Fluorescence In Situ Hybridization-Based Chromosome Aberration Analysis Unveils the Mechanistic Basis for Boron-Neutron Capture Therapy’s Radiobiological Effectiveness
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture Maintenance
2.2. X-Ray Irradiation
2.3. BPA Preparation and Cell Treatment for BNCT Irradiation
2.4. BNCT Irradiation
Verification of Boron Uptake in Cells
2.5. Cytogenetic Preparation
2.6. Chromosome Hybridization
2.6.1. WCP
2.6.2. m-FISH
2.7. Scoring and Analysis of CAs
3. Results
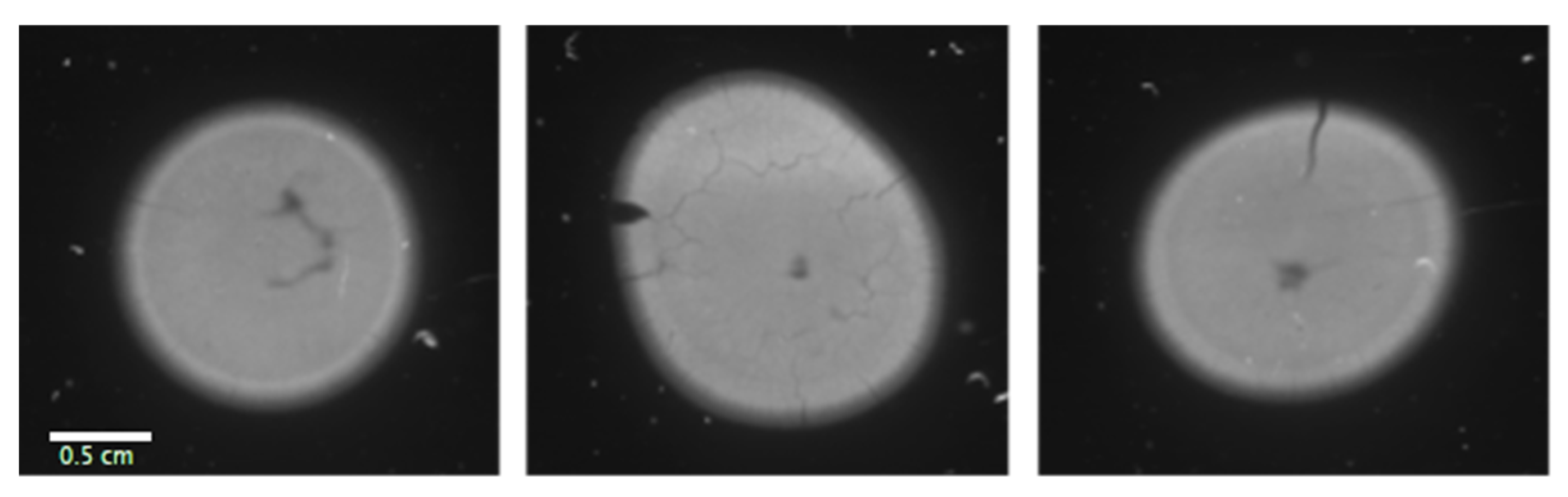
3.1. Boron Distribution Imaging
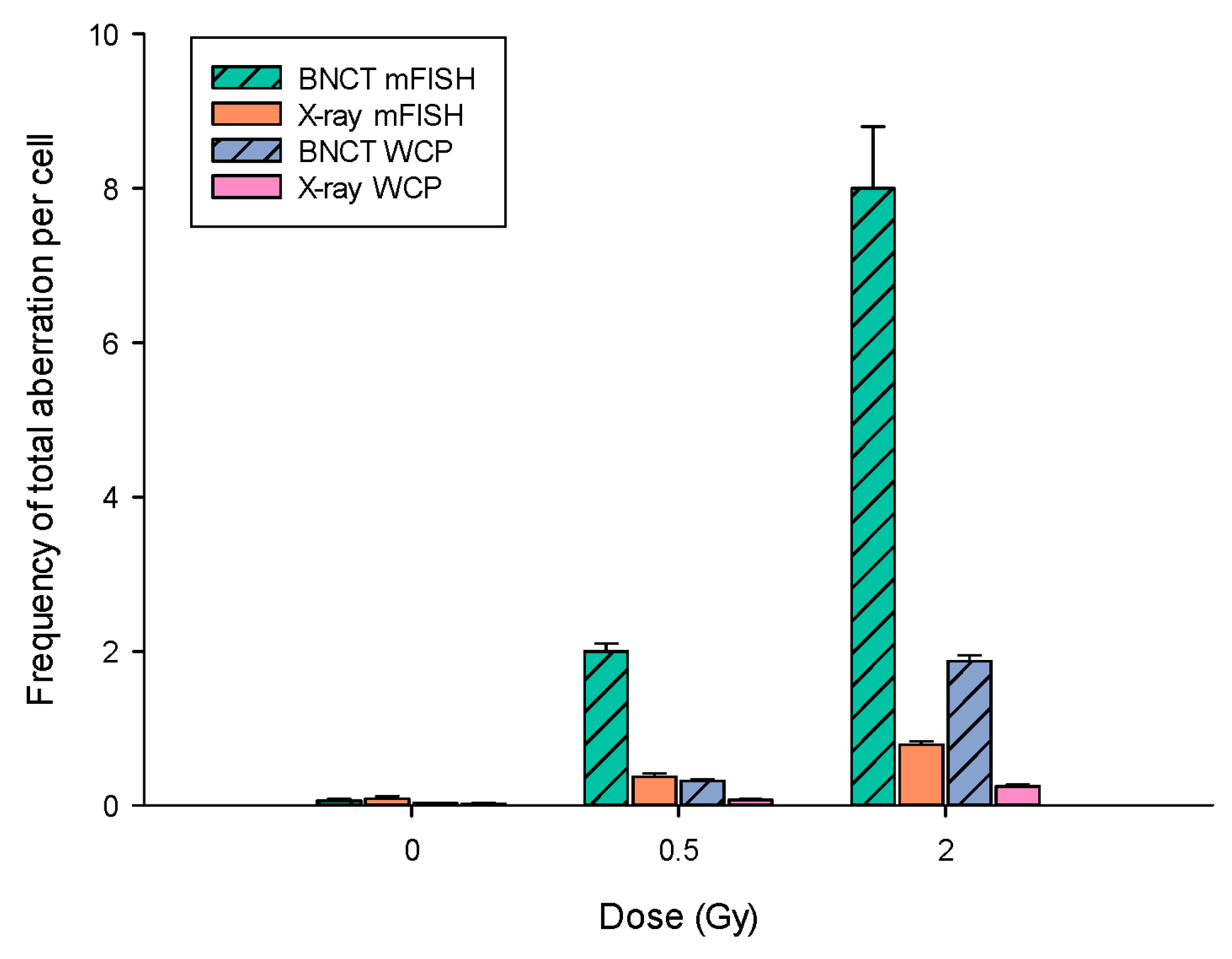
3.2. BNCT Is More Effective Than X-rays at Causing DNA Damage
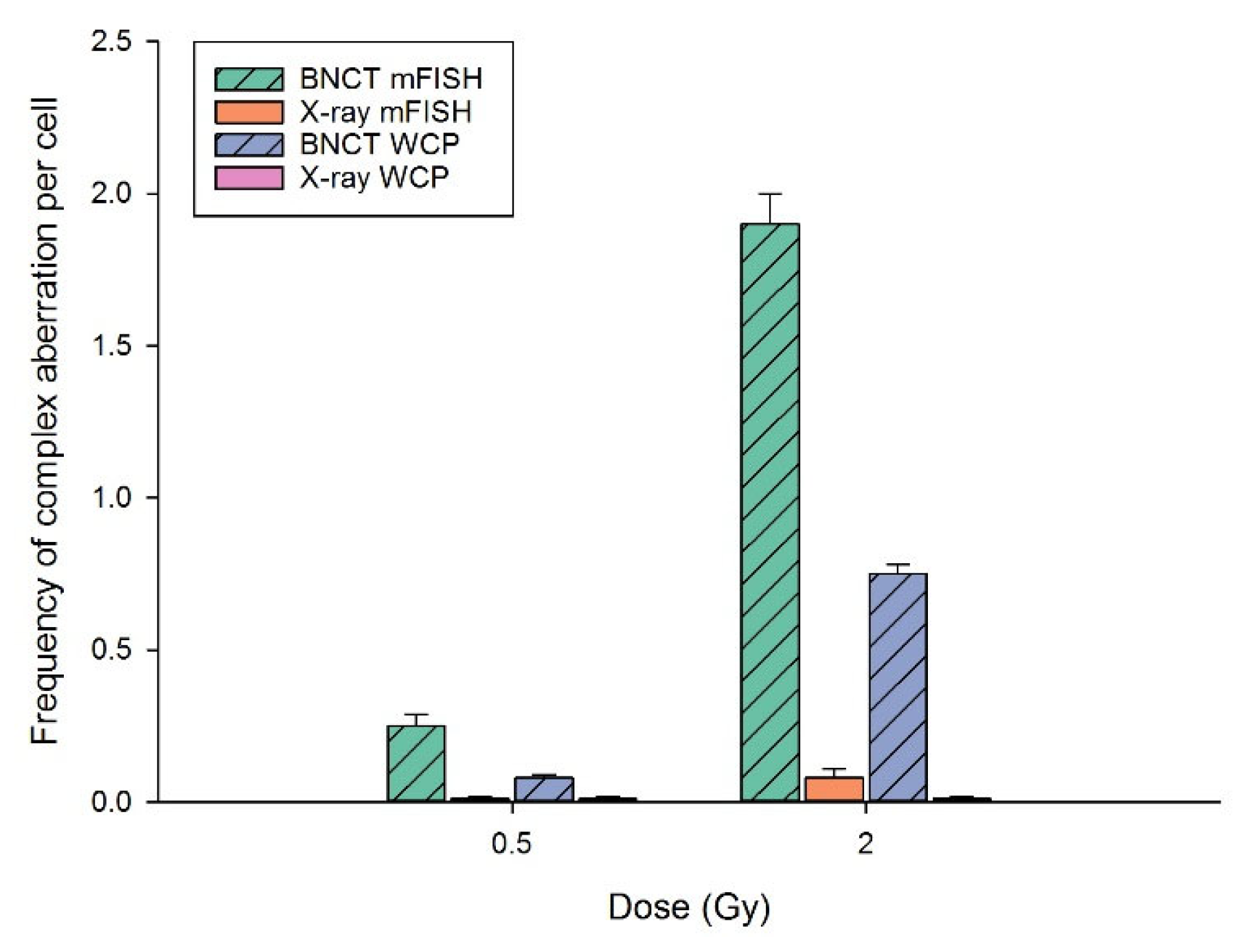
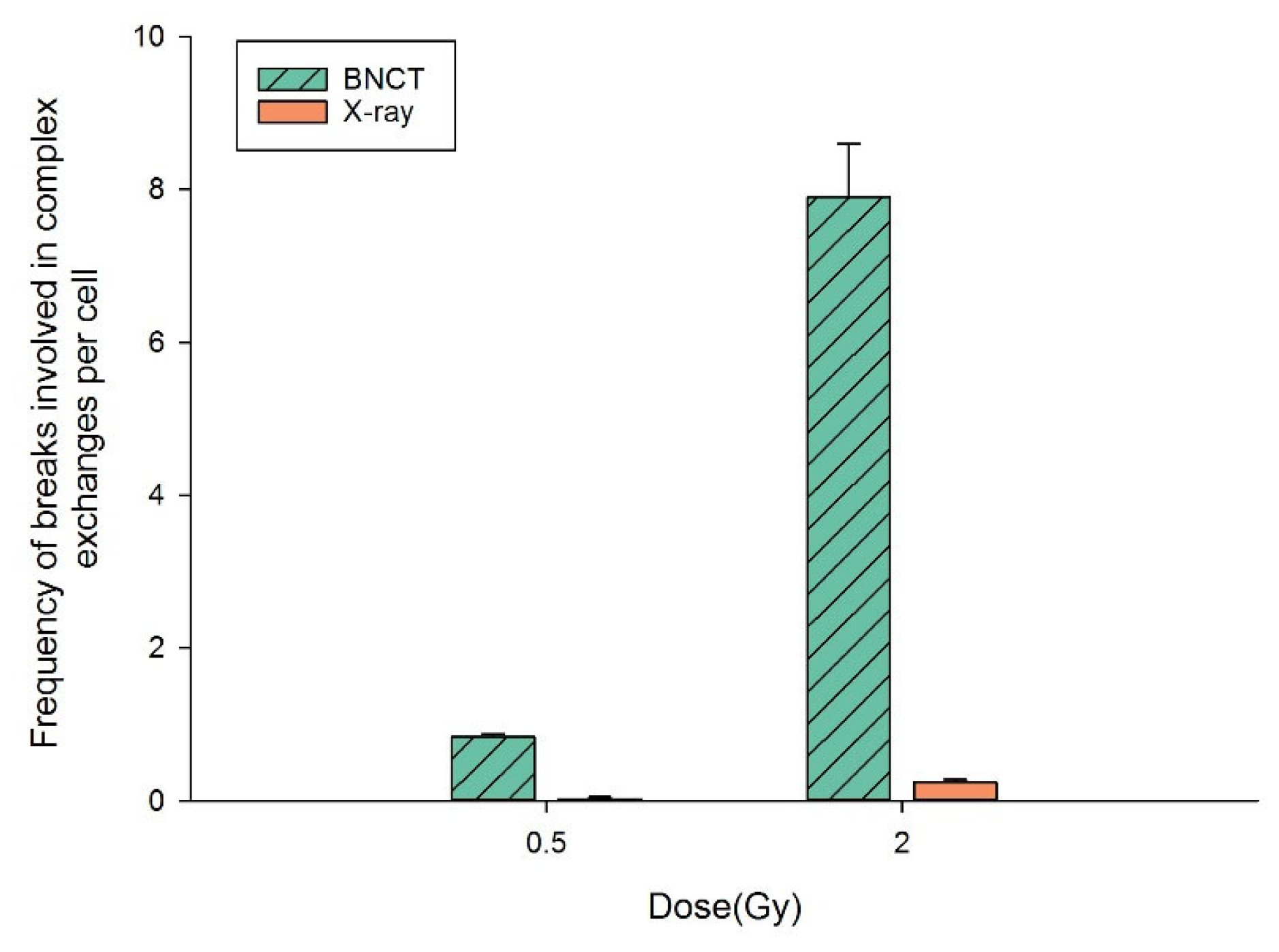
3.3. BNCT Causes More Complex DNA Damage Than X-rays
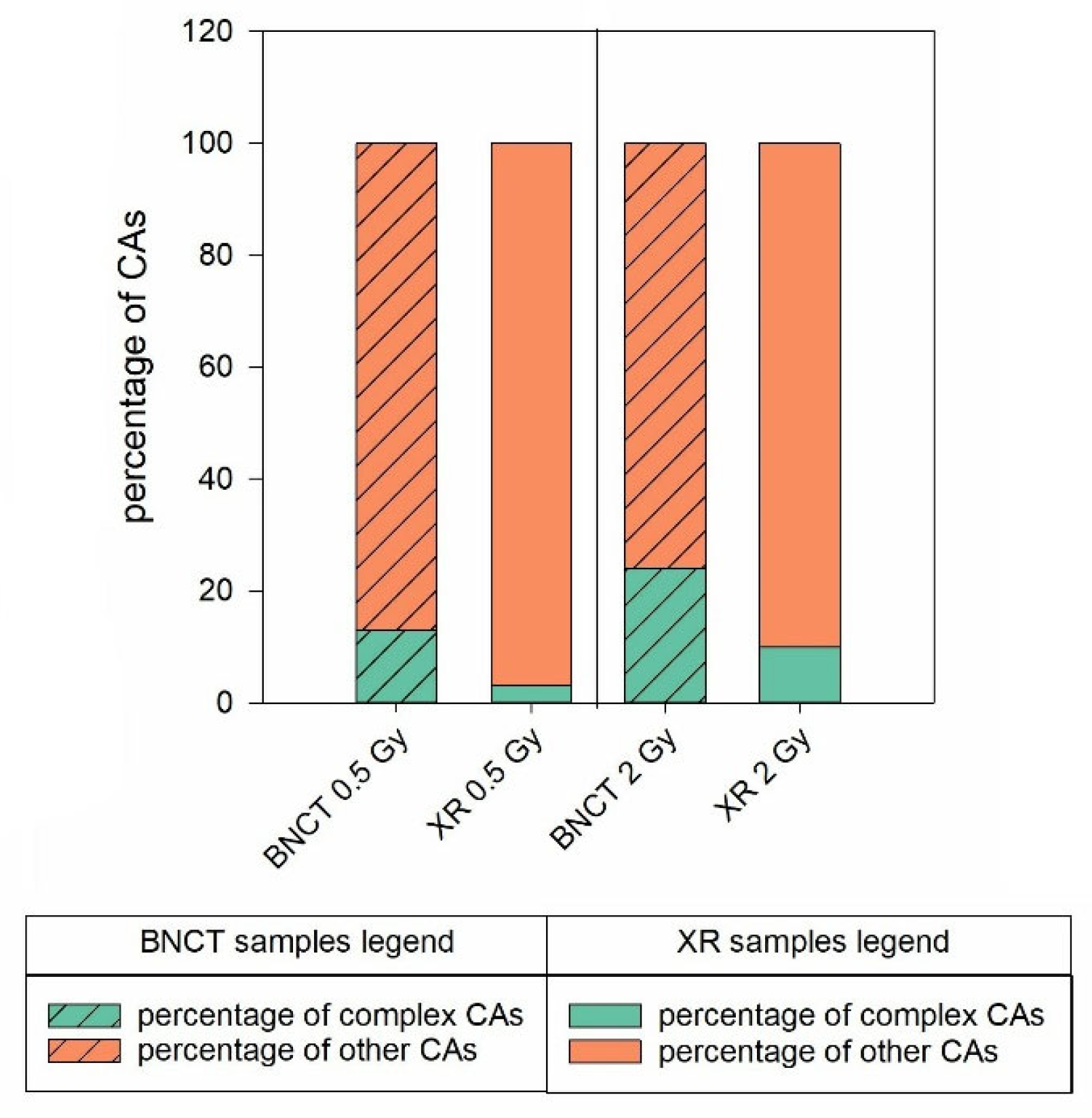
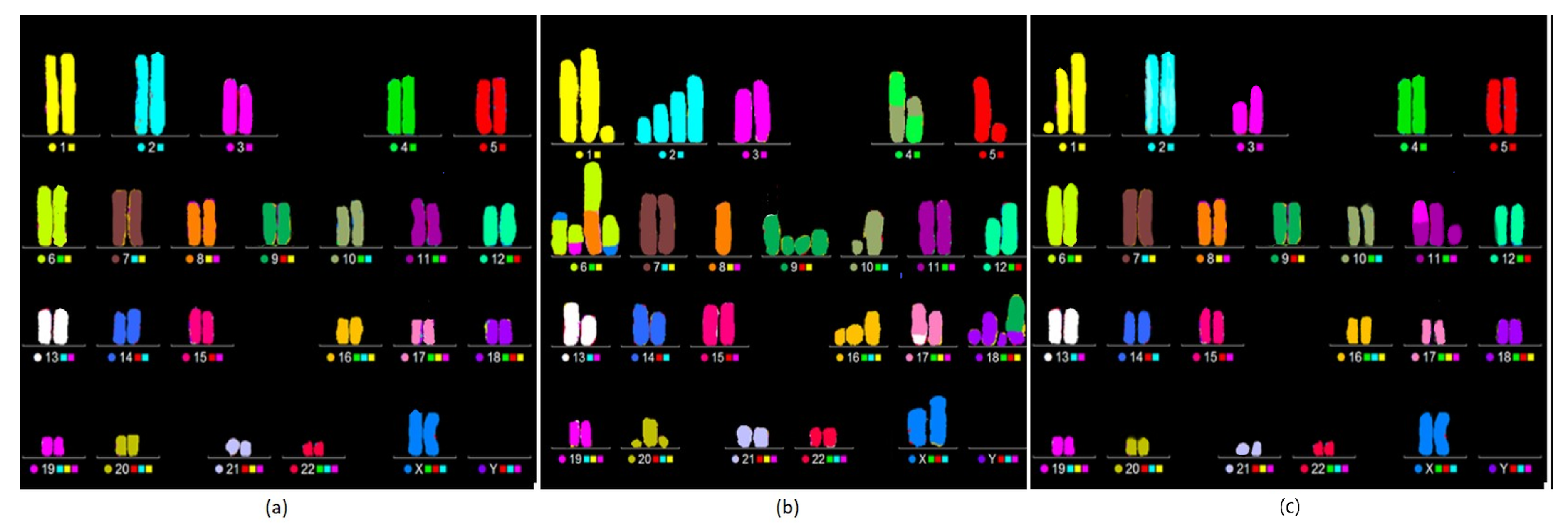
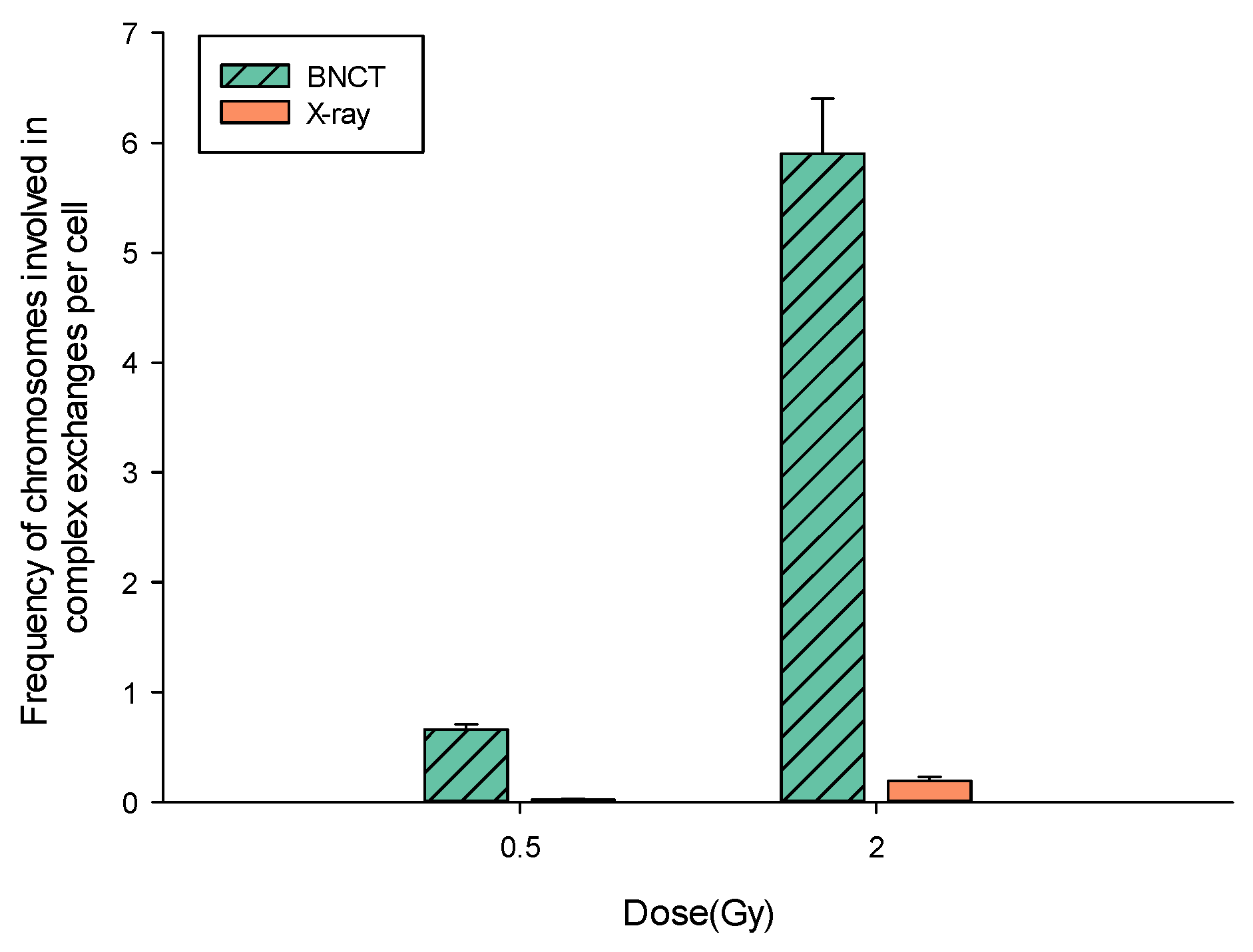
BNCT Greatly Enhances the Degree of Complexity Associated with Complex Aberrations Detected by m-FISH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiorino, C.; Guckemberger, M.; Schwarz, M.; van der Heide, U.A.; Heijmen, B. Technology-Driven Research for Radiotherapy Innovation. Mol. Oncol. 2020, 14, 1500–1513. [Google Scholar] [CrossRef]
- Eaton, B.R.; MacDonald, S.M.; Yock, T.I.; Tarbell, N.J. Secondary Malignancy Risk Following Proton Radiation Therapy. Front. Oncol. 2015, 5, 261. [Google Scholar] [CrossRef]
- Berrington de González, A.; Gibson, T.M.; Lee, C.; Albert, P.S.; Griffin, K.T.; Kitahara, C.M.; Liu, D.; Mille, M.M.; Shin, J.; Bajaj, B.V.M.; et al. The Pediatric Proton and Photon Therapy Comparison Cohort: Study Design for a Multicenter Retrospective Cohort to Investigate Subsequent Cancers After Pediatric Radiation Therapy. Adv. Radiat. Oncol. 2023, 8, 101273. [Google Scholar] [CrossRef]
- Lazar, A.A.; Schulte, R.; Faddegon, B.; Blakely, E.A.; Roach, M., 3rd. Clinical Trials Involving Carbon-Ion Radiation Therapy and the Path Forward. Cancer 2018, 124, 4467–4476. [Google Scholar] [CrossRef]
- Bláha, P.; Feoli, C.; Agosteo, S.; Calvaruso, M.; Cammarata, F.P.; Catalano, R.; Ciocca, M.; Cirrone, G.A.P.; Conte, V.; Cuttone, G.; et al. The Proton-Boron Reaction Increases the Radiobiological Effectiveness of Clinical Low- and High-Energy Proton Beams: Novel Experimental Evidence and Perspectives. Front. Oncol. 2021, 11, 682647. [Google Scholar] [CrossRef]
