Improved Production of Astaxanthin from Haematococcus pluvialis Using a Hybrid Open–Closed Cultivation System
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgal Strain and Culture
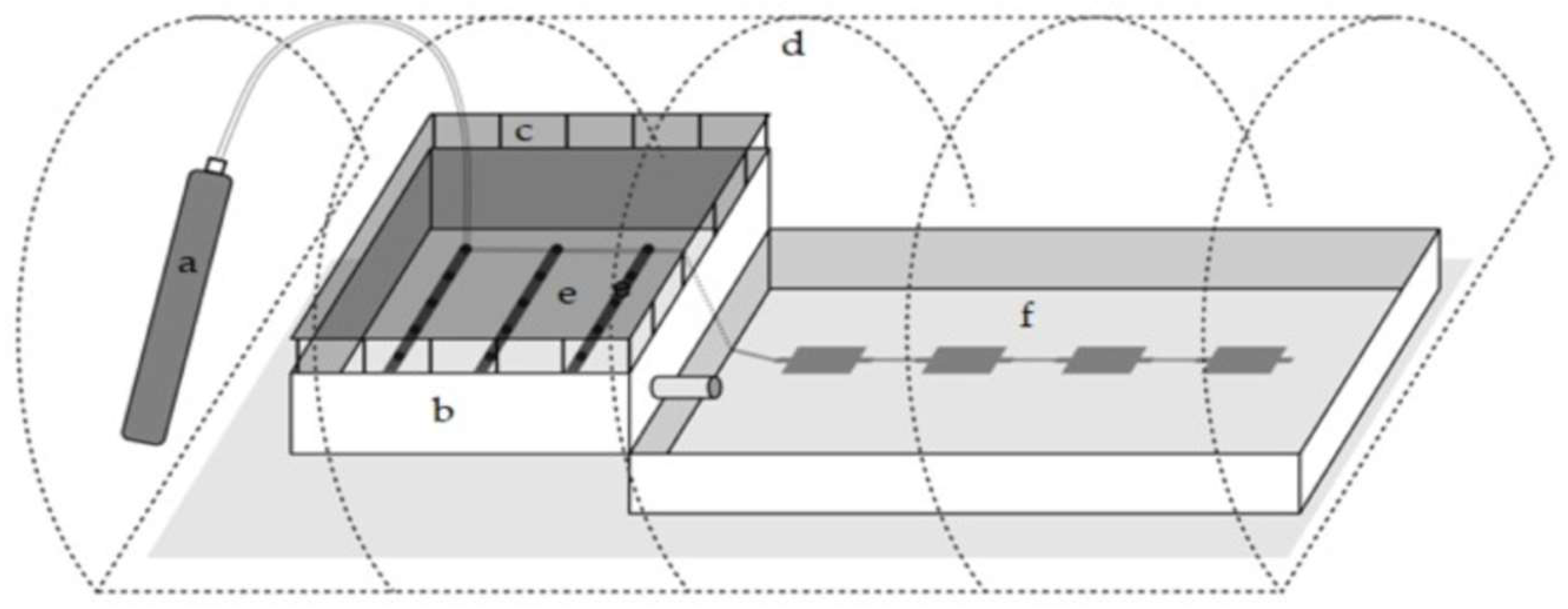
2.2. Design and Construction of a Hybrid System for H. pluvialis Mass Cultivation
2.3. Conditions for H. pluvialis Growth (Nitrogen-Depleted Conditions)
2.4. Measurement of Light Intensity
2.5. Estimation of Cell Density
2.6. Extraction and Estimation of Chlorophyll (Chl) and Total Carotenoid Contents
2.7. Cell Growth Calculation
2.8. Statistical Analysis
3. Results
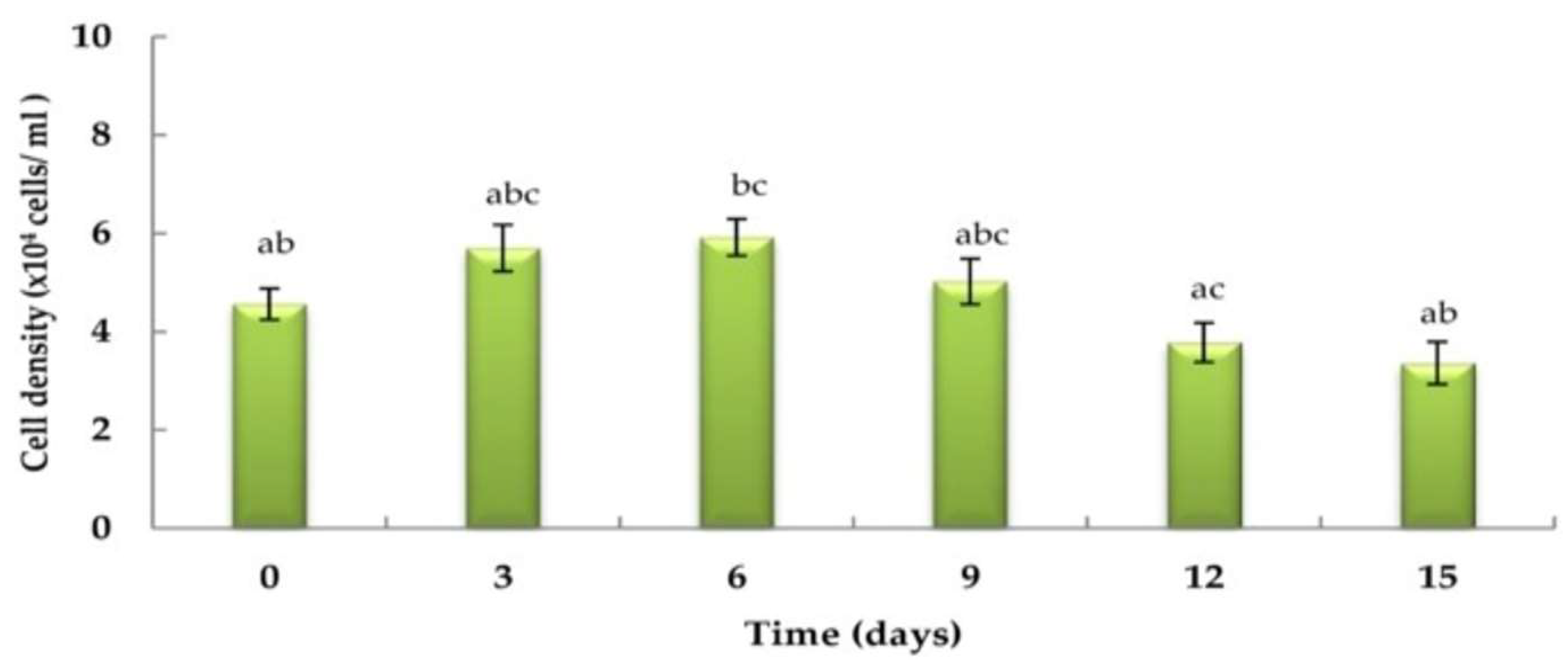
3.1. Haematococcus pluvialis Growth Curve
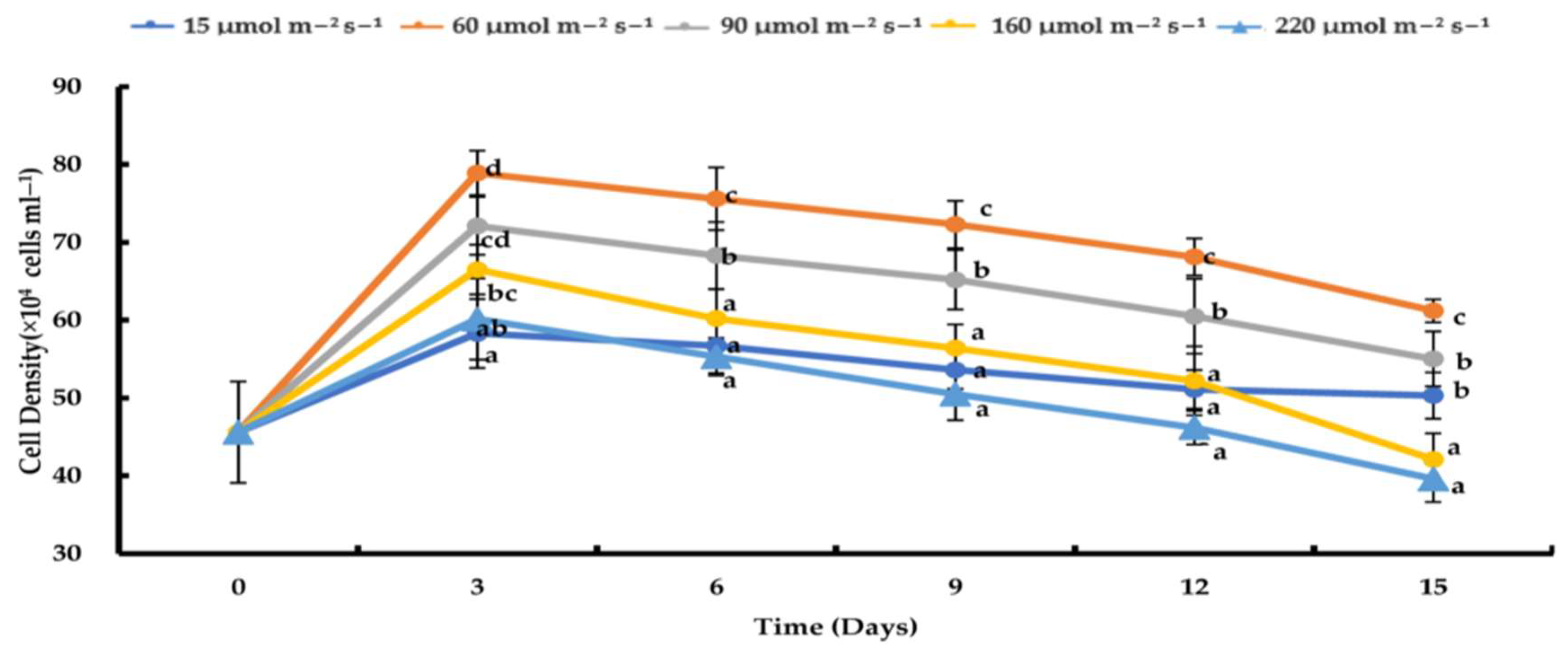
3.2. Effects of Varying Light Intensities on H. pluvialis Growth under Nitrogen-Depleted Conditions
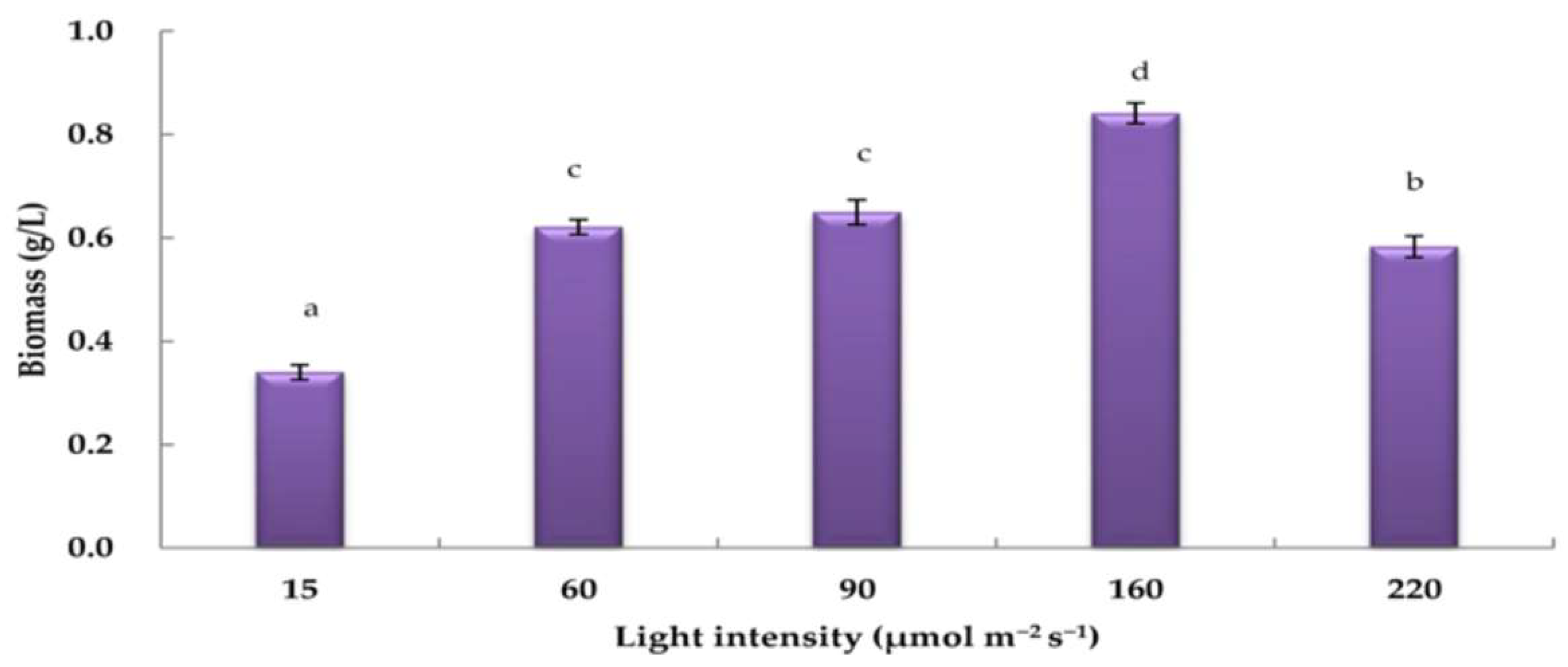
3.3. Effects of Varying Light Intensities on H. pluvialis Biomass under Nitrogen-Depleted Conditions
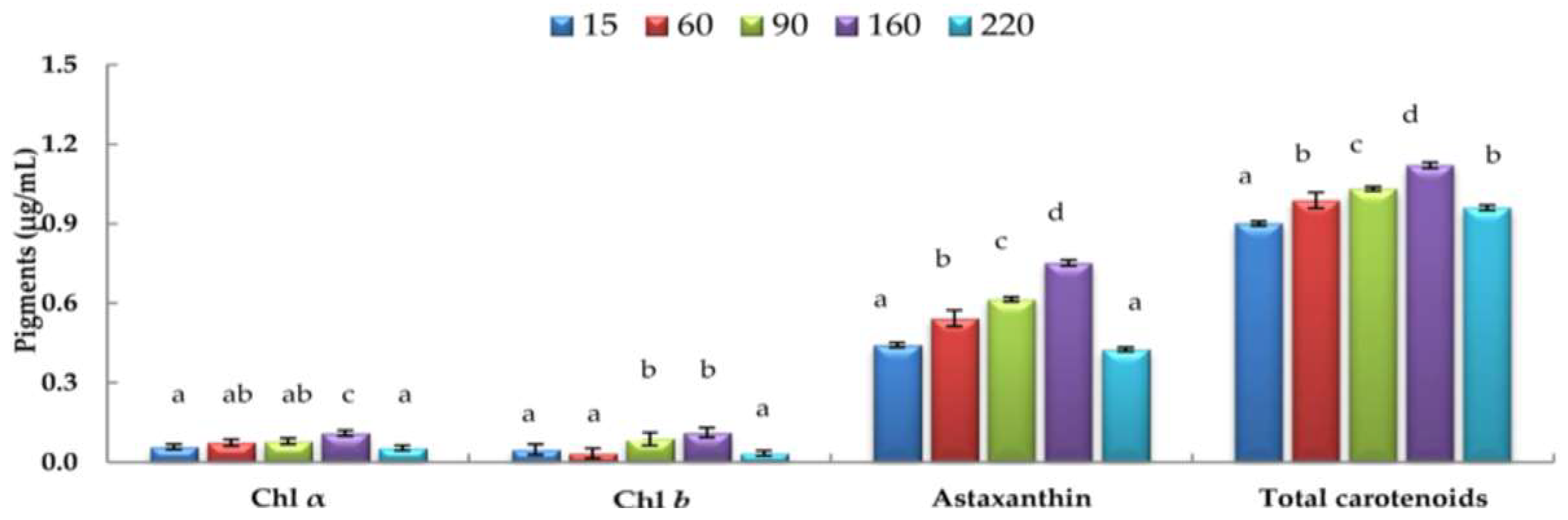
3.4. Effects of Varying Light Intensities on H. pluvialis Chl a, Chl b, and Carotenoid Contents under Nitrogen-depleted Conditions
3.5. Effects of 25 µmol m−2 s−1 Light Intensity on Cell Density
3.6. Effects of 160 µmol m−2 s−1 Light Intensity on Cell Density
3.7. Biomass and Chl a, Chl b, Total Carotenoid, and Astaxanthin Contents Produced under Laboratory Culture and Hybrid Outdoor Mass Culture Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Udayan, A.; Sirohi, R.; Sreekumar, N.; Sang, B.I.; Sim, S.J. Mass cultivation and harvesting of microalgal biomass: Current trends and future perspectives. Bioresour. Technol. 2022, 344 Pt B, 126406. [Google Scholar] [CrossRef]
- Karthikeyan, D.; Muthukumaran, M.; Balakumar, B.S. Mass cultivation of microalgae in open raceway pond for biomass and biochemicals production. Int. J. Adv. Res. Biol. Sci. 2016, 3, 247–260. [Google Scholar]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- Hong, J.W.; Jo, S.W.; Yoon, H.S. Research and development for algae-based technologies in Korea: A review of algae biofuel production. Photosynth. Res. 2015, 123, 297–303. [Google Scholar] [CrossRef]
