Abstract
Proteins undergo a series of conformational changes when affected by the applied electric field, which changes their functions and properties. The conformational changes in proteins in various electric fields are different due to their internal structures. This study simulates the molecular dynamics of proteins in different amounts and directions of electric fields with gromacs software. According to the root mean square deviation, hydrogen bond, dipole moment, and solvent accessible surface area, it is proved that the conformation change in proteins is more drastic under the simultaneous action of multiple electric fields under various directions, and different fragments unfold with divergent electric fields combined, which is of great importance to control protein function, improve biochemical research and production efficiency in the food and drug safety field.
1. Introduction
Nowadays, proteins are widely applied in different areas. Proteins are indispensable for physiological health [1], food production [2], industrial manufacturing [3] and biomedical research [4]. The secondary structure of proteins plays an important role in framework and conformational stability. It has been found that altering the secondary structure of a protein affects its higher structure, which, in turn, affects its activity and properties to varying degrees [5]. Taking proteases as an example, proteases are a type of catalyst that affects the degree and speed of protein decomposition and are divided into the following two categories: endopeptidase [6] and telpeptidase [7] according to the way they degrade polypeptides. Because of their high efficiency, specificity, and gentleness, proteases have become the catalyst of choice in biochemical research. By stimulating proteases according to this property to change their secondary structure and, thus, affect the function or properties of proteases, the catalytic process can be precisely controlled to achieve the goals of clinical precision treatment and maximizing production benefits. Many factors affect the structure of proteins, and a large number of studies have shown that exogenous factors such as pressure [8], temperature [9,10,11], ion concentration [11], irradiation [12], PH [13], the electric field [14,15,16], and magnetic field [17] affect protein conformation stability.
External electrostatic field stimulation is a popular direction for protein conformation studies. Studies have confirmed that the degree of unfolding of proteins placed in the electric field is closely related to the strength of the electric field [14]. The electrical effects of proteins are often studied in tandem with thermal effects. Studies have shown that external electric field stimulation causes significant conformational changes in proteins, and the charged regions are arranged in the direction of the electric field. Different temperatures also have different effects on the speed and degree of development of secondary protein structures [18], guiding maximum nutrient retention and cost control in food industry production. The idea of modulating protein behavior (velocity, selectivity, etc.) by applying an electrostatic field of a certain intensity without affecting protein deformation has also been confirmed [19]. And this finding could bring important contributions to applications such as protein isolation in biochemical reactions and the controlled release of targeted drugs.
The existing research on protein conformational changes mostly uses Raman spectroscopy9, terahertz dielectric spectroscopy [19], Fourier infrared spectroscopy [20], and other techniques for observation and analysis. Compared to these techniques, molecular dynamics (MD) simulation phases can be more easily analyzed at the atomic level, and it is more intuitive to observe conformational changes in proteins. The molecular dynamic simulation is a simulation scientific method specializing in the multidimensional reaction between nanoatoms and other molecular nanoscales, which can accurately predict the dynamic change characteristics of various chemicals larger than the nanometer scale and simulate various basic processes closely related to the path of atomic motion.
In earlier studies, the authors found that applying an electric field of 0.5 v/nm in the x direction can cause significant conformational changes in trypsin inhibitors, resulting in changes in their properties and function. Trypsin inhibitors can inhibit the absorption and utilization of food proteins and, thus, achieve the purpose of antimicrobial and anti-insect pests, regulating the growth and development of organisms and even the apoptosis of cells. In addition, trypsin inhibitors also have broad research and application space in the prevention of diseases (pancreatitis, etc.), such as antiviral and anti-cancer. Based on this study, the molecular dynamics simulation is used to further explore the conformational changes of trypsin inhibitors under the simultaneous action of multiple exogenous electric fields, which has practical value for food production and pharmaceutical research.
2. Materials and Methods
In this study, molecular dynamics simulation methods are used to study secondary structural changes in trypsin inhibitors in an electric field at the molecular level [21]. And the Groningen Chemical Simulation Machine (GROMACS 18.8) software is used to simulate protein kinetics to establish an SPC water model [22], using the Gromos 54a7 force field [23] to apply different amounts of electric fields in the direction of x, y, and z, respectively, [24] and to observe the secondary structure of the protein under different conditions of the electric field and analyze its hydrogen bond, root mean square deviation (RMSD), dipole moment and other data. Trypsin inhibitors are derived from the Protein Database, protein PDB number 4H9W. We placed the selected protein in a cube water box with a size of 6.68 × 6.68 × 6.68 nm, which avoided mirroring and satisfied the periodic boundary conditions. There were 8864 water molecules in the box. In addition, 5 sodium ions (NA+) were introduced to neutralize the entire system.
The downloaded protein molecule consists of 180 residues, with two disulfide bonds between cysteine residues 40–84 and 134–143 [25]. First of all, we used the convergence criterion of 10 kJ/nm/mol maximum force and eliminated close contact via the fastest descent method of 5000 steps so as to minimize the energy of the system. A 50 ns equilibrium simulation was then performed under constant temperature and pressure (NPT) and constant temperature and capacity (NYT) systems so that the water molecules were completely relaxed inside the box to avoid problems in the long-term simulation. The temperature was controlled at 300 K, the temperature control time constant was 0.2 ps, the pressure was maintained at 1 bar, and the pressure control time constant was 2 ps. In all simulations, time steps of 2 fs are used. For the non-bond interaction calculation setting, the cutoff value was set to 1 nm. The electrostatic interaction adopts the PME [26] algorithm, and the calculation of the Van der Waals force interaction uses the cutoff method. Trypsin inhibitors are subjected to different amounts and directions of electrostatic fields to explore the effects of applied electric fields on the secondary structure of proteins [27]. In addition, the RMSD, solvent accessible surface area (SASA), dipole moment, and hydrogen bonds inside the protein and between the protein and water are calculated to analyze the protein’s effect in the electrostatic field.
