Presence of Quercus Suber Soft-Leaf Defoliators on Trees with Distinct Foliar Monoterpene Emission Profiles
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
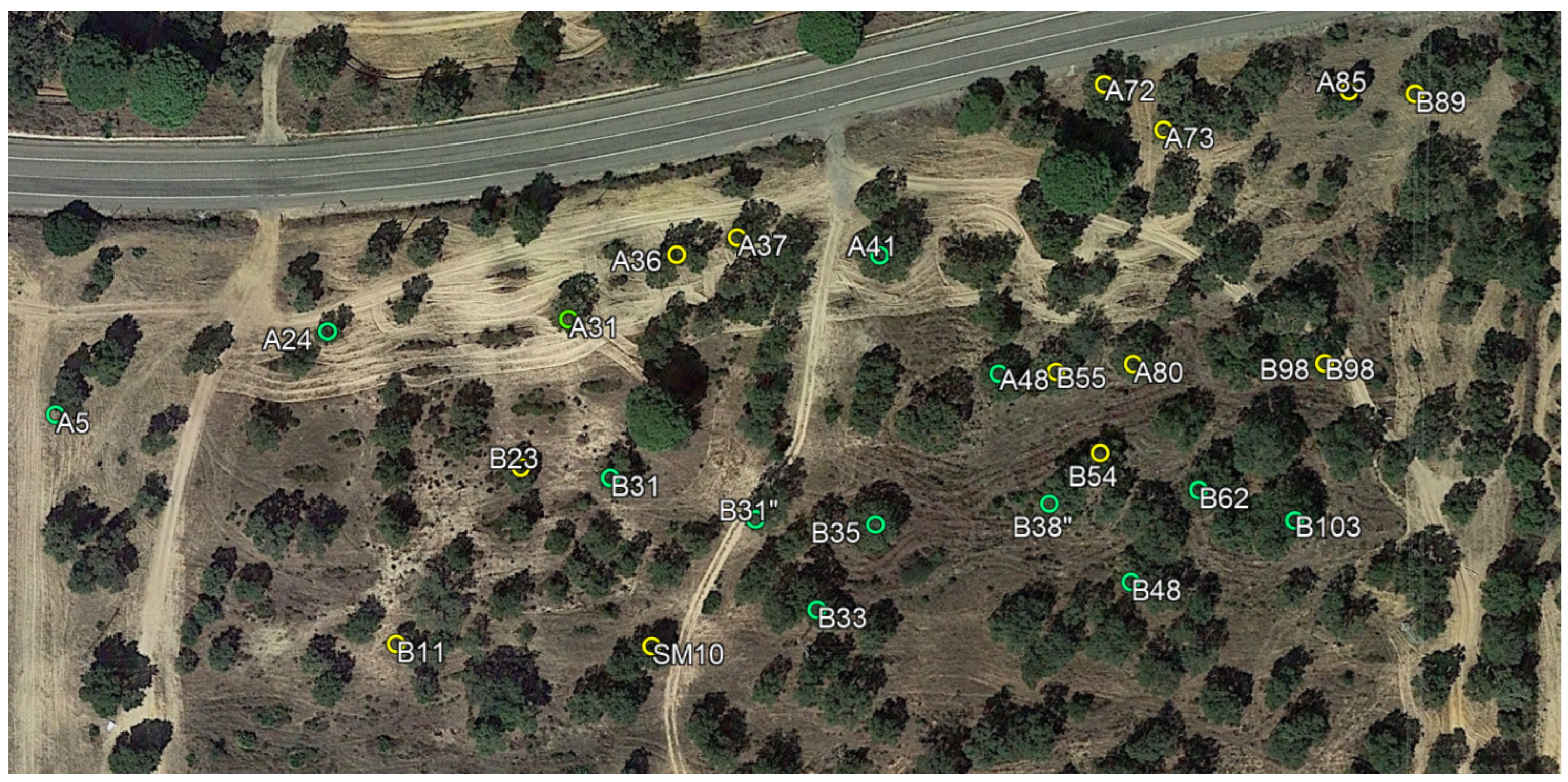
2.1. Trees under Study
2.2. Insect Sampling
2.3. Field Estimation of Defoliation
2.4. Statistical Analysis
3. Results
3.1. Defoliating Species
3.2. Relationship between the Presence of Defoliators and Tree Emission Profile
3.3. Relationship between Defoliation Damage and Tree Emission Profile
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- San Miguel, A. The Spanish Dehesa. Origin, Typology, Characteristics and Management (La Dehesa Española. Origen, Tipología, Características y Gestión); Conde del Valle de Salazar Foundation: Madrid, Spain, 1994; p. 2. [Google Scholar]
- Alejano, R.; Domingo, J.M.; Fernández, M. Manual for the Sustainable Management of Andalusian Dehesas (in Spanish) Forum for the Defense and Conservation of the Dehesa “Encinal”; University of Huelva: Huelva, Spain, 2011. [Google Scholar]
- Summerville, K.; Marquis, R. Comparing the responses of larval and adult lepidopteran communities to timber harvest using long-term, landscape-scale studies in oak-hickory forests. Forest. Ecol. Manag. 2016, 387, 64–72. [Google Scholar] [CrossRef]
- Antonietty, C.A. Design of an Integrated Management Plan for Tortrix viridana L. (Lepidoptera: Tortricidae) (Diseño de un Plan de Manejo Integrado para Tortrix viridana L. (Lepidoptera: Tortricidae)). Ph.D. Thesis, University of Sevilla, Sevilla, Spain, 2013. [Google Scholar]
- Luciano, P.; Roversi, P. Oak Defoliators in Italy; Industria Grafica Poddighe s.r.l.: Sassari, Italy, 2001. [Google Scholar]
- Toimil, F.J. Some lepidopterans that defoliate holm oak (Q. ilex L.) and cork oak (Q. suber L.), in the province of Huelva (Algunos lepidópteros defoliadores de la encina (Q. ilex L.) y alcornoque (Q. suber L.), en la provincia de Huelva). Bol. San. Veg. Plagas. 1987, 13, 331–346. [Google Scholar]
- Monreal, J.A.; Salvador, D.; Mansilla, J. Contribution to the knowledge of holm oak defoliating insects (Q. ilex L.), in the province of Albacete. Bol. San. Veg. Plagas. 1992, 18, 395–405. (In Spanish) [Google Scholar]
- Tiberi, R.; Branco, M.; Bracalini, M.; Croci, F.; Panzavolta, T. Cork oak pests: A review of insect damage and management. Ann. For. Sci. 2016, 73, 219–232. [Google Scholar] [CrossRef]