- Cirrone, G.A.P.; Manti, L.; Margarone, D.; Petringa, G.; Giuffrida, L.; Minopoli, A.; Picciotto, A.; Russo, G.; Cammarata, F.; Pisciotta, P.; et al. First Experimental Proof of Proton Boron Capture Therapy (PBCT) to Enhance Protontherapy Effectiveness. Sci. Rep. 2018, 8, 1141. [Google Scholar] [CrossRef]
- Barth, R.F.; Zhang, Z.; Liu, T. A Realistic Appraisal of Boron Neutron Capture Therapy as a Cancer Treatment Modality. Cancer Commun. 2018, 38, 36. [Google Scholar] [CrossRef]
- Barth, R.F.; Mi, P.; Yang, W. Boron Delivery Agents for Neutron Capture Therapy of Cancer. Cancer Commun. 2018, 38, 35. [Google Scholar] [CrossRef]
- Barth, R.F.; Coderre, J.A.; Graça, M.; Vicente, H.; Blue, T.E. Boron Neutron Capture Therapy of Cancer: Current Status and Future Prospects. Clin. Cancer Res. 2005, 11, 3987–4002. [Google Scholar] [CrossRef]
- Hopewell, J.W.; Morris, G.M.; Schwint, A.; Coderre, J.A. The Radiobiological Principles of Boron Neutron Capture Therapy: A Critical Review. Appl. Radiat. Isot. 2011, 69, 1756–1759. [Google Scholar] [CrossRef]
- Dymova, M.A.; Taskaev, S.Y.; Richter, V.A.; Kuligina, E.V. Boron Neutron Capture Therapy: Current Status and Future Perspectives. Cancer Commun. 2020, 40, 406–421. [Google Scholar] [CrossRef]
- Suzuki, M. Boron Neutron Capture Therapy (BNCT): A Unique Role in Radiotherapy with a View to Entering the Accelerator-Based BNCT Era. Int. J. Clin. Oncol. 2020, 25, 43–50. [Google Scholar] [CrossRef]
- Coderre, J.A.; Morris, G.M. The Radiation Biology of Boron Neutron Capture Therapy. Radiat. Res. 1999, 151, 1–18. [Google Scholar] [CrossRef]
- Skwierawska, D.; López-Valverde, J.A.; Balcerzyk, M.; Leal, A. Clinical Viability of Boron Neutron Capture Therapy for Personalized Radiation Treatment. Cancers 2022, 14, 2865. [Google Scholar] [CrossRef]
- Schwint, A.E.; Monti Hughes, A.; Garabalino, M.A.; Pozzi, E.C.C.; Heber, E.M.; Trivillin, V.A. Translational Radiobiological Boron Neutron Capture Therapy (BNCT) Studies for the Treatment of Different Pathologies: A Bench to Bedside Approach. Austin J. Nanomed. Nanotechnol. 2018, 6, 1049. [Google Scholar]