3. Results and Discussions
3.1. VMD Conformational Observation
VMD (Visual Molecular Dynamics) is a molecule-based visualization application that displays, animates, and analyzes a variety of large-scale biomolecular systems through 3D images and built-in scripts [28]. We obtained Figure 1 by observing the molecular structure and molecular trajectory of proteins through VMD. In previous studies, authors have found that the secondary structure of the protein begins to change after applying an electric field of 0.5 v/nm in intensity in the x-axis direction, and the residues 4–19 gradually unfold and stabilize around 30 ns, and this study also successfully reproduced this conclusion [29]. Under the action of the 0.5 v/nm intensity electric field in the y-axis direction, the residues 4–19 of the protein also unfolded around 10ns, and the residues 139–149 at about 20 ns also began to deform until they were stable at about 60 ns. When the electric field acted in the z-axis direction, the protein conformation changed the same as the former except in the direction. When the 0.354 v/nm electric field was applied simultaneously in the x and y axis directions (the combined field strength was 0.5 v/nm, and the direction was 45 degrees from the x-axis to the y-axis), the protein also expanded the residues for the 61–88 and 169–180 base on the residues 4–19 and 139–149 and rotated because the VMD observation was the same when 0.5 v/nm was applied in each direction; therefore, the situation of 0.5 v/nm of the unidirectional electric field is not discussed in this article. When a 0.5 v/nm electric field is applied simultaneously on the x-axis and z-axis, the protein immediately expands the residues 4–19 at the beginning of the reaction, and the residues 139–149 and 61–88 are gradually expanded but do not rotate for about 30 ns. When the 0.5 v/nm intensity electric field is applied to both the y-axis and the z-axis electric field, the residues 4–19 and 139–149 are deployed along the y-z-axis, and the protein rotates, but the residues 61–88 have no clear conformational changes. When an electric field of the same size acts simultaneously in the direction of the three coordinate axes, the above three fragments have clear conformational changes and rotation.
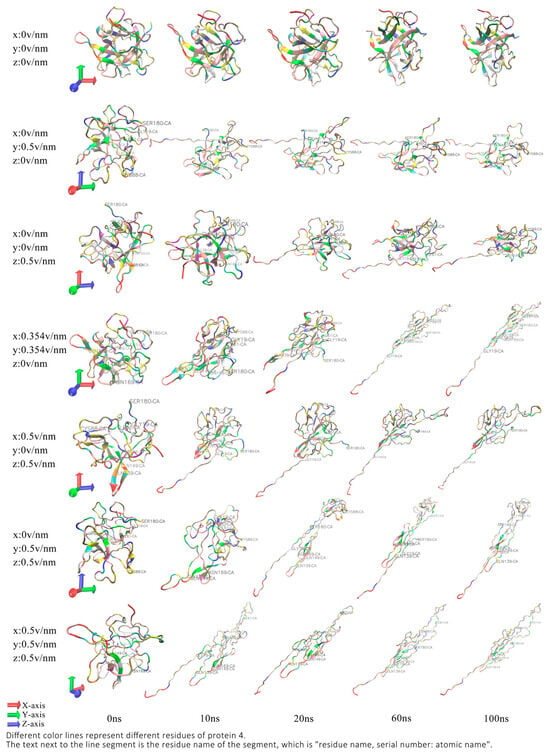
Figure 1.
Conformational changes in proteins at different amounts and directions of electric fields.
3.2. Root Mean Square Deviation (RMSD) Analysis
RMSD is an important parameter for observing and analyzing the degree of conformational change and stability of proteins, and its calculation formula is as follows:
In the above equation, A represents the number of atoms, Natm represents the number of atoms in the selected range, and the superscript ref represents the reference structure.
In this paper, the RMSD of the whole and partially changing fragments of the simulated protein under the action of electric fields in different directions is calculated, as shown in Figure 2, to study the conformational changes, stability, and convergence of the proteins during the simulation [30]. Firstly, we calculate the RMSD value of the protein in the 310 K, 0 v/nm state and set it as the reference value. According to Figure 2a, the RMSD reference value of the protein fluctuated at around 0.25 nm, and the overall dynamic was stable. After applying the electric field, the RMSD curve of the protein could be seen to shift, and the change was clear. This phenomenon indicates that the protein changes its original spatial position and the conformation changes under the action of the electrostatic field. In the above analysis, we observed that the secondary structure of some regions of the protein except residues 4–19 (residues 61–88, 139–149, 169–180 residues) also changed significantly. So, the RMSD parameter graph of residues 61–88, 139–149, and 169–180 under the action of electric fields in different directions was drawn.
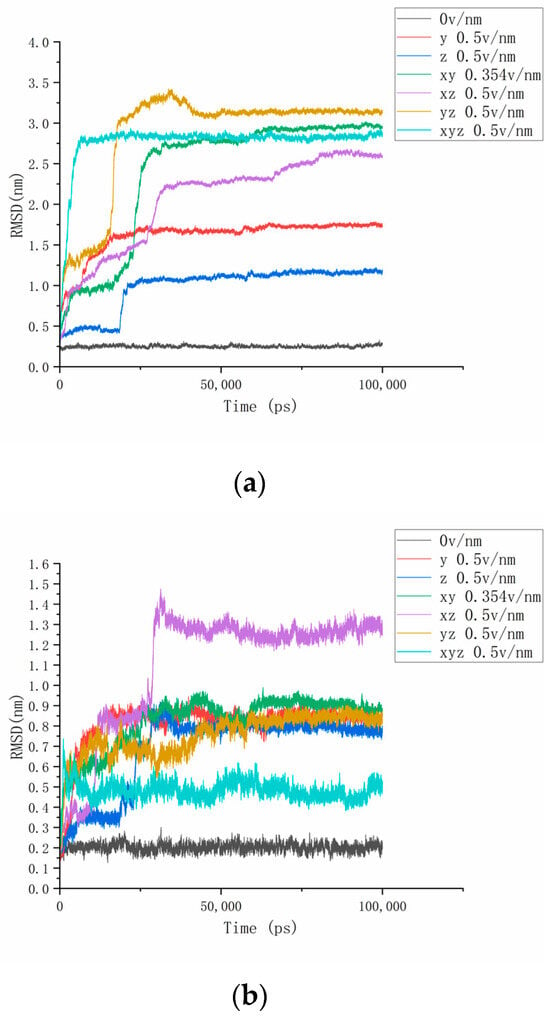
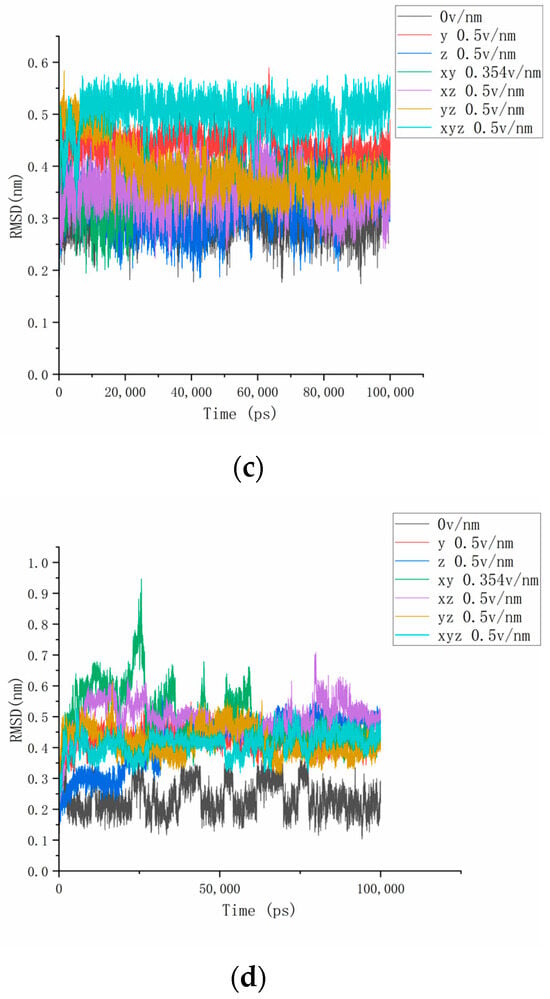
Figure 2.
