Computational Design of a Novel Dithranol–Salicylic Acid Antipsoriatic Prodrug for Esterase-Activated Topical Drug Delivery
Abstract
1. Introduction
- Red patches: the affected areas of the skin are red and inflamed.
- Silvery scales: overlying the red patches, there are often silvery or white scales.
- Itching and discomfort: psoriasis plaques can be itchy, and the skin may feel sore or painful.
- Nail changes: psoriasis can also affect the nails, causing changes such as pitting, discoloration, and separation from the nail bed.
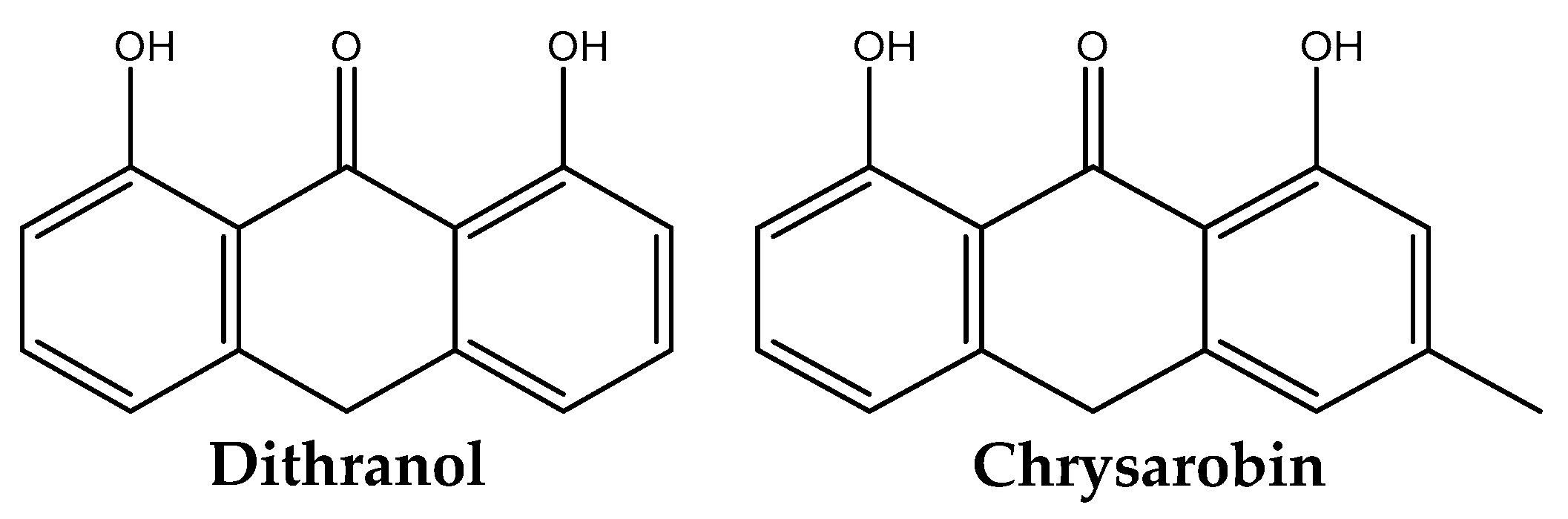
