Balneotherapy (Mud-Bath Therapy) with a Peloid Enriched with Rosmarinic Acid Enhances Clinical Outcomes and Innate Immune Benefits in Elderly Patients with Osteoarthritis: A Pilot Study
Abstract
Featured Application
Abstract
1. Introduction
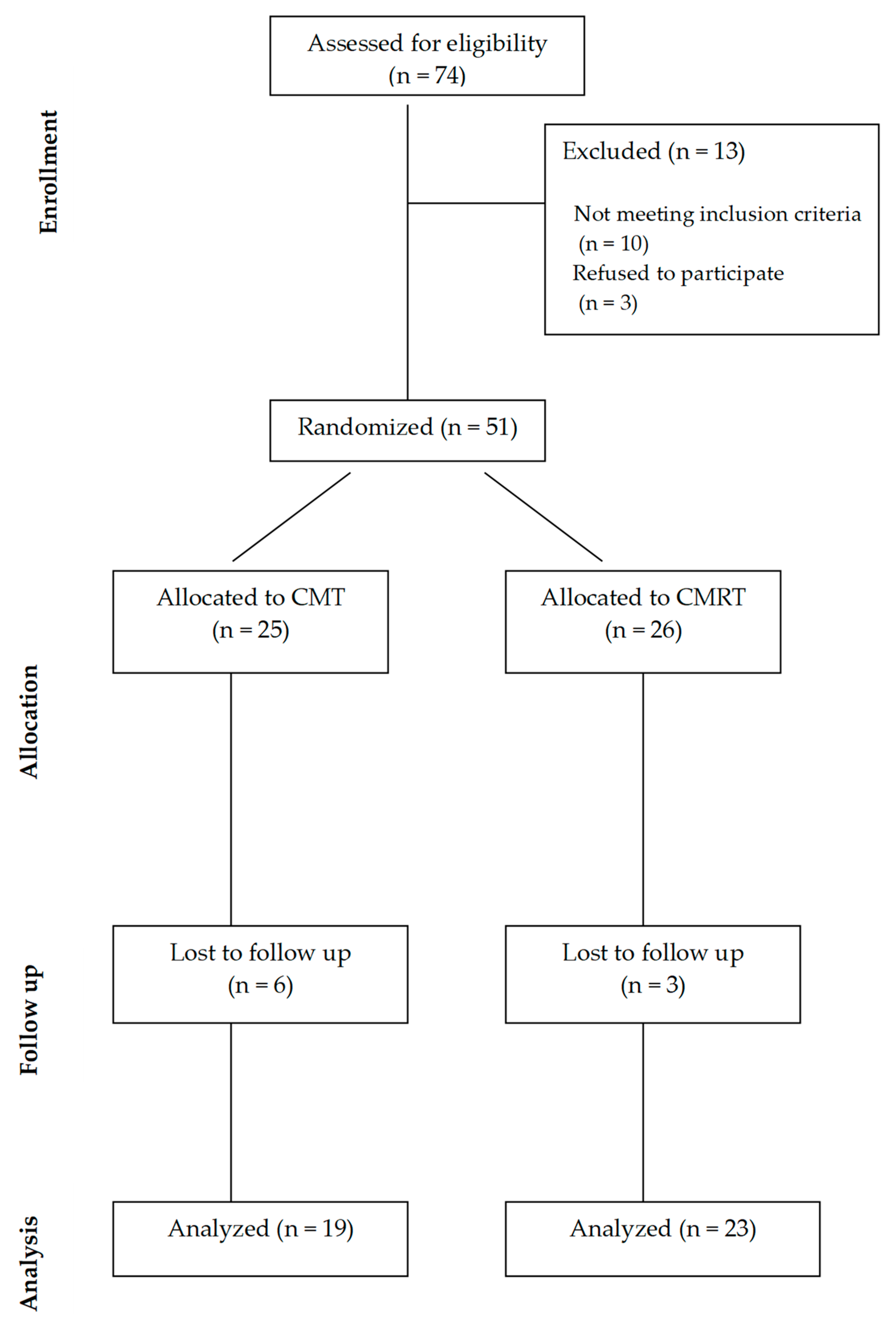
2. Materials and Methods
2.1. Intervention
2.2. Anthropometric and Clinical Data
2.3. Whole Blood Extraction and Evaluation of Neutrophil Phagocytic and Microbicidal Capacity
2.4. Statistical Analysis
3. Results
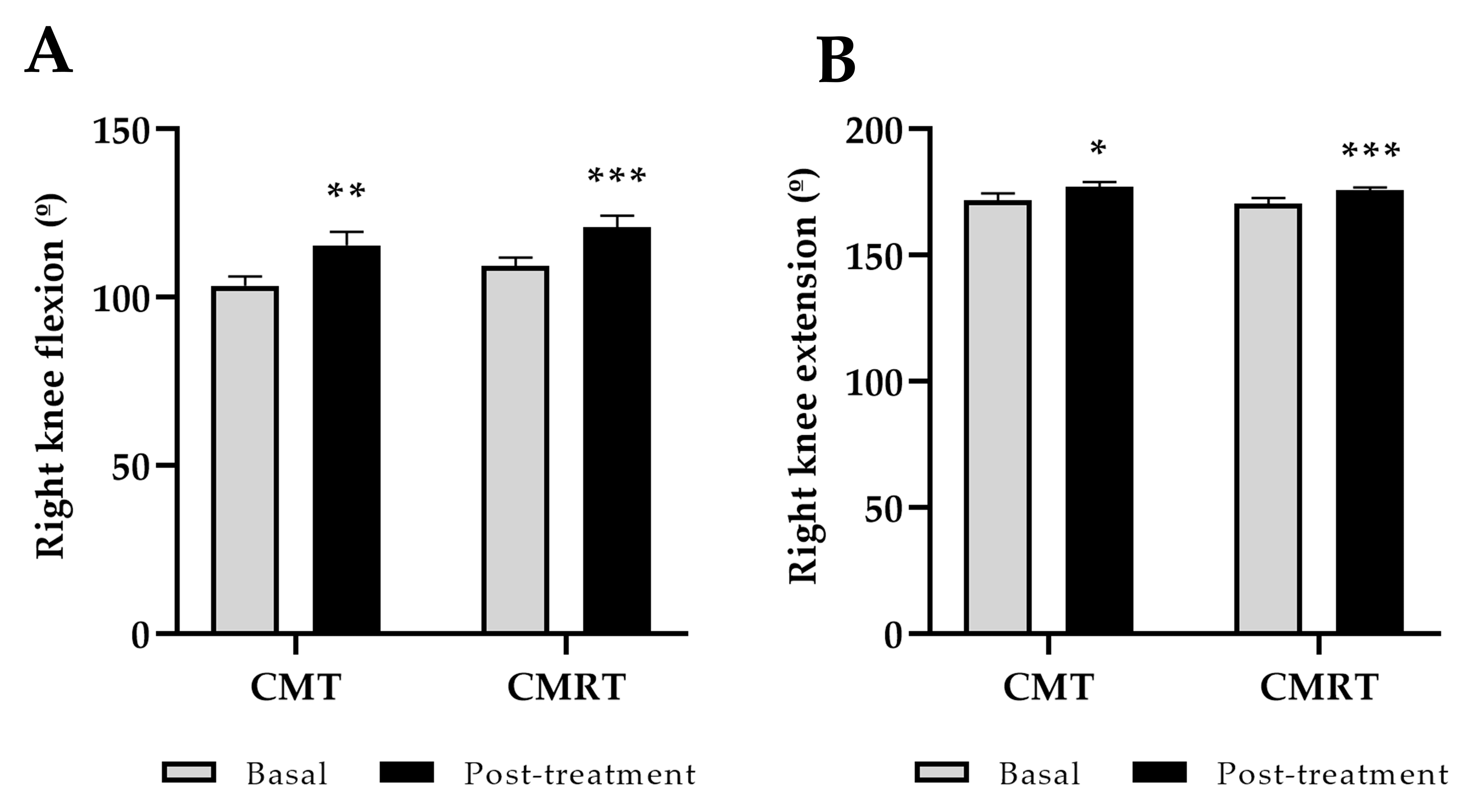
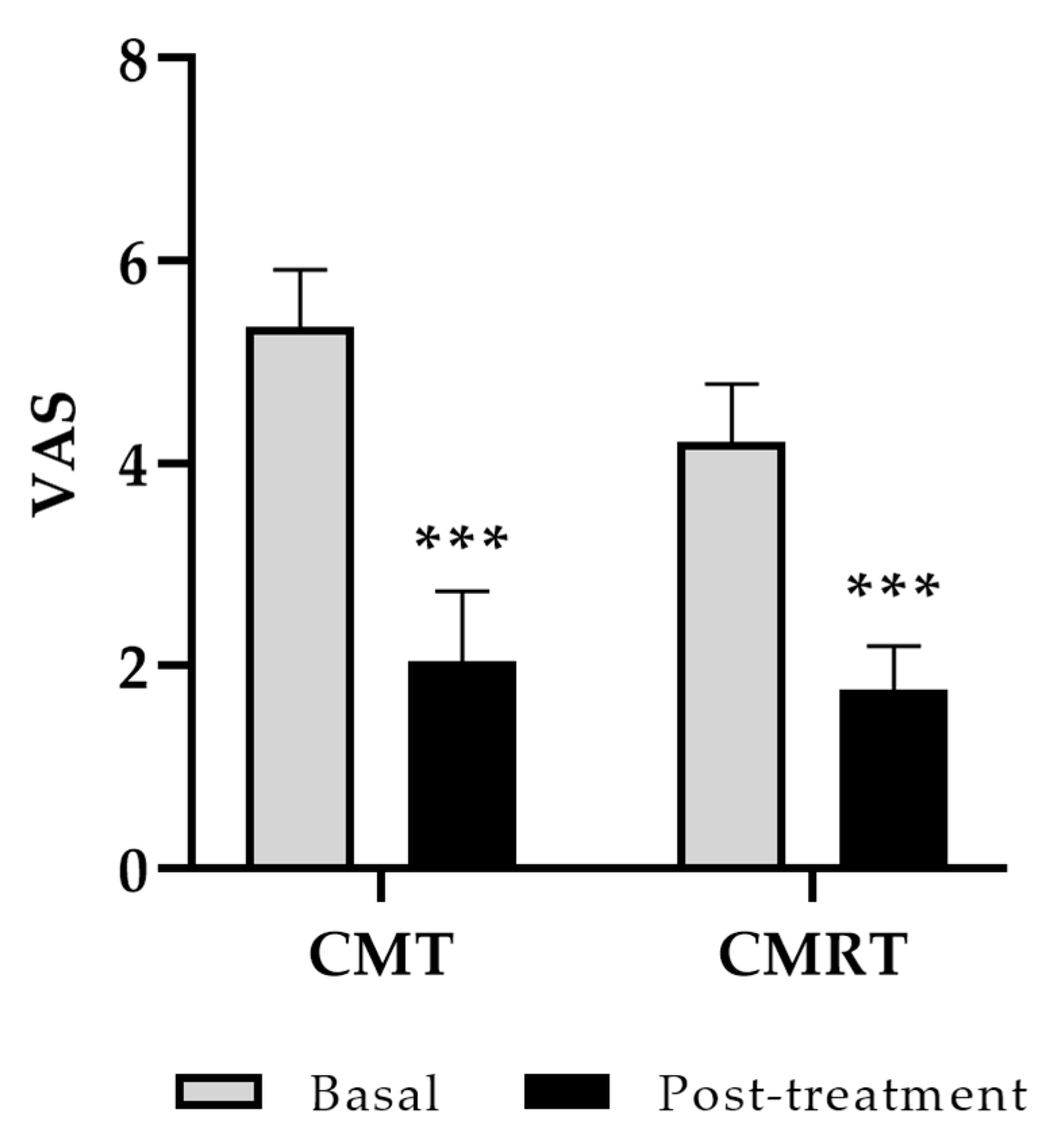
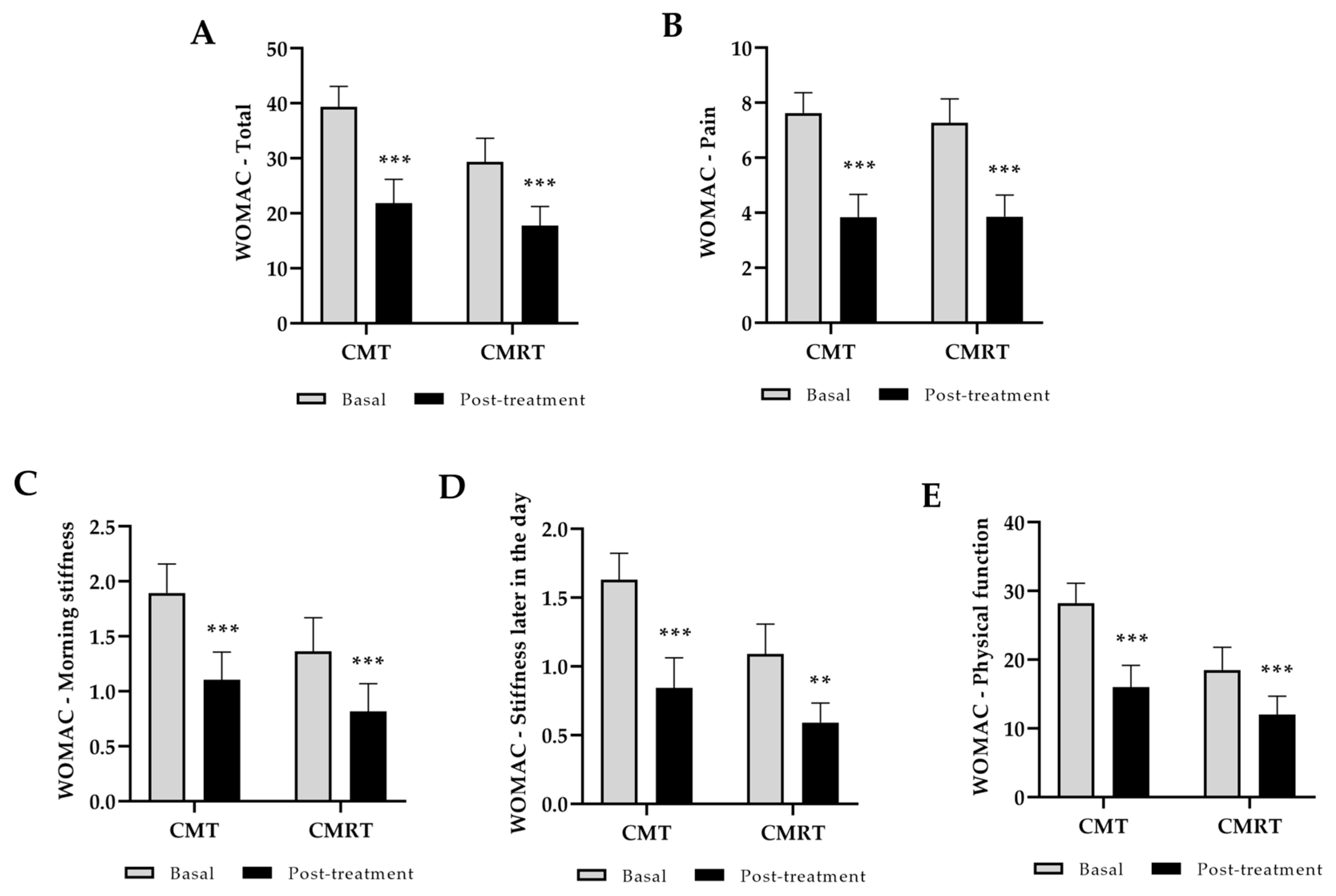
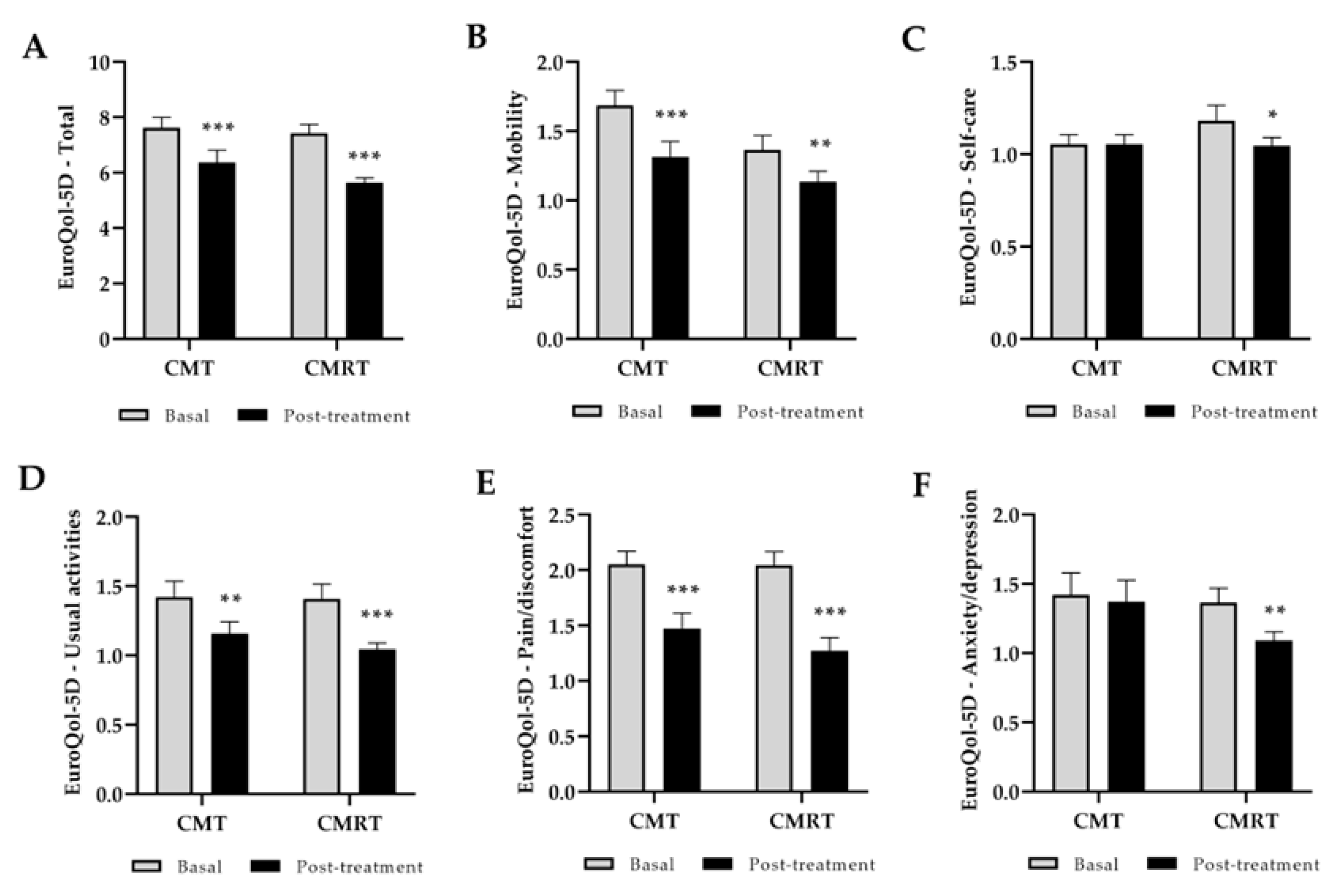
3.1. Clinical Outcome: Function, Pain, Stiffness, and Quality of Life
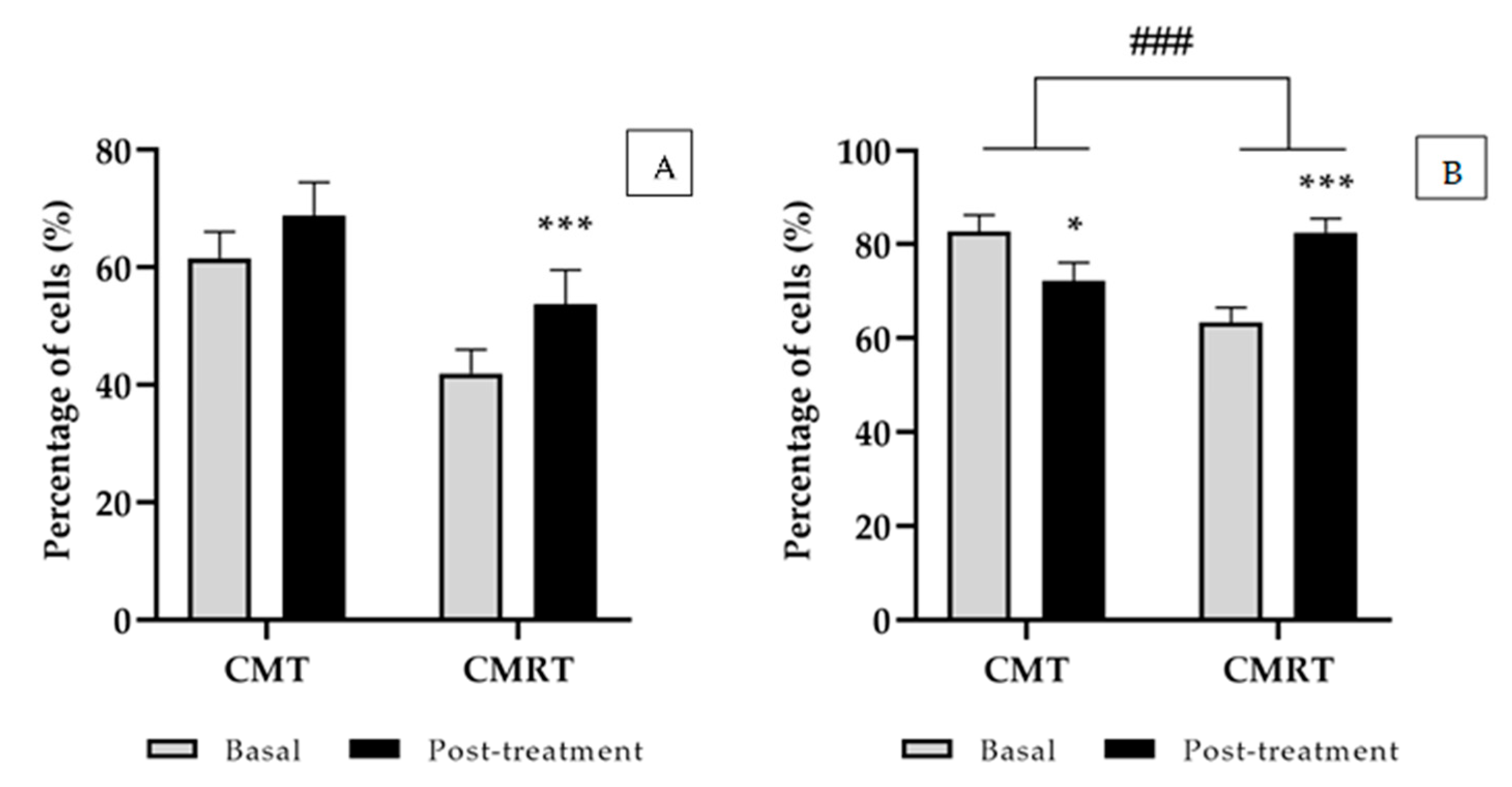
3.2. Innate Immune Response
4. Discussion
Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pereira, D.; Ramos, E.; Branco, J. Osteoarthritis. Acta Med. Port. 2015, 28, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.E.; Brandt, K.; Hawker, G.; Peeva, E.; Schreyer, E.; Tsuji, W.; Hochberg, M.C. OARSI-FDA initiative: Defining the disease state of osteoarthritis. Osteoarthr. Cartil. 2011, 19, 478–482. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, M.H.J.; van Lent, P.L.E.M.; van der Kraan, P.M. Identifying effector molecules, cells, and cytokines of innate immunity in OA. Osteoarthr. Cartil. 2020, 28, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.W.; Schlüter-Brust, K.U.; Eysel, P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch. Arztebl. Int. 2010, 107, 152–162. [Google Scholar] [CrossRef]
