Abstract
Bone density at the implant site is correlated to the success of osseointegration. The objective of this in vitro study was to evaluate the efficacy of osseodensification burs in increasing bone density using a solid polyurethane foam block model. The osseodensification burs kit was used to perform 48 osteotomies on a rigid polyurethane foam test ground. Burs were utilized on a TMM2 implant motor for data collection. The osteotomies were divided into two study groups (A and C) in which implant sites, extended 12 and 14 mm deep, respectively, were prepared using the drills to a compaction rotation; two control groups, B and D, represented the osteotomies for which the drills were used in cutting direction. A 3.8 × 12 mm conical implant was inserted into each site; for each implant, data were collected on the peak torque (Cp), mean torque (Cm), and integral depth curve (I). The implants underwent resonance frequency analysis (RFA) to assess the implant stability quotient (ISQ). Correlation analysis was performed between I, Cm, Cp and ISQ. One-way analysis of variance (ANOVA) was used to identify statistically significant differences between groups. Group C, representing osteotomies prepared at 14 mm with osseodensification burs, showed a significantly higher value for each parameter. Implants at sites obtained with osteocondensation drills and prepared at greater depth for autologous particle grafting showed significant increases in each implant stability parameter.
1. Introduction
Over the last 25 years, osseointegrated oral implantology has had a significant impact on the practice of dentistry, particularly in the field of prosthetic rehabilitation. In implant-prosthetic rehabilitation, the evaluation of bone density is always considered a key element in achieving primary implant stability [1]. Osseodensification burs are proposed in the scientific community as a surgical technique to increase the bone density in edentulous areas [2]. Osseointegration is a functional biological–mechanical interaction determined at the bone–implant interface level, avoiding the interposition of poorly organized fibroblastic tissue. The neoformation of compact and organized lamellar bone enables implant integration and defines its secondary stability [3]. The biological processes underlying osseointegration significantly depend on the primary implant stability achieved during fixture insertion, defined as the biomechanical engagement between bone and implant. This allows the implant to mechanically interlock with the bone tissue until secondary stability is attained. The role of osseodensification in dental implant stability has gained increasing attention, as evidenced by recent studies. Barberá-Millán et al. (2021) and Cáceres et al. (2020) emphasized its effectiveness in enhancing primary stability, especially in low-density bone [4,5]. This is complemented by findings from Feher et al. (2021) and Romeo et al. (2023), who demonstrated the correlation between drilling protocols and implant stability [6,7].
During the preparation of osteotomy, the preservation and maintenance of bone play critical roles in achieving improved primary mechanical stability and bone–implant contact (BIC), ultimately enhancing the implant’s secondary stability [5]. The attainment of primary stability is directly influenced by factors such as implant macro-geometry, the surgical technique employed, and the quality of the bone in the edentulous site, including bone density and trabecular architecture. While the operator has control over the first two factors, bone quality and density significantly impact the achievement of primary stability [8].
To validate implant stability, various commonly used indicators can be employed, including peak torque values (Cp) and primary implant stability values (ISQ). The insertion torque of the implant can be measured by equipping the insertion micromotor with a torque gauge, which indicates the resistance encountered during the implant’s insertion into the bone tissue. Values approaching 35 Ncm are considered functional and promote predictable osseointegration [9]. Another method for assessing implant stability is the use of resonance frequency analysis (RFA), which provides the implant stability quotient (ISQ). The ISQ is obtained by analyzing the resonance frequency response of the implant, offering valuable information about its stability.
RFA is a method that measures the amplitude of lateral oscillation of an implant within bone when stimulated electromagnetically. The resulting resonance frequency, measured in kHz, has been clinically validated, and values between 55 and 85 are considered acceptable within a reliable stability range [10]. The implant stability quotient (ISQ) obtained from RFA is closely linked to the level of bonding between the implant surface and the surrounding bone, which is directly influenced by bone–implant contact (BIC). Therefore, the ISQ is highly dependent on BIC. Resonance frequency analysis is a valuable tool for assessing implant stability not only at the time of insertion (time zero), but also during subsequent follow-up periods [11]. By monitoring the resonance frequency over time, clinicians can evaluate the stability of the implant and track its progress.
Meredith N. et al. used an Osstell™ device (Ab Integration Diagnostics, Gothenburg, Sweden) to evaluate the initial stability of a dental implant. This device aids in monitoring the long-term stability of the implant, allowing for the differentiation between clinical success and potential implant failure. Furthermore, this analysis can provide valuable insights regarding the selection of appropriate loading protocols [12].