- Topical treatments: these include corticosteroid creams, vitamin D analogues, retinoids, dithranol, coal tar, and moisturizers.
- Phototherapy: exposure to ultraviolet light can be beneficial for some individuals with psoriasis.
- Systemic medications: In cases of moderate-to-severe psoriasis, oral or injectable medications (e.g., methotrexate) can be used.
- Biologics: these are a newer class of medications that target specific components of the immune system involved in the development of psoriasis (e.g., IL 12/23 blocker–ustekinumab and anti-IL-17–secukinumab).
2. Computational Methods
3. Results and Discussion
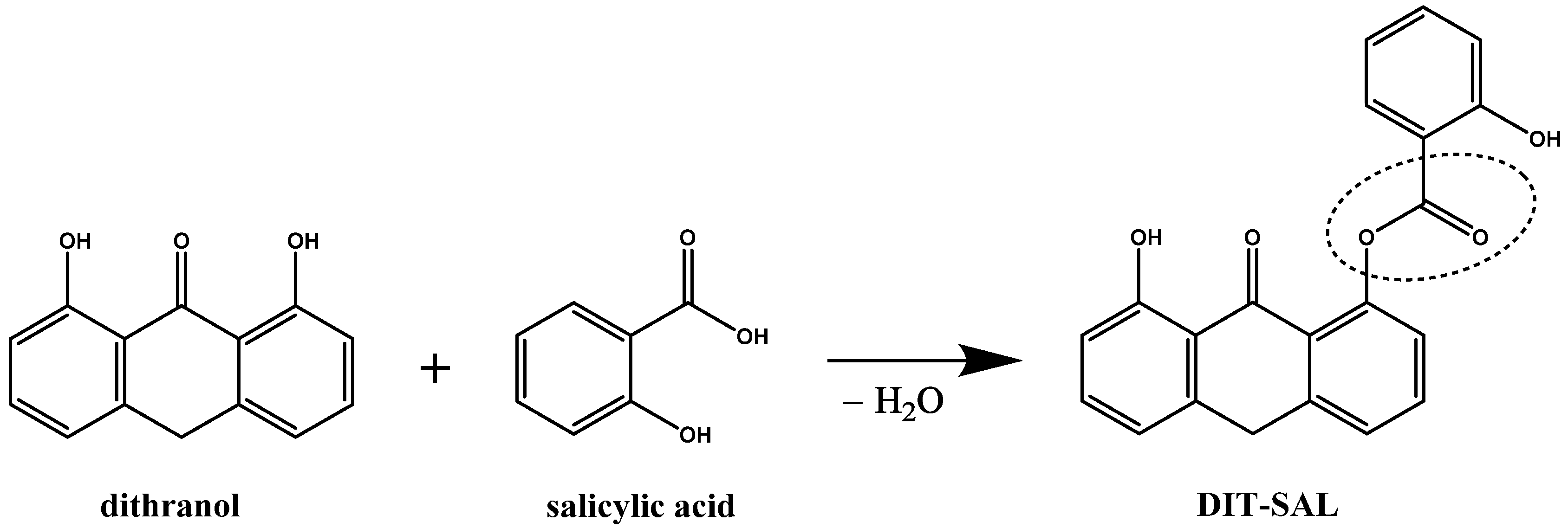
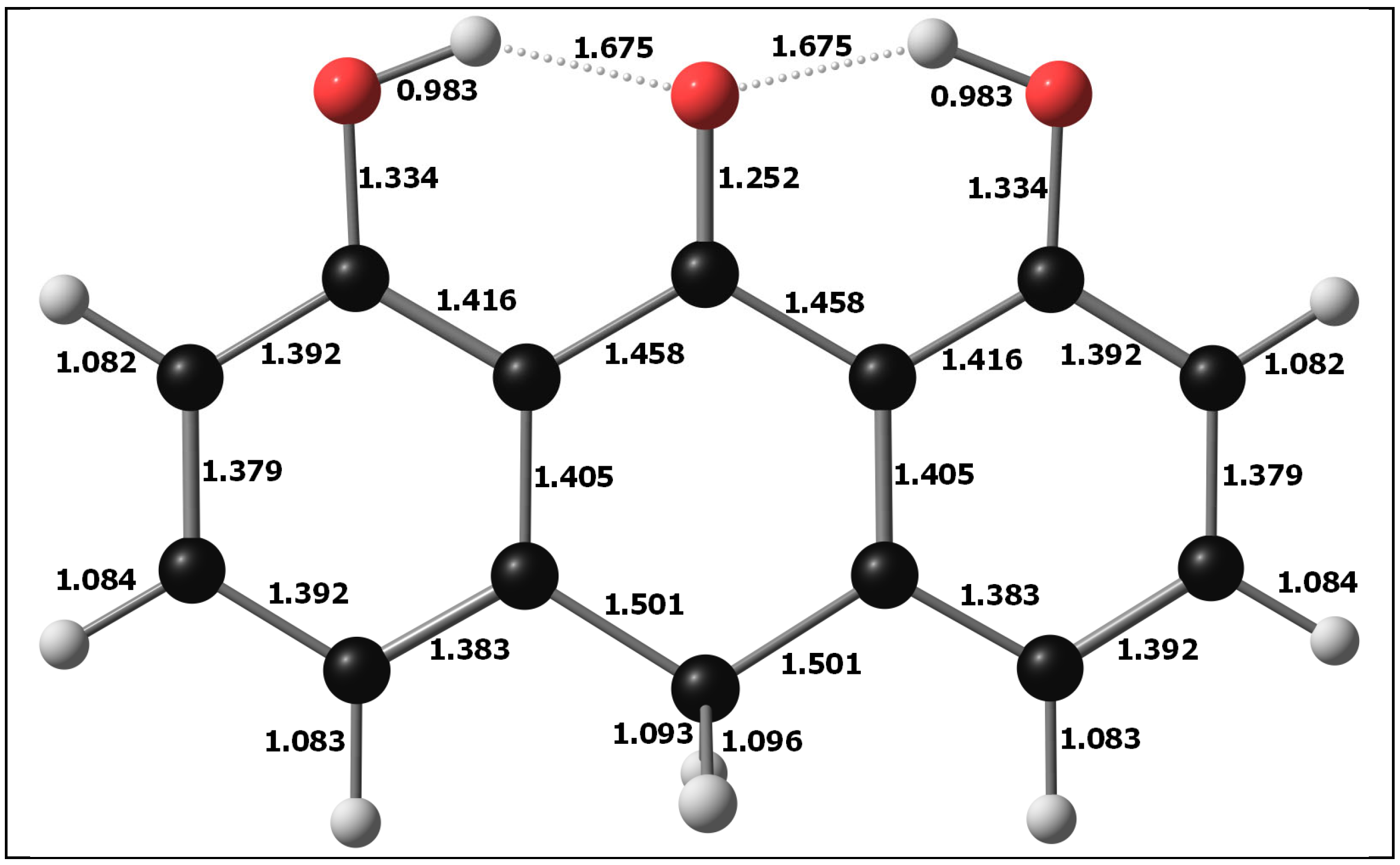
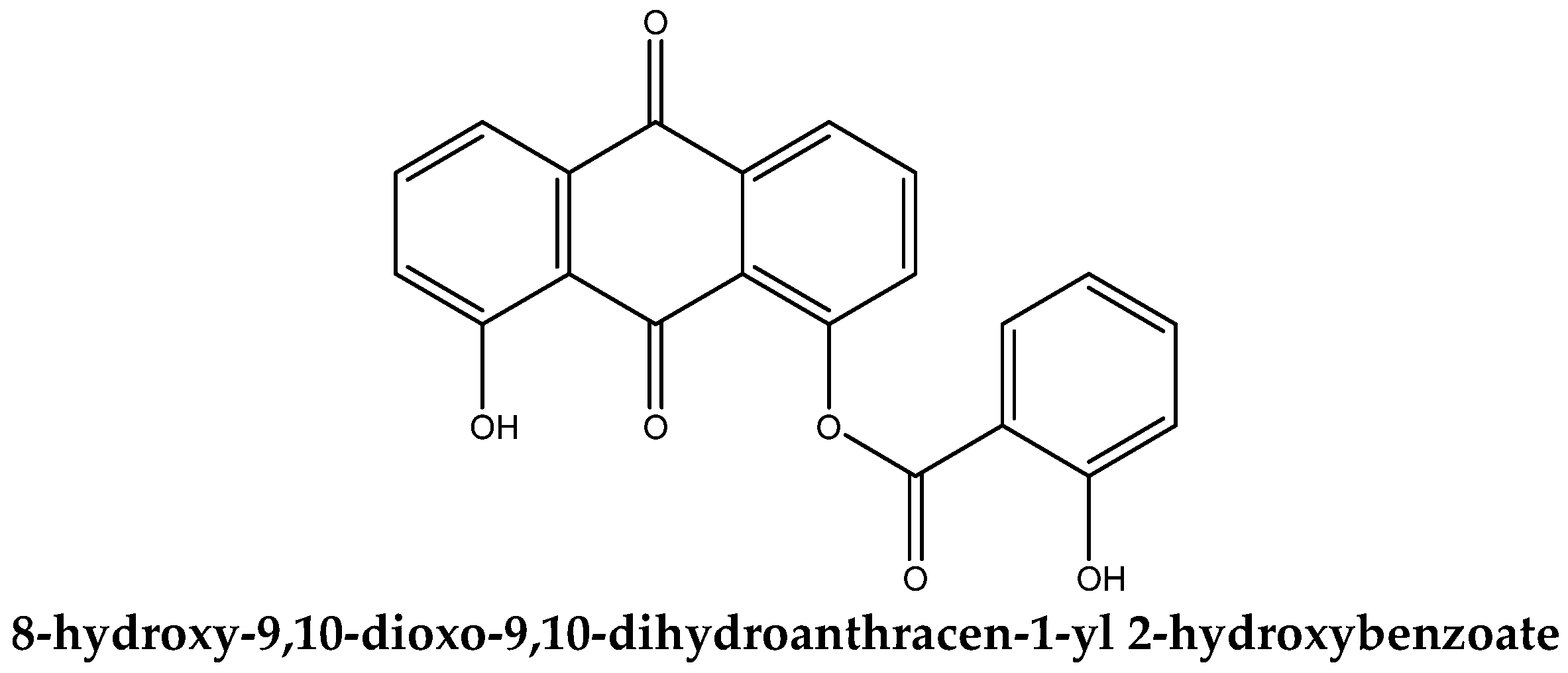
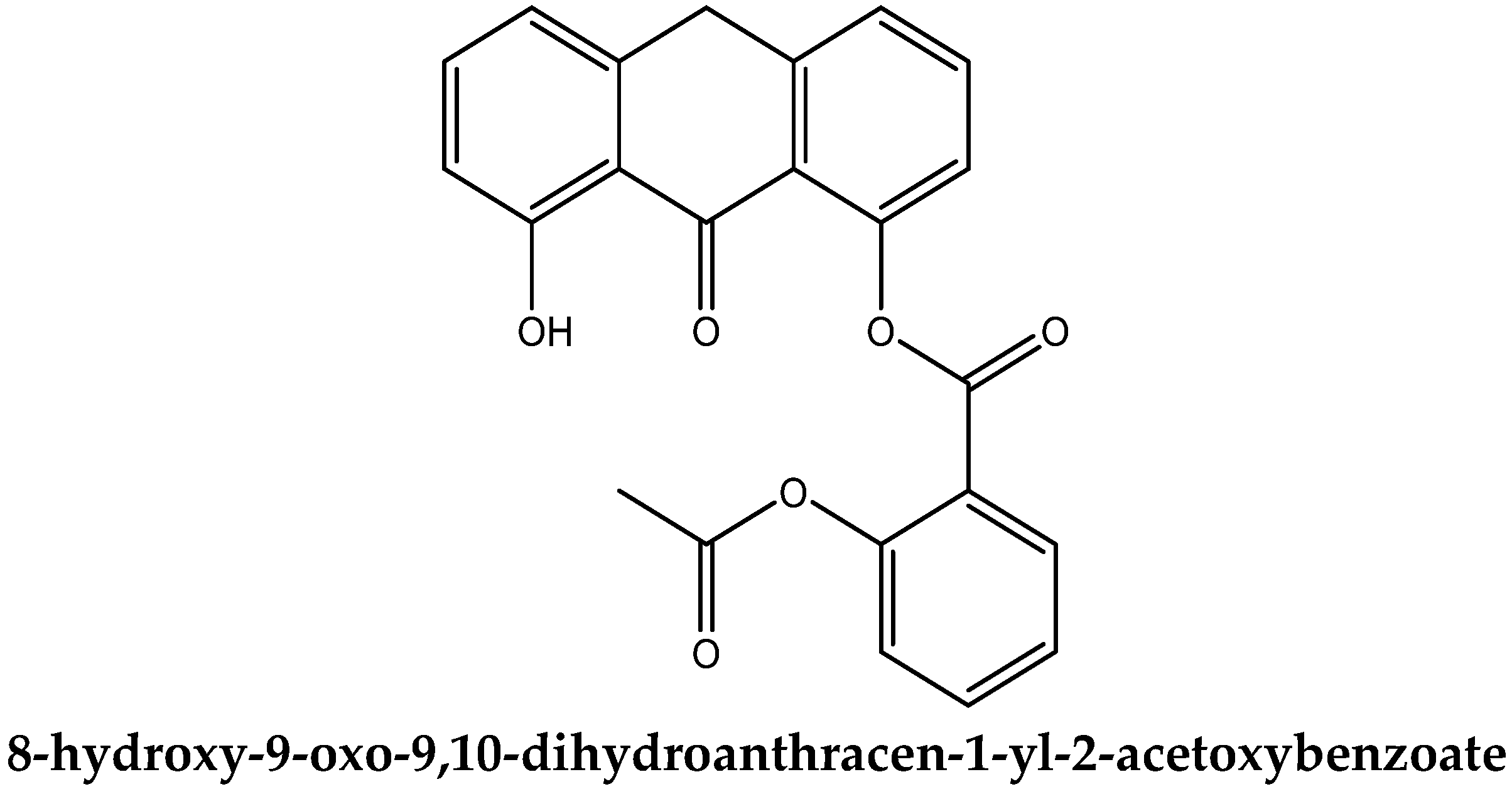
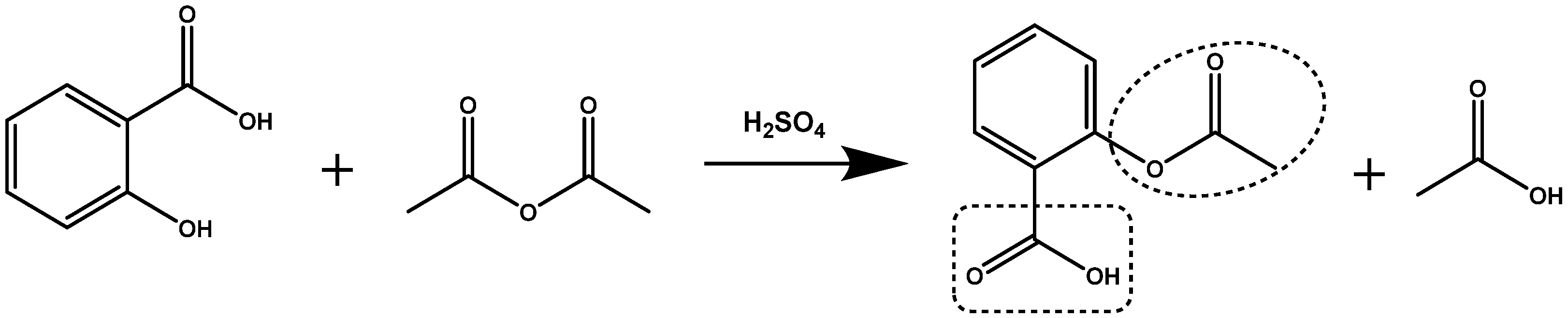
3.1. Molecular Structure of DIT-SAL
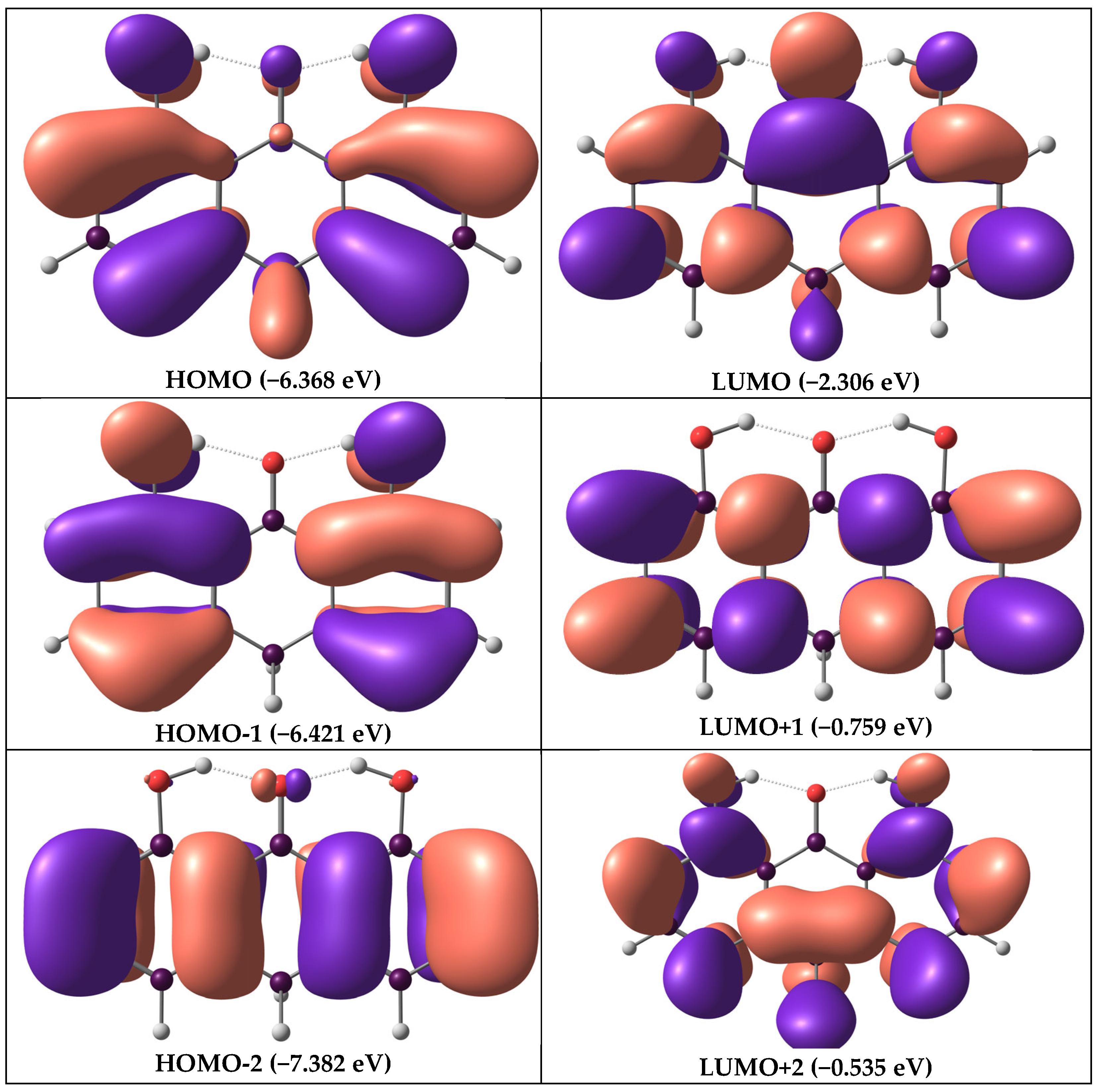
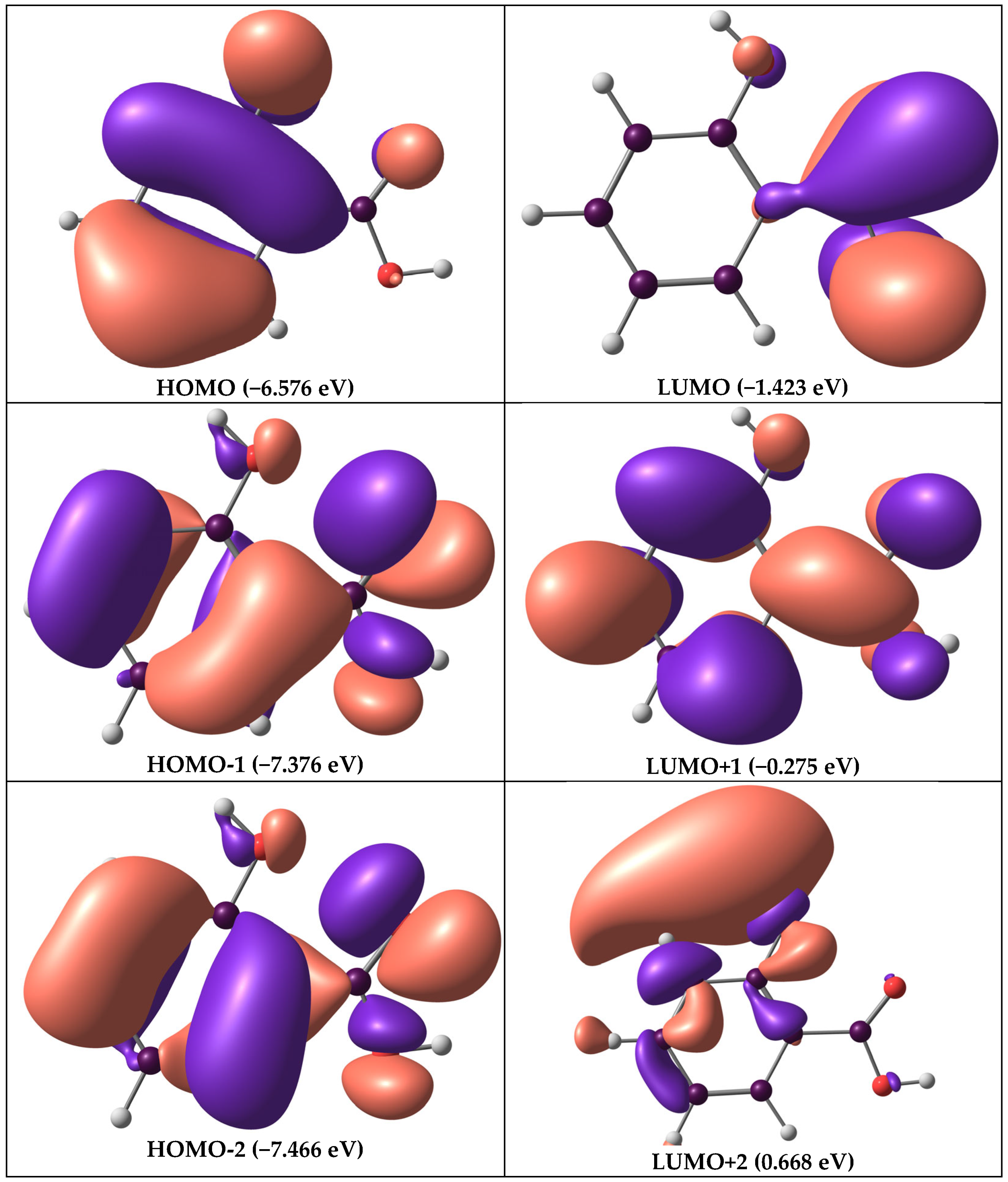
3.2. Frontier Molecular Orbitals
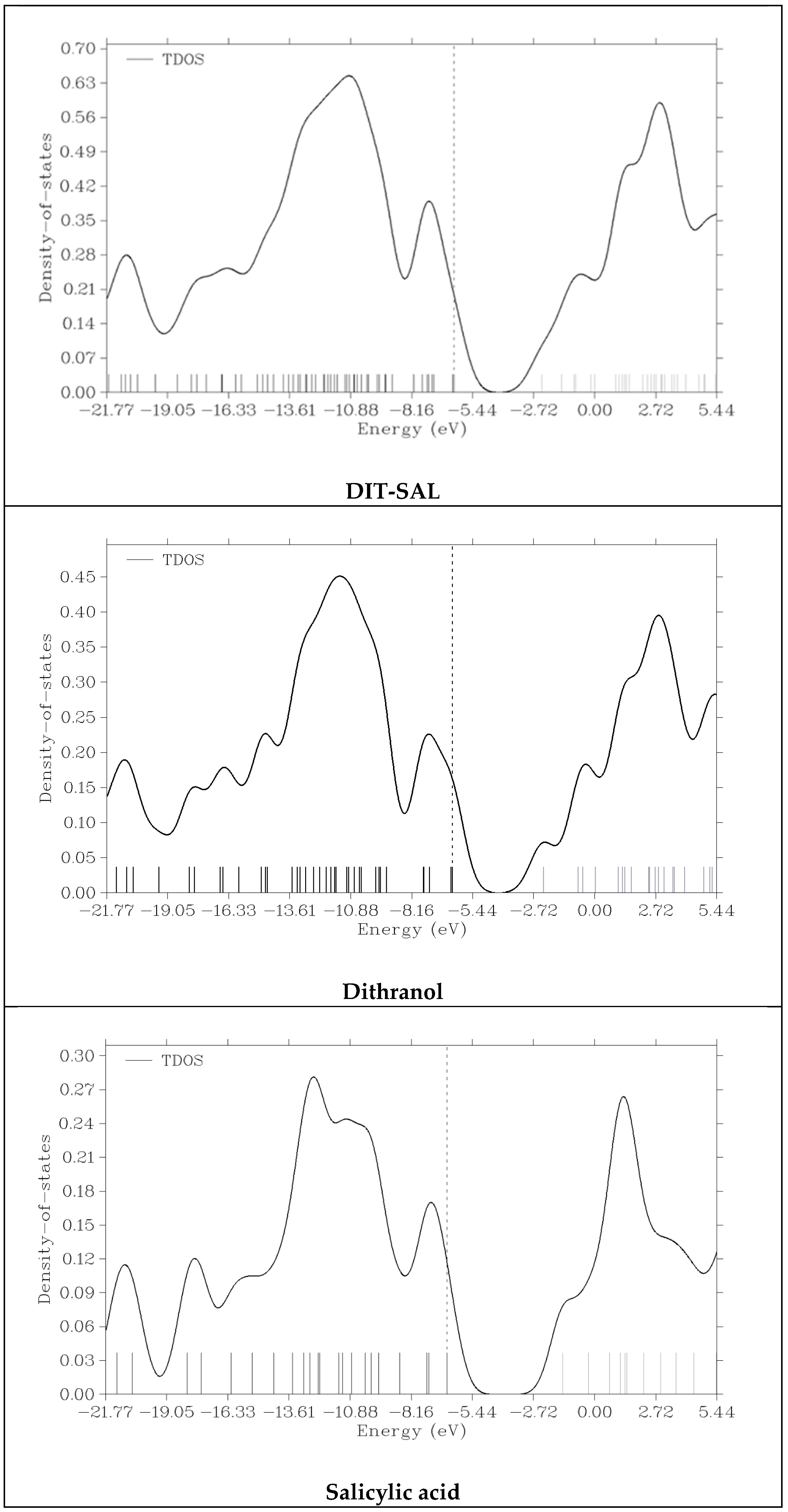
3.3. TDOS (Total Density of States) Calculation
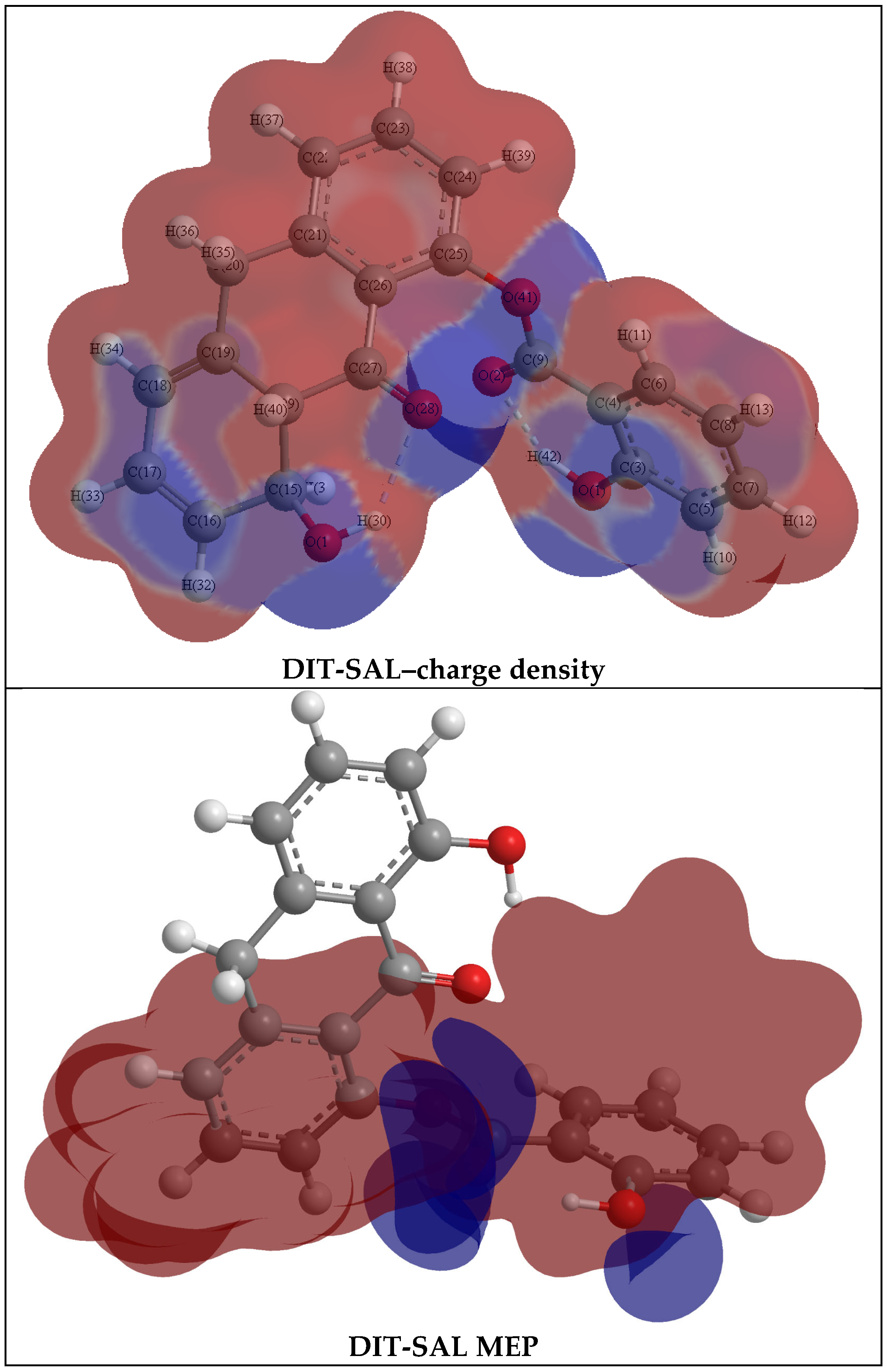