- Park, K.C.; Whitney, C.; McNichol, J.C.; Dickinson, K.E.; MacQuarrie, S.; Skrupski, B.P.; Zou, J.; Wilson, K.E.; O’Leary, S.J.B.; McGinn, P.J. Mixotrophic and photoautotrophic cultivation of 14 microalgae isolates from Saskatchewan, Canada: Potential applications for wastewater remediation for biofuel production. J. Appl. Phycol. 2012, 24, 339–348. [Google Scholar] [CrossRef]
- Wegeberg, S. Algae Biomass for Bioenergy in Denmark Biological/Technical Challenges and Opportunities; DONG Energy: Fredericia, Denmark, 2010. [Google Scholar]
- Gómez, P.I.; Inostroza, I.; Pizarro, M.; Pérez, J. From genetic improvement to commercial-scale mass culture of a Chilean strain of the green microalga Haematococcus pluvialis with enhanced productivity of the red ketocarotenoid astaxanthin. AoB Plants 2013, 5, plt026. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Hong, J.W.; Kim, O.H.; Kim, H.; Jo, S.W.; Cho, H.W.; Yoon, H.S. Mass Cultivation from a Korean Raceway Pond System of Indigenous Microalgae as Potential Biofuel Feedstock. Oil Gas. Res. 2016, 2, 108. [Google Scholar]
- Nishida, Y.; Berg, P.C.; Shakersain, B.; Hecht, K.; Takikawa, A.; Tao, R.; Kakuta, Y.; Uragami, C.; Hashimoto, H.; Misawa, N.; et al. Astaxanthin: Past, Present, and Future. Mar. Drugs 2023, 21, 514. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef] [PubMed]
- Jannel, S.; Caro, Y.; Bermudes, M.; Petit, T. Novel Insights into the Biotechnological Production of Haematococcus pluvial-is-Derived Astaxanthin: Advances and Key Challenges to Allow Its Industrial Use as Novel Food Ingredient. J. Mar. Sci. Eng. 2020, 8, 789. [Google Scholar] [CrossRef]
- Rizzo, A.; Ross, M.E.; Norici, A.; Jesus, B. A Two-Step Process for Improved Biomass Production and Non-Destructive Astaxanthin and Carotenoids Accumulation in Haematococcus pluvialis. Appl. Sci. 2022, 12, 1261. [Google Scholar] [CrossRef]