Over the years, various surgical techniques have been developed to enhance primary implant stability, particularly in areas with low bone density. Among these techniques, bone densification of the implant site has demonstrated notable benefits in achieving improved primary implant stability. This is particularly relevant in edentulous areas characterized by low bone density, where bone densification techniques can significantly contribute to enhancing the stability of the implants [13,14]. The under-preparation of the implant site to increase primary stability is widely used today. In this technique, the final drilling step involves using a drill with a reduced diameter compared with the size of the implant [15]. In areas with poor bone quality, the under-preparation of the site, up to 10%, is enough to increase primary stability. However, reducing the diameter further does not result in higher primary stability values. Instead, it can introduce excessive stress along the osteotomy, potentially causing microcracks that impede the natural healing and osseointegration processes. Therefore, it is important to strike a balance between under-preparation and avoiding excessive reduction in diameter to ensure optimal implant stability and support proper healing [16,17].
In the 1990s, Summers introduced the concept of osteocondensation, which involved the use of manual osteotomes activated by a dedicated hammer [18].
In 2014, Salah Huwais and colleagues developed a novel line of drills with distinctive geometries that not only create implant sites, but also densify the bone in the edentulous area. These drills, known as osteocondensation drills, are conical in shape and come in different diameters to accommodate varying rehabilitation needs. They are designed with cutting planes featuring negative angles, which compact the bone during rotation, and a chisel tip that facilitates the drill’s penetration into the bone. Interestingly, these osteocondensation drills can also function in the opposite manner. By reversing the process, the same drills can cut bone using their cutting planes and remove local bone tissue, thereby creating an implant site through a conventional osteo-subtractive mechanism [2,16].
Osteocondensation drills have demonstrated their ability to enhance bone quality by preserving local bone tissue, facilitating elastic expansion of the bone, and promoting autologous grafting of the particulate matter generated during the osteotomy phase. This particulate matter is compacted along the periphery and apical portion of the implant site [19]. The biomechanical efficiency of osseodensification drills lies in their ability to leverage the inherent elasticity and plasticity of the bone. By applying appropriate stress or force, these drills induce plastic deformation that compacts the bone particulate within the trabecular space instead of removing it.
Furthermore, osteocondensation technique exhibits a detectable response at the bone–implant interface due to the reverse compression exerted by the bone on the elastic rebound resulting from residual elastic deformation during osteotomy. This reverse compression helps to prevent excessive stress. Simultaneously, the irrigation of the site with saline serves a functional purpose by generating continuous hydrodynamic pressure that facilitates the compaction of bone chips. The process of bone compaction and autografting achieved through osteocondensation cutters enables the implant to be placed in an area of the bone matrix characterized by increased density and enhanced mechanical stability [20]. Consequently, higher values of implant stability can be observed during implant insertion due to improved interlocking between the implant geometry and the bone matrix, resulting in greater contact between the bone and implant surface (thus increasing BIC).
The increased contact between the implant and bone promotes bone healing and remodeling processes, resulting in advantages such as improved secondary stability and osseointegration [21]. Additionally, the autograft generated during drilling facilitates the retention of autologous bone particulate matter, which serves as a source of mineralization nuclei at the periphery of the implant site. This has been confirmed through histomorphometric analyses conducted in vivo on sheep and bovine bone [22].
The objective of this in vitro study was to assess the implant stability values associated with site preparation using osteocondensation drills, specifically comparing the osteocondensation direction with the cutting direction. The use of osseodensification drilling protocols in the preparation of implant sites is hypothesized to significantly enhance the primary stability of dental implants. A secondary objective was to evaluate the influence of the depth of implant site preparation on implant stability.
The evaluation of bone density at the implant site enhanced by osteodensification drills was conducted using parameters such as the integral depth curve (I), Cp, average torque (Cm), and ISQ.
2. Methods
An experimental in vitro study model was designed using a solid polyurethane foam block with specific characteristics: bone density type III-IV, as described by Lekholm and Zarb, measuring 13 × 20 × 4 cm [4]. The block represents an alternative to animal or corpse bone and exhibits common mechanical properties according to standards defined by the manufacturer (ASTM F-1839-08 [23]) (Figure 1). This sample takes advantage of the homogeneity and uniformity of the physical properties of polyurethane that can be repeated in every point of the block: this reduces the variables, alterations, and deformations found using cadaver bone or in animal bone. Moreover, it enables subsequent analysis, which is repeatable at any time.

Figure 1.
Image of the 13 × 20 × 4 cm polyurethane foam blocks used.