- Malouff, T.D.; Seneviratne, D.S.; Ebner, D.K.; Stross, W.C.; Waddle, M.R.; Trifiletti, D.M.; Krishnan, S. Boron Neutron Capture Therapy: A Review of Clinical Applications. Front. Oncol. 2021, 11, 601820. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Miao, L.; Li, Y. Boron Neutron Capture Therapy: Current Status and Challenges. Front. Oncol. 2022, 12, 788770. [Google Scholar] [CrossRef]
- Monti Hughes, A. Importance of Radiobiological Studies for the Advancement of Boron Neutron Capture Therapy (BNCT). Expert Rev. Mol. Med. 2022, 24, e14. [Google Scholar] [CrossRef]
- Wang, P.; Zhen, H.; Jiang, X.; Zhang, W.; Cheng, X.; Guo, G.; Mao, X.; Zhang, X. Boron Neutron Capture Therapy Induces Apoptosis of Glioma Cells through Bcl-2/Bax. BMC Cancer 2010, 10, 661. [Google Scholar] [CrossRef]
- Chen, K.H.; Lai, Z.Y.; Li, D.Y.; Lin, Y.C.; Chou, F.I.; Chuang, Y.J. Analysis of DNA Damage Responses after Boric Acid-Mediated Boron Neutron Capture Therapy in Hepatocellular Carcinoma. Anticancer Res. 2019, 39, 6661–6671. [Google Scholar] [CrossRef]
- Ferrari, C.; Bakeine, J.; Ballarini, F.; Boninella, A.; Bortolussi, S.; Bruschi, P.; Cansolino, L.; Clerici, A.M.; Coppola, A.; Di Liberto, R.; et al. In Vitro and In Vivo Studies of Boron Neutron Capture Therapy: Boron Uptake/Washout and Cell Death. Radiat. Res. 2011, 175, 452–462. [Google Scholar] [CrossRef]
- Okamoto, E.; Yamamoto, T.; Nakai, K.; Yoshida, F.; Matsumura, A. Detection of DNA Double-Strand Breaks in Boron Neutron Capture Reaction. Appl. Radiat. Isot. 2015, 106, 185–188. [Google Scholar] [CrossRef]
- Fujita, Y.; Kato, I.; Iwai, S.; Ono, K.; Suzuki, M.; Sakurai, Y.; Ohnishi, K.; Ohnishi, T.; Yura, Y. Role of P53 Mutation in the Effect of Boron Neutron Capture Therapy on Oral Squamous Cell Carcinoma. Radiat. Oncol. 2009, 4, 63. [Google Scholar] [CrossRef]
- Maliszewska-Olejniczak, K.; Kaniowski, D.; Araszkiewicz, M.; Tymińska, K.; Korgul, A. Molecular Mechanisms of Specific Cellular DNA Damage Response and Repair Induced by the Mixed Radiation Field During Boron Neutron Capture Therapy. Front. Oncol. 2021, 11, 676575. [Google Scholar] [CrossRef]
- Kinashi, Y.; Takahashi, S.; Kashino, G.; Okayasu, R.; Masunaga, S.; Suzuki, M.; Ono, K. DNA Double-Strand Break Induction in Ku80-Deficient CHO Cells Following Boron Neutron Capture Reaction. Radiat. Oncol. 2011, 6, 106. [Google Scholar] [CrossRef]