- Dawidziuk, A.; Popiel, D.; Luboinska, M.; Grzebyk, M.; Wisniewski, M.; Koczyk, G. Assessing contamination of microalgal astaxanthin producer Haematococcus cultures with high-resolution melting curve analysis. J. Appl. Genet. 2017, 58, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sommerfeld, M.R.; Lu, C.; Hu, Q. Combined effect of initial biomass density and nitrogen concentration on growth and astaxanthin production of Haematococcus pluvialis (Chlorophyta) in outdoor cultivation. Algae 2013, 28, 193–202. [Google Scholar] [CrossRef]
- Bao, Y.Z.; Ya, H.G.; Zhong, K.L.; Hong, J.H.; Ye, G.L. Production of astaxanthin from Haematococcus in open pond by two-stage growth one-step process. Aquaculture 2009, 295, 275–281. [Google Scholar]
- Oslan, S.N.H.; Shoparwe, N.F.; Yusoff, A.H.; Rahim, A.A.; Chang, C.S.; Tan, J.S.; Oslan, S.N.; Arumugam, K.; Ariff, A.B.; Sulaiman, A.Z.; et al. A Review on Haematococcus pluvialis Bioprocess Optimization of Green and Red Stage Culture Conditions for the Production of Natural Astaxanthin. Biomolecules 2021, 11, 256. [Google Scholar] [CrossRef]
- Dechatiwongse, P.; Choorit, W. Mixotrophic Growth of Astaxanthin-Rich Alga Haematococcus pluvialis using Refined Crude Glycerol as Carbon Substrate: Batch and Fed-Batch Cultivations. Walailak J. Sci. Technol. (WJST) 2021, 18, 7354. [Google Scholar] [CrossRef]
- Solovchenko, A.E. Recent breakthroughs in the biology of astaxanthin accumulation by microalgal cell. Photosynth. Res. 2015, 125, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Herrero, C.; Orosa, M.; Abalde, J.; Rioboo, C.; Cid, A. Astaxanthin Production in Cysts and Vegetative Cells of the Microalga Haematococcus Pluvialis Flotow. In Microalgae: Biotechnology, Microbiology and Energy; Johnsen, M.N., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2012; pp. 359–371. [Google Scholar]