Forty-eight implant sites were prepared using osseodensification drills (Model Compact Drill, provided by FMD (Falappa Medical Devices, Rome, Italy)). The procedure was performed according to the sequence recommended by the manufacturer and considered a series of four steps for each osteotomy, starting with a pilot bur with a diameter of 1.7 mm. This was followed by the passage of the first conical bur of 1.5–2.3 mm diameter, with a pumping movement until the expected working depth was reached. We proceeded with the passage of progressive cutters, such as 1.7–2.5 mm and 2.0–2.8 mm, to end with the passage of the last cutter: 2.4–3.2 mm. The simultaneous irrigation of the site (90 mL/min with distilled water at room temperature (20 ± 1 °C)) with saline during the preparation of the osteotomies avoided alterations of the foam block due to the thermal damage induced by mechanical overheating; it is also functional in the formation of a continuous hydrodynamic pressure wave that favors the compaction of the bone chips. The steps were the same for all groups and both techniques. The same operator performed every osteotomy (Figure 2).

Figure 2.
Representative images of the osseodensification bur kit.
The implant osteotomy site was prepared for the experimental groups with the burs running in a clockwise direction (densifying mode) with two different target depths of 12 and 14 mm. For the control groups, the osteotomies were performed with the same burs but running in the counterclockwise direction (cutting mode) at the same two targets of depths (12 and 14 mm).
In the present study, osteotomies of groups A and B were prepared at the same length as the implants (12 mm), according to an under/standard preparation rationale. The osteotomies of groups C and D were performed with an overpreparation (14 mm) in which the space between the apex of the implant and the bone mimicked a healing chamber to facilitate the positioning of the clot, or possibly autologous particulate matter, as autografting produced during the drilling phase by osseodensification burs.
A TMM2® surgical micromotor (IDI Evolution) with an integrated instantaneous torque measurement system was used for drilling. Through the device, it was possible to collect data related to implant stability, such as Cm and I, or the average torque of forces and the integral relative to the depth curve. The device determined the average torque necessary to keep the probe at a constant rotational speed; because friction varies with bone density, the average torque differs accordingly. It is possible to obtain a density–depth graph for each point of the implant tunnel. During implant insertion, the micromotor, through digital software, performs a high-frequency measurement sample of the instantaneous torque (IT) necessary to enable positioning. It simultaneously records the depth reached by the implant. The device also describes, as an output, a torque/depth graph that shows instantaneous torque variations as a function of the depth of the implant. The implant motor then acquires and records the average insertion torque (Cm), the maximum torque (IT), and the integral values relating to the depth torque/curve (I).
Forty-eight conical implants, 3.8 × 12 mm with 0.75 single pitch with internal hexagon connection, were inserted at a constant speed of 35 rpm (Figure 3), and the following data were collected: Cp, Cm, and I (Figure 4) [24]. Then, the ISQ values were assessed using the Ostell ISQ® instrument (Ostell, Gothenburg, Sweden), registering the value on the four faces of each implant (anterior, posterior, medial, and lateral, concerning the orientation of the block), and the average value of these measurements was assigned to each implant.
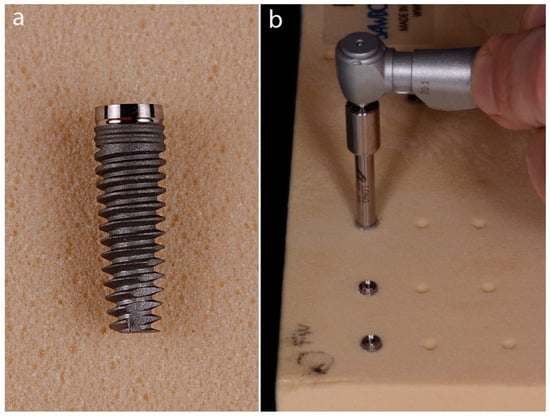
Figure 3.
(a) The 3.8 × 12 mm conical implant used. (b) Implant positioning in the polyurethane foam block.
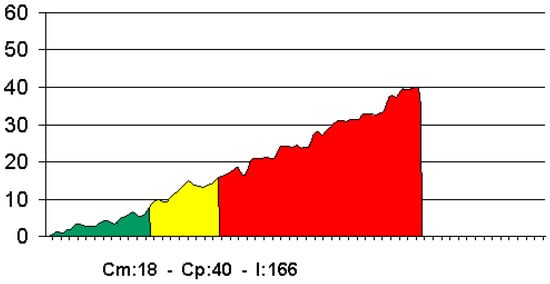
Figure 4.
Example of a torque–depth plot displayed by the micromotor at implant insertion.
