Cmpk2 Gene and Protein Expression in Saliva or Salivary Glands of Dyslipidemic Mice
Abstract
1. Introduction
2. Materials and Methods
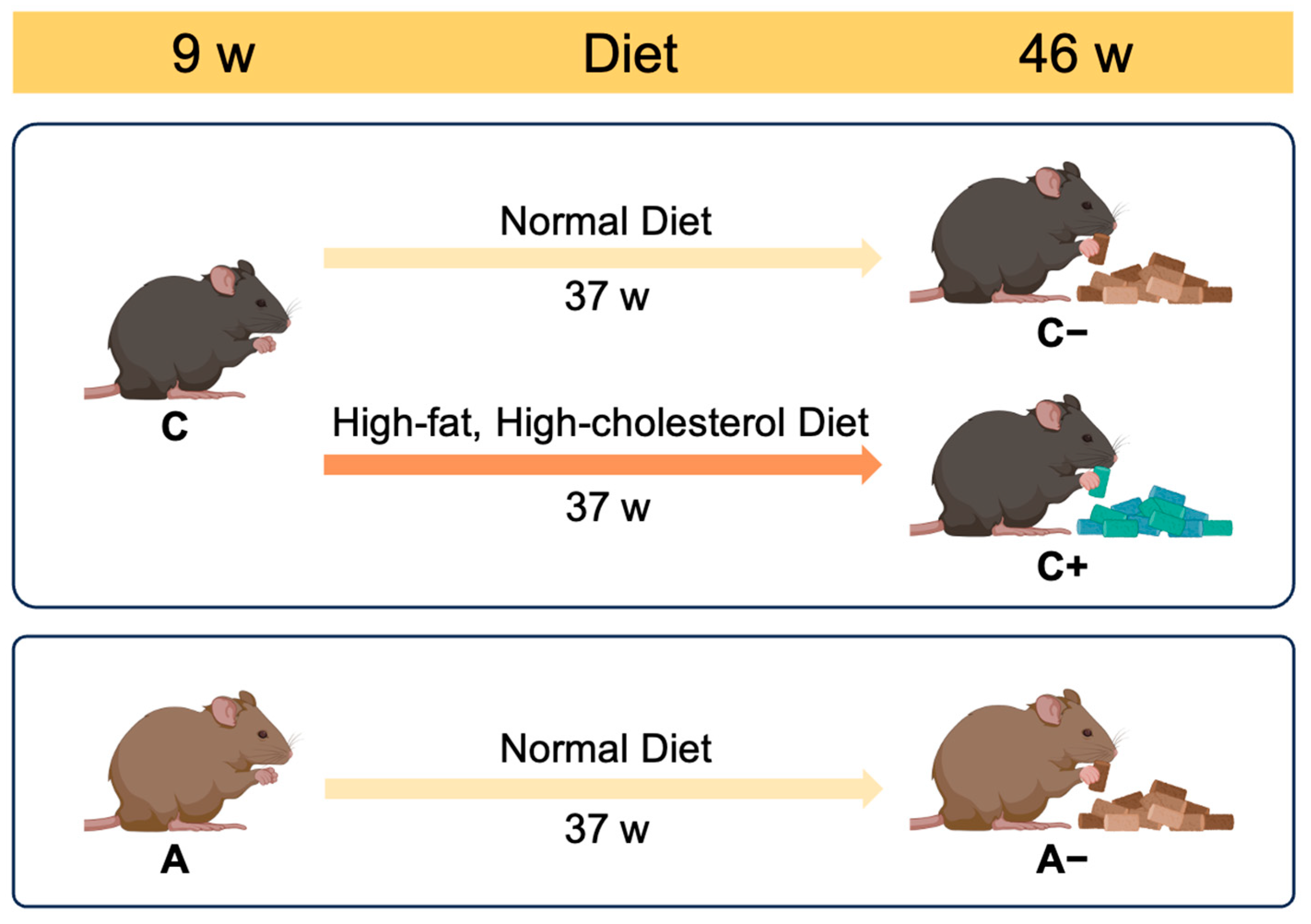
2.1. Animals
2.2. Assessment of Lipid Metabolism and Obesity-Related Parameters
2.3. Saliva Sample Collection
2.4. Saliva RNA Isolation and qRT-PCR
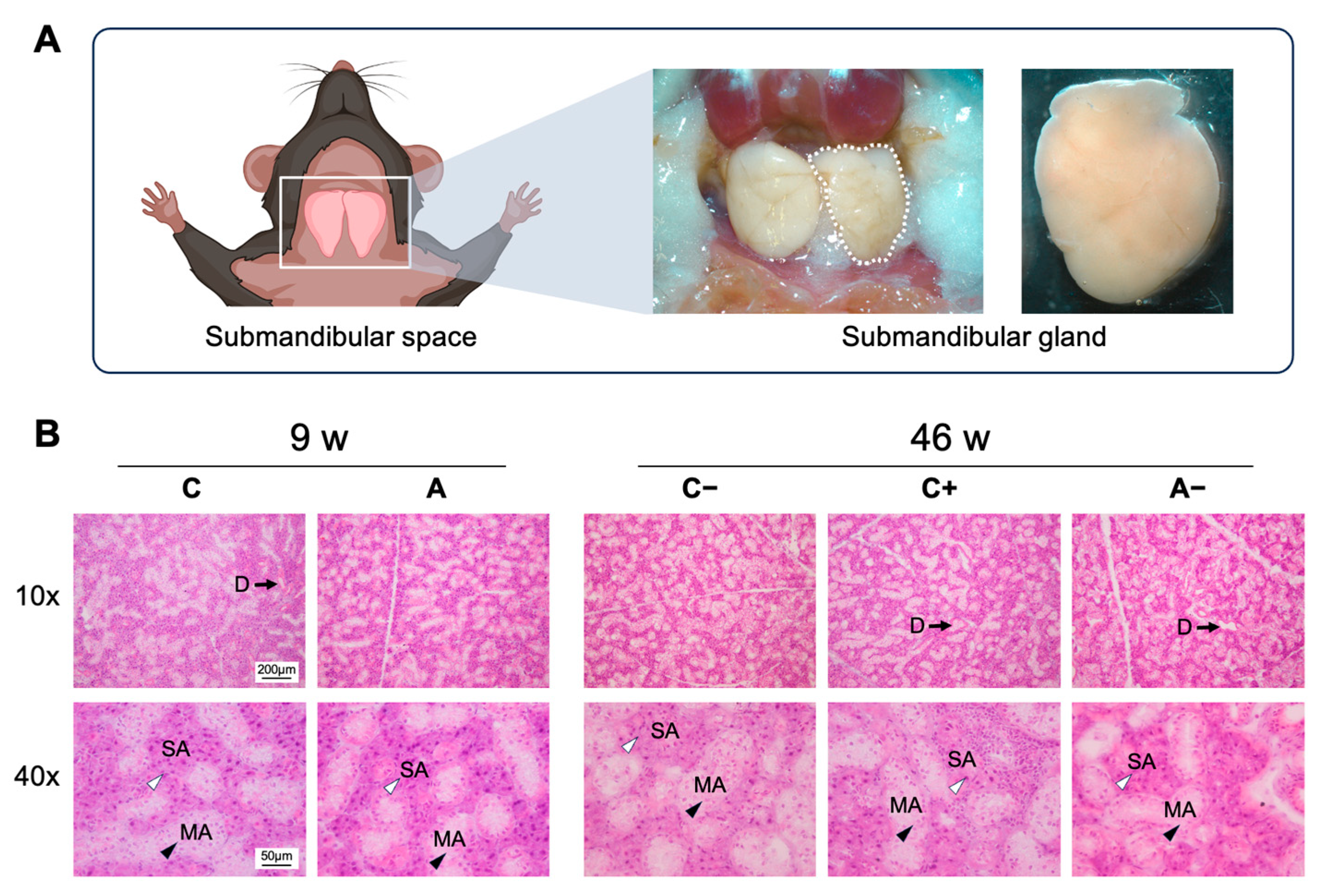
2.5. Histological Staining
2.6. Statistical Analysis and English Editing
3. Results
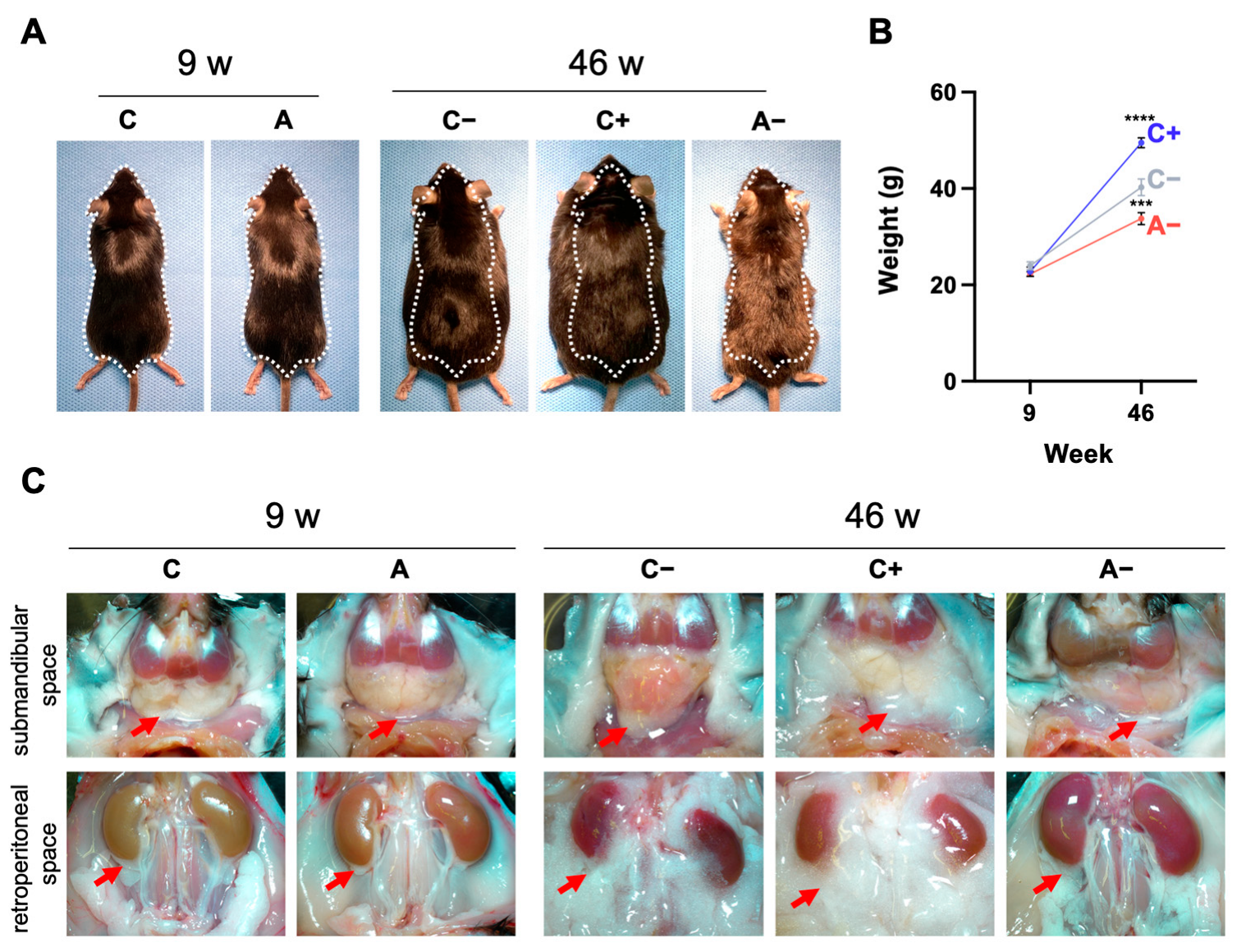
3.1. Body Weight Changes and Visceral Fat Accumulation in Mice
3.2. Serum Lipid Levels
3.3. Comparison of the Quality of RNA Extracted from Saliva Using Different Reagents
3.4. Cmpk2 Gene Expression in the Saliva of Dyslipidemic Mice
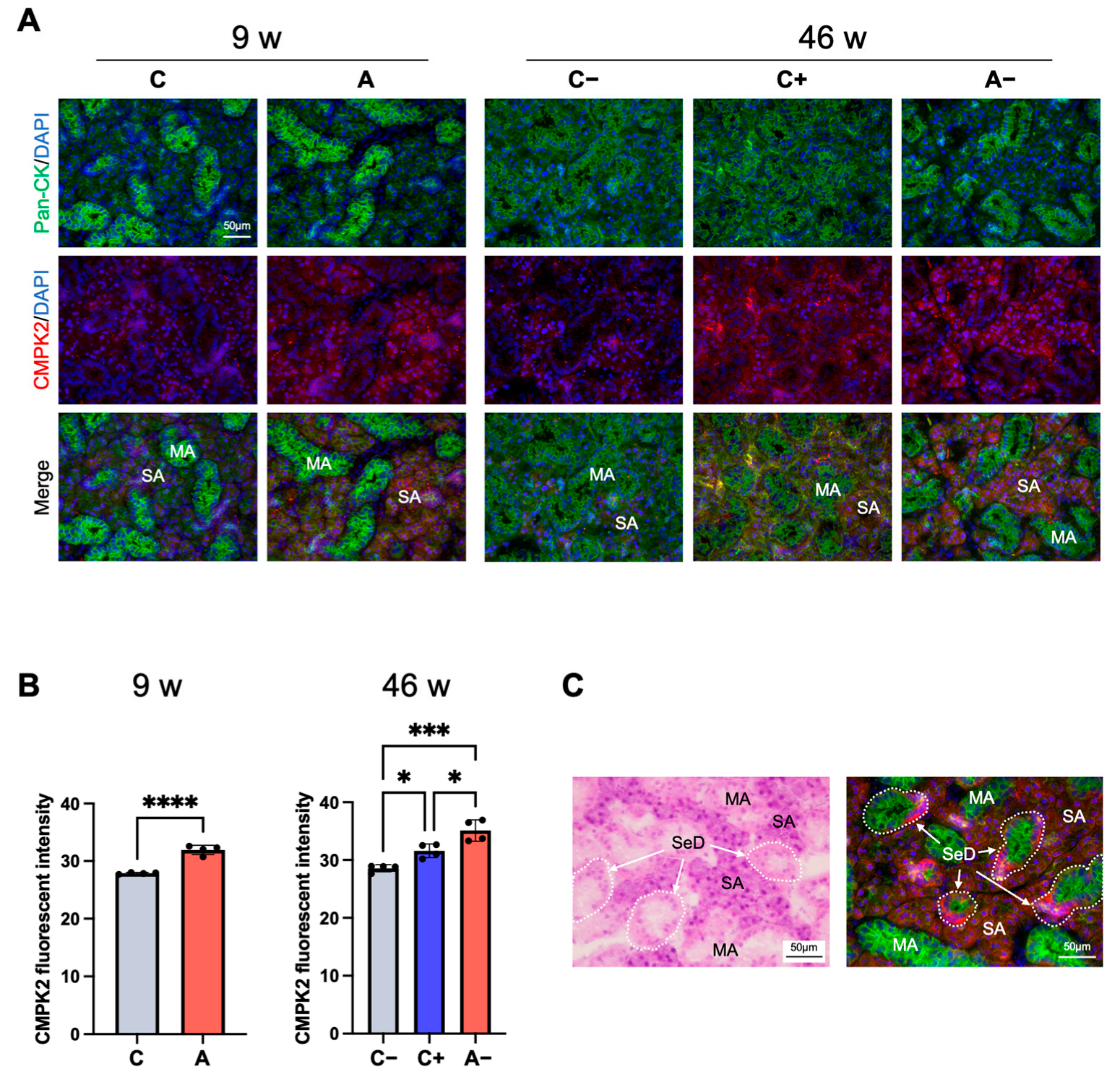
3.5. CMPK2 Immunofluorescence Staining in the Submandibular Gland of Dyslipidemic Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malik, V.S.; Willett, W.C.; Hu, F.B. Global obesity: Trends, risk factors and policy implications. Nat. Rev. Endocrinol. 2013, 9, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Tsukamoto, K.; Arai, H.; Fujioka, Y.; Ishigaki, Y.; Koba, S.; Ohmura, H.; Shoji, T.; Yokote, K.; Yoshida, H.; et al. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2022. J. Atheroscler. Thromb. 2024, 31, 657. [Google Scholar] [CrossRef]
- Sarwar, N.; Danesh, J.; Eiriksdottir, G.; Sigurdsson, G.; Wareham, N.; Bingham, S.; Boekholdt, S.M.; Khaw, K.T.; Gudnason, V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007, 115, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Do, R.; Willer, C.J.; Schmidt, E.M.; Sengupta, S.; Gao, C.; Peloso, G.M.; Gustafsson, S.; Kanoni, S.; Ganna, A.; Chen, J.; et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat. Genet. 2013, 45, 1345–1352. [Google Scholar] [CrossRef]
- Turner, R.C.; Millns, H.; Neil, H.A.; Stratton, I.M.; Manley, S.E.; Matthews, D.R.; Holman, R.R. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom prospective diabetes study (UKPDS: 23). BMJ 1998, 316, 823–828. [Google Scholar] [CrossRef]
- Djelilovic-Vranic, J.; Alajbegovic, A.; Zelija-Asimi, V.; Niksic, M.; Tiric-Campara, M.; Salcic, S.; Celo, A. Predilection role diabetes mellitus and dyslipidemia in the onset of ischemic stroke. Med. Arch. 2013, 67, 120–123. [Google Scholar] [CrossRef]