(a–d) Represents the RMSD curves of protein No. 4H9W, residues 61–88, residues 139–149, and residues 169–180 under different numbers and directions of electric fields.
From Figure 2b–d, it can be seen that the above three segment residues changed significantly under the action of electrostatic fields in different directions. After applying an electric field of 0.5 v/nm at different amounts and directions, the RMSD curve of residues 61–88 increased to a certain height sharply and maintained dynamic stability. This indicates that the secondary structure of the protein fragment changes under the influence of the electric field and deviates significantly from the initial position, maintaining the current conformation to maintain equilibrium after a period of deformation. When the 0.5 v/nm electrostatic field was applied to the z axis and x, z-axis, respectively, the RMSD of residues 61–88 underwent a second mutation at about 20 ns to 30 ns at the start of the reaction and remained dynamically stable after the second mutation. This is because protein molecules have an interaction force that maintains the initial structure, and the destruction of this force requires a certain period of continuous action, sometimes divided into several stages. In addition, the RMSD value of residues 61–88 was finally dynamically stabilized at about 0.5 nm after the electric field was applied to the z-axis alone, while the final value was about 1.3 nm when the electric field was applied to the x and z-axis simultaneously. It can be seen that the stability of protein molecules is anisotropy, and the application of electric fields in different directions has different effects on the protein structure. It can be found from the changes in the RMSD curve in Figure 2c for residues 139–149 that although the RMSD value is significantly higher than the reference value under the action of the electric field, the curves of each group do not undergo clear bending or rising but increase as a whole. This phenomenon shows that the structure of residues 139–149 is very stable, and the movement of protein molecules is intensified under the action of the electric field, but no clear deformation occurs. In addition, the different structures of proteins in different directions have different effects on the reaction time and degree, just like residues 169–180. Compared with other directions, when the 0.354 v/nm electric field is applied to both the x and y axes, the RMSD value of residues 169~180 increases sharply to 0.85 nm within 25 ns and then drops rapidly to about 0.5 nm, which also proves this conclusion. The RMSD curve can reflect the conformational changes in proteins under the action of the electric field, and these proteins can be controlled to achieve a different deformation according to the difference between electric fields.
3.3. Dipole Moment Analysis
In the study of changes in protein conformation via external electrostatic fields, the dipole moment parameter analysis of proteins is a very simple and intuitive method of analysis [31]. The dipole moment of a protein molecule is the product of the distance between the centers of positive and negative charges and the electricity carried by the center of the charge, which is a vector representing the polarity of the molecule. In the natural state, the molecular dipole moment presents as a random, disordered distribution state in the system. In this study, we applied an external electrostatic field so that the dipole moment of the protein molecule could couple with the direction of the applied electric field. From Figure 3, we can see that the dipole moment of the protein molecule without the action of the reference group (310 K, 0 v/nm) was stable at about 400–500 Debye. When the instantaneous dipole moment of the electric field was applied simultaneously in the x, y, x, and z directions, it increased rapidly until the dynamic stability was about 2800 Debye and 2600 Debye, respectively, about 30 ns after the simulation began. The results show that there was a significant coupling between the dipole moment and the direction of the electric field, which affected the behavior and conformational change in the protein in the electric field. When an electric field of 0.5 v/nm was applied to the x, y, and z axes at the same time, the growth amplitude and speed of the dipole moment increased significantly, and the reaction was complete within about 10 ns from the beginning of the simulation and remained dynamically stable at about 2250 Debye. It can be inferred that the electric field intensity had an effect on the coupling reaction speed of the electric field to the dipole moment. When the electric field intensity was strong, the coupling speed clearly accelerated, and the electric field with the same intensity in different directions had different effects. It is worth noting that when an electric field of 0.5 v/nm in size was applied to the y and z axes, respectively, or in the direction of both axes, the total dipole moment of the protein molecule decreased and fluctuated around the value of 0. The final equilibrium position was even negative when applied in both directions at the same time: a phenomenon that indicates that the protein may have a certain force in the y and z directions in the natural state. The intensity of the electric field in both directions does not cause a coupling reaction with a significant dipole moment, and some fragments may be more compact under the electric field. Combined with the different dipole moment convergence values of other experimental groups, it is not difficult to find that there are differences in forces and stability in different directions within the protein [32].
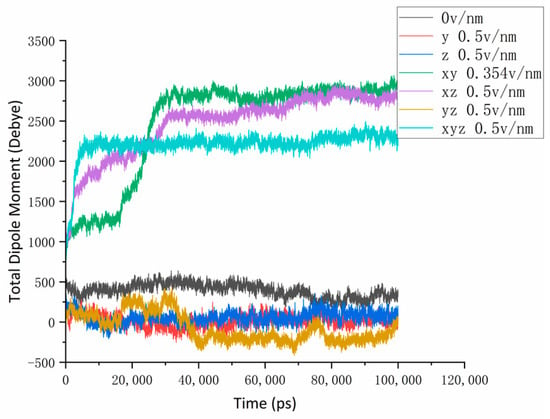
Figure 3.
Protein dipole moment curves under different numbers and directional electric fields.