- Kapoor, M.; Mahomed, N.N. Osteoarthritis: Pathogenesis, Diagnosis, Available Treatments, Drug Safety, Regenerative and Precision Medicine; Adis International: Lausanne, Switzerland, 2015. [Google Scholar]
- Fioravanti, A.; Cantarini, L.; Bacarelli, M.R.; de Lalla, A.; Ceccatelli, L.; Blardi, P. Effects of spa therapy on serum leptin and adiponectin levels in patients with knee osteoarthritis. Rheumatol. Int. 2011, 31, 879–882. [Google Scholar] [CrossRef]
- Bender, T.; Karagülle, Z.; Bálint, G.P.; Gutenbrunner, C.; Bálint, P.V.; Sukenik, S. Hydrotherapy, balneotherapy, and spa treatment in pain management. Rheumatol. Int. 2005, 25, 220–224. [Google Scholar] [CrossRef]
- Gutenbrunner, C.; Bender, T.; Cantista, P.; Karagülle, Z. A proposal for a worldwide definition of health resort medicine, balneology, medical hydrology, and climatology. Int. J. Biometeorol. 2010, 54, 495–507. [Google Scholar] [CrossRef]
- Tognolo, L.; Coraci, D.; Fioravanti, A.; Tenti, S.; Scanu, A.; Magro, G.; Maccarone, M.C.; Masiero, S. Clinical Impact of Balneotherapy and Therapeutic Exercise in Rheumatic Diseases: A Lexical Analysis and Scoping Review. Appl. Sci. 2022, 12, 7379. [Google Scholar] [CrossRef]
- Maccarone, M.C.; Magro, G.; Solimene, U.; Masiero, S. From in vitro research to real life studies: An extensive narrative review of the effects of balneotherapy on human immune response. Sport. Sci. Health 2021, 17, 817–835. [Google Scholar] [CrossRef] [PubMed]
- Giannitti, C.; De Palma, A.; Pascarelli, N.A.; Cheleschi, S.; Giordano, N.; Galeazzi, M.; Fioravanti, A. Can balneotherapy modify microRNA expression levels in osteoarthritis? A comparative study in patients with knee osteoarthritis. Int. J. Biometeorol. 2017, 61, 2153–2158. [Google Scholar] [CrossRef]