- Kumar, K.; Mishra, S.K.; Shrivastav, A.; Park, M.S.; Yang, J.-W. Recent trends in the mass cultivation of algae in raceway ponds. Renew. Sustain. Energy Rev. 2015, 51, 875–885. [Google Scholar] [CrossRef]
- Yadala, S.; Cremaschi, S. A Dynamic Optimization Model for Designing Open-Channel Raceway Ponds for Batch Production of Algal Biomass. Processes 2016, 4, 10. [Google Scholar] [CrossRef]
- Gateau, H.; Solymosi, K.; Marchand, J.; Schoefs, B. Carotenoids of Microalgae Used in Food Industry and Medicine. Mini Rev Med Chem. 2017, 17, 1140–1172. [Google Scholar] [CrossRef]
- Li, Y.; Gong, F.; Guo, S.; Yu, W.; Liu, J. Adonis amurensis Is a Promising Alternative to Haematococcus as a Resource for Natural Esterified (3S,3′S)-Astaxanthin Production. Plants 2021, 10, 1059. [Google Scholar] [CrossRef]
- Kopecky, J.; Schoefs, B.; Loest, K.; Stys, D.; Pulz, O. Microalgae as a source for secondary carotenoid production: A screening study. Algological Studies, Int. J. Phycol. Res. 2000, 133, 153–168. [Google Scholar] [CrossRef]
- Schoefs, B.; Rmiki, N.; Rachadi, J.; Lemoine, Y. Astaxanthin accumulation in Haematococcus requires a cytochrome P450 hydroxylase and an active synthesis of fatty acids. FEBS Lett. 2001, 500, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Borowiak, D.; Pstrowska, K.; Wiśniewski, M.; Grzebyk, M. Propagation of Inoculum for Haematococcus pluvialis Microalgae Scale-Up Photobioreactor Cultivation System. Appl. Sci. 2020, 10, 6283. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Navid, R.M. Open pond culture systems. In Algae Biofuels Energy; Springer: Dordrecht, The Netherlands, 2013; pp. 133–152. [Google Scholar]
- Qin, S.; Wang, K.; Gao, F.; Ge, B.; Cui, H.; Li, W. Biotechnologies for bulk production of microalgal biomass: From mass cultivation to dried biomass acquisition. Biotechnol. Biofuels 2023, 16, 131. [Google Scholar] [CrossRef] [PubMed]
- Narala, R. Comparison of Microalgae Cultivation in Photobioreactor, Open Raceway Pond, and a Two-Stage Hybrid System. Front. Energy Res. 2016, 4, 29. [Google Scholar] [CrossRef]