- Jacobs, J.M.; Adkins, J.N.; Qian, W.J.; Liu, T.; Shen, Y.; Camp, D.G.; Smith, R.D. Utilizing human blood plasma for proteomic biomarker discovery. J. Proteome Res. 2005, 4, 1073–1085. [Google Scholar] [CrossRef]
- Cen, G.; Wang, J.; Wang, X.; Song, Y.; Chen, S.; Li, J.; Liang, Z. Pathogenesis and biomarkers of cancer-related ischemic stroke. J. Inflamm. Res. 2024, 17, 8589–8597. [Google Scholar] [CrossRef]
- Pisitkun, T.; Johnstone, R.; Knepper, M.A. Discovery of urinary biomarkers. Mol. Cell. Proteom. 2006, 5, 1760–1771. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, G.W.; Hwang, H.S.; Kim, Y.G.; Moon, J.Y.; Lee, S.H.; Seok, J.; Tae, D.; Jeong, K.H. Urinary sediment mRNA as a potent biomarker of IgA nephropathy. BMC Nephrol. 2024, 25, 401. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, C.E.; Tumani, H.; Engelborghs, S.; Mollenhauer, B. Biobanking of CSF: International standardization to optimize biomarker development. Clin. Biochem. 2014, 47, 288–292. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, H.; Zhou, H.; Santiago, S.; Lee, J.M.; Garon, E.B.; Yang, J.; Brinkmann, O.; Yan, X.; Akin, D.; et al. Development of transcriptomic biomarker signature in human saliva to detect lung cancer. Cell. Mol. Life Sci. 2012, 69, 3341–3350. [Google Scholar] [CrossRef]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Mal, M. Noninvasive metabolic profiling for painless diagnosis of human diseases and disorders. Future Sci. OA 2016, 2, FSO106. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; St John, M.A.; Zhou, X.; Kim, Y.; Sinha, U.; Jordan, R.C.; Eisele, D.; Abemayor, E.; Elashoff, D.; Park, N.H.; et al. Salivary transcriptome diagnostics for oral cancer detection. Clin. Cancer Res. 2004, 10, 8442–8450. [Google Scholar] [CrossRef]
- Katakura, A.; Yamamoto, N.; Sakuma, T.; Sugahara, K.; Onda, T.; Noguchi, S.; Shibahara, T. A screening test for oral cancer using saliva samples: Proteomic analysis of biomarkers in whole saliva. J. Oral Maxillofac. Surg. Med. Pathol. 2015, 27, 1–5. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, S.; Koh, D.; Hsu, C.Y. Salivary biomarkers for dental caries. Periodontology 2000 2016, 70, 128–141. [Google Scholar] [CrossRef]
