From Waste to Strength: Applying Wastepaper, Fungi and Bacteria for Soil Stabilization
Abstract
1. Introduction
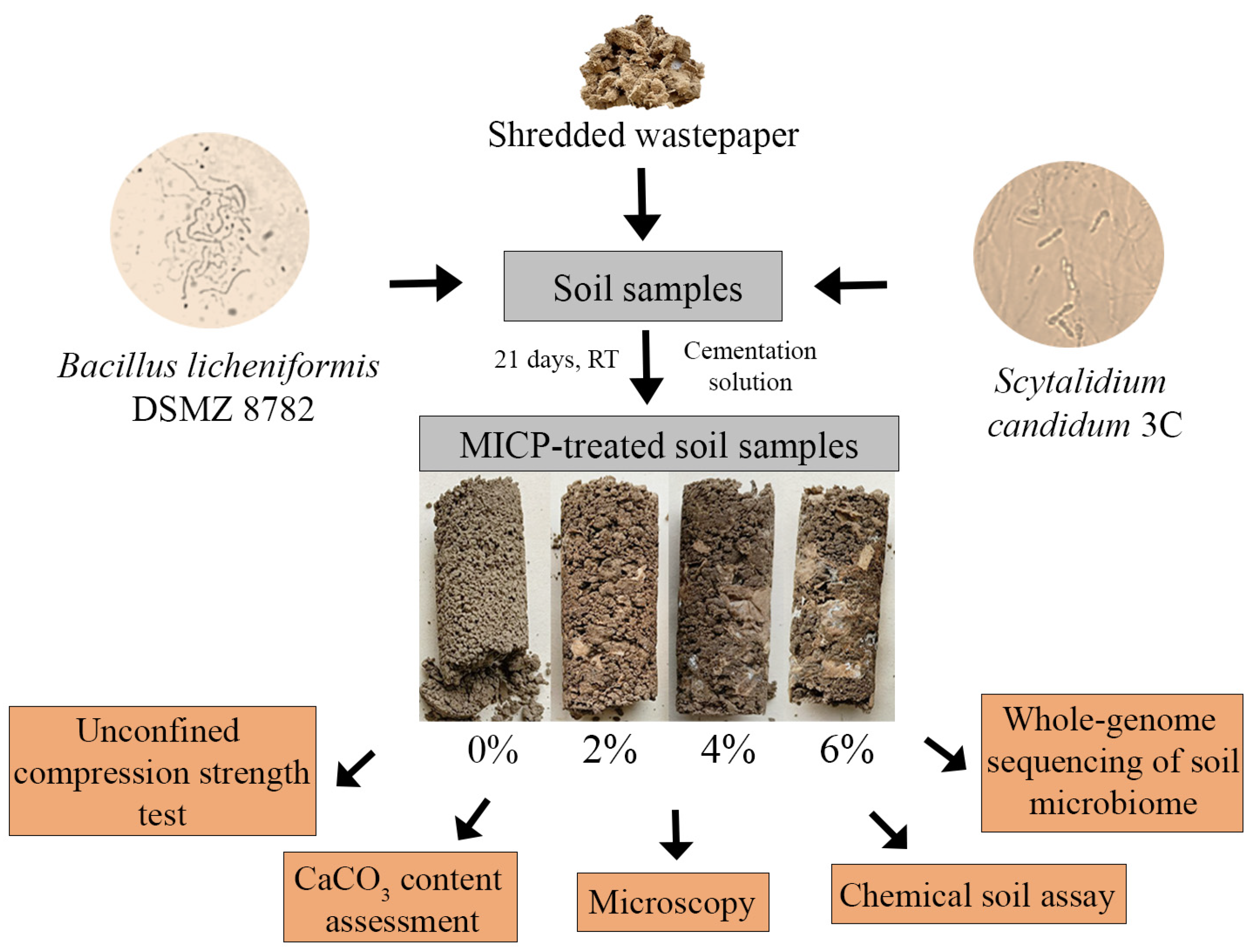
2. Materials and Methods
2.1. Soil and Wastepaper
2.1.1. Soil Characteristics
2.1.2. Wastepaper
2.2. Microorganisms and Cultivation Conditions
2.2.1. Bacterial Strain
2.2.2. Fungal Strain
2.3. Soil Stabilization Using Microorganisms
2.3.1. Sample Preparation
- Bacterial suspension (4 mL)—sample Bact;
- Fungal biomass (10 g wet biomass)—sample Fng;
- Bacterial suspension (4 mL) и fungal wet biomass (10 g)—sample Mix.