- Branco, M.; Ramos, P. Coping with pests and diseases. In Cork Oak Woodlands on the Edge: Ecology, Adaptive Management, and Restoration; Aronson, J., Pereira, J.S., Pausas, J.G., Eds.; Island Press: Washington, DC, USA, 2009; pp. 103–111. [Google Scholar]
- Pereira, P.; Godinho, C.; Roque, I.; Marques, A.; Branco, M.; Rabaça, J.E. Time to rethink the management intensity in a Mediterranean oak woodland: The response of insectivorous birds and leaf-chewing defoliators as key groups in the forest ecosystem. Ann. For. Sci. 2012, 71, 25–32. [Google Scholar] [CrossRef]
- Teodorescu, I.; Simionescu, A. Dynamics of defoliating Lepidoptera attacks and the control measures in Romania deciduous forests, 1953–1990. Ambio. 1994, 23, 260–266. [Google Scholar]
- Torrent, J.A. Montaneras en los últimos diez años (1953-62). Bol. Serv. Plag. For. 1963, 11, 73–77. [Google Scholar]
- Thomas, F. Recent advances in cause-effect research on oak decline in Europe. Agric. Vet. Sci. Nutr. Nat. Resour. 2008, 3, 1–12. [Google Scholar] [CrossRef]
- Dicke, M.; Baldwin, I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Grote, R.; Monson, R.; Niinemets, Ü. Leaf-level models of constitutive and stress-driven volatile organic compound emissions. In Biology, Controls and Models of Tree Volatile Organic Compound Emission; Niinemets, Ü., Monson, R.K., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2013; pp. 315–355. [Google Scholar]
- Pearse, I.S.; Gee, W.S.; Beck, J.J. Headspace Volatiles from 52 oak species advertise induction, species identity, and evolution, but not Defense. J. Chem. Ecol. 2013, 9, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Pio, C.A.; Silva, P.A.; Cerqueira, M.A.; Nunes, T.V. Diurnal and seasonal emissions of volatile organic compounds from cork oak (Quercus suber) trees. Atmos. Environ. 2005, 39, 1817–1827. [Google Scholar] [CrossRef]
- Lavoir, A.V.; Duffet, C.; Mouillot, F.; Rambal, S.; Ratte, J.P.; Schnitzler, J.P.; Staudt, M. Scaling-up leaf monoterpene emissions from a water limited Quercus ilex woodland. Atmos. Environ. 2011, 45, 2888–2897. [Google Scholar] [CrossRef]
- Gols, R. Direct and indirect chemical defences against insects in a multitrophic framework: Plant chemical defences against insects. Plant Cell Environ. 2012, 37, 1741–1752. [Google Scholar] [CrossRef]