- Chawda, J.G.; Chaduvula, N.; Patel, H.R.; Jain, S.S.; Lala, A.K. Salivary SIgA and dental caries activity. Indian Pediatr. 2011, 48, 719–721. [Google Scholar] [CrossRef]
- Ghallab, N.A. Diagnostic potential and future directions of biomarkers in gingival crevicular fluid and saliva of periodontal diseases: Review of the current evidence. Arch. Oral Biol. 2018, 87, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Terada, K.; Katamine, S.; Eguchi, K.; Moriuchi, R.; Kita, M.; Shimada, H.; Yamashita, I.; Iwata, K.; Tsuji, Y.; Nagataki, S. Prevalence of serum and salivary antibodies to HTLV-1 in Sjögren’s syndrome. Lancet 1994, 344, 1116–1119. [Google Scholar] [CrossRef]
- Javaid, M.A.; Ahmed, A.S.; Durand, R.; Tran, S.D. Saliva as a diagnostic tool for oral and systemic diseases. J. Oral Biol. Craniofacial Res. 2016, 6, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Rammos, A.; Bechlioulis, A.; Kalogeras, P.; Tripoliti, E.E.; Goletsis, Y.; Kalivi, A.; Blathra, E.; Salvo, P.; Trivella, M.G.; Lomonaco, T.; et al. Salivary biomarkers for diagnosis and therapy monitoring in patients with heart failure. A systematic review. Diagnostics 2021, 11, 824. [Google Scholar] [CrossRef] [PubMed]
- Bahbah, E.I.; Noehammer, C.; Pulverer, W.; Jung, M.; Weinhaeusel, A. Salivary biomarkers in cardiovascular disease: An insight into the current evidence. FEBS J. 2021, 288, 6392–6405. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Blackburn, C.; Mohamed, M.; Sivagami, A.V.; Blum, J. Literature-based discovery of salivary biomarkers for type 2 diabetes mellitus. Biomark. Insights 2015, 10, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Sabaei, M.; Rahimian, S.; Haj Mohamad Ebrahim Ketabforoush, A.; Rasoolijazi, H.; Zamani, B.; Hajiakhoundi, F.; Soleimani, M.; Shahidi, G.; Faramarzi, M. Salivary levels of disease-related biomarkers in the early stages of Parkinson’s and Alzheimer’s disease: A cross-sectional study. IBRO Neurosci. Rep. 2023, 14, 285–292. [Google Scholar] [CrossRef]