- Fioravanti, A.; Giannitti, C.; Cheleschi, S.; Simpatico, A.; Pascarelli, N.A.; Galeazzi, M. Circulating levels of adiponectin, resistin, and visfatin after mud-bath therapy in patients with bilateral knee osteoarthritis. Int. J. Biometeorol. 2015, 59, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Bellometti, S.; Richelmi, P.; Tassoni, T.; Bertè, F. Production of matrix metalloproteinases and their inhibitors in osteoarthritic patients undergoing mud bath therapy. Int. J. Clin. Pharmacol. Res. 2005, 25, 77–94. [Google Scholar] [PubMed]
- Berenbaum, F.; Eymard, F.; Houard, X. Osteoarthritis, inflammation and obesity. Curr. Opin. Rheumatol. 2013, 25, 114–118. [Google Scholar] [CrossRef]
- Oyama, J.; Kudo, Y.; Maeda, T.; Node, K.; Makino, N. Hyperthermia by bathing in a hot spring improves cardiovascular functions and reduces the production of inflammatory cytokines in patients with chronic heart failure. Heart Vessels 2013, 28, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Oláh, M.; Koncz, A.; Fehér, J.; Kálmánczhey, J.; Oláh, C.; Balogh, S.; Nagy, G.; Bender, T. The effect of balneotherapy on C-reactive protein, serum cholesterol, triglyceride, total antioxidant status and HSP-60 levels. Int. J. Biometeorol. 2010, 54, 249–254. [Google Scholar] [CrossRef]
- Oláh, M.; Koncz, Á.; Fehér, J.; Kálmánczhey, J.; Oláh, C.; Nagy, G.; Bender, T. The effect of balneotherapy on antioxidant, inflammatory, and metabolic indices in patients with cardiovascular risk factors (hypertension and obesity)—A randomised, controlled, follow-up study. Contemp. Clin. Trials 2011, 32, 793–801. [Google Scholar] [CrossRef]