3.4. The Molecular Electrostatic Potential (MEP)
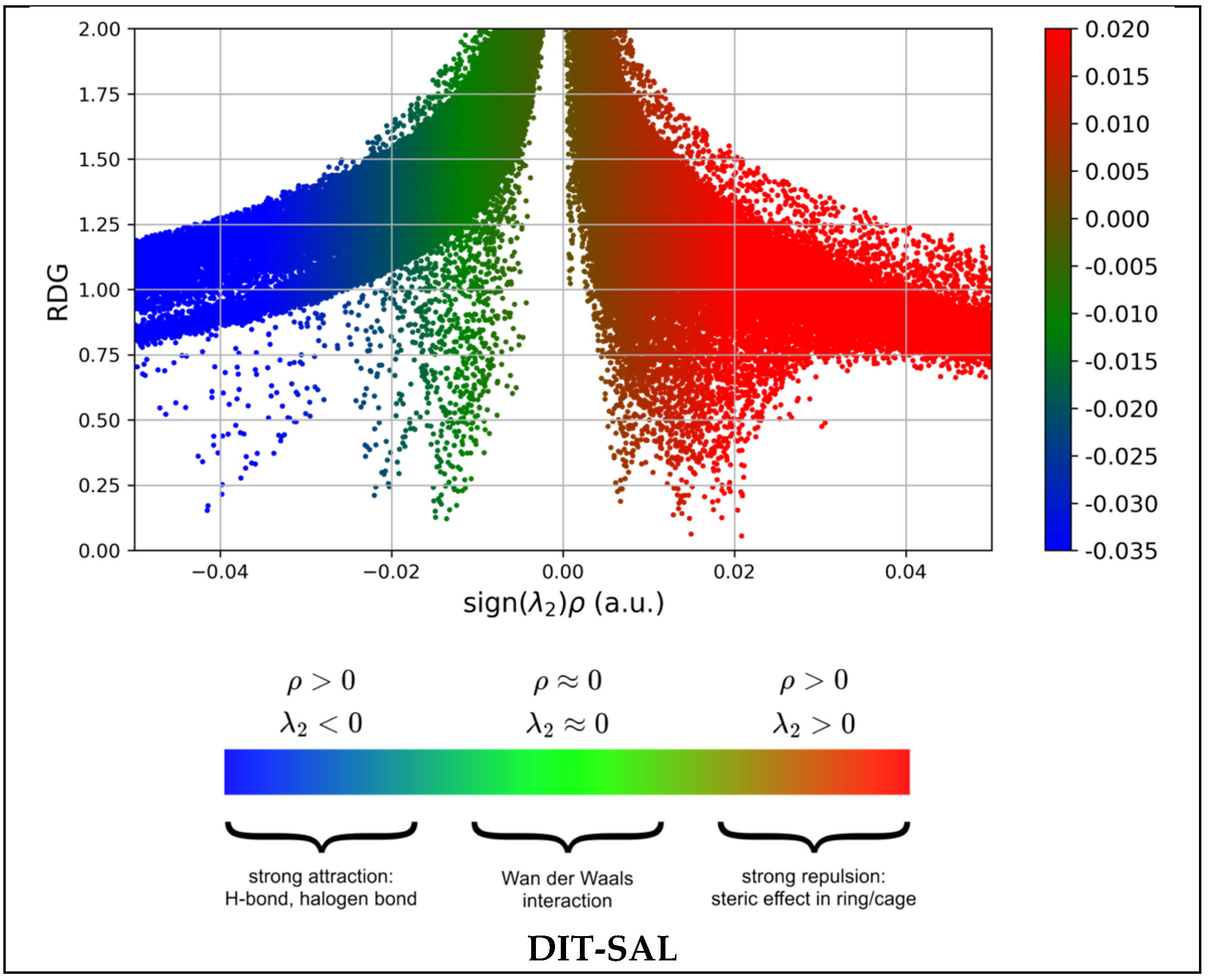
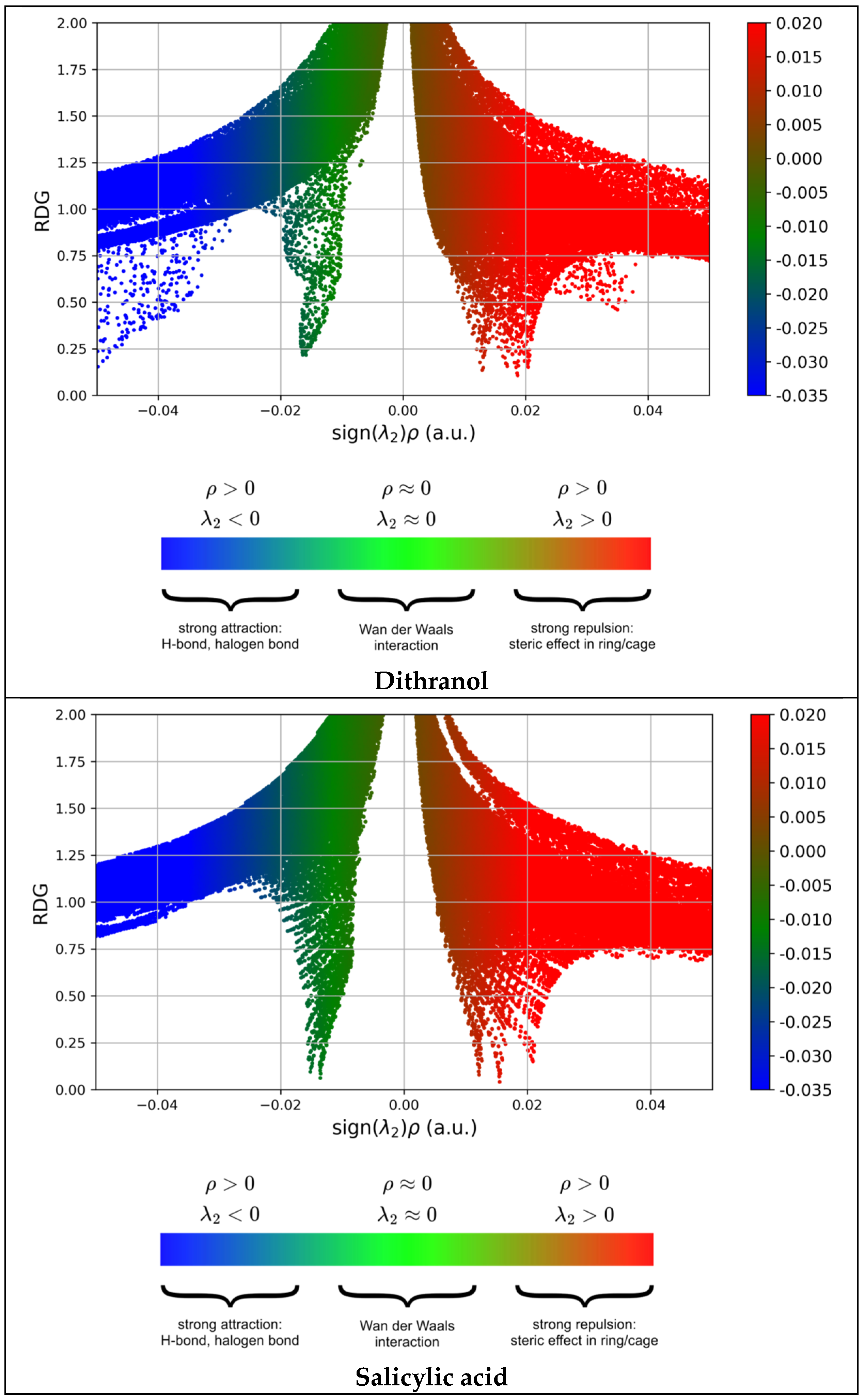
3.5. Non-Covalent Interaction-Reduced Density Gradient (NCI-RDG)
- (sign λ2)ρ > 0: repulsive/steric effects;
- (sign λ2)ρ < 0: attractive interactions (hydrogen bonding);
- (sign λ2)ρ ≃ 0: van der Waals forces, which arise from overlapping electron clouds and occur over larger distances. The colored RDG scatter plots in Figure 16 were prepared using Multiwfn 3.7 software and plotted using Gnuplot version 5.4.