- Sabbagh, M.N.; Shi, J.; Lee, M.; Arnold, L.; Al-Hasan, Y.; Heim, J.; McGeer, P. Salivary beta amyloid protein levels are detectable and differentiate patients with Alzheimer’s disease dementia from normal controls: Preliminary findings. BMC Neurol. 2018, 18, 155. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.L.; Pacheco, V.B.; Borges, L.; Athwal, H.K.; de Paula Eduardo, F.; Bezinelli, L.; Correa, L.; Jimenez, M.; Dame-Teixeira, N.; Lombaert, I.M.A.; et al. Saliva in the diagnosis of COVID-19: A review and new research directions. J. Dent. Res. 2020, 99, 1435–1443. [Google Scholar] [CrossRef]
- Rayment, S.A.; Liu, B.; Soares, R.V.; Offner, G.D.; Oppenheim, F.G.; Troxler, R.F. The effects of duration and intensity of stimulation on total protein and mucin concentrations in resting and stimulated whole saliva. J. Dent. Res. 2001, 80, 1584–1587. [Google Scholar] [CrossRef]
- Ostheim, P.; Tichy, A.; Sirak, I.; Davidkova, M.; Stastna, M.M.; Kultova, G.; Paunesku, T.; Woloschak, G.; Majewski, M.; Port, M.; et al. Overcoming challenges in human saliva gene expression measurements. Sci. Rep. 2020, 10, 11147. [Google Scholar] [CrossRef]
- Hamada, Y.; Honda, Y.; Kawamoto, A.; Shimizu, H.; Takahashi, K. Detection of biomarkers on aging and vascular senescence in saliva. J. Osaka Dent. Univ. 2020, 54, 117–125. [Google Scholar] [CrossRef]
- Bhamidimarri, P.M.; Fuentes, D.; Salameh, L.; Mahboub, B.; Hamoudi, R. Assessing the impact of storage conditions on RNA from human saliva and its application to the identification of mRNA biomarkers for asthma. Front. Mol. Biosci. 2024, 11, 1363897. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Johansson, M.; Karlsson, A. Human UMP-CMP kinase 2, a novel nucleoside monophosphate kinase localized in mitochondria. J. Biol. Chem. 2008, 283, 1563–1571. [Google Scholar] [CrossRef]
- Zhong, Z.; Liang, S.; Sanchez-Lopez, E.; He, F.; Shalapour, S.; Lin, X.J.; Wong, J.; Ding, S.; Seki, E.; Schnabl, B.; et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 2018, 560, 198–203. [Google Scholar] [CrossRef]
- Natarajan, N.; Florentin, J.; Johny, E.; Xiao, H.; O’Neil, S.P.; Lei, L.; Shen, J.; Ohayon, L.; Johnson, A.R.; Rao, K.; et al. Aberrant mitochondrial DNA synthesis in macrophages exacerbates inflammation and atherosclerosis. Nat. Commun. 2024, 15, 7337. [Google Scholar] [CrossRef]
- Lai, J.H.; Wu, D.W.; Wu, C.H.; Hung, L.F.; Huang, C.Y.; Ka, S.M.; Chen, A.; Chang, Z.F.; Ho, L.J. Mitochondrial CMPK2 mediates immunomodulatory and antiviral activities through IFN-dependent and IFN-independent pathways. iScience 2021, 24, 102498. [Google Scholar] [CrossRef]