Statistical Analyses
The required sample size was calculated using statistics software (GPower 3.1.9.2, Heinrich-Heine-Universität, Düsseldorf, Germany). Power analysis using one-way ANOVA, an alpha level of 0.05, and an effect size of f = 0.68 showed that 48 implants would be adequate to obtain 95% power in detecting a statistical difference. The power calculation was based on the mean values of the variable with the lowest difference (Cm) between the first 5 osteotomies for each group.
Data were evaluated using standard statistical analysis software (version 20.0, Statistical Package for the Social Sciences, IBM Corporation, Armonk, NY, USA). Descriptive statistics, including mean ± SD values, were calculated for the variables Cp, Cm, I, and ISQ. The Shapiro–Wilk test was used to determine whether the data conformed to a normal distribution. One-way ANOVA was used to identify statistically significant differences for each variable between four different groups:
- Group A (implants positioned in an osteotomic site prepared at 12 mm depth in densifying mode).
- Group B (implants positioned in an osteotomic site prepared at 12 mm depth in cutting mode).
- Group C (implants positioned in an osteotomic site prepared at 14 mm depth in densifying mode).
- Group D (implants positioned in an osteotomic site prepared at 14 mm depth in cutting mode).
Pairwise comparisons were performed with Bonferroni correction for multiple comparisons. In each test, the cut-off for statistical significance was p ≤ 0.05.
3. Results
In total, 48 implant sites (12 for each group) were prepared, and 48 fixtures were inserted to evaluate the Cp, Cm, I, and ISQ. An expert investigator performed the implant placement, blinded to other study aspects. All implants in group A failed to complete positioning inside the osteotomic site, with the neck of implants remaining outside the foam blocks (Figure 3b). The highest values of implant stability parameters were reported from Group C (implants positioned in an osteotomic site prepared at 14 mm depth in densifying mode). In contrast, the lowest values were reported in Group A (implants positioned in an osteotomic site prepared at 12 mm depth in densifying mode). The results of the descriptive analysis are summarized in Table 1.

Table 1.
Description of the implant stability (ISQ), average torque (Cm), peak torque (Cp), and integral depth curve (I) values for each group.
No outliers were detected, and the assumption of normality, assessed by the Shapiro–Wilk test, was not violated. Pairwise comparisons with Bonferroni correction for multiple analyses revealed statistical differences in the ISQ between groups A and C (p = 0.001) and A and D (p = 0.035); in the Cm between groups A and B (p = 0.014), A and C (p < 0.001), A and D (p < 0.001), groups B and C (p < 0.001) and groups C and D (p = 0.04); in the Cp between groups A and B (p < 0.001), A and C (p < 0.001), groups B and D (p < 0.001), and groups C and D (p = 0.040); and in the I between groups A and B (p = 0.005), A and C (p < 0.001), A and D (p < 0.001), groups B and C (p < 0.001), and groups C and D (p = 0.006) (Table 2).

Table 2.
One-way ANOVA comparing the mean values (ISQ), average torque (Cm), peak torque (Cp), and integral depth curve (I) in the four groups.
4. Discussion
Primary stability plays a crucial role in the success of dental implants and influences the choice of prosthetic loading [25]. In cases where the bone is poorly mature, such as in the posterior areas of the maxilla and mandible, or in elderly and osteopenic patients, bone densification techniques can be employed to increase bone density [26]. Different bone compaction techniques are utilized to achieve effective densification of the implant site; each method comes with its own set of advantages and disadvantages [19].
Osteocondensation burs offer a notable advantage in preserving autologous bone tissue within the implant bed area. This is achieved through the compaction of bone particles along the perimeter of the osteotomy, facilitated by the negative cutting angle blades of the burs. The compaction of the bone surrounding the implant site enhances bone mineral density, positively impacting primary stability and subsequently promoting the osteointegration process [27].
The presence of autologous particulate within the implant site, being living bone material, has the potential to serve as a catalyst for bone formation. It can stimulate the processes of bone neoformation by facilitating the creation of mineralization nuclei. These mineralization nuclei play a significant role in accelerating the healing and reorganization of bone tissue surrounding the implant threads, ultimately promoting the attainment of both primary and secondary stability of the implant.
Histomorphometric analyses conducted by Trisi et al. have highlighted the mineralization nuclei derived from the compacted autologous particulate [22]. The impact of the compacted particulate is evident both during implant insertion, as indicated by the IT value, and in terms of the implant’s stability, assessed by the ISQ. In contrast to osteosubtractive techniques, where the particulate generated during osteotomy is typically removed, osteocondensation burs operate through a bone tissue expansion mechanism. This expansion causes the bone to spring back, potentially leading to increased implant stability.
One advantage of using osteocondensation drills is that they facilitate the preparation of implant sites in thin crests of bone. By avoiding the excessive removal of bone, these drills limit the formation of microcracks and fracture fissures that could compromise the overall stability of the implant site.