- Iliakis, G.; Mladenov, E.; Mladenova, V. Necessities in the Processing of DNA Double Strand Breaks and Their Effects on Genomic Instability and Cancer. Cancers 2019, 11, 1671. [Google Scholar] [CrossRef]
- Georgakilas, A.G.; O’Neill, P.; Stewart, R.D. Induction and Repair of Clustered DNA Lesions: What Do We Know so Far? Radiat. Res. 2013, 180, 100–109. [Google Scholar] [CrossRef]
- Hada, M.; Georgakilas, A.G. Formation of Clustered DNA Damage after High-LET Irradiation: A Review. J. Radiat. Res. 2008, 49, 203–210. [Google Scholar] [CrossRef]
- Ballarini, F.; Bakeine, J.; Bortolussi, S.; Bruschi, P.; Cansolino, L.; Clerici, A.M.; Ferrari, C.; Protti, N.; Stella, S.; Zonta, A.; et al. Cell Death Following BNCT: A Theoretical Approach Based on Monte Carlo Simulations. Appl. Radiat. Isot. 2011, 69, 1745–1747. [Google Scholar] [CrossRef]
- Anderson, R.M.; Marsden, S.J.; Wright, E.G.; Kadhim, M.A.; Goodhead, D.T.; Griffin, C.S. Complex Chromosome Aberrations in Peripheral Blood Lymphocytes as a Potential Biomarker of Exposure to High-LET α-Particles. Int. J. Radiat. Biol. 2000, 76, 31–42. [Google Scholar] [CrossRef]
- Manti, L.; Durante, M.; Grossi, G.; Ortenzia, O.; Pugliese, M.; Scampoli, P.; Gialanella, G. Measurements of Metaphase and Interphase Chromosome Aberrations Transmitted through Early Cell Replication Rounds in Human Lymphocytes Exposed to Low-LET Protons and High-LET 12C Ions. Mutat. Res. 2006, 596, 151–165. [Google Scholar] [CrossRef]
- Kawata, T.; Ito, H.; George, K.; Wu, H.; Cucinotta, F.A. Chromosome Aberrations Induced by High-LET Radiations. Biol. Sci. Space 2004, 18, 216–223. [Google Scholar] [CrossRef]
- Savage, J.R.; Simpson, P.J. FISH “Painting” Patterns Resulting from Complex Exchanges. Mutat. Res. 1994, 312, 51–60. [Google Scholar] [CrossRef]
- Cornforth, M.N. Analyzing Radiation-Induced Complex Chromosome Rearrangements by Combinatorial Painting. Radiat. Res. 2001, 155, 643–659. [Google Scholar] [CrossRef]
- Anderson, R. Multiplex Fluorescence in Situ Hybridization (M-FISH). Methods Mol. Biol. 2010, 659, 83–97. [Google Scholar] [CrossRef]
- Anderson, R.M.; Stevens, D.L.; Goodhead, D.T. M-FISH Analysis Shows That Complex Chromosome Aberrations Induced by Alpha-Particle Tracks Are Cumulative Products of Localized Rearrangements. Proc. Natl. Acad. Sci. USA 2002, 99, 12167–12172. [Google Scholar] [CrossRef]