- Bellometti, S.; Galzigna, L. Serum levels of a prostaglandin and a leukotriene after thermal mud pack therapy. J. Investig. Med. 1998, 46, 140–145. [Google Scholar]
- Gálvez, I.; Torres-Piles, S.; Ortega-Rincón, E. Balneotherapy, immune system, and stress response: A hormetic strategy? Int. J. Mol. Sci. 2018, 19, 1687. [Google Scholar] [CrossRef]
- Cheleschi, S.; Tenti, S.; Seccafico, I.; Gálvez, I.; Fioravanti, A.; Ortega, E. Balneotherapy year in review 2021: Focus on the mechanisms of action of balneotherapy in rheumatic diseases. Environ. Sci. Pollut. Res. Int. 2022, 29, 8054–8073. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and Its New Insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef]
- Lee, K.H.; Yang, M.C.; Kim, K.H.; Kwon, H.C.; Choi, S.U.; Lee, K.R. A new phenolic amide from the roots of Paris verticillata. Molecules 2008, 13, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, N.; Takano, H.; Sanbongi, C.; Yasuda, A.; Yanagisawa, R.; Inoue, K.; Yoshikawa, T. Anti-inflammatory and anti-allergic effect of rosmarinic acid (RA); inhibition of seasonal allergic rhinoconjunctivitis (SAR) and its mechanism. Biofactors 2004, 21, 127–131. [Google Scholar] [CrossRef]
- Connelly, A.E.; Tucker, A.J.; Tulk, H.; Catapang, M.; Chapman, L.; Sheikh, N.; Yurchenko, S.; Fletcher, R.; Kott, L.S.; Duncan, A.M.; et al. High-rosmarinic acid spearmint tea in the management of knee osteoarthritis symptoms. J. Med. Food 2014, 17, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Sirše, M. Effect of Dietary Polyphenols on Osteoarthritis-Molecular Mechanisms. Life 2022, 12, 436. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M.; et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Arthritis Rheum. 1986, 29, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Carretero, M.I.; Pozo, M.; Martín-Rubí, J.A.; Pozo, E.; Maraver, F. Mobility of elements in interaction between artificial sweat and peloids used in Spanish spas. Appl. Clay Sci. 2010, 48, 506–515. [Google Scholar] [CrossRef]
- Pozo, M.; Carretero, M.I.; Maraver, F.; Martín-Rubí, J.A. Composition and physico-chemical properties of peloids used in Spanish spas: A comparative study. Appl. Clay Sci. 2013, 83–84, 270–279. [Google Scholar] [CrossRef]
- Scott, J.; Huskisson, E.C. Graphic representation of pain. Pain 1976, 2, 175–184. [Google Scholar] [CrossRef]
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt, L.W. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1988, 15, 1833–1840. [Google Scholar] [PubMed]
- Williams, A. The EuroQol Group. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar]
- Galvez, I.; Torres-Piles, S.; Hinchado, M.D.; Alvarez-Barrientos, A.; Torralbo-Jimenez, P.; Guerrero, J.; Martin-Cordero, L.; Ortega, E. Immune-Neuroendocrine Dysregulation in Patients with Osteoarthritis: A Revision and a Pilot Study. Endocr. Metab. Immune Disord. Drug Targets 2017, 17, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Evcik, D.; Kavuncu, V.; Yeter, A.; Yigit, I. The efficacy of balneotherapy and mud-pack therapy in patients with knee