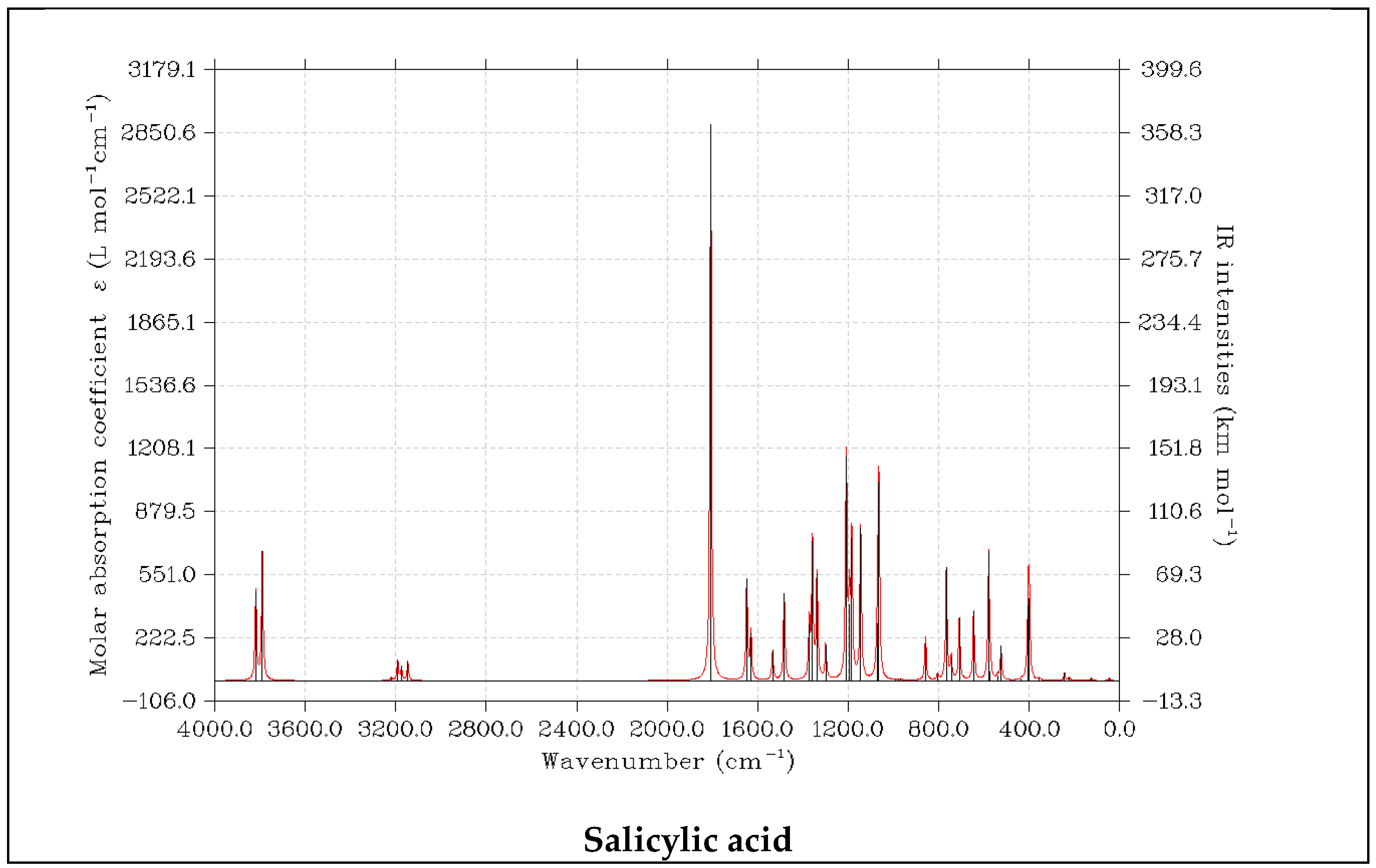
3.6. UV-VIS Spectra of DIT-SAL Degradation Products
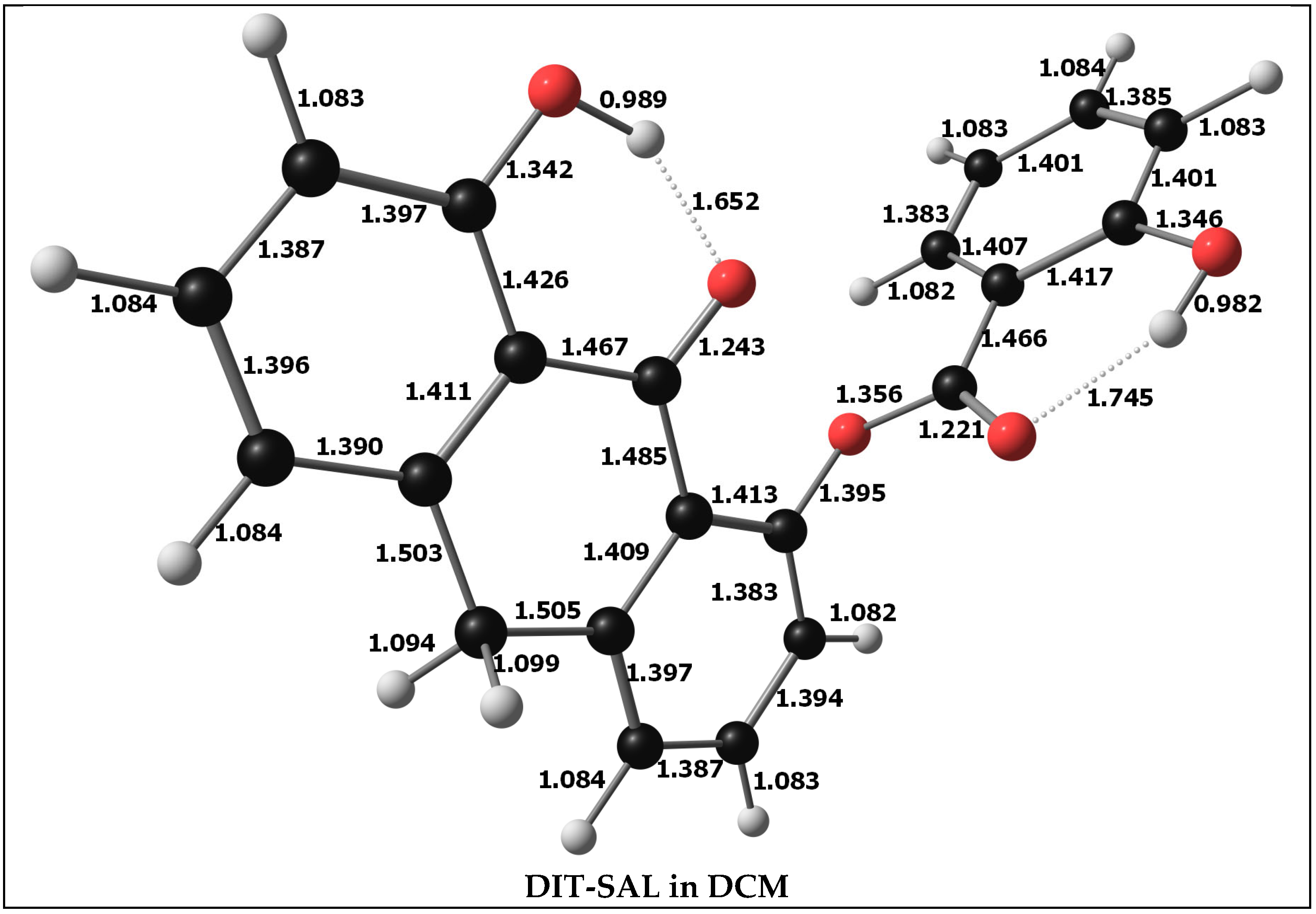
3.7. Structure of DIT-SAL in Dichloromethane
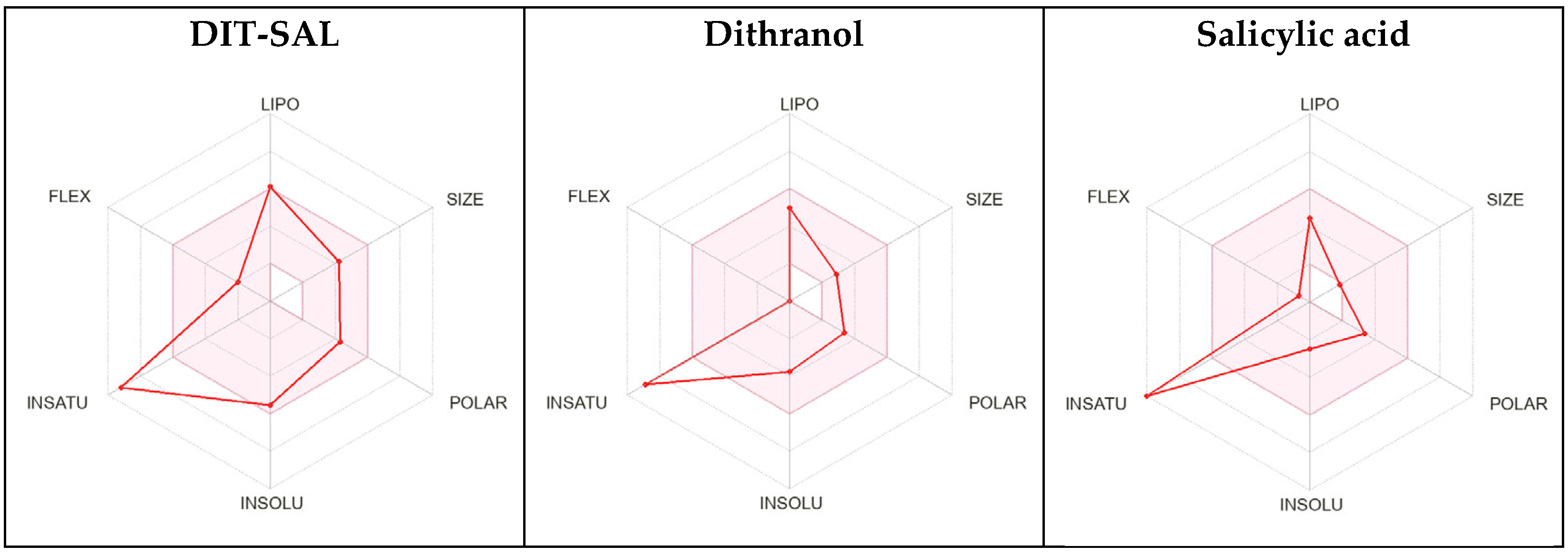
3.8. ADME In Silico Modelling of DIT-SAL
3.9. Further Molecular Alternatives to DIT-SAL for Therapy of Psoriasis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kocaaga, A.; Kocaaga, M. Psoriasis: An Immunogenetic Perspective. Glob. Med. Genet. 2022, 9, 82–89. [Google Scholar] [CrossRef]
- Šimaljaková, M.; Buchvald, D. Dermatovenereology; Comenius University Press: Bratislava, Slovakia, 2019. [Google Scholar]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Šimaljaková, M. Psoriasis—Etiopathogenesis, clinical picture and current therapy options. Dermatol. Prax 2008, 2, 50–55. [Google Scholar]
- Ali, Z.; Robert Zibert, J.; Dahiya, P.; Bachdal Johansen, C.B.; Grønlund Holm, J.; Ravn Jørgensen, A.H.; Manole, J.; Suru, A.; Egeberg, A.; Francis Thomsen, S.; et al. Mild-to-moderate severity of psoriasis may be assessed remotely based on photographs and self-reported extent of skin involvement. JAAD Int. 2023, 11, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Jin, H. Research progress of metabolomics in psoriasis. Chin. Med. J. 2023, 136, 1805–1816. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Mohammed, A.A.; Algahtani, M.S.; Mishra, A.; Ahmad, J. Nanoscale Topical Pharmacotherapy in Management of Psoriasis: Contemporary Research and Scope. J. Funct. Biomater. 2022, 14, 19. [Google Scholar] [CrossRef]
- Babincová, N.; Jirsák, O.; Babincová, M.; Babinec, P.; Šimaljaková, M. Remote magnetically controlled drug release from electrospun composite nanofibers: Design of a smart platform for therapy of psoriasis. Z. Naturforsch. 2020, 75, 587–591. [Google Scholar] [CrossRef]
- Andrýsková, N.; Sourivong, P.; Babincová, M.; Šimaljaková, M. Controlled Release of Tazarotene from Magnetically Responsive Nanofiber Patch: Towards More Efficient Topical Therapy of Psoriasis. Appl. Sci. 2021, 11, 11022. [Google Scholar] [CrossRef]
- Sehgal, V.N.; Verma, P.; Khurana, A. Anthralin/dithranol in dermatology. Int. J. Dermatol. 2014, 53, 449–460. [Google Scholar] [CrossRef]
- Ashton, R.E.; Andre, P.; Lowe, N.J.; Whitefield, M. Anthralin: Historical and current perspectives. J. Am. Acad. Dermatol. 1983, 9, 173–192. [Google Scholar] [CrossRef]
- Seville, R.H. Advances in the use of anthralin. J. Am. Acad. Dermatol. 1981, 5, 319–321. [Google Scholar] [CrossRef]
- Kadian, V.; Kumar, S.; Saini, K.; Kakkar, V.; Rao, R. Dithranol: An Insight into its Novel Delivery Cargos for Psoriasis Management. Curr. Drug. Res. Rev. 2020, 12, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Andrýsková, N.; Sourivong, P.; Babincová, M.; Babinec, P.; Šimaljaková, M. Electrospun PCL/PVA Coaxial Nanofibers with Embedded Titanium Dioxide and Magnetic Nanoparticles for Stabilization and Controlled Release of Dithranol for Therapy of Psoriasis. Magnetochemistry 2023, 9, 187. [Google Scholar] [CrossRef]
- de Mare, S.; Calis, N.; den Hartog, G.; van Erp, P.E.; van de Kerkhof, P.C. The relevance of salicylic acid in the treatment of plaque psoriasis with dithranol creams. Skin Pharmacol. 1988, 1, 259–264. [Google Scholar] [CrossRef] [PubMed]
- van de Kerkhof, P.C.; van der Valk, P.G.; Swinkels, O.Q.; Kucharekova, M.; de Rie, M.A.; de Vries, H.J.; Damstra, R.; Oranje, A.P.; de Waard-van der Spek, F.B.; van Neer, P.; et al. A comparison of twice-daily calcipotriol ointment with once-daily short-contact dithranol cream therapy: A randomized controlled trial of supervised treatment of psoriasis vulgaris in a day-care setting. Br. J. Dermatol. 2006, 155, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Unna, P.G. Cignolin als Heilmittel der Psoriasis. Dermatol. Wochenschr. 1916, 7, 150–163. [Google Scholar]
- Benezeder, T.; Gehad, A.; Patra, V.; Clark, R.; Wolf, P. Induction of IL-1β and antimicrobial peptides as a potential mechanism for topical dithranol. Exp. Dermatol. 2021, 30, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Behrangi, E.; Roohaninasab, M.; Sadeghzadeh-Bazargan, A.; Najar Nobari, N.; Ghassemi, M.; Seirafianpour, F.; Goodarzi, A.; Dodangeh, M. A systematic review on the treatment of pediatric severe alopecia areata by topical immunotherapy or anthralin (contact sensitization) or low-level light/laser therapy (LLLT): Focus on efficacy, safety, treatment duration, recurrence, and follow-up based on clinical studies. J. Cosmet. Dermatol. 2022, 21, 2727–2741. [Google Scholar] [CrossRef] [PubMed]