- Jerney, J.; Spilling, K. Large Scale Cultivation of Microalgae: Open and Closed Systems. Methods Mol. Biol. 2020, 1980, 1–8. [Google Scholar] [PubMed]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Li, F.; Cai, M.; Wu, Y.; Lian, Q.; Qian, Z.; Luo, J.; Zhang, N.; Li, C.; Huang, X. Effects of Nitrogen and Light Intensity on the Astaxanthin Accumulation in Motile Cells of Haematococcus pluvialis. Front. Mar. Sci. 2022, 9, 909237. [Google Scholar] [CrossRef]
- Han, D.; Li, Y.; Hu, Q. Astaxanthin in microalgae: Pathways, functions and biotechnological implications. Algae 2013, 28, 131–147. [Google Scholar] [CrossRef]
- Li, J.; Zhu, D.; Niu, J.; Shen, S.; Wang, G. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol. Adv. 2011, 29, 568–574. [Google Scholar] [CrossRef]
- Wang, F.; Gao, B.; Wu, M.; Huang, L.; Zhang, C. A novel strategy for the hyper-production of astaxanthin from the newly isolated microalga Haematococcus pluvialis JNU35. Algal Res. 2019, 39, 101466. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Z.; Xu, X.; Cheng, J.; Chen, S.; Tian, J.; Yang, W.; Crocker, M. Simultaneous promotion of photosynthesis and astaxanthin accumulation during two stages of Haematococcus pluvialis with ammonium ferric citrate. Sci. Total Environ. 2020, 750, 141689. [Google Scholar] [CrossRef] [PubMed]
- Rushan, N.H.; Said, F.M. The effect of culture medium on the oil yield and fatty acid methyl ester of freshwater microalgae Chlorella vulgaris. Chem. Eng. Commun. 2021, 208, 592–600. [Google Scholar] [CrossRef]
- Byeon, H.; An, Y.; Kim, T.; Rayamajhi, V.; Lee, J.; Shin, H.; Jung, S. Effects of Four Organic Carbon Sources on the Growth and Astaxanthin Accumulation of Haematococcus lacustris. Life 2024, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Cheng, J.; Li, K.; Yang, Z.; Zhou, J.; Cen, K. Enhancing the growth rate and astaxanthin yield of Haematococcus pluvialis by nuclear irradiation and high concentration of carbon dioxide stress. Bioresour. Technol. 2016, 204, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Goksan, T.; Gokpinar, S. An alternative approach to the traditional mixotrophic cultures of Haematococcus pluvialis Flotow (Chlorophyceae). J. Microbiol. Biotechnol. 2010, 20, 1276–1282. [Google Scholar] [CrossRef]