- Bu, F.; Qin, X.; Wang, T.; Li, N.; Zheng, M.; Wu, Z.; Ma, K. Unlocking potential biomarkers bridging coronary atherosclerosis and pyrimidine metabolism-associated genes through an integrated bioinformatics and machine learning approach. BMC Cardiovasc. Disord. 2024, 24, 148. [Google Scholar] [CrossRef]
- Jin, L.; Chen, Q.; Hu, K.; Fan, D.; Zhang, H.; Deng, J.; Qi, W.; Yu, Q. The FTO-CMPK2 pathway in fibroblast-like synoviocytes modulates rheumatoid arthritis synovial inflammation and cartilage homeostasis via mtDNA regulation. Int. J. Biol. Sci. 2024, 20, 1617–1633. [Google Scholar] [CrossRef]
- Lai, J.H.; Hung, L.F.; Huang, C.Y.; Wu, D.W.; Wu, C.H.; Ho, L.J. Mitochondrial protein CMPK2 regulates IFN alpha-enhanced foam cell formation, potentially contributing to premature atherosclerosis in SLE. Arthritis Res. Ther. 2021, 23, 120. [Google Scholar] [CrossRef]
- Guan, X.; Zhu, S.; Song, J.; Liu, K.; Liu, M.; Xie, L.; Wang, Y.; Wu, J.; Xu, X.; Pang, T. Microglial CMPK2 promotes neuroinflammation and brain injury after ischemic stroke. Cell Rep. Med. 2024, 5, 101522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, H.; Huang, P.; Wu, G.; Wang, Q.; Luan, X.; Zhang, H.; Yu, D.; Wang, H.; Lu, D.; et al. Dracorhodin targeting CMPK2 attenuates inflammation: A novel approach to sepsis therapy. Clin. Transl. Med. 2023, 13, e1449. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zhao, Y.; Sun, C.; Ou Yang, Z.; Chen, F.; Hu, W.; Zhang, H.; Wang, Y.; Zhu, R.; Cheng, Y.; et al. ASIC1a-CMPK2-mediated M1 macrophage polarization exacerbates chondrocyte senescence in osteoarthritis through IL-18. Int. Immunopharmacol. 2023, 124, 110878. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Hu, J.; Wu, Y.; Yang, J.; Fan, H.; Li, L.; Luo, D.; Ye, Y.; Gao, Y.; et al. A link between mitochondrial dysfunction and the immune microenvironment of salivary glands in primary Sjogren’s syndrome. Front. Immunol. 2022, 13, 845209. [Google Scholar] [CrossRef]