2.3.2. Sample Composition Analysis
2.3.3. Assessment of Unconfined Compression Strength
2.3.4. Assessment of the Calcium Carbonate Content
2.3.5. DNA Extraction, Preparation and Sequencing of Whole-Genome Libraries
2.3.6. Bioinformatics Analysis
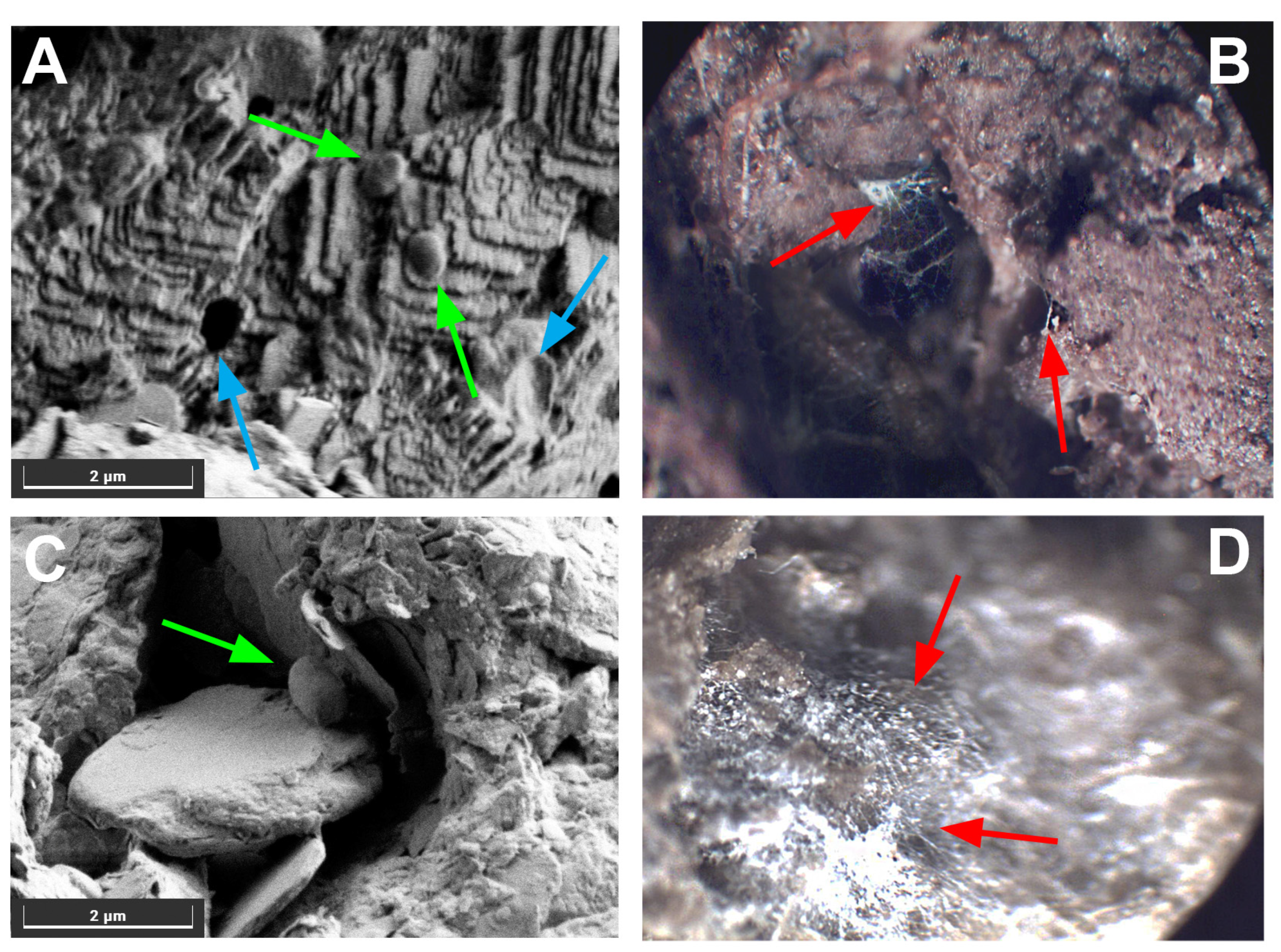
2.3.7. Microscopy
2.4. Statistics
3. Results and Discussion
3.1. Properties of MICP-Treated Soil Samples with Wastepaper Additives
3.1.1. Compressive Strength of Soil Samples with Different Wastepaper Contents Treated with Bacterial and Fungal Biomasses
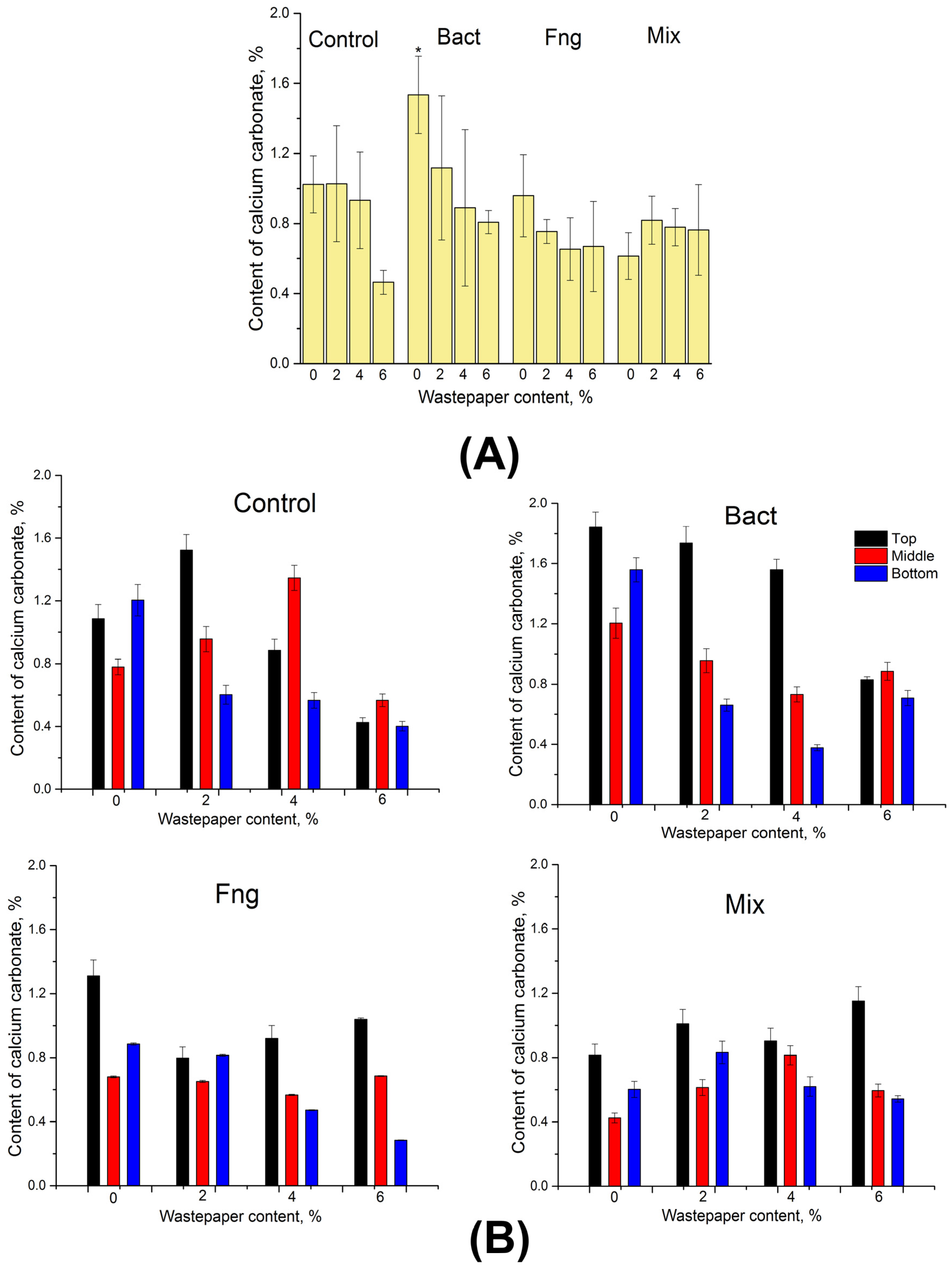
3.1.2. Formation of Calcium Carbonate in Soil Samples with Different Wastepaper Contents, Treated with Bacterial and Fungal Biomasses
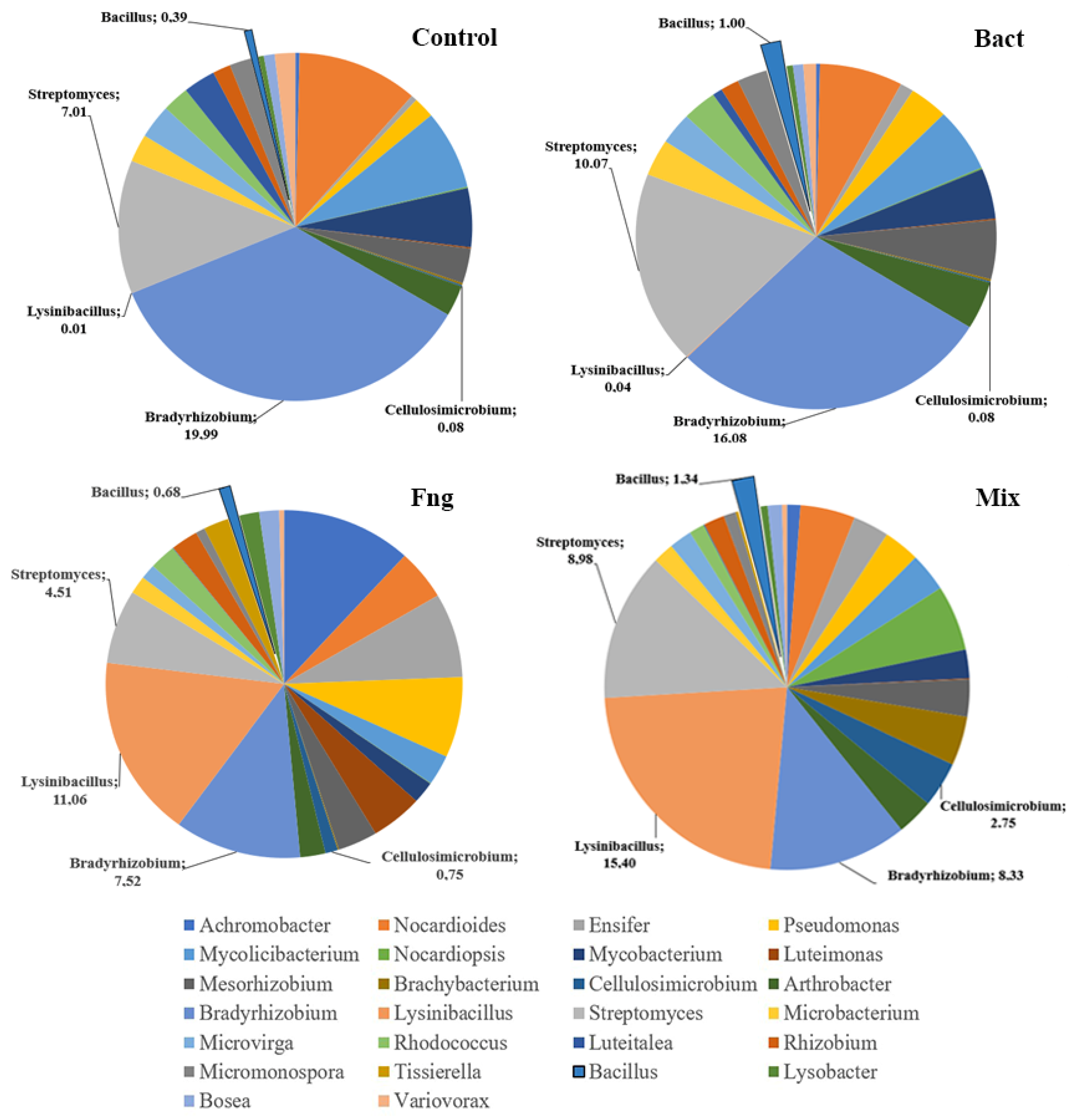