osteoarthritis. Jt. Bone Spine 2007, 74, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, A.; Cantarini, L.; Guidelli, G.M.; Galeazzi, M. Mechanisms of action of spa therapies in rheumatic diseases: What scientific evidence is there? Rheumatol. Int. 2011, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ortega, E.; Gálvez, I.; Hinchado, M.D.; Guerrero, J.; Martín-Cordero, L.; Torres-Piles, S. Anti-inflammatory effect as a mechanism of effectiveness underlying the clinical benefits of pelotherapy in osteoarthritis patients: Regulation of the altered inflammatory and stress feedback response. Int. J. Biometeorol. 2017, 61, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Antúnez, L.E.; Puértolas, B.C.; Burgos, B.I.; Payán, J.M.P.; Piles, S.T.T. Effects of mud therapy on perceived pain and quality of life related to health in patients with knee osteoarthritis. Reumatol. Clin. 2013, 9, 156–160. [Google Scholar]
- Carretero, M.I. Clays in Pelotherapy. A Review. Part I: Mineralogy, Chemistry, Physical and Physicochemical Properties. Appl. Clay Sci. 2020, 189, 105526. [Google Scholar] [CrossRef]
- Gomes, C.S.F.; Rautureau, M.; Gomes, J.H.C.; Silva, E.A.F. Interactions of Clay and Clay Minerals with Human Health. In Minerals Latu Sensu and Human Health; Gomes, C., Rautureau, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 271–375. [Google Scholar] [CrossRef]
- Maraver Eyzaguirre, F.M. Antecedentes históricos de la peloterapia. An. Hidrol. Méd. 2006, 1, 17–42. [Google Scholar]
