solution by anilinepropylsilica xerogel” by Pavan FA, Dias SLP, Lima EC, Benvenutti EV. Dyes and Pigments 2008;76:64–9. Dye. Pigm. 2008, 77, 481–482. [Google Scholar] [CrossRef]
- Bewick, V.; Cheek, L.; Ball, J. Statistics review 9: One-way analysis of variance. Crit Care 2004, 8, 130–136. [Google Scholar] [CrossRef]
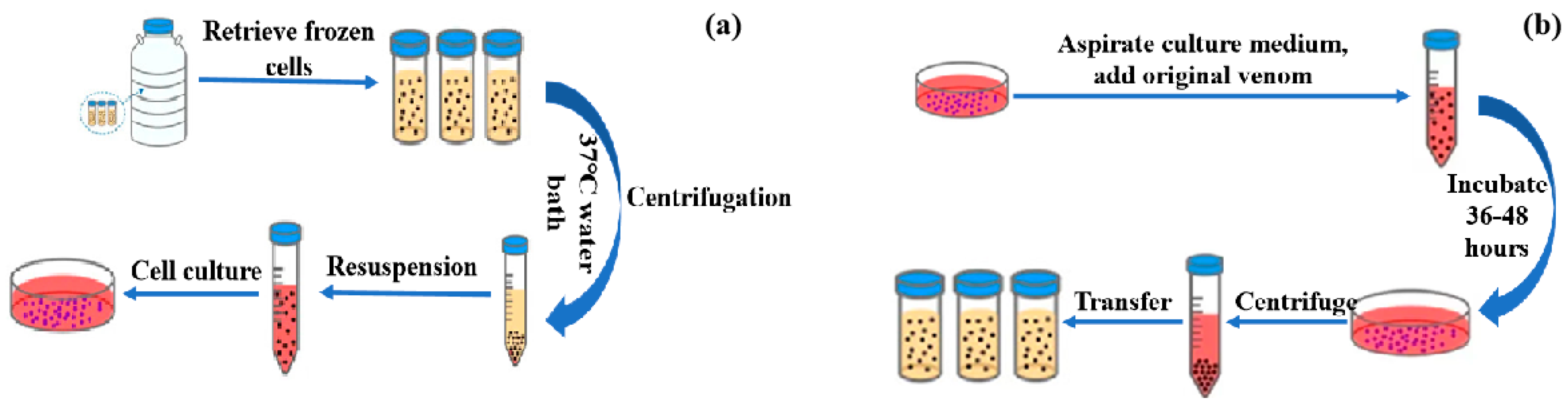

| Number | Factors | Sediment Particle | Temperature (°C) | pH | Humic Acid (mg/L) | Virus Concentration (TCID50/mL) |
|---|---|---|---|---|---|---|
| 1 | Sediment particle | Clay | 10 | 6.5 | 0 | 1 × 108 |
| 2 | Silt | 10 | 6.5 | 0 | ||
| 3 | Fine sand | 10 | 6.5 | 0 | ||
| 4 | Medium sand | 10 | 6.5 | 0 | ||
| 5 | Coarse sand | 10 | 6.5 | 0 | ||
| 6 | Temperature | Clay | 10 | 6.5 | 0 | 2.15 × 108 |
| 7 | Clay | 25 | 6.5 | 0 | ||
| 8 | pH | Clay | 10 | 5.5 | 0 | 1.7 × 108 |
| 9 | Clay | 10 | 6.5 | 0 | ||
| 10 | Clay | 10 | 8.5 | 0 | ||
| 11 | Clay | 10 | 9.5 | 0 | ||
| 12 | Humic acid | Clay | 10 | 6.5 | 0 | 6.4 × 107 |
| 13 | Clay | 10 | 6.5 | 5 | ||
| 14 | Clay | 10 | 6.5 | 10 | ||
| 15 | Virus concentration | Clay | 10 | 6.5 | 0 | 1 × 108 |
| 16 | Clay | 10 | 6.5 | 0 | 1 × 107 | |
| 17 | Clay | 10 | 6.5 | 0 | 4.6 × 106 | |
| 18 | Clay | 10 | 6.5 | 0 | 2.1 × 105 | |
| 19 | Clay | 10 | 6.5 | 0 | 4.6 × 104 |
| Kinetic Model | Adjusted R2 | p Value | ||||
|---|---|---|---|---|---|---|
| Factor | First-Order | Secondary-Order | Pseudo-First-Order | Pseudo-Second-Order | ||
| Sediment particle | Clay | 0.9602 | 0.8597 | 0.9707 | 0.9985 | <0.05 * |
| Silt | 0.9637 | 0.8580 | 0.9644 | 0.9974 | ||
| Fine sand | 0.9345 | 0.9734 | 0.9749 | 0.9977 | ||
| Medium sand | 0.8975 | 0.9857 | 0.9471 | 0.9985 | ||
| Coarse sand | 0.8509 | 0.9790 | 0.9069 | 0.9987 | ||
| Temperature (°C) | 10 | 0.9383 | 0.9405 | 0.9728 | 0.9985 | <0.05 * |
| 25 | 0.9320 | 0.9275 | 0.9648 | 0.9982 | ||
| pH | 5.5 | 0.7247 | 0.9647 | 0.8735 | 0.9983 | <0.05 * |
| 6.5 | 0.8864 | 0.9678 | 0.9362 | 0.9964 | ||
| 8.5 | 0.8497 | 0.9776 | 0.8960 | 0.9999 | ||
| 9.5 | 0.8467 | 0.9854 | 0.9030 | 0.9977 | ||
| Humic acid (mg/L) | 0 | 0.9703 | 0.7438 | 0.9662 | 0.9908 | <0.05 * |
| 5 | 0.9643 | 0.8703 | 0.9697 | 0.9870 | ||
| 10 | 0.9018 | 0.9664 | 0.9527 | 0.9994 | ||
| Adsorption Isotherms | Model Equation | Adjusted R2 | p Value |
|---|---|---|---|
| Freundlich | 0.8748 | <0.05 * | |
| Langmuir | 0.9826 | <0.05 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Zhang, X.; Su, W.; Cai, F.; Lan, T.; Dai, Z. Adsorption of Coxsackievirus in Sediments: Influencing Factors, Kinetics, and Isotherm Modeling. Appl. Sci. 2024, 14, 1480. https://doi.org/10.3390/app14041480
Li M, Zhang X, Su W, Cai F, Lan T, Dai Z. Adsorption of Coxsackievirus in Sediments: Influencing Factors, Kinetics, and Isotherm Modeling. Applied Sciences. 2024; 14(4):1480. https://doi.org/10.3390/app14041480
Chicago/Turabian StyleLi, Mengyu, Xiaoying Zhang, Weiheng Su, Fangfei Cai, Tianshan Lan, and Zhenxue Dai. 2024. "Adsorption of Coxsackievirus in Sediments: Influencing Factors, Kinetics, and Isotherm Modeling" Applied Sciences 14, no. 4: 1480. https://doi.org/10.3390/app14041480
APA StyleLi, M., Zhang, X., Su, W., Cai, F., Lan, T., & Dai, Z. (2024). Adsorption of Coxsackievirus in Sediments: Influencing Factors, Kinetics, and Isotherm Modeling. Applied Sciences, 14(4), 1480. https://doi.org/10.3390/app14041480