- Hindson, C.; Diffey, B.; Lawlor, F.; Downey, A. Dithranol-UV-A phototherapy (DUVA) for psoriasis: A treatment without dressings. Br. J. Dermatol. 1983, 108, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Kussini, J.; Charalambous, A.; Sachsenweger, F.; Steinert, M. Successful removal of anthralin staining from facial skin. Int. J. Dermatol. 2023, 62, 21–22. [Google Scholar] [CrossRef] [PubMed]
- Thoma, K.; Holzmann, C. Photostability of dithranol. Eur. J. Pharm. Biopharm. 1998, 46, 201–208. [Google Scholar] [CrossRef]
- Savian, A.L.; Rodrigues, D.; Weber, J.; Ribeiro, R.F.; Motta, M.H.; Schaffazick, S.R.; Adams, A.I.; de Andrade, D.F.; Beck, R.C.; da Silva, C.B. Dithranol-loaded lipid-core nanocapsules improve the photostability and reduce the in vitro irritation potential of this drug. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 46, 69–76. [Google Scholar] [CrossRef]
- Melo, T.S.; Dubertret, L.; Prognon, P.; Gond, A.; Mahuzier, G.; Santus, R. Physicochemical properties and stability of anthralin in model systems and human skin. J. Investig. Dermatol. 1983, 80, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mahrle, G.; Bonnekoh, B.; Ghyczy, M.; Wiegrebe, W. Stability of anthralin in liposomal phospholipids. Arch. Dermatol. Res. 1991, 283, 483–484. [Google Scholar] [CrossRef][Green Version]
- Herman, J.; Remon, J.P.; De Bersagues, J. Influence of storage conditions on the stability of anthralin in the presence of coal tar and salicylic acid in a white soft paraffin base. J. Am. Acad. Dermatol. 1988, 18, 750–751. [Google Scholar] [CrossRef]
- Kruszewski, F.H.; DiGiovanni, J. Alterations in epidermal polyamine levels and DNA synthesis following topical treatment with chrysarobin in SENCAR mice. Cancer Res. 1988, 48, 6390–6395. [Google Scholar]
- Müller, K. Antipsoriatic anthrones: Aspects of oxygen radical formation, challenges and prospects. Gen. Pharmacol. 1996, 27, 1325–1335. [Google Scholar] [CrossRef]
- Müller, K.; Gawlik, I. Novel 10-substituted antipsoriatic anthrones as inhibitors of epidermal 12-lipoxygenase and lipid peroxidation in membranes. Biochem. Pharmacol. 1995, 50, 2077–2083. [Google Scholar] [CrossRef]
- Müller, K.; Gürster, D.; Piwek, S.; Wiegrebe, W. Antipsoriatic anthrones with modulated redox properties. 1. Novel 10-substituted 1,8-dihydroxy-9(10H)-anthracenones as inhibitors of 5-lipoxygenase. J. Med. Chem. 1993, 36, 4099–4107. [Google Scholar] [CrossRef]
- Müller, K.; Leukel, P.; Ziereis, K.; Gawlik, I. Antipsoriatic anthrones with modulated redox properties. 2. Novel derivatives of chrysarobin and isochrysarobin--antiproliferative activity and 5-lipoxygenase inhibition. J. Med. Chem. 1994, 37, 1660–1669. [Google Scholar] [CrossRef]
- Prinz, H.; Wiegrebe, W.; Müller, K. Syntheses of Anthracenones. 3. Revised Preparative Route to 10-Benzoyl-1,8-dihydroxy-9(10H)-anthracenones. J. Org. Chem. 1996, 61, 2861–2864. [Google Scholar] [CrossRef]
- Müller, K.; Reindl, H. Cornified envelope formation by anthralin, simple analogues, and related anthracenones. Arch. Pharm. 2001, 334, 86–92. [Google Scholar] [CrossRef]
- Arif, T. Salicylic acid as a peeling agent: A comprehensive review. Clin. Cosmet. Investig. Dermatol. 2015, 8, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Lavis, L.D. Ester bonds in prodrugs. ACS. Chem. Biol. 2008, 3, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Liederer, B.M.; Borchardt, R.T. Enzymes involved in the bioconversion of ester-based prodrugs. J. Pharm. Sci. 2006, 95, 1177–1195. [Google Scholar] [CrossRef]
- Lau, W.M.; White, A.W.; Heard, C.M. Topical delivery of a naproxen-dithranol co-drug: In vitro skin penetration, permeation, and staining. Pharm. Res. 2010, 27, 2734–2742. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.M.; Heard, C.M.; White, A.W. Design, synthesis and in vitro degradation of a novel co-drug for the treatment of psoriasis. Pharmaceutics 2013, 5, 232–245. [Google Scholar] [CrossRef]
- Hsieh, P.W.; Aljuffali, I.A.; Fang, C.L.; Chang, S.H.; Fang, J.Y. Hydroquinone-salicylic acid conjugates as novel anti-melasma actives show superior skin targeting compared to the parent drugs. J. Dermatol. Sci. 2014, 76, 120–131. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09, Revision, D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013.