- Gálvez, I.; Torres-Piles, S.; Ortega, E. Innate/inflammatory bioregulation and clinical effectiveness of whole-body hyperthermia (balneotherapy) in elderly patients with osteoarthritis. Int. J. Hyperth. 2018, 35, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, I.; Torres-Piles, S.; Ortega, E. Effect of mud-bath therapy on the innate/inflammatory responses in elderly patients with osteoarthritis: A discussion of recent results and a pilot study on the role of the innate function of monocytes. Int. J. Biometeorol. 2020, 64, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, I.; Hinchado, M.D.; Otero, E.; Navarro, M.C.; Ortega-Collazos, E.; Martín-Cordero, L.; Torres-Piles, S.T.; Ortega, E. Circulating serotonin and dopamine concentrations in osteoarthritis patients: A pilot study on the effect of pelotherapy. Int. J. Biometeorol. 2024, 68, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Aydın, N.G.; Karagülle, M.; Koçak, E.N.; Karagülle, M.Z. Evaluation of balneological treatment on pain, function, and quality of life in knee osteoarthritis: A randomized, controlled, single-blinded trial. J. Ist. Fac. Med. 2024, 87, 165–175. [Google Scholar] [CrossRef]
| Controlled Mud Therapy (CMT) | Controlled Mud with Rosmarinic Acid Therapy (CMRT) | |||
|---|---|---|---|---|
| N | 19 | 23 | ||
| Sex (%) | Women (63%) | Women (60%) | ||
| Ethnic group (%) | Caucasian (100%) | Caucasian (100%) | ||
| Age (years) | 71.7 ± 2.51 | 70.2 ± 1.61 | ||
| Basal | Post-treatment | Basal | Post-treatment | |