- Liu, L.; Ying, K.; Wu, K.; Tang, S.; Zhou, J.; Cai, Z. Promoting the Growth of Haematococcus lacustris under High Light In-tensity through the Combination of Light/Dark Cycle and Light Color. J. Mar. Sci. Eng. 2022, 10, 839. [Google Scholar] [CrossRef]
- Thanh-Tri, D.; Bich-Huy, T.T.; Binh-Nguyen, O.; Tuan-Loc, L.; Thanh-Cong, N.; Quoc-Dang, Q.; Thuong-Chi, L.; Dai-Long, T.; Melkonian, M.; Hoang-Dung, T. Effects of red and blue light emitting diodes on biomass and astaxanthin of Haematococcus pluvialis in pilot scale angled twin-layer porous substrate photobioreactors. Vietnam J. Sci. Technol. Eng. 2022, 63, 81–88. [Google Scholar]
- Li, K.; Ye, Q.; Li, Q.; Xia, R.; Guo, W.; Cheng, J. Effects of the spatial and spectral distribution of red and blue light on Haematococcus pluvialis growth. Algal Res.-Biomass Biofuels Bioprod. 2020, 51, 102045. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Wantip, K.; Chai-issarapap, N.; Maneechote, W.; Pekkoh, J.; Duangjan, K.; Ruangrit, K.; Pumas, C.; Pathom-aree, W.; Srinuanpan, S. Enhanced production of astaxanthin and co-bioproducts from microalga Haematococcus sp. integrated with valorization of industrial wastewater under two-stage LED light illumination strategy. Environ. Technol. Innov. 2022, 28, 102620. [Google Scholar] [CrossRef]
- Ma, R.; Thomas-Hall, S.R.; Chua, E.T.; Eltanahy, E.; Netzel, M.E.; Netzel, G.; Lu, Y.; Schenk, P.M. Blue Light Enhances Astaxanthin Biosynthesis Metabolism and Extraction Efficiency in Haematococcus Pluvialis by Inducing Haematocyst Ger-mination. Algal Res. 2018, 35, 215–222. [Google Scholar] [CrossRef]