- Kim, H.; Subbannayya, Y.; Humphries, F.; Skejsol, A.; Pinto, S.M.; Giambelluca, M.; Espevik, T.; Fitzgerald, K.A.; Kandasamy, R.K. UMP-CMP kinase 2 gene expression in macrophages is dependent on the IRF3-IFNAR signaling axis. PLoS ONE 2021, 16, e0258989. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.W.; Heldwein, K.A.; Means, T.K.; Saukkonen, J.J.; Fenton, M.J. Differential roles of Toll-like receptors in the elicitation of proinflammatory responses by macrophages. Ann. Rheum. Dis. 2001, 60 (Suppl. S3), iii6–iii12. [Google Scholar] [CrossRef]
- Fessler, M.B.; Rudel, L.L.; Brown, J.M. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr. Opin. Lipidol. 2009, 20, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Ittichaicharoen, J.; Apaijai, N.; Tanajak, P.; Sa-nguanmoo, P.; Chattipakorn, N.; Chattipakorn, S.C. Impaired mitochondria and intracellular calcium transients in the salivary glands of obese rats. Appl. Physiol. Nutr. Metab. 2017, 42, 420–429. [Google Scholar] [CrossRef]
- Division of Laboratory Animal Medicine. Serum Chemistry Reference Ranges for Mice. Available online: https://labs.dgsom.ucla.edu/dlam/files/view/docs/diagnostic-lab-services/private/serum_chemistry_reference_ranges_mice.pdf (accessed on 13 December 2024).
- Zheng, C.; Liu, Y.; Xu, C.; Zeng, S.; Wang, Q.; Guo, Y.; Li, J.; Li, S.; Dong, M.; Luo, X.; et al. Association between obesity and the prevalence of dyslipidemia in middle-aged and older people: An observational study. Sci. Rep. 2024, 14, 11974. [Google Scholar] [CrossRef]
- Bays, H.E.; Toth, P.P.; Kris-Etherton, P.M.; Abate, N.; Aronne, L.J.; Brown, W.V.; Gonzalez-Campoy, J.M.; Jones, S.R.; Kumar, R.; La Forge, R.; et al. Obesity, adiposity, and dyslipidemia: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 304–383. [Google Scholar] [CrossRef] [PubMed]
- Mathew, H.; Farr, O.M.; Mantzoros, C.S. Metabolic health and weight: Understanding metabolically unhealthy normal weight or metabolically healthy obese patients. Metabolism 2016, 65, 73–80. [Google Scholar] [CrossRef]
- Bluher, M. Metabolically healthy obesity. Endocr. Rev. 2020, 41, bnaa004. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.; Plump, A.S.; Raines, E.W.; Breslow, J.L.; Ross, R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb. 1994, 14, 133–140. [Google Scholar] [CrossRef]