| BMI (kg/m2) | 30.1 ± 0.9 | 30.2 ± 0.8 | 29.4 ± 0.8 | 29.1 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Collazos, E.; Hinchado, M.D.; Otero, E.; López-Jurado, C.F.; Gálvez, I.; Legido, J.L.; Muñoz-Torrero, J.F.S.; Ortega, E.; Torres-Piles, S. Balneotherapy (Mud-Bath Therapy) with a Peloid Enriched with Rosmarinic Acid Enhances Clinical Outcomes and Innate Immune Benefits in Elderly Patients with Osteoarthritis: A Pilot Study. Appl. Sci. 2024, 14, 12017. https://doi.org/10.3390/app142412017
Ortega-Collazos E, Hinchado MD, Otero E, López-Jurado CF, Gálvez I, Legido JL, Muñoz-Torrero JFS, Ortega E, Torres-Piles S. Balneotherapy (Mud-Bath Therapy) with a Peloid Enriched with Rosmarinic Acid Enhances Clinical Outcomes and Innate Immune Benefits in Elderly Patients with Osteoarthritis: A Pilot Study. Applied Sciences. 2024; 14(24):12017. https://doi.org/10.3390/app142412017
Chicago/Turabian StyleOrtega-Collazos, Eduardo, María Dolores Hinchado, Eduardo Otero, Casimiro Fermín López-Jurado, Isabel Gálvez, José Luis Legido, Juan Francisco Sánchez Muñoz-Torrero, Eduardo Ortega, and Silvia Torres-Piles. 2024. "Balneotherapy (Mud-Bath Therapy) with a Peloid Enriched with Rosmarinic Acid Enhances Clinical Outcomes and Innate Immune Benefits in Elderly Patients with Osteoarthritis: A Pilot Study" Applied Sciences 14, no. 24: 12017. https://doi.org/10.3390/app142412017
APA StyleOrtega-Collazos, E., Hinchado, M. D., Otero, E., López-Jurado, C. F., Gálvez, I., Legido, J. L., Muñoz-Torrero, J. F. S., Ortega, E., & Torres-Piles, S. (2024). Balneotherapy (Mud-Bath Therapy) with a Peloid Enriched with Rosmarinic Acid Enhances Clinical Outcomes and Innate Immune Benefits in Elderly Patients with Osteoarthritis: A Pilot Study. Applied Sciences, 14(24), 12017. https://doi.org/10.3390/app142412017