- Li, J.; Valimaki, S.; Shi, J.; Zong, S.; Luo, Y.; Heliovaara, K. Attraction of the gypsy moth to volatile organic compounds (VOCs) of damaged dahurian larch. Z. Naturforsch. C. J. Biosci. 2012, 67, 437–444. [Google Scholar] [CrossRef]
- Solla, A.; Milanović, S.; Gallardos, A.; Bueno, A.; Corcobado, T.; Cáceres, Y.; Morcuende, D.; Quesada, A.; Moreno, G.; Pulido, F. Genetic determination of tannins and herbivore resistance in Quercus ilex. Tree Gen. Genom. 2016, 12, 117. [Google Scholar] [CrossRef]
- Sánchez-Osorio, I.; López-Pantoja, G.; Tapias, R.; Pareja-Sánchez, E.; Domínguez, L. Monoterpene emission of Quercus suber L. highly infested by Cerambyx welensii Küster. Ann. For. Sci. 2019, 76, 98. [Google Scholar] [CrossRef]
- Staudt, M.; Mir, C.; Joffre, R.; Rambal, S.; Bonin, A.; Landais, D.; Lumaret, R. Isoprenoid emissions of Quercus spp. (Q. suber and Q. ilex) in mixed stands contrasting in interspecific genetic introgression. New Phytol. 2004, 163, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Birgersson, G.; Zhu, J.; Lofstedt, C.; Lofqvist, J.; Schlyter, F. Leaf volatiles from nonhost deciduous trees: Variation by tree species, season and temperature, and electrophysiological activity in Ips typographus. J. Chem. Ecol. 1999, 8, 1923–1943. [Google Scholar] [CrossRef]
- Granados, C.; Ramírez, D.; Sánchez, I.; López, G.; Vázquez, E. Holm oak defoliators in Andévalo Occidental, in the province of Huelva. Comparison between two specific situations: The period 1985–1988 and the year 2000 (Defoliadores de encinar en el Andévalo Occidental de la provincia de Huelva. Comparación entre dos situaciones puntuales: El período 1985–1988 y el año 2000). In Mountains for the Society of the New Millennium, Proceedings of the III Spanish Forestry Congress, Granada, Spain, 25–28 September 2001; Spanish Society of Forest Sciences: Granada, Spain, 2000; pp. 59–61. [Google Scholar]
- Mair, P.; Wilcox, R.R. Robust Statistical Methods in R Using the WRS2 Package. Behav. Res. Methods. 2020, 52, 464–488. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D. snpar: Supplementary Non-Parametric Statistics Methods. R package version 1.0. 2014. Available online: https://CRAN.R-project.org/package=snpar (accessed on 2 July 2019).