- Frisch, A.E.; Keith, T.A.; Dennington, R.D. GaussView Reference; Semichem, Inc.: Irvine, CA, USA, 2003. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An Integrated Online Platform for Accurate and Comprehensive Predictions of ADMET Properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef] [PubMed]
- Rubab, S.L.; Raza, A.R.; Nisar, B.; Ashfaq, M.; Altaf, Y.; Hussain, R.; Sajjad, N.; Akram, M.S.; Tahir, M.N.; Shaheen, M.A.; et al. Synthesis, Crystal Structure, DFT Calculations, Hirshfeld Surface Analysis and In Silico Drug-Target Profiling of (R)-2-(2-(1,3-Dioxoisoindolin-2-yl)propanamido)benzoic Acid Methyl Ester. Molecules 2023, 28, 4375. [Google Scholar] [CrossRef] [PubMed]
- Assad, M.; Paracha, R.N.; Siddique, A.B.; Shaheen, M.A.; Ahmad, N.; Mustaqeem, M.; Kanwal, F.; Mustafa, M.Z.U.; Rehman, M.F.U.; Fatima, S.; et al. In Silico and In Vitro Studies of 4-Hydroxycoumarin-Based Heterocyclic Enamines as Potential Anti-Tumor Agents. Molecules 2023, 28, 5828. [Google Scholar] [CrossRef] [PubMed]
- Akman, F.; Demirpolat, A.; Kazachenko, A.S.; Kazachenko, A.S.; Issaoui, N.; Al-Dossary, O. Molecular Structure, Electronic Properties, Reactivity (ELF, LOL, and Fukui), and NCI-RDG Studies of the Binary Mixture of Water and Essential Oil of Phlomis bruguieri. Molecules 2023, 28, 2684. [Google Scholar] [CrossRef] [PubMed]
- Kazachenko, A.S.; Tanış, E.; Akman, F.; Medimagh, M.; Issaoui, N.; Al-Dossary, O.; Bousiakou, L.G.; Kazachenko, A.S.; Zimonin, D.; Skripnikov, A.M. A Comprehensive Study of N-Butyl-1H-Benzimidazole. Molecules 2022, 27, 7864. [Google Scholar] [CrossRef] [PubMed]
- Akman, F. Spectroscopic investigation, HOMO–LUMO energies, natural bond orbital (NBO) analysis and thermodynamic properties of two-armed macroinitiator containing coumarin with DFT quantum chemical calculations. Can. J. Phys. 2016, 94, 583–593. [Google Scholar] [CrossRef]
- Abraham, C.S.; Prasana, J.C.; Muthu, S. Quantum mechanical, spectroscopic and docking studies of 2-Amino-3-bromo-5-nitropyridine by Density Functional Method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 181, 153–163. [Google Scholar] [CrossRef]
- Arulaabaranam, K.; Muthu, S.; Mani, G.; Ben Geoffrey, A.S. Speculative assessment, molecular composition, PDOS, topology exploration (ELF, LOL, RDG), ligand-protein interactions, on 5-bromo-3-nitropyridine-2-carbonitrile. Heliyon 2021, 7, e07061. [Google Scholar] [CrossRef]
- Ahmed, F.R. The correct structural formula for anthralin. Acta Crystallogr. B 1980, 36, 3184–3186. [Google Scholar] [CrossRef]
- Andersen, K.B.; Spanget-Larsen, J. Electronic transitions and intramolecular hydrogen bonding in anthralin. UV_VIS linear dichroism spectroscopy and quantum chemical calculations. Spectrochim. Acta 1997, 53, 2615–2625. [Google Scholar] [CrossRef]
- Holder, A.J.; Upadrashta, S.M. A semiempirical computational investigation of the antipsoriatic drug anthralin. J. Pharm. Sci. 1992, 81, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E.S.; MacHale, L.T.; Szilagyi, R.K.; DuBois, J.L. How Chemical Environment Activates Anthralin and Molecular Oxygen for Direct Reaction. J. Org. Chem. 2020, 85, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Czerwinska, M.; Sikora, A.; Szajerski, P.; Zielonka, J.; Adamus, J.; Marcinek, A.; Piech, K.; Bednarek, P.; Bally, T. Anthralin: Primary products of its redox reactions. J. Org. Chem. 2006, 71, 5312–5319. [Google Scholar] [CrossRef] [PubMed]
- Maheshwary, S.; Lourderaj, U.; Sathyamurthy, N. Ab initio quantum chemical investigation of the ground and excited states of salicylic acid dimer. J. Phys. Chem. A 2006, 110, 12662–12669. [Google Scholar] [CrossRef] [PubMed]
- Raeker, T.; Hartke, B. Full-Dimensional Excited-State Intramolecular Proton Transfer Dynamics of Salicylic Acid. J. Phys. Chem. A 2017, 121, 5967–5977. [Google Scholar] [CrossRef]
- Suresh, S.; Gunasekaran, S.; Srinivasan, S. Spectroscopic (FT-IR, FT-Raman, NMR and UV-Visible) and quantum chemical studies of molecular geometry, Frontier molecular orbital, NLO, NBO and thermodynamic properties of salicylic acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 132, 130–141. [Google Scholar] [CrossRef]
- Yu, J.; Su, N.Q.; Yang, W. Describing Chemical Reactivity with Frontier Molecular Orbitalets. JACS 2022, 2, 1383–1394. [Google Scholar] [CrossRef]