3.4. Hydrogen Bond Analysis
Proteins have a secondary structure because the peptide backbone hovers or folds according to a certain law in space, which is manifested as the α-helix, β-folding, β-corner, and Ω-rings and other structural conformations. Hydrogen bonds are molecular forces stronger than van der Waals’ forces formed via the covalent bonding of hydrogen atoms and electronegativity atoms in proteins with secondary structures. Since changes in the secondary structure of proteins lead to changes in hydrogen bonds inside and outside the proteins, this paper provides lateral proofs of conformational changes in proteins by analyzing and comparing the changes in the number and length of hydrogen bonds inside the proteins and between proteins and water after an applied electric field [33]. As can be seen from Figure 4, the number of hydrogen bonds inside the reference group (310 K, 0 v/nm) protein without an electric field is stable at around 120. The number of hydrogen bonds inside the protein decreases significantly after the electrostatic field is applied [24], which indicates that the original internal structure of the protein changes under the action of the electric field, and the hydrogen bond between the secondary structure begins to break. The change in the number of hydrogen bonds between the protein and water can be observed in Figure 5, and it is not difficult to find that the number of hydrogen bonds in this part changed and the number of hydrogen bonds in the protein changed in a complementary relationship. Taking the simultaneous application of the 0.5 v/nm electric field in the x, y, and z axes as an example, the number of intermolecular hydrogen bonds of proteins decreased to the minimum when the simulation started at about 10 ns. Correspondingly, the number of hydrogen bonds between protein molecules and water molecules increased to the maximum at about 10 ns; that is, the number of hydrogen bonds inside the protein decreased, and the hydrogen bond increased with the water because more hydrogen bonds are formed with the water molecules after the hydrogen bond breaks under the action of the electric field. In addition, the electric field’s strength also has an impact on the speed at which hydrogen bonds break and form. From Figure 4, it can be found that the drop time of the number of hydrogen bonds when applying a 0.5 v/nm electric field in one direction is around 30 ns. As the number of electric fields increases, the strength of the combined field becomes larger, the time for the hydrogen bond change in the protein is also shortened, and the decline time of the number of hydrogen bonds inside the protein is only about 20 ns when the electric field is applied to the two sides, while the decline time when the electric field is applied at the same time in the direction of the x, y, z coordinate axis is only 5–10 ns, and it can be seen that the strength of the electric field has a great impact on the conformational change rate of the protein.

Figure 4.
(a,b) Represents the curves of change in the number and length of hydrogen bonds inside proteins under different amounts and directional electric fields.
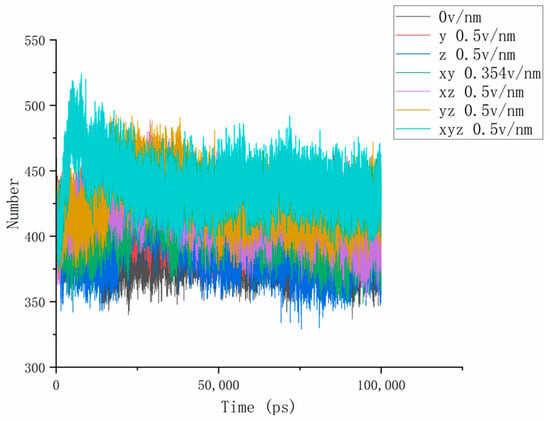
Figure 5.
The curves of the change in the number of hydrogen bonds between proteins and water under different amount and directional electric fields.
To accurately describe the conformational changes in proteins, we then calculated and analyzed the number of hydrogen bonds between the three residues selected above and the water and the hydrogen bond length inside the residues to obtain Figure 6. It was then found that the number of hydrogen bonds between residues 61 and 88 and water under the action of the electric field increased significantly and, combined with the above parameter analysis, it can be seen that the secondary structure of this area changed, and the contact area with water increased while more hydrogen bonds were formed with water molecules [34]. In addition, the change in the number of hydrogen bonds of different residues also reflected the diversity and difference in the internal structure of the protein. Unlike residues 61–88, it can be seen from Figure 7 that the number of hydrogen bonds between residues 139 and 149 and water was relatively stable after different electric fields were applied, and this change was not clear. Combined with the calculation and analysis of the RMSD parameters of residues 139–149 above, the RMSD curve of this part of the residue moved upward but did not significantly jump under the action of the electric field, and the change amplitude only increased from an initial 0.3 nm to about 0.5 nm, indicating that residues 139–149 became active under the action of the current electric field but did not undergo significant structural changes. This result confirms that the β folding structure of this fragment region is stable, and the conformational changes are subtle. When the electric field is applied simultaneously in the x, y, and z directions, the number of hydrogen bonds increases as a whole, indicating that the conformation of residues 139–149 does change under the action of the electric field, and more hydrogen bonds are formed with water. In addition, the change in the number of hydrogen bonds between residues 169 and 180 and water in Figure 8 also proves the difference within the protein, and it can be found that the hydrogen bond between residues 169 and 180 and water is significantly reduced under the action of the unidirectional electric field, and the number of hydrogen bonds is closer to the reference value after the electric field is increased. This indicates that the internal structure of the protein fragment folds more under a one-way electric field [35], and the hydrogen bond with the water is broken. Combined with VMD images and RMSD parameter analysis, the changes in the number of hydrogen bonds in the three-segment residue confirmed the conformational changes in proteins. We define the hydrogen bond length as the distance between the donor and the receiver. From Figure 4b, we can find that the internal hydrogen bond length of the protein is elongated but not clear under the action of the electric field. We analyzed this phenomenon for the following reasons: First, the hydrogen bonding force inside the protein is strong, and the electric field applied by the experiment is not strong enough to completely prevent the hydrogen bond at all. Second, the residue region analyzed in this article has a strong dipole moment; in addition, because residues 139–149 contain disulfide bonds, which are offset by the electric field coupling of proteins, skeletal changes are also limited. Combined with the previous analysis, we correctly characterized the conformational changes of proteins by analyzing the number and length of the hydrogen bonds of proteins.
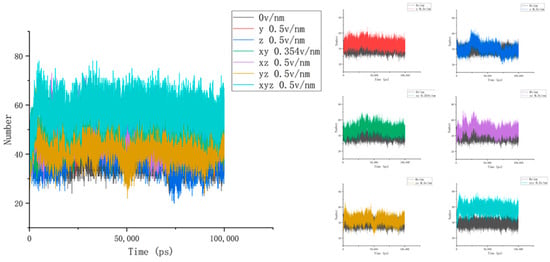
Figure 6.
The change curves of the number of hydrogen bonds between residues 61 and 88 and water under different amounts and directions of electric fields.
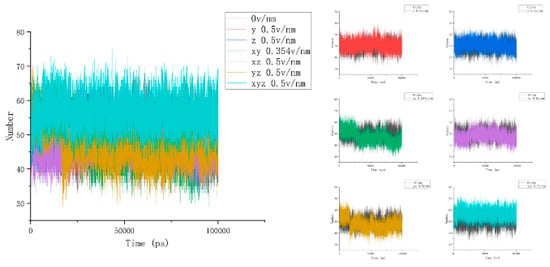
Figure 7.
The change curves of the number of hydrogen bonds between residues 139 and 149 and water under different amounts and directional electric fields.