- Debnath, J.; Muthuswamy, S.K.; Brugge, J.S. Morphogenesis and Oncogenesis of MCF-10A Mammary Epithelial Acini Grown in Three-Dimensional Basement Membrane Cultures. Methods 2003, 30, 256–268. [Google Scholar] [CrossRef]
- Bortolussi, S.; Protti, N.; Ferrari, M.; Postuma, I.; Fatemi, S.; Prata, M.; Ballarini, F.; Carante, M.P.; Farias, R.; González, S.J.; et al. Neutron flux and gamma dose measurement in the BNCT irradiation facility at the TRIGA reactor of the University of Pavia. Nucl. Instrum. Methods Phys. Res. Sect. B 2018, 414, 113–120. [Google Scholar] [CrossRef]
- Viegas, A.M.D.; Postuma, I.; Bortolussi, S.; Guidi, C.; Riback, J.S.; Provenzano, L.; Marcaccio, B.; Rossini, A.E.; Ferrari, C.; Cansolino, L.; et al. Detailed Dosimetry Calculation for In-Vitro Experiments and Its Impact on Clinical BNCT. Phys. Med. 2021, 89, 282–292. [Google Scholar] [CrossRef]
- Postuma, I.; Bortolussi, S.; Protti, N.; Ballarini, F.; Bruschi, P.; Ciani, L.; Ristori, S.; Panza, L.; Ferrari, C.; Cansolino, L.; et al. An Improved Neutron Autoradiography Set-up for 10B Concentration Measurements in Biological Samples. Rep. Pract. Oncol. Radiother. 2016, 21, 123–128. [Google Scholar] [CrossRef]
- Manti, L.; Braselmann, H.; Calabrese, M.L.; Massa, R.; Pugliese, M.; Scampoli, P.; Sicignano, G.; Grossi, G. Effects of Modulated Microwave Radiation at Cellular Telephone Frequency (1.95 GHz) on X-ray-Induced Chromosome Aberrations in Human Lymphocytes in Vitro. Radiat. Res. 2008, 169, 575–583. [Google Scholar] [CrossRef]
- Durante, M.; Furusawa, Y.; Gotoh, E. A Simple Method for Simultaneous Interphase-Metaphase Chromosome Analysis in Biodosimetry. Int. J. Radiat. Biol. 1998, 74, 457–462. [Google Scholar] [CrossRef]
- Manti, L.; Durante, M.; Cirrone, G.A.P.; Grossi, G.; Lattuada, M.; Pugliese, M.; Sabini, M.G.; Scampoli, P.; Valastro, L.; Gialanella, G. Modelled Microgravity Does Not Modify the Yield of Chromosome Aberrations Induced by High-Energy Protons in Human Lymphocytes. Int. J. Radiat. Biol. 2005, 81, 147–155. [Google Scholar] [CrossRef]
- Savage, J.R. Classification and Relationships of Induced Chromosomal Structural Changes. J. Med. Genet. 1976, 13, 103–122. [Google Scholar] [CrossRef]