- Oksanen, F.; Blanchet, G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B. Vegan: Community Ecology Package. R package version 2.2-1. 2015. Available online: http://CRAN.R-project.org/package=vegan (accessed on 2 July 2019).
- Extremera, F.M.; Cobo, A.; Pérez, M.C.; Pérez-Guerrero, S.; Vargas-Osuna, E. The Quercus defoliating lepidopteran complex in the province of Córdoba (El complejo de lepidópteros defoliadores de Quercus en la provincia de Córdoba). Bol. San. Veg. Plagas. 2004, 30, 203–209. [Google Scholar]
- Ivashov, A.V.; Boyko, G.E.; Simchuk, A.P. The role of host plant phenology in the development of the oak leaf roller moth, Tortrix viridana L. (Lepidoptera: Tortricidae). For. Ecol. Manag. 2002, 157, 7–14. [Google Scholar] [CrossRef]
- Sánchez-Herrera, F.; Soria, S. The problem of monitoring and controlling harmful lepidopterans in holm oak forests, special reference to the holm oak dehesa in Madrid (La problemática del seguimiento y control de lepidópteros nocivos del encinar, especial referencia al encinar adehesado madrileño). Bol. San. Veg. Plagas. 1987, 13, 213–224. [Google Scholar]
- Carrasco, D.; Larsson, M.C.; Anderson, P. Insect host plant selection in complex environments. Curr. Opin. Insect Sci. 2015, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Conchou, L.; Lucas, P.; Meslin, C.; Proffit, M.; Staudt, M.; Renou, M. Insect odorscapes: From plant volatiles to natural olfactory scenes. Front. Physiol. 2019, 10, 972. [Google Scholar] [CrossRef]
- Staudt, M.; Jackson, B.; El-Aouni, H.; Buatois, B.; Lacroze, J.P.; Poëssel, J.L.; Sauge, M.H. Volatile organic compound emissions induced by the aphid Myzus persicae differ among resistant and susceptible peach cultivars and a wild relative. Tree Physiol. 2010, 30, 1320–1334. [Google Scholar] [CrossRef]
- Srinivasan, R. Introduction: Host plant choice and feeding ecology of insects. Entomol. Exp. Appl. 2019, 167, 288–291. [Google Scholar] [CrossRef]
- Sangüesa-Barreda, G.; Camarero, J.J.; García-Martín, A.; Hernández, R.; de la Riva, J. Remote-sensing and tree-ring based characterization of forest defoliation and growth loss due to the Mediterranean pine processionary moth. For. Ecol. Manag. 2014, 320, 171–181. [Google Scholar] [CrossRef]
- Kneeshaw, D.; Sturtevant, B.R.; Cooke, B.; Work, T.; Pureswaran, D.; DeGrandpre, L.; MacLean, D. Insect disturbances in forest ecosystems. In Routledge Handbook of Forest Ecology; Peh, K.S.-H., Corlett, R.T., Bergeron, Y., Eds.; Taylor & Francis Group: New York, NY, USA, 2015; pp. 93–132. [Google Scholar]
- Bowsher, C.; Steer, M.; Tobin, A. Plant Biochemistry; Taylor & Francis Group: New York, NY, USA, 2008; p. 320. [Google Scholar]
- Kigathi, R.N.; Unsicker, S.B.; Reichelt, M.; Kesselmeier, J.; Gershenzon, J.; Weisser, W.W. Emission of volatile organic compounds after herbivory from Trifolium pratense (L.) under laboratory and field conditions. J. Chem. Ecol. 2009, 35, 1335–1348. [Google Scholar] [CrossRef]
- Henneken, J.; Goodger, J.Q.D.; Jones, T.M.; Elgar, M.A. Diet-mediated pheromones and signature mixtures can enforce signal reliability. Front. Ecol. Evol. 2017, 4, 145. [Google Scholar] [CrossRef]
- Ando, T. List of Lepidopteran Sex Pheromones and Attractants. Available online: https://lepipheromone.sakura.ne.jp/index_eng.html (accessed on 3 July 2019).