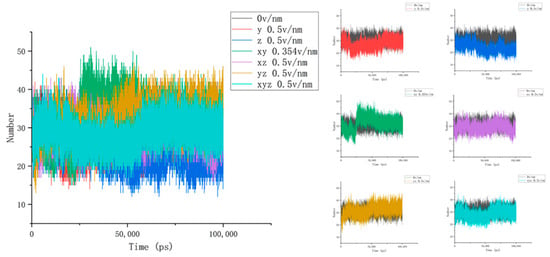
Figure 8.
The curves of the number of hydrogen bonds between residues 169 and 180 and water under different amounts and directional electric fields.
3.5. Solvent Accessible Surface Area (SASA) Analysis
The previous article explored the formation of more hydrogen bonds between the hydrogen bond breaks inside the protein and the water molecule under the action of the electric field and found that the contact area between the protein and the water may change during the reaction. To explore the interaction between proteins and solvents, we continued to study the SASA of proteins to analyze changes in the protein–water contact area. We performed this by calculating the contact area change chart between the No. 4H9W protein and the three-segment residue (residues 61–88, residues 139–149, residues 169–180) and the water solvent, as shown in Figure 9. As can be seen from Figure 9a, the SASA value of the protein of the reference group (310 K, 0 v/nm) of this experiment was stable at about 100 nm2 without significant change, which proves that the protein has a certain force inside, so that it can maintain structural stability in the natural state and, thus, stabilize the contact area with water. Under the action of the electric field, the contact area of the protein as a whole with the water solvent increases sharply, and it is dynamically balanced at a certain value after 25 ns. It can be seen that the internal structure of the protein changes significantly under the action of the external electric field, and some areas become stretched or rotated from curling up to expanding so that the contact area with the water solvent increases. Since most of them are distributed on the surface of the protein, more active sites are exposed [36]. In addition, as the strength of the combined electric field increases, the change time of the contact area is significantly shortened, and the area growth rate increases significantly, which also matches the results of the previous analysis of the RMSD, dipole moment, and hydrogen bond. In combination with the protein RMSD curve in Figure 2a, it is not difficult to find that the curves of different parameters under the same electric field change at the same speed. Taking the electric field of 0.5 v/nm applied simultaneously in the x, y, and z axes as an example, the RMSD and SASA parameter curves of the proteins both increased to the highest within 10ns after the simulation began and then tended to be stable. Figure 9b–d calculates the SASA values of residues 61–88, 139–149, and 169–180, respectively, and we found that the SASA parameter changes in some protein fragments were slightly different from the overall characteristics discussed above. Taking residues 61–88 as an example, we could see that the contact surface of this segment of the residue with the aqueous solvent mostly significantly increased after different electric fields were applied, indicating that the deformation of this protein fragment under the action of the electric field exposed more hydrophilic regions. In particular, when an electric field of 0.354 v/nm was applied simultaneously in the x, y-axis direction, the SASA value of this fragment dropped to a certain extent before it began to grow and exceed the reference value, indicating that residues 61–88 in this state may shrink to a certain extent and then expand. In the RMSD and hydrogen bond parameter curves of residues 139–149, the parameters in this direction electric field were the most active. By observing Figure 9c, it can be found that the SASA change in residues 139–149 under the action of the electric field is not drastic, and the same change in the number of hydrogen bonds is also the overall upward movement of the curve, which proves that the conformational change in this protein fragment is not clear. In contrast, the SASA value of residues 169–180 in Figure 9d did not increase but decreased under the action of some electric fields, which also confirmed the anisotropy inside the protein and the influence of disulfide bonds.
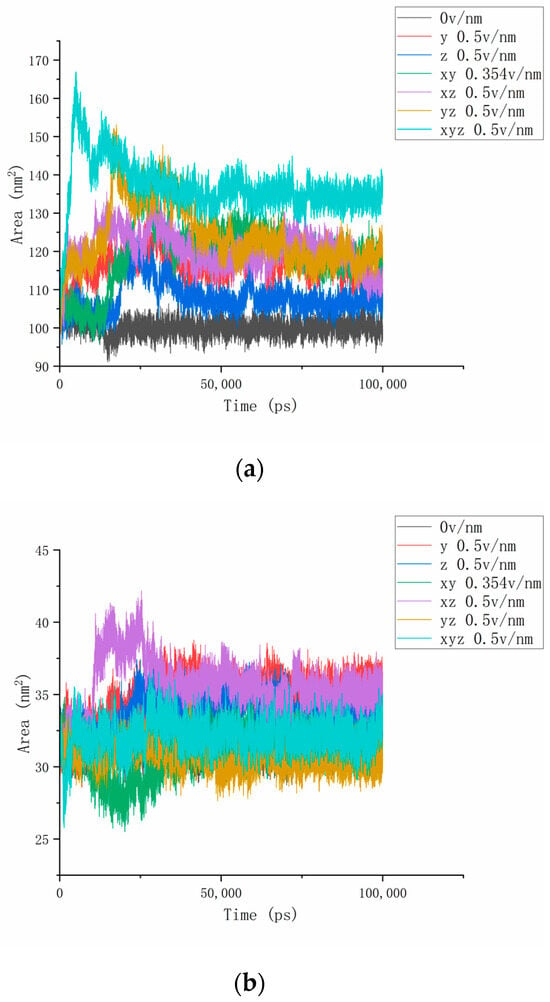
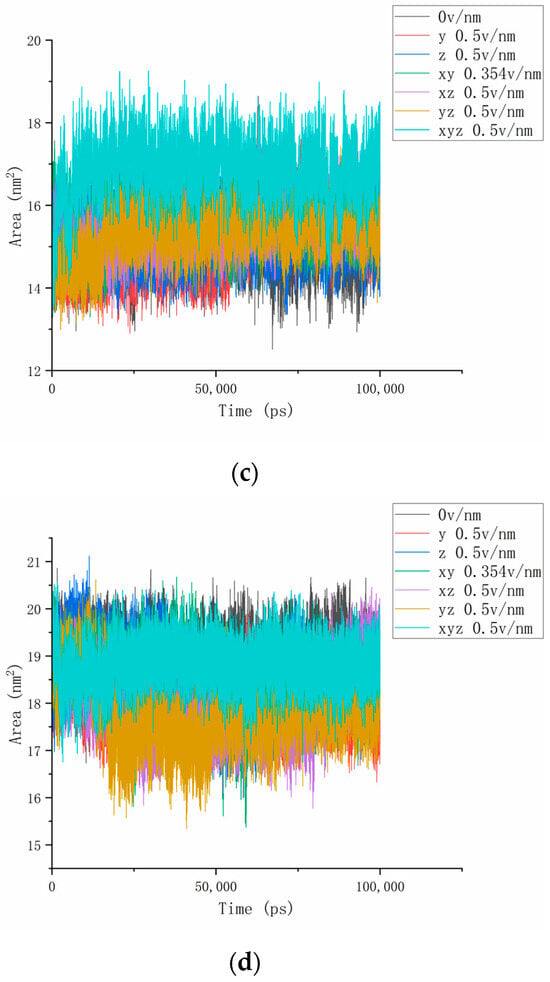
Figure 9.