Implants inserted in osteotomic sites prepared through osteodensification and extended to accommodate the particulate graft exhibit a significant increase in insertion torque values, as well as parameters such as I and ISQ values (Table 1). Both IT and ISQ are important clinical parameters used to assess implant stability [9].
Implant stability depends on the direct contact between the implant surface and the surrounding bone tissue [28]. The amount of micromovement is influenced by bone density. In cases where the bone density is low, preparing the implant site using the osteosubtractive method leads to lower insertion torque values and further reductions in bone density. These implant micromovements can contribute to the formation of poorly organized tissue with the presence of fibrous matrix interposition, resulting in a reduced percentage of osseointegration [29]. The use of osteocondensation burs has been documented in preliminary studies by Huwais et al., where the authors demonstrated how densification increases the bone formation rate on the implant surface by enhancing the bone mineral density in the peri-implant site. Histomorphological analysis of bone samples from sheep confirmed that compacted bone maintains its histological structure [2].
In a separate in vivo study conducted by Trisi et al., the effectiveness of osteodensification drills in improving primary implant stability and preserving long-term secondary stability was demonstrated. The study compared parameters such as bone–implant contact (BIC) and bone volume (BV) between the test group, which underwent osteocondensation preparation, and the control group, which received the osteosubtractive technique. The results highlighted the advantages of osteocondensation in terms of achieving superior BIC and BV outcomes compared with the osteosubtractive technique.
The study reported a statistically significant difference in the percentage of BIC between the two groups, with the tester group achieving higher BIC values compared with the control group. Additionally, the BV in the tester group showed an increase of approximately 30% compared with the control group. Histological analysis of the samples supported the findings of Huwais et al., indicating that the healing process is facilitated by bone compaction and that bone density can increase around the implant’s surface, particularly in the coronal portion. The test group exhibited a high presence of mineralization nuclei surrounded by osteoid tissue and osteoblasts. This evidence suggests that, thanks to the mineralization nuclei, the bone may have the ability to increase its density over the long term, thus contributing to the preservation of secondary implant stability [22]. In an in vivo study conducted on sheep by Lahens et al., the effectiveness of osteocondensation drills was emphasized. The study paid specific attention to histomorphometric analysis, which revealed a percentage increase in values such as the BIC and bone area fraction occupied (BAFO) when the osteocondensation technique was utilized [30].
Barbera-Chàvarri et al. evaluated 110 osteotomies performed on 30 coronal sections of pig tibiae (Maxylar®, Girona, Spain) using two different techniques: under-preparation (UD) and osteocondensation (OD). The authors compared various parameters associated with implant stability, including insertion torque (IT) and implant stability quotient (ISQ). The study found that implants inserted using the osteocondensation technique (OD) exhibited significantly higher values of primary stability when compared with the under-preparation technique (UD). The average implant insertion torque in the osteodensification group was 8.87 ± 6.17 N/cm in the control group (UD) and 21.72 ± 17.14 Ncm in the test group (OD). Similarly, the mean ISQ values were 65.16 ± 7.45 kHz in the control group and 69.75 ± 6.79 kHz in the test group. The study reported significant differences between the groups in terms of ISQ values and implant insertion torque [4].
In a study conducted by Bergamo et al. in 2020, a single-blind, multi-center controlled clinical study was carried out with 56 enrolled patients requiring prosthetic rehabilitation with a minimum of two implants. The study involved the placement of implants of various lengths and types (narrow, regular, and wide) in the anterior and posterior regions of the maxilla and mandible. The preparation of implant sites in each patient was performed using two different techniques: the osteodensifying technique and the osteosubtractive technique. The study evaluated the values of IT at the time of implant placement and ISQ recorded using RFA at three stages: immediately after surgery, at 3 weeks, and at 6 weeks.
The statistical analysis revealed a significant increase in the implant IT value when the site preparation was performed using the osteocondensation technique (60 ± 3.4 Ncm) compared with the osteosubtractive preparation (35 ± 3.4 Ncm). Additionally, ISQ data showed higher values for the experimental group at all evaluation time points. Specifically, the experimental group had higher ISQ values (I: 73 ± 2.0, 3 W: 70 ± 2.0, and 6 W: 74 ± 1.5) compared with the control group (I: 62 ± 2.0, 3 W: 59 ± 2.0, and 6 W: 66 ± 1.5), regardless of the time of evaluation [31].
Evidence from various related studies demonstrates the significant advantages of using osteocondensation drills. These advantages are observed across different parameters and histomorphometric analyses, with all values in experimental groups utilizing the osteodensification technique showing a significant increase compared with control groups [2,4,22,27,30].