- Ertl, P.; Gerebtzoff, G.; Lewis, R.; Muenkler, H.; Schneider, N.; Sirockin, F.; Stiefl, N.; Tosco, P. Chemical Reactivity Prediction: Current Methods and Different Application Areas. Mol. Inform. 2022, 41, 2100277. [Google Scholar] [CrossRef] [PubMed]
- Kohn, W.; Becke, A.D.; Parr, R.G. Density functional theory of electronic structure. J. Phys. Chem. 1996, 100, 12974–12980. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Pérez, P.; Contreras, R. Quantitative character-ization of the global electrophilicity power of common diene/dienophile pairs in Diels-Alder reactions. Tetrahedron 2002, 58, 4417–4423. [Google Scholar] [CrossRef]
- Contreras, P.; Seijas, L.; Osorio, D. TDOS quantum mechanical visual analysis for single molecules. Can. J. Pure Appl. Sci. 2021, 15, 5239–5245. [Google Scholar]
- Sjoberg, P.; Politzer, P. Use of the electrostatic potential at the molecular surface to interpret and predict nucleophilic processes. J. Phys. Chem. 1990, 94, 3959–3961. [Google Scholar] [CrossRef]
- Politzer, P.; Laurence, P.R.; Jayasuriya, K. Molecular electrostatic potentials: An effective tool for the elucidation of biochemical phenomena. Environ. Health Perspect. 1985, 61, 191–202. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Hobza, P. Calculations on noncovalent interactions and databases of benchmark interaction energies. Acc. Chem. Res. 2012, 45, 663–672. [Google Scholar] [CrossRef]
- Alqahtani, S. In silico ADME-Tox modeling: Progress and prospects. Expert. Opin. Drug Metab. Toxicol. 2017, 13, 1147–1158. [Google Scholar] [CrossRef]
- Filho, R.P.; Polli, M.C.; Filho, S.B.; Garcia, M.; Ferreira, E.I. Prodrugs available on the Brazilian pharmaceutical market and their corresponding bioactivation pathways. Braz. J. Pharm. Sci. 2010, 46, 393–420. [Google Scholar] [CrossRef]
- Montinari, M.R.; Minelli, S.; De Caterina, R. The first 3500 years of aspirin history from its roots—A concise summary. Vascul. Pharmacol. 2019, 113, 1–8. [Google Scholar] [CrossRef] [PubMed]


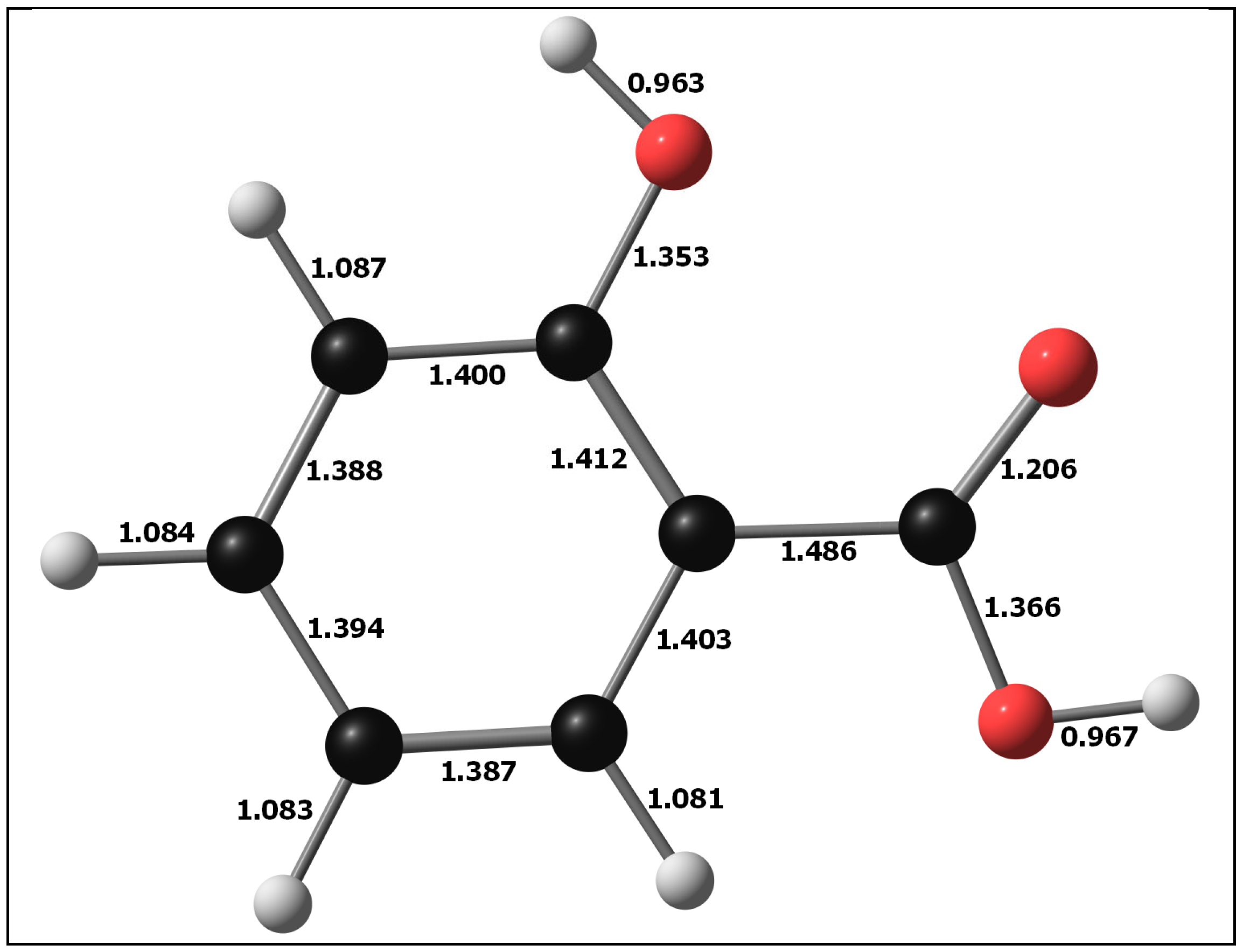
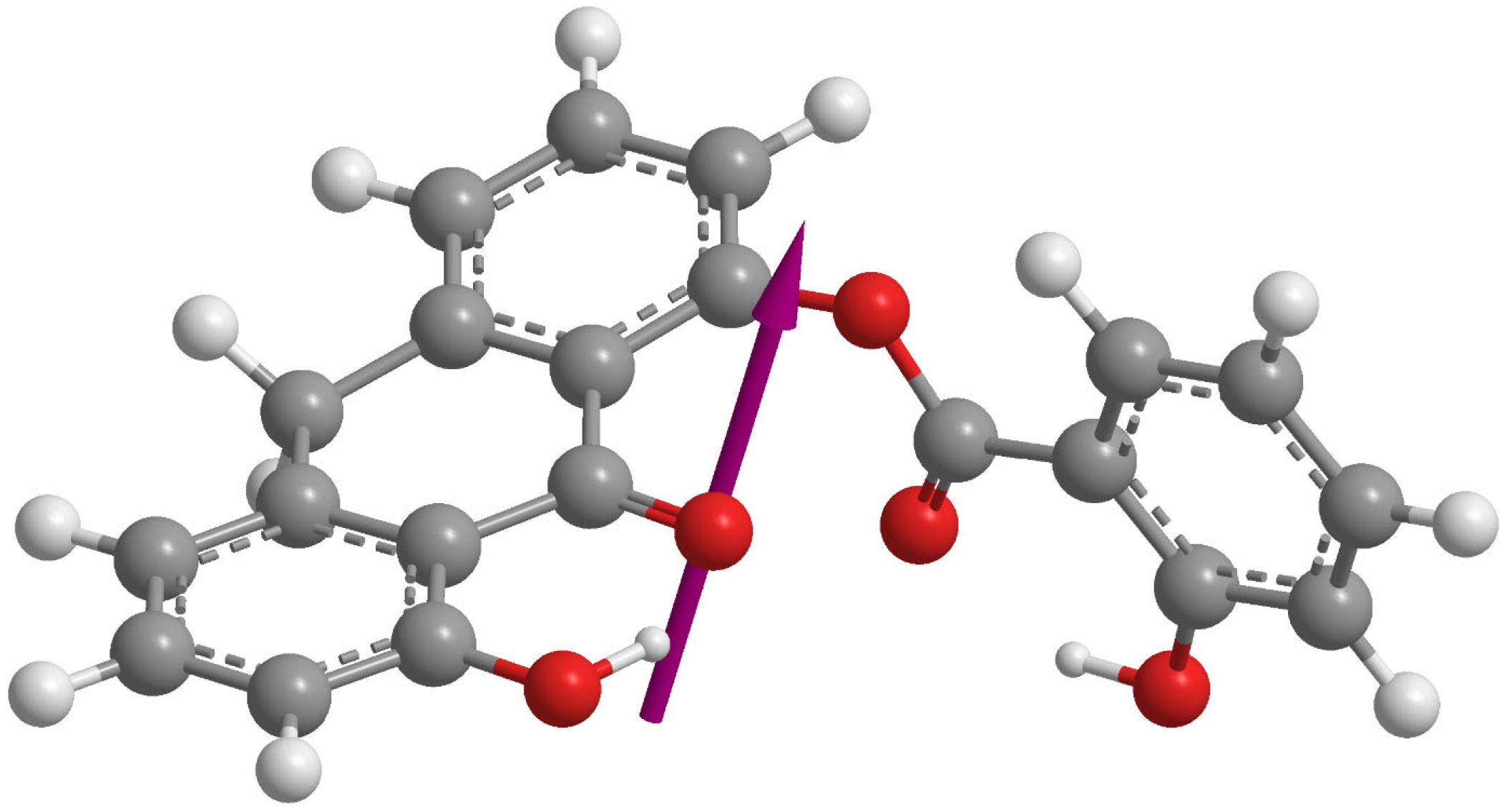




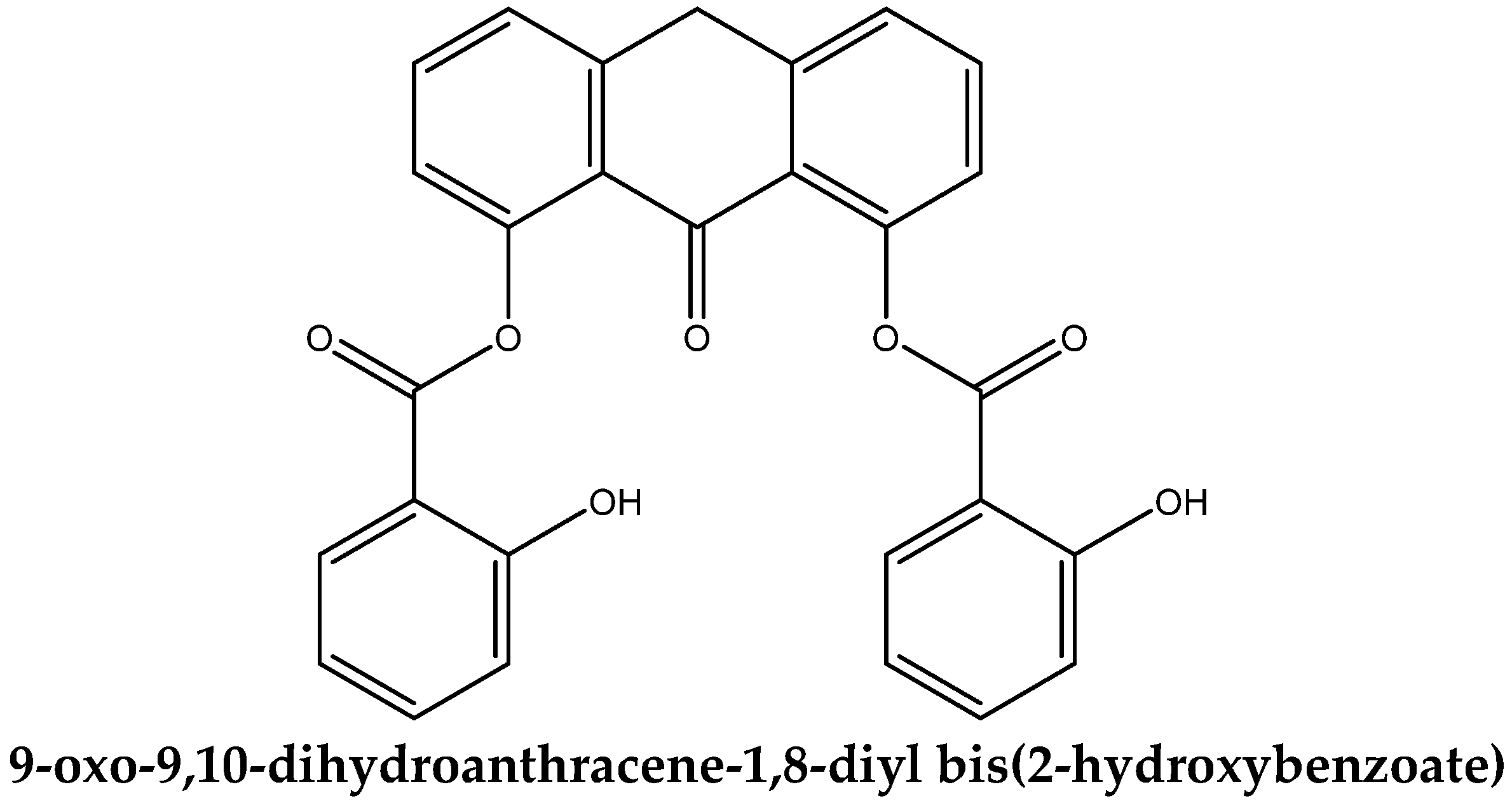
| Dipole Moment = (3.2541, 3.1276, −1.6948) 4.8211 D |
| Entropy = 148.643 cal/mol.K |
| Heat Capacity = 79.744 cal/mol.K |
| Molecular Mass = 346.08412 |
| SCF Energy = −743680.78 kcal/mol |
| Thermodynamic Energy = 202.013 kcal/mol |
| Zero-Point Energy = 189.608733 kcal/mol |
| Molecule | Orbital | Energy (eV) | Energy Gap (Eg) (eV) | Electron Affinity (EA) (eV) | Ionization Potential (IP) (eV) | Chemical Hardness (η) (eV) | Chemical Softness (ζ) (eV−1) | Chemical Potential (μ) (eV) | Electrophile (ω) (eV) |
|---|---|---|---|---|---|---|---|---|---|
| SAL-DIT | EHOMO | −6.280 | 3.915 | 2.365 | 6.280 | 1.958 | 0.511 | −4.323 | 4.772 |
| ELUMO | −2.365 | ||||||||
| Dithranol | EHOMO | −6.368 | 4.062 | 2.306 | 6.368 | 2.031 | 0.492 | −4.337 | 4.631 |
| ELUMO | −2.306 | ||||||||
| Salicylic acid | EHOMO | −6.576 | 5.153 | 1.423 | 6.576 | 2.568 | 0.389 | −4.000 | 3.115 |
| ELUMO | −1.423 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrýsková, N.; Motyčka, J.; Babincová, M.; Babinec, P.; Šimaljaková, M. Computational Design of a Novel Dithranol–Salicylic Acid Antipsoriatic Prodrug for Esterase-Activated Topical Drug Delivery. Appl. Sci. 2024, 14, 1094. https://doi.org/10.3390/app14031094
Andrýsková N, Motyčka J, Babincová M, Babinec P, Šimaljaková M. Computational Design of a Novel Dithranol–Salicylic Acid Antipsoriatic Prodrug for Esterase-Activated Topical Drug Delivery. Applied Sciences. 2024; 14(3):1094. https://doi.org/10.3390/app14031094
Chicago/Turabian StyleAndrýsková, Natália, Jozef Motyčka, Melánia Babincová, Peter Babinec, and Mária Šimaljaková. 2024. "Computational Design of a Novel Dithranol–Salicylic Acid Antipsoriatic Prodrug for Esterase-Activated Topical Drug Delivery" Applied Sciences 14, no. 3: 1094. https://doi.org/10.3390/app14031094
APA StyleAndrýsková, N., Motyčka, J., Babincová, M., Babinec, P., & Šimaljaková, M. (2024). Computational Design of a Novel Dithranol–Salicylic Acid Antipsoriatic Prodrug for Esterase-Activated Topical Drug Delivery. Applied Sciences, 14(3), 1094. https://doi.org/10.3390/app14031094