- Kaewpintong, K.; Shotipruk, A.; Powtongsook, S.; Pavasant, P. Photoautotrophic High-Density Cultivation of Vegetative Cells of Haematococcus Pluvialis in Airlift Bioreactor. Bioresour. Technol. 2007, 98, 288–295. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, B.; Hu, Q.; Sommerfeld, M.; Li, Y.; Han, D. A new paradigm for producing astaxanthin from the unicellular green alga Haematococcus pluvialis. Biotechnol. Bioeng. 2016, 113, 2088–2099. [Google Scholar] [CrossRef]
- Oncel, S.S.; Imamoglu, E.; Gunerken, E.; Sukan, F.V. Comparison of different cultivation modes and light intensities using mono-cultures and co-cultures of Haematococcus pluvialis and Chlorella zofingiensis. J. Chem. Technol. Biotechnol. 2011, 86, 414–420. [Google Scholar] [CrossRef]
- Wen, X.; Wang, Z.; Ding, Y.; Geng, Y.; Li, Y. Enhancing the production of astaxanthin by mixotrophic cultivation of Haematococcus pluvialis in open raceway ponds. Aquac. Int. 2020, 28, 625–638. [Google Scholar] [CrossRef]
- Lam, T.P.; Lee, T.M.; Chen, C.Y.; Chang, J.S. Strategies to control biological contaminants during microalgal cultivation in open ponds. Bioresour. Technol. 2018, 252, 180–187. [Google Scholar] [CrossRef]
- Hu, J.; Nagarajan, D.; Zhang, Q.; Chang, J.S.; Lee, D.J. Heterotrophic cultivation of microalgae for pigment production: A review. Biotechnol. Adv. 2018, 36, 54–67. [Google Scholar] [CrossRef]
- Middelburg, J.J.; Nieuwenhuize, J. Nitrogen uptake by heterotrophic bacteria and phytoplankton in the nitrate-rich Thames estuary. Mar. Ecol. Prog. Progress. Ser. 2000, 203, 13–21. [Google Scholar] [CrossRef]
- Deschênes, J.S.; Boudreau, A.; Tremblay, R. Mixotrophic production of microalgae in pilot-scale photobioreactors: Practicability and process considerations. Algal Res. 2015, 10, 80–86. [Google Scholar] [CrossRef]
- Zhao, K.; Li, Y.; Yan, H.; Hu, Q.; Han, D. Regulation of Light Spectra on Cell Division of the Unicellular Green Al-ga Haematococcus pluvialis: Insights from Physiological and Lipidomic Analysis. Cells 2022, 11, 1956. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, L.; Yamada, H.; Katsuda, T.; Yamaji, H. Effects of temperature on the astaxanthin productivity and light harvesting characteristics of the green alga Haematococcus pluvialis. J. Biosci. Bioeng. 2015, 119, 345–350. [Google Scholar] [CrossRef]