- Lee, R.; Sommer, S.; Hartel, C.; Nasonova, E.; Durante, M.; Ritter, S. Complex Exchanges Are Responsible for the Increased Effectiveness of C-Ions Compared to X-rays at the First Post-Irradiation Mitosis. Mutat. Res. 2010, 701, 52–59. [Google Scholar] [CrossRef]
- Couto, M.; Alamón, C.; Nievas, S.; Perona, M.; Dagrosa, M.A.; Teixidor, F.; Cabral, P.; Viñas, C.; Cerecetto, H. Bimodal Therapeutic Agents against Glioblastoma, One of the Most Lethal Forms of Cancer. Chemistry 2020, 26, 14335–14340. [Google Scholar] [CrossRef]
- Schwint, A.E.; Garabalino, M.A.; Hughes, A.M.; Pozzi, E.C.C.; Heber, E.M.; Palmieri, M.A.; Trivillin, V.A. Teachings of Our Translational Studies on Boron Neutron Capture Therapy (BNCT): Thinking “Outside the Box”. Ther. Radiol. Oncol. 2019, 3, 20. [Google Scholar] [CrossRef]
- Nikitaki, Z.; Velalopoulou, A.; Zanni, V.; Tremi, I.; Havaki, S.; Kokkoris, M.; Gorgoulis, V.G.; Koumenis, C.; Georgakilas, A.G. Key Biological Mechanisms Involved in High-LET Radiation Therapies with a Focus on DNA Damage and Repair. Expert Rev. Mol. Med. 2022, 24, e15. [Google Scholar] [CrossRef]
- Kawabata, S.; Suzuki, M.; Hirose, K.; Tanaka, H.; Kato, T.; Goto, H.; Narita, Y.; Miyatake, S.I. Accelerator-Based BNCT for Patients with Recurrent Glioblastoma: A Multicenter Phase II Study. Neurooncol. Adv. 2021, 3, vdab067. [Google Scholar] [CrossRef]
- Rodriguez, C.; Carpano, M.; Curotto, P.; Thorp, S.; Casal, M.; Juvenal, G.; Pisarev, M.; Dagrosa, M.A. In Vitro Studies of DNA Damage and Repair Mechanisms Induced by BNCT in a Poorly Differentiated Thyroid Carcinoma Cell Line. Radiat. Environ. Biophys. 2018, 57, 143–152. [Google Scholar] [CrossRef]
- Schipler, A.; Iliakis, G. DNA Double-Strand-Break Complexity Levels and Their Possible Contributions to the Probability for Error-Prone Processing and Repair Pathway Choice. Nucleic Acids Res. 2013, 41, 7589–7605. [Google Scholar] [CrossRef]
- Murmann-Konda, T.; Soni, A.; Stuschke, M.; Iliakis, G. Analysis of Chromatid-Break-Repair Detects a Homologous Recombination to Non-Homologous End-Joining Switch with Increasing Load of DNA Double-Strand Breaks. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2021, 867, 503372. [Google Scholar] [CrossRef]