- Frerot, B.; Renou, M.; Gallois, M.; Descoins, C. A sex attractant for Archips xylosteana L. (Lepid., Tortricidae, Tortricinae). Agron. 1983, 3, 173–178. [Google Scholar] [CrossRef]
- Reed, D.W.; Chisholm, M.D. Attraction of moth species of Tortricidae, Gelechiidae, Geometridae, Drepanidae, Pyralidae, and Gracillariidae families to field traps baited with conjugated dienes. J. Chem. Ecol. 1985, 11, 1645–1657. [Google Scholar] [CrossRef] [PubMed]
- Arn, H.; Tóth, M.; Priesner, E. List of Sex Pheromones of Lepidoptera and Related Attractants; Springer: Paris, France, 1986; p. 123. [Google Scholar]
- Oehlke, B. Catocala Grynea. Available online: http://www.silkmoths.bizland.com/Catocala/catgrynea.htm (accessed on 2 February 2020).
- Niinemets, U.; Seufert, G.; Steinbrecher, R.; Tenhunen, J.D. A model coupling foliar monoterpene emissions to leaf photosynthetic characteristics in Mediterranean evergreen Quercus species. New Phytol. 2002, 153, 257–275. [Google Scholar] [CrossRef]


| Family | Species (Abbreviation) | N (%) |
|---|---|---|
| Noctuidae | Catocala nymphagoga Esper (Catnym) | 40.8 |
| Bena bicolorana L. (Benbic) | 11.0 | |
| Lycaenidae | Syntaurucus pirithous L. (Synpir) | 0.7 |
| Satyrium esculi Hb. (Satesc) | 1.1 | |
| Tenthredinidae | Periclista andrei Konow. (Perand) | 27.6 |
| Tortricidae | Archips xylosteana L. (Arcxyl) | 2.2 |
| Drepanidae | Drepana uncinula Bkh. (Dreunc) | 2.2 |
| Lasiocampidae | Lasiocampa trifolii D. and S. (Lastri) | 0.4 |
| Geometridae | Cyclophora punctaria L. (Cycpun) | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Osorio, I.; Robles, D.; Tapias, R. Presence of Quercus Suber Soft-Leaf Defoliators on Trees with Distinct Foliar Monoterpene Emission Profiles. Appl. Sci. 2024, 14, 1112. https://doi.org/10.3390/app14031112
Sánchez-Osorio I, Robles D, Tapias R. Presence of Quercus Suber Soft-Leaf Defoliators on Trees with Distinct Foliar Monoterpene Emission Profiles. Applied Sciences. 2024; 14(3):1112. https://doi.org/10.3390/app14031112
Chicago/Turabian StyleSánchez-Osorio, Israel, Daniel Robles, and Raúl Tapias. 2024. "Presence of Quercus Suber Soft-Leaf Defoliators on Trees with Distinct Foliar Monoterpene Emission Profiles" Applied Sciences 14, no. 3: 1112. https://doi.org/10.3390/app14031112
APA StyleSánchez-Osorio, I., Robles, D., & Tapias, R. (2024). Presence of Quercus Suber Soft-Leaf Defoliators on Trees with Distinct Foliar Monoterpene Emission Profiles. Applied Sciences, 14(3), 1112. https://doi.org/10.3390/app14031112