(a–d) Represents the SASA numerical curves of protein No. 4H9W, residues 61–88, residues 139–149, and residues 169–180 under different amounts and directional electric fields.
3.6. Cell Pulse Electrical Breakdown Experiment
In order to study the changes in the protein under the action of the electric field in the real environment, the lightning surge generator (SUG61005TB, Prima, Shanghai, China) was used as the pulse power supply in this project, as shown in Figure 10. The myeloma cells (SP/20) were removed from the liquid nitrogen tank and melted in a water bath at 37 °C, then centrifuged at 1000 r/min for 5 min before the removed supernatant; then, they were re-suspended with an SP/20 cell culture medium and spread in a 10 mL cell culture dish, and then cultured in a cell incubator. After the cells grew normally, they multiplied for 2 to 3 generations and multiplied the cells once the day before the experiment, controlling the cell density by 80% to 90%. An an ppropriate amount of myeloma cell suspension was dropped into the electric cup, and the pulse electric field voltage was set as 0.2 kV, 0.4 kV, 0.6 kV, 0.8 kV, and 1 kV, respectively. The pulse number was 50, and the pulse width was fixed as 12.8 μs. The electrode plate spacing of the rotating cup was 0.4 cm. The control group was cells in the natural environment, and the experiment times in the pulsed electric field environment were five times, respectively. After the completion of each experiment, cell samples were taken and placed on a slide of a biological microscope (Olympus CX43, Olympus Corporation, Tokyo, Japan) to observe the changes in cell structure.
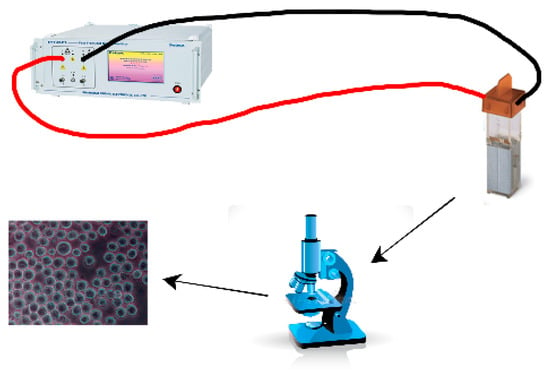
Figure 10.
Experimental apparatus for the effect of the pulsed electric field on biological tissue.
In this paper, the structure of myeloma cells was observed many times under different levels of electric field intensity. The control group did not apply the pulsed electric field, and the five experimental groups applied a pulsed electric field of 0.5 kV/cm, 1 kV/cm, 1.5 kV/cm, 2 kV/cm, and 2.5 kV/cm. Under the electric field intensity of 0.5 kV/cm, 1 kV/cm, and 1.5 kV/cm, the structure of myeloma cells did not change significantly, as shown in Figure 11. When the pulsed electric field intensity was 2 kV/cm and 2.5 kV/cm, the cell membrane structure of some myeloma cells was broken. The results show that when the pulse electric field intensity reaches 2 kV/cm, the electric field has a clear effect on the phospholipid membrane structure.
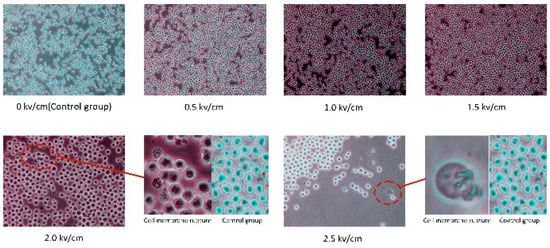
Figure 11.
Myeloma cells’ state under different levels of electric field intensity.
4. Summary
In this study, we discuss the conformational changes in trypsin inhibitors at different amounts and different directions of applied electrostatic fields using a molecular dynamics simulation to find the change law of the protein’s secondary structure, and the simulation results found that the external electrostatic field had a huge impact on protein conformation changes. When an electric field of 0.5 v/nm was applied to a one-way electric field, the chain region of the protein (residues 4–19) and the folding region (residues 139–149) began to deform, and the latter changed insignificantly. When an electric field of 0.5 v/nm (0.354 v/nm) was applied simultaneously in multiple directions, the new folding region (residues 61–88) and the chain region (residues 169–180) also changed successively. In the next three segments, residues 61–88 and residues 139–149 were expanded to a certain extent in molecular dynamic simulations, and residues 169–180 gradually expanded into flat protein chains. We calculated parameters such as RMSD, dipole moment analysis, hydrogen bonding, SASA, etc. The analysis of the number and length of hydrogen bonds showed that some of the structures inside the protein were deconstructed under the action of the electric field; the hydrogen bonds between some protein molecules were broken, and new hydrogen bonds formed with the water molecules. The numerical changes in SASA also confirmed this conclusion, and under the action of the electric field, the area of hydrophilic regions and the hydrophobic regions of proteins increased due to conformational changes. RSMD indicates that the protein as a whole and the analyzed fragments spatially shifted after energization, and the conformation changes deviated from their original positions. The analysis of dipole moments shows that proteins are coupled to the electric field direction under the action of the electric field, and the coupling effect and speed of each direction are different. In summary, the direction and quantity of the electric field have different effects on the secondary structure of proteins. The greater the electric field intensity, the more intense the movement of protein molecules and the more likely conformational changes occur. While the electric field intensity is the same, the reaction results of protein molecule motion under the action of the electric field in different directions are also different. In the actual experiment of myeloma cells under a pulsed electric field, the experimental results show that the phospholipid membrane structure has a certain resistance to the pulsed electric field below 1.5 kV/cm. When the electric field intensity is higher than this value, the pulsed electric field causes irreversible damage to the membrane structure. Due to the complex structure of phospholipids and proteins, the specific molecules destroyed by a pulsed electric field need to be further studied. The subject of this paper is a trypsin inhibitor, which has the effect of controlling the catalytic rate and effect of trypsin. Through the study and calculation of the behavior of trypsin inhibitors under the electric field, it is possible to accurately control the conformational changes in trypsin inhibitors, thereby changing the properties of proteins and achieving accurate control of the catalytic process of trypsin, which is of great value for antimicrobial and insect pests [37], food and drug production [38], health maintenance [39], and disease prevention [15].