- Abbotts, R.; Wilson, D.M., 3rd. Coordination of DNA Single Strand Break Repair. Free Radic. Biol. Med. 2017, 107, 228–244. [Google Scholar] [CrossRef]
- Mladenova, V.; Mladenov, E.; Chaudhary, S.; Stuschke, M.; Iliakis, G. The High Toxicity of DSB-Clusters Modelling High-LET-DNA Damage Derives from Inhibition of c-NHEJ and Promotion of Alt-EJ and SSA despite Increases in HR. Front. Cell Dev. Biol. 2022, 10, 1016951. [Google Scholar] [CrossRef]
- Schmid, T.E.; Canella, L.; Kudejova, P.; Wagner, F.M.; Röhrmoser, A.; Schmid, E. The Effectiveness of the High-LET Radiations from the Boron Neutron Capture [10B(n,α)7Li] Reaction Determined for Induction of Chromosome Aberrations and Apoptosis in Lymphocytes of Human Blood Samples. Radiat. Environ. Biophys. 2015, 54, 91–102. [Google Scholar] [CrossRef]
- Ritter, S.; Nasonova, E.; Furusawa, Y.; Ando, K. Relationship between Aberration Yield and Mitotic Delay in Human Lymphocytes Exposed to 200 MeV/u Fe-Ions or X-rays. J. Radiat. Res. 2002, 43, S175–S179. [Google Scholar] [CrossRef]
- Cornforth, M.N.; Bedford, J.S. A Quantitative Comparison of Potentially Lethal Damage Repair and the Rejoining of Interphase Chromosome Breaks in Low Passage Normal A Quantitative Comparison of Potentially Lethal Damage Repair and the Rejoining of Interphase Chromosome Breaks in Low Passage Normal Human Fibroblasts. Radiat. Res. 1987, 111, 385–405. [Google Scholar]
- Konings, K.; Vandevoorde, C.; Baselet, B.; Baatout, S.; Moreels, M. Combination Therapy with Charged Particles and Molecular Targeting: A Promising Avenue to Overcome Radioresistance. Front. Oncol. 2020, 10, 128. [Google Scholar] [CrossRef]
- Ricciardi, V.; Portaccio, M.; Piccolella, S.; Manti, L.; Pacifico, S.; Lepore, M. Study of SH-SY5Y Cancer Cell Response to Treatment with Polyphenol Extracts Using FT-IR Spectroscopy. Biosensors 2017, 7, 57. [Google Scholar] [CrossRef]
- Cirrone, G.A.P.; Margarone, D.; Maggiore, M.; Anzalone, A.; Borghesi, M.; Jia, S.B.; Bulanov, S.S.; Bulanov, S.; Carpinelli, M.; Cavallaro, S.; et al. ELIMED: A new hadron therapy concept based on laser driven ion beams. In Proceedings of the SPIE, The International Society for Optical Engineering, Prague, Czech Republic, 15–18 April 2013; Volume 8779. [Google Scholar] [CrossRef]
- Ban, H.S.; Nakamura, H. Boron-Based Drug Design. Chem. Rec. 2015, 15, 616–635. [Google Scholar] [CrossRef]