- Diaz-MacAdoo, D.; Mata, M.T.; Riquelme, C. Influence of Irradiance and Wavelength on the Antioxidant Activity and Carotenoids Accumulation in Muriellopsis sp. Isolated from the Antofagasta Coastal Desert. Molecules 2022, 27, 2412. [Google Scholar] [CrossRef]



| Laboratory Culture | Outdoor Culture | |
|---|---|---|
| Biomass (g L−1 DW) | 0.71 ± 0.020 * | 0.51 ± 0.130 |
| Chl a (μg mL−1) | 0.110 ± 0.010 | 0.090 ± 0.012 |
| Chl b (μg mL−1) | 0.112 ± 0.024 | 0.090 ± 0.014 |
| Total carotenoid content (μg mL−1) | 1.125 ± 0.011 * | 0.841 ± 0.240 |
| Astaxanthin content (μg mL−1) | 0.752 ± 0.014 * | 0.521 ± 0.171 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, Y.; Kim, T.; Byeon, H.; Rayamajhi, V.; Lee, J.; Jung, S.; Shin, H. Improved Production of Astaxanthin from Haematococcus pluvialis Using a Hybrid Open–Closed Cultivation System. Appl. Sci. 2024, 14, 1104. https://doi.org/10.3390/app14031104
An Y, Kim T, Byeon H, Rayamajhi V, Lee J, Jung S, Shin H. Improved Production of Astaxanthin from Haematococcus pluvialis Using a Hybrid Open–Closed Cultivation System. Applied Sciences. 2024; 14(3):1104. https://doi.org/10.3390/app14031104
Chicago/Turabian StyleAn, Yunji, Taesoo Kim, Huijeong Byeon, Vijay Rayamajhi, Jihyun Lee, SangMok Jung, and HyunWoung Shin. 2024. "Improved Production of Astaxanthin from Haematococcus pluvialis Using a Hybrid Open–Closed Cultivation System" Applied Sciences 14, no. 3: 1104. https://doi.org/10.3390/app14031104
APA StyleAn, Y., Kim, T., Byeon, H., Rayamajhi, V., Lee, J., Jung, S., & Shin, H. (2024). Improved Production of Astaxanthin from Haematococcus pluvialis Using a Hybrid Open–Closed Cultivation System. Applied Sciences, 14(3), 1104. https://doi.org/10.3390/app14031104