The results from the in vitro comparative analysis further support this notion, indicating that the preparation of implant sites using the osteodensification technique with osteocondensation drills can substantially enhance the indicator values of implant stability, reflecting a measurable increase in peri-implant bone mineral density. This study investigated the use of osteocondensation drills in the preparation of the implant site through an in vitro analysis.
The study compared implant stability parameters between two test groups (A and C) and two control groups (B and D). The implants in the test groups were inserted into osteotomic sites using osteocondensation drills, employing an osteocompacting rotation technique.
In the control groups (B and D), the osteotomies were prepared using the same drills but with a reverse rotation, employing osteosubtractive mechanics. The relationship between osteocondensation drills and increased preparation depth was investigated for the first time. Group A’s osteotomies were performed using the same method but with an extended depth of 12 mm, matching the length of the inserted implants. However, the implant stability values in group A were lower than those observed in all other groups in the study.
During the realization phase of the osteotomies, it became evident that implant insertion in group A sites prevented them from reaching the intended preparation depth. This incomplete insertion of the implant could be attributed to the presence of trabecular substance around the walls and apex of the preparation site. In group A, the implant fixture did not make full contact with the bone along its entire length, resulting in a fraction of the crestal module of the implant emerging from the surface of the sample block (Figure 3b). Consequently, the ISQ values decreased, indicating reduced bone-to-implant contact and stability. In control group B, the osteotomies were prepared to a depth of 12 mm, matching the length of the implant fixture. In contrast, in group D, the osteotomies were extended to 14 mm. The Cp parameter showed an increase in group B compared with group D, but remained lower than in group C. On the other hand, the ISQ value of group B was lower than the same parameter in group D, highlighting the influence of osteotomy depth on the ISQ value.
Additional studies have demonstrated that overpreparation of the implant site leads to faster healing and bone formation around the implant surface. This is attributed to reduced bone compression, increased cell viability, enhanced osteogenesis, and an improved implant stability ratio over time [32]. Furthermore, our findings are in line with those of Barberá-Millán et al., who demonstrated that osseodensification significantly enhances primary stability in low-density bone, as evidenced by higher insertion torque and RFA values. This supports our observations of increased stability with deeper implant placement using osseodensification drills [4].
A significant limitation of this study stems from employing a polyurethane sample block for experimental purposes. This model lacks the cortical bone layer typically present in human anatomy. The absence of this cortical layer omits a critical aspect of bone structure, notably the transition from dense cortical to spongy cancellous bone, which is pivotal in understanding implant dynamics in a natural physiological environment. Consequently, the findings, especially those relating to the performance and impact of osteocondensation drills, should be cautiously interpreted, considering this simplification of bone anatomy. Furthermore, the study did not include the factors influencing implant stability in a clinical setting. Critical variables such as patient-specific differences, which exhibit variability in bone quality, age-related changes, and individual healing capacities, were not accounted for. The impact of the surgeon’s skill and experience was also not reflected in this in vitro model. In practice, the complexity of clinical cases can significantly affect the results, a variable not captured in this controlled environment. Moreover, future studies should compare the osteocondensation method with other advanced and commonly employed dental implant techniques, limiting the breadth of its applicability. Considering these limitations is essential for comprehensively understanding the study’s findings and applicability in clinical practice.
5. Conclusions
Within the limitations of this study, it was observed that the use of osteocondensation drills and an increase in the depth of preparation resulted in improvements in all variables related to implant stability: Cp, Cm, I, and ISQ. This study’s findings demonstrate the effectiveness of deeper implant site preparation using osteocondensation drills. These results could encourage clinicians to adopt these techniques, particularly for patients with challenging bone densities, potentially leading to higher implant stability and osseointegration success rates. However, further studies are required to analyze and understand the relationship between the effect of osteocondensation drills and the depth of preparation. These future investigations can provide more insights into the optimal usage of osteocondensation drills and the impact of preparation depth on implant stability.