- Nuez-Martinez, M.; Pinto, C.I.G.; Guerreiro, J.F.; Mendes, F.; Marques, F.; Muñoz-Juan, A.; Xavier, J.A.M.; Laromaine, A.; Bitonto, V.; Protti, N.; et al. Cobaltabis(Dicarbollide) ([o-Cosan]−) as Multifunctional Chemotherapeutics: A Prospective Application in Boron Neutron Capture Therapy (BNCT) for Glioblastoma. Cancers 2021, 13, 6367. [Google Scholar] [CrossRef]
- Matsumura, A.; Asano, T.; Hirose, K.; Igaki, H.; Kawabata, S.; Kumada, H. Initiatives Toward Clinical Boron Neutron Capture Therapy in Japan. Cancer Biother. Radiopharm. 2023, 38, 201–207. [Google Scholar] [CrossRef]
| Absorbed Dose [10−6 Gy s−1 kW−1] | |||
|---|---|---|---|
| 10B(n,alpha)7Li per ug/g of 10B | 14N(n,p)14C | H (n,n’) H | gamma |
| 5.07 ± 0.01 | 8.56 ± 0.01 | 3.02 ± 0.03 | 17.68 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elia, V.C.; Fede, F.; Bortolussi, S.; Cansolino, L.; Ferrari, C.; Formicola, E.; Postuma, I.; Manti, L. Fluorescence In Situ Hybridization-Based Chromosome Aberration Analysis Unveils the Mechanistic Basis for Boron-Neutron Capture Therapy’s Radiobiological Effectiveness. Appl. Sci. 2024, 14, 1171. https://doi.org/10.3390/app14031171
Elia VC, Fede F, Bortolussi S, Cansolino L, Ferrari C, Formicola E, Postuma I, Manti L. Fluorescence In Situ Hybridization-Based Chromosome Aberration Analysis Unveils the Mechanistic Basis for Boron-Neutron Capture Therapy’s Radiobiological Effectiveness. Applied Sciences. 2024; 14(3):1171. https://doi.org/10.3390/app14031171
Chicago/Turabian StyleElia, Valerio Cosimo, Francesca Fede, Silva Bortolussi, Laura Cansolino, Cinzia Ferrari, Emilia Formicola, Ian Postuma, and Lorenzo Manti. 2024. "Fluorescence In Situ Hybridization-Based Chromosome Aberration Analysis Unveils the Mechanistic Basis for Boron-Neutron Capture Therapy’s Radiobiological Effectiveness" Applied Sciences 14, no. 3: 1171. https://doi.org/10.3390/app14031171
APA StyleElia, V. C., Fede, F., Bortolussi, S., Cansolino, L., Ferrari, C., Formicola, E., Postuma, I., & Manti, L. (2024). Fluorescence In Situ Hybridization-Based Chromosome Aberration Analysis Unveils the Mechanistic Basis for Boron-Neutron Capture Therapy’s Radiobiological Effectiveness. Applied Sciences, 14(3), 1171. https://doi.org/10.3390/app14031171