3.1.3. Results of Whole-Genome Sequencing of the Microbiome of Soil Samples Treated with Bacterial and Fungal Biomasses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Environment Programme. Emissions Gap Report 2023: Broken Record—Temperatures Hit New Highs, Yet World Fails to Cut Emissions (Again); United Nations Environment Programme: Nairobi, Kenya, 2023; ISBN 9789280740981. [Google Scholar]
- Chavan, S.; Yadav, B.; Atmakuri, A.; Tyagi, R.D.; Wong, J.W.C.; Drogui, P. Bioconversion of Organic Wastes into Value-Added Products: A Review. Bioresour. Technol. 2022, 344, 126398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Tang, C.-S.; Jiang, N.-J.; Pan, X.-H.; Liu, B.; Wang, Y.-J.; Shi, B. Microbial-induced Carbonate Precipitation (MICP) Technology: A Review on the Fundamentals and Engineering Applications. Environ. Earth Sci. 2023, 82, 229. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Oualha, M.; Ashfaq, M.Y.; Suleiman, M.T.; Zouari, N. Isolation, Differentiation and Biodiversity of Ureolytic Bacteria of Qatari Soil and Their Potential in Microbially Induced Calcite Precipitation (MICP) for Soil Stabilization. RSC Adv. 2018, 8, 5854–5863. [Google Scholar] [CrossRef]
- Han, J.; Lian, B.; Ling, H. Induction of Calcium Carbonate by Bacillus Cereus. Geomicrobiol. J. 2013, 30, 682–689. [Google Scholar] [CrossRef]
- Krajewska, B. Urease-Aided Calcium Carbonate Mineralization for Engineering Applications: A Review. J. Adv. Res. 2018, 13, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Du, K.; Wen, K.; Huang, W.; Amini, F.; Li, L. Sandy Soil Improvement