Author Contributions
Conceptualization, N.P. and G.L.M.; methodology, N.P. and F.D.A.; software, M.A.B.; validation, N.P., I.V. and S.D.C.; formal analysis, F.D.A.; investigation, G.L.M. and S.G.F.; resources, S.D.C.; data curation, S.G.F. and A.M.; writing—original draft preparation, N.P. and S.G.F.; writing—review and editing, N.P., F.D.A. and E.B.; visualization, A.M.; supervision, N.P.; project administration, N.P.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article and available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Albrektsson, T.; Branemark, P.I.; Hansson, H.A.; Lindstrom, J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone to implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Huwais, S.; Meyer, E.G. A Novel Osseous Densification Approach in Implant Osteotomy Preparation to Increase Biomechanical Primary Stability, Bone Mineral Density, and Bone-to-Implant Contact. Int. J. Oral Maxillofac. Implant. 2017, 32, 27–36. [Google Scholar] [CrossRef]
- Javed, F.; Romanos, G.E. The role of primary stability for successful immediate loading of dental implants. A literature review. J. Dent. 2010, 38, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Barberá-Millán, J.; Larrazábal-Morón, C.; Enciso-Ripoll, J.J.; Pérez-Pevida, E.; Chávarri-Prado, D.; Gómez-Adrián, M.D. Evaluation of the primary stability in dental implants placed in low density bone with a new drilling technique, Osseodensification: An in vitro study. Med. Oral Patol. Oral Cir. Bucal 2021, 26, e361–e367. [Google Scholar] [CrossRef]
- Cáceres, F.; Troncoso, C.; Silva, R.; Pinto, N. Effects of osseodensification protocol on insertion, removal torques, and resonance frequency analysis of BioHorizons® conical implants. An ex vivo study. J. Oral Biol. Craniofac Res. 2020, 10, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Feher, B.; Frommlet, F.; Gruber, R.; Hirtler, L.; Ulm, C.; Kuchler, U. Resonance frequency analysis of implants placed in condensed bone. Clin. Oral Implant. Res. 2021, 32, 1200–1208. [Google Scholar] [CrossRef]
- Romeo, D.; Chochlidakis, K.; Barmak, A.B.; Agliardi, E.; Lo Russo, L.; Ercoli, C. Insertion and removal torque of dental implants placed using different drilling protocols: An experimental study on artificial bone substitutes. J. Prosthodont. 2023, 32, 633–638. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Bone Quality and Quantity and Dental Implant Failure: A Systematic Review and Meta-analysis. Int. J. Prosthodont. 2017, 30, 219–237. [Google Scholar] [CrossRef]
- Atsumi, M.; Park, S.H.; Wang, H.L. Methods used to assess implant stability: Current status. Int. J. Oral Maxillofac. Implant. 2007, 22, 743–754. [Google Scholar]
- Yang, S.M.; Shin, S.Y.; Kye, S.B. Relationship between implant stability measured by resonance frequency analysis (RFA) and bone loss during early healing period. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 105, e12–e19. [Google Scholar] [CrossRef]
- Degidi, M.; Daprile, G.; Piattelli, A. Primary stability determination by means of insertion torque and RFA in a sample of 4.135 implants. Clin. Implant. Dent. Relat. Res. 2012, 14, 501–507. [Google Scholar] [CrossRef]
- Meredith, N.; Books, K.; Friberg, B.; Jemt, T.; Sennerby, L. Resonance frequency measurements of implant stability in vivo. A cross-sectional and longitudinal study of resonance frequency measurements on implants in the edentulous and partially dentate maxilla. Clin. Oral Implant. Res. 1997, 8, 226–233. [Google Scholar] [CrossRef]
- Cavallaro, J., Jr.; Greenstein, B.; Greenstein, G. Clinical methodologies for achieving primary dental implant stability: The effects of alveolar bone density. J. Am. Dent. Assoc. 2009, 140, 1366–1372. [Google Scholar] [CrossRef]
- Tabassum, A.; Meijer, G.J.; Walboomers, X.F.; Jansen, J.A. Evaluation of primary and secondary stability of titanium implants using different surgical techniques. Clin. Oral Implant. Res. 2014, 25, 487–492. [Google Scholar] [CrossRef]
- Alghamdi, H.; Anand, P.S.; Anil, S. Undersized implant site preparation to enhance primary implant stability in poor bone density: A prospective clinical study. J. Oral Maxillofac. Surg. 2011, 69, e506–e512. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Daprile, G.; Piattelli, A. Influence of underpreparation on primary stability of implants inserted in poor quality bone sites: An in vitro study. J. Oral Maxillofac. Surg. 2015, 73, 1084–1088. [Google Scholar] [CrossRef]