Author Contributions
M.H.: Multidirection complex electric field simulation of protein; Calculation and analysis of protein parameters such as RMSD and hydrogen bond; Writing—Original Draft; K.Z.: Simulation experiments of different electric fields in X-axis direction of protein No. 4H9W, Writing—Original Draft; F.C.: VMD visualization of protein molecular motion trajectories; Y.J.: Search and evaluation of protein file No. 4; Supervise the simulation experiment process; C.Y.: Teaching and supervision of gromacs software; Validation; C.J.: Repeated validation of protein parameters; Writing—Review and Editing; L.X.: Funding Acquisition; Resources; Supervision; Writing—Review and Editing; Corresponding author. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Natural Science Foundation of China (62105196) and State Grid Shaanxi Electric Power Company Technology Project (No. 5226KY2000ID).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in [GitHub] at: https://github.com/xueliangokay/XL/tree/Molecular-study-on-conformational-changesof-trypsin-inhibitors-in-multidirectional-electrostatic-fields (accessed on 23 July 2023).
Conflicts of Interest
The authors declare that this study received funding from the National Natural Science Foundation of China (62105196) and State Grid Shaanxi Electric Power Company Technology Project (No. 5226KY2000ID). The funder had the following involvement with the study: Fund support, Sample provision.
References
- Wu, N.; Yang, M.Y.; Gaur, U.; Xu, H.L.; Yao, Y.F.; Li, D.Y. Alpha-Ketoglutarate: Physiological Functions and Applications. Biomol. Ther. 2016, 24, 1–8. [Google Scholar] [CrossRef]
- Pojic, M.; Misan, A.; Tiwari, B. Eco-innovative technologies for extraction of proteins for human consumption from renewable protein sources of plant origin. Trends Food Sci. Technol. 2018, 75, 93–104. [Google Scholar] [CrossRef]
- Kumar, M.; Selvasekaran, P.; Kapoor, S.; Barbhai, M.D.; Lorenzo, J.M.; Saurabh, V.; Potkule, J.; Changan, S.; ElKelish, A.; Selim, S.; et al. Moringa oleifera Lam. seed proteins: Extraction, preparation of protein hydrolysates, bioactivities, functional food properties, and industrial application. Food Hydrocoll. 2022, 131, 107791. [Google Scholar] [CrossRef]
- Da Cunha, M.; Caracciolo, P.C.; Abraham, G.A. Latest advances in electrospun plant-derived protein scaffolds for biomedical applications. Curr. Opin. Biomed. Eng. 2021, 18, 100243. [Google Scholar]
- Fenwick, R.B.; Oyen, D.; van den Bedem, H.; Dyson, H.J.; Wright, P.E. Modeling of Hidden Structures Using Sparse Chemical Shift Data from NMR Relaxation Dispersion. Biophys. J. 2021, 120, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Krupina, N.A.; Bogdanova, N.G.; Khlebnikova, N.N.; Zolotov, N.N.; Kryzhanovskii, G.N. Benzyloxycarbonyl-Methionyl-2(S)-Cyanopyrrolidine, a Prolyl Endopeptidase Inhibitor, Modulates Depression-Like Behavior of Rats in Forced Swimming Test and Activities of Proline-Specific Peptidases in the Brain Structures. Bull. Exp. Biol. Med. 2013, 154, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Nishie, M.; Sasaki, M.; Nagao, J.; Zendo, T.; Nakayama, J.; Sonomoto, K. Lantibiotic Transporter Requires Cooperative Functioning of the Peptidase Domain and the ATP Binding Domain. J. Biol. Chem. 2011, 286, 11163–11169. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Y.; Zhang, L.L.; Wang, Y.C.; Li, Z.R.; Wang, Z.H.; Han, J.C. Effect of high hydrostatic pressure on solubility and conformation changes of soybean protein isolate glycated with flaxseed gum. Food Chem. 2020, 333, 127530. [Google Scholar] [CrossRef] [PubMed]
- Polokhin, A.A.; Savelyev, M.S.; Podgaetsky, V.M.; Neklyudov, I.A.; Pavlov, A.A. Study of Structural Changes of Bovine Serum Albumin Occurring as a Result of Heating Using Methods of Raman Spectroscopy. In Proceedings of the IEEE Russia Section Young Researchers in Electrical and Electronic Engineering Conference (EIConRus), St. Petersburg Electrotechnical University LETI, St. Petersburg, Russia, 1–3 February 2017; pp. 56–57. [Google Scholar]
- Ur, V.F.; Kokurina, N.Y. Effect of Water on the Temperatures of Human Immunoglobulin Conformation Transitions. Russ. J. Phys. Chem. A 2013, 87, 1632–1637. [Google Scholar]
- Leon, D.; Vermeuel, M.P.; Gupta, P.; Bunagan, M.R. The effect of salt and temperature on the conformational changes of P1LEA-22, a repeat unit of plant Late Embryogenesis Abundant proteins. J. Pept. Sci. 2020, 26, e3247. [Google Scholar] [CrossRef]
- Liu, G.X.; Li, J.; Tu, Z.C.; Sha, X.M.; Wang, H.; Wang, Z.X. Investigation of conformation change of glycated ovalbumin obtained by Co-60 gamma-ray irradiation under drying treatment. Innov. Food Sci. Emerg. Technol. 2018, 47, 286–291. [Google Scholar] [CrossRef]
- Mir, S.; Ashraf, S.; Saeed, M.; Rahman, A.U.; Ul-Haq, Z. Protonation states at different pH, conformational changes and impact of glycosylation in synapsin Ia. Phys. Chem. Chem. Phys. 2021, 23, 16718–16729. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.M.; Avelar, Z.; Vicente, A.A.; Petersen, S.B.; Pereira, R.N. Influence of moderate electric fields in beta-lactoglobulin thermal unfolding and interactions. Food Chem. 2020, 304, 125442. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.S.; Suresh, A.; Pirogova, E. Effects of oscillating electric fields on conotoxin peptide conformation: A molecular dynamic simulation study. J. Mol. Graph. Model. 2021, 103, 107799. [Google Scholar] [CrossRef]
- Xie, Y.; Pan, Y.F.; Zhang, R.; Liang, Y.; Li, Z.C. Modulating protein behaviors on responsive surface by external electric fields: A molecular dynamics study. Appl. Surf. Sci. 2015, 326, 55–65. [Google Scholar] [CrossRef]