- Liang, H.; Jiang, F.; Cheng, R.; Luo, Y.; Wang, J.; Luo, Z.; Li, M.; Shen, X.; He, F. A high-fat diet and high-fat and high-cholesterol diet may affect glucose and lipid metabolism differentially through gut microbiota in mice. Exp. Anim. 2021, 70, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Meyerson, N.R.; Paige, C.L.; Morrison, J.H.; Clark, S.K.; Fattor, W.T.; Decker, C.J.; Steiner, H.R.; Lian, E.; Larremore, D.B.; et al. Human mRNA in saliva can correctly identify individuals harboring acute infection. mBio 2023, 14, 01712–01723. [Google Scholar] [CrossRef]
- Nonaka, T.; Wong, D.T.W. Saliva Diagnostics. Annu. Rev. Anal. Chem. 2022, 15, 107–121. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Morton, D.W.; Smirnov, V.; Petukhov, A.; Gegechkori, V.; Kuzina, V.; Gorpinchenko, N.; Ramenskaya, G. Analytical Strategies in Lipidomics for Discovery of Functional Biomarkers from Human Saliva. Dis. Markers 2019, 2019, 6741518. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gao, P.; Wang, X.; Duan, Y. The early diagnosis and monitoring of squamous cell carcinoma via saliva metabolomics. Sci. Rep. 2014, 4, 6802. [Google Scholar] [CrossRef] [PubMed]
- Unger, C.; Lokmer, N.; Lehmann, D.; Axmann, I.M. Detection of phenol contamination in RNA samples and its impact on qRT-PCR results. Anal. Biochem. 2019, 571, 49–52. [Google Scholar] [CrossRef]
- Arumugam, P.; Chauhan, M.; Rajeev, T.; Chakraborty, R.; Bisht, K.; Madan, M.; Shankaran, D.; Ramalingam, S.; Gandotra, S.; Rao, V. The mitochondrial gene-CMPK2 functions as a rheostat for macrophage homeostasis. Front. Immunol. 2022, 13, 935710. [Google Scholar] [CrossRef]
- Madenspacher, J.H.; Draper, D.W.; Smoak, K.A.; Li, H.; Griffiths, G.L.; Suratt, B.T.; Wilson, M.D.; Rudel, L.L.; Fessler, M.B. Dyslipidemia induces opposing effects on intrapulmonary and extrapulmonary host defense through divergent TLR response phenotypes. J. Immunol. 2010, 185, 1660–1669. [Google Scholar] [CrossRef]
- Nakamura, K.; Fuster, J.J.; Walsh, K. Adipokines: A link between obesity and cardiovascular disease. J. Cardiol. 2014, 63, 250–259. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.; Sorensen, C.E.; Proctor, G.B.; Carpenter, G.H. Salivary functions in mastication, taste and textural perception, swallowing and initial digestion. Oral Dis. 2018, 24, 1399–1416. [Google Scholar] [CrossRef]