- Coelho, P.G.; Marin, C.; Teixeira, H.S.; Campos, F.E.; Gomes, J.B.; Guastaldi, F.; Anchieta, R.B.; Silveira, L.; Bonfante, E.A. Biomechanical evaluation of undersized drilling on implant biomechanical stability at early implantation times. J. Oral Maxillofac. Surg. 2013, 71, e69–e75. [Google Scholar] [CrossRef]
- Summers, R.B. A new concept in maxillary implant surgery: The osteotome technique. Compendium 1994, 15, 152, 154–156, 158 passim; quiz 162. [Google Scholar] [PubMed]
- Mullings, O.; Tovar, N.; Abreu de Bortoli, J.P.; Parra, M.; Torroni, A.; Coelho, P.G.; Witek, L. Osseodensification versus subtractive drilling techniques in bone healing and implant osseointegration: Ex vivo histomorphologic/histomorphometric analysis in a low-density bone ovine model. Int. J. Oral Maxillofac. Implant. 2021, 36, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Da Rosa, J.C.M.; De Oliveira Rosa, A.C.P.; Huwais, S. Use of the Immediate Dentoalveolar Restoration Technique combined with osseodensification in periodontally compromised extraction sites. Int. J. Periodontics Restor. Dent. 2019, 39, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Mercier, F.; Bartala, M.; Ella, B. Evaluation of the osseodensification technique in implant primary stability: Study on cadavers. Int. J. Oral Maxillofac. Implant. 2022, 37, 593–600. [Google Scholar] [CrossRef]
- Trisi, P.; Berardini, M.; Falco, A.; Vulpiani, M.P. New osseodensification implant site preparation method to increase bone density in low-density bone: In vivo evaluation in sheep. Implant. Dent. 2016, 25, 24–31. [Google Scholar] [CrossRef]
- ASTM F1839; Standard Specification for Rigid Polyurethane Foam for Use as a Standard Material for Testing Orthopaedic Devices and Instruments. American Society for Testing and Materials ASTM: West Conshohocken, PA, USA, 2008.
- Capparé, P.; Vinci, R.; Di Stefano, D.A.; Traini, T.; Pantaleo, G.; Gherlone, E.F.; Gastaldi, G. Correlation between initial BIC and the insertion torque/depth integral recorded with an instantaneous torque-measuring implant motor: An in vivo study. Clin. Implant. Dent. Relat. Res. 2015, 17 (Suppl. S2), e613–e620. [Google Scholar] [CrossRef]
- Molly, L. Bone density and primary stability in implant therapy. Clin. Oral Implant. Res. 2006, 17 (Suppl. S2), 124–135. [Google Scholar] [CrossRef]
- Ribeiro-Rotta, R.F.; De Oliveira, R.C.; Dias, D.R.; Lindh, C.; Leles, C.R. Bone tissue microarchitectural characteristics at dental implant sites part 2: Correlation with bone classification and primary stability. Clin. Oral Implant. Res. 2014, 25, e47–e53. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Xhajanka, E.; Romeo, D.M.; Romeo, M.; Zappone, C.M.F.; Malcangi, G.; Scarano, A.; Lorusso, F.; et al. The Effectiveness of Osseodensification Drilling Protocol for Implant Site Osteotomy: A Systematic Review of the Literature and Meta-Analysis. Materials 2021, 14, 1147. [Google Scholar] [CrossRef]
- Brizuela-Velasco, A.; Álvarez-Arenal, Á.; Gil-Mur, F.J.; Herrero-Climent, M.; Chávarri-Prado, D.; Chento-Valiente, Y.; Dieguez-Pereira, M. Relationship between Insertion Torque and Resonance Frequency Measurements, Performed by Resonance Frequency Analysis, in Micromobility of Dental Implants: An In Vitro Study. Implant. Dent. 2015, 24, 607–611. [Google Scholar] [CrossRef]
- Trisi, P.; Perfetti, G.; Baldoni, E.; Berardi, D.; Colagiovanni, M.; Scogna, G. Implant micromotion is related to peak insertion torque and bone density. Clin. Oral Implant. Res. 2009, 20, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Lahens, B.; Neiva, R.; Tovar, N.; Alifarag, A.M.; Jimbo, R.; Bonfante, E.A.; Bowers, M.M.; Cuppini, M.; Freitas, H.; Witek, L.; et al. Biomechanical and histologic basis of osseodensification drilling for endosteal implant placement in low density bone. An experimental study in sheep. J. Mech. Behav. Biomed. Mater. 2016, 63, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, E.T.P.; Zahoui, A.; Barrera, R.B.; Huwais, S.; Coelho, P.G.; Karateew, E.D.; Bonfante, E.A. Osseodensification effect on implants primary and secondary stability: Multicenter controlled clinical trial. Clin. Implant. Dent. Relat. Res. 2021, 23, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Cohen, O.; Ormianer, Z.; Tal, H.; Rothamel, D.; Weinreb, M.; Moses, O. Differences in crestal bone-to-implant contact following an under-drilling compared to an over-drilling protocol. A study in the rabbit tibia. Clin. Oral Investig. 2016, 20, 2475–2480. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).