- Hayashi, S.; Kakikawa, M. Exposure to 60 Hz magnetic field can affect membrane proteins and membrane potential in human cancer cells. Electromagn. Biol. Med. 2021, 40, 459–466. [Google Scholar] [CrossRef]
- Baruah, I.; Borgohain, G. Structural and functional changes of the protein beta-lactoglobulin under thermal and electrical processing conditions. Biophys. Chem. 2020, 267, 106479. [Google Scholar] [CrossRef]
- Zang, Z.Y.; Yan, S.H.; Hana, X.H.; Wei, D.S.; Cui, H.L.; Du, C.L. Temperature- and pH-dependent protein conformational changes investigated by terahertz dielectric spectroscopy. Infrared Phys. Technol. 2019, 98, 260–265. [Google Scholar] [CrossRef]
- Wei, W.; Hu, W.; Zhang, X.Y.; Zhang, F.P.; Sun, S.Q.; Liu, Y.; Xu, C.H. Analysis of protein structure changes and quality regulation of surimi during gelation based on infrared spectroscopy and microscopic imaging. Sci. Rep. 2018, 8, 5566. [Google Scholar] [CrossRef]
- Budi, A.; Legge, S.; Treutlein, H.; Yarovsky, I. Effect of external stresses on protein conformation: A computer modelling study. Eur. Biophys. J. 2004, 33, 121–129. [Google Scholar] [CrossRef]
- Huang, I.S.; Tsai, M.K. Interplay between Polarizability and Hydrogen Bond Network of Water: Reparametrizing the Flexible Single-Point-Charge Water Model by the Nonlinear Adaptive Force Matching Approach. J. Phys. Chem. A 2018, 122, 4654–4662. [Google Scholar] [CrossRef]
- Lynn, S.; Silva, Y.R.E.; Diambra, L.; McCarthy, A.N.; Liping, L.; Ru, B.; Román, C.L.; Maiztegui, B.; Flores, L.E.; Gagliardino, J.J. A new analogue of islet neogenesis associated protein with higher structural and plasma stability. J. Biomol. Struct. Dyn. 2021, 39, 766–776. [Google Scholar] [CrossRef]
- Muller, W.A.; Sarkis, J.R.; Marczak, L.D.F.; Muniz, R. Molecular dynamics study of the effects of static and oscillating electric fields in ovalbumin. Innov. Food Sci. Emerg. Technol. 2022, 75, 102911. [Google Scholar] [CrossRef]
- Vagadia, B.H.; Vanga, S.K.; Singh, A.; Raghavan, V. Effects of thermal and electric fields on soybean trypsin inhibitor protein: A molecular modelling study. Innov. Food Sci. Emerg. Technol. 2016, 35, 9–20. [Google Scholar] [CrossRef]
- Robertson, A.; Luttmann, E.; Pande, V.S. Effects of long-range electrostatic forces on simulated protein folding kinetics. J. Comput. Chem. 2008, 29, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.T.; You, L.; Dou, W.H.; Sun, T.T.; Xu, P. Effects of an Electric Field on the Conformational Transition of the Protein: A Molecular Dynamics Simulation Study. Polymers 2019, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, H.S.; Sousa, S.F.; Cerqueira, N. VMD Store-A VMD Plugin to Browse, Discover, and Install VMD Extensions. J. Chem. Inf. Model. 2019, 59, 4519–4523. [Google Scholar] [CrossRef] [PubMed]
- Lynn, S.; Silva, Y.R.E.; Diambra, L.; McCarthy, A.N.; Liping, L.; Ru, B.; Román, C.L.; Maiztegui, B.; Flores, L.E.; Gagliardino, J.J. The Effect of External Electric Field on the Conformational Integrity of Trypsin Inhibitor: A Molecular Model Study. Russ. J. Phys. Chem. A 2022, 96, 2533–2540. [Google Scholar]
- Cazals, F.; Tetley, R. Characterizing molecular flexibility by combining least root mean square deviation measures. Proteins-Struct. Funct. Bioinform. 2019, 87, 380–389. [Google Scholar] [CrossRef]
- De Visser, S.P.; Mukherjee, G.; Ali, H.S.; Sastri, C.V.; Distributions, L.C.; Moments, E.D. and Local Electric Fields Influence Reactivity Patterns and Guide Regioselectivities in alpha-Ketoglutarate-Dependent Non-heme Iron Dioxygenases. Acc. Chem. Res. 2022, 55, 65–74. [Google Scholar] [CrossRef]
- Chen, J.H.; Gathiaka, S.; Wang, Z.J.; Thuo, M. Role of Molecular Dipoles in Charge Transport across Large Area Molecular Junctions Delineated Using Isomorphic Self-Assembled Monolayers. J. Phys. Chem. C 2017, 121, 23931–23938. [Google Scholar] [CrossRef]
- Nakagawa, H.; Tamada, T. Hydration and its Hydrogen Bonding State on a Protein Surface in the Crystalline State as Revealed by Molecular Dynamics Simulation. Front. Chem. 2021, 9, 738077. [Google Scholar] [CrossRef] [PubMed]
- Fulara, A.; Wojcik, S.; Loksztejn, A.; Dzwolak, W. De novo refolding and aggregation of insulin in a nonaqueous environment: An inside out protein remake. J. Phys. Chem. B 2008, 112, 8744–8747. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Li, Y.X.; He, X.; Chen, S.D.; Zhang, J.Z.H. Effect of Strong Electric Field on the Conformational Integrity of Insulin. J. Phys. Chem. A 2014, 118, 8942–8952. [Google Scholar] [CrossRef] [PubMed]
- Sraphet, S.; Javadi, B. Application of Hierarchical Clustering to Analyze Solvent-Accessible Surface Area Patterns in Amycolatopsis lipases. Biology 2022, 11, 652. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.S.P.; Meriño-Cabrera, Y.B.; Mantilla-Afanador, J.G.; Lima, G.D.A.; Barbosa, S.L.; Vital, C.E.; Barros, R.; Rodrigues-Junior, N.; Oliveira, E.E.; Oliveira, M.G.A. Proteolytic enzymes in the salivary glands of the Neotropical brown stink bug Euschistus heros: Reduced activities in imidacloprid-resistant strains. Ann. Appl. Biol. 2021, 179, 85–95. [Google Scholar] [CrossRef]
- Stanojevic, S.P.; Barac, M.B.; Kostic, A.Z.; Pesic, M.B. Trypsin inhibitor content and activity of soaking water whey as waste in soy milk processing. J. Environ. Sci. Health Part B-Pestic. Food Contam. Agric. Wastes 2021, 56, 292–296. [Google Scholar] [CrossRef]
- Serquiz, A.C.; Machado, R.J.A.; Serquiz, R.P.; Lima, V.C.O.; de Carvalho, F.M.C.; Carneiro, M.A.A.; Maciel, B.L.L.; Uchôa, A.F.; Santos, E.A.; Morais, A.H.A. Supplementation with a new trypsin inhibitor from peanut is associated with reduced fasting glucose, weight control, and increased plasma CCK secretion in an animal model. J. Enzym. Inhib. Med. Chem. 2016, 31, 1261–1269. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).