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Lindgren, A.G.; Srivastava, A.S.; Clark, A.T.; Banerjee, U. Mitochondrial function controls proliferation and early differentiation potential of embryonic stem cells. Stem Cells 2011, 29, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Ligtenberg, A.J.M.; Meuffels, M.; Veerman, E.C.I. Effects of environmental temperature on saliva flow rate and secretion of protein, amylase and mucin 5B. Arch. Oral Biol. 2020, 109, 104593. [Google Scholar] [CrossRef]
- Hardt, M.; Witkowska, H.E.; Webb, S.; Thomas, L.R.; Dixon, S.E.; Hall, S.C.; Fisher, S.J. Assessing the effects of diurnal variation on the composition of human parotid saliva: Quantitative analysis of native peptides using iTRAQ reagents. Anal. Chem. 2005, 77, 4947–4954. [Google Scholar] [CrossRef]
- Maruyama, C.L.; Monroe, M.M.; Hunt, J.P.; Buchmann, L.; Baker, O.J. Comparing human and mouse salivary glands: A practice guide for salivary researchers. Oral Dis. 2019, 25, 403–415. [Google Scholar] [CrossRef]
- Blanchard, A.A.; Ezzati, P.; Shamshurin, D.; Nistor, A.C.; Leygue, E.; Wilkins, J.A.; Myal, Y. Towards further defining the proteome of mouse saliva. Proteome Sci. 2015, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Loo, J.A.; Yan, W.; Ramachandran, P.; Wong, D.T. Comparative human salivary and plasma proteomes. J. Dent. Res. 2010, 89, 1016–1023. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Kawamoto, A.; Nakagawa, M.; Honda, Y.; Takahashi, K. Cmpk2 Gene and Protein Expression in Saliva or Salivary Glands of Dyslipidemic Mice. Appl. Sci. 2024, 14, 12004. https://doi.org/10.3390/app142412004
Zhang B, Kawamoto A, Nakagawa M, Honda Y, Takahashi K. Cmpk2 Gene and Protein Expression in Saliva or Salivary Glands of Dyslipidemic Mice. Applied Sciences. 2024; 14(24):12004. https://doi.org/10.3390/app142412004
Chicago/Turabian StyleZhang, Baiyan, Akiyo Kawamoto, Masato Nakagawa, Yoshitomo Honda, and Kazuya Takahashi. 2024. "Cmpk2 Gene and Protein Expression in Saliva or Salivary Glands of Dyslipidemic Mice" Applied Sciences 14, no. 24: 12004. https://doi.org/10.3390/app142412004
APA StyleZhang, B., Kawamoto, A., Nakagawa, M., Honda, Y., & Takahashi, K. (2024). Cmpk2 Gene and Protein Expression in Saliva or Salivary Glands of Dyslipidemic Mice. Applied Sciences, 14(24), 12004. https://doi.org/10.3390/app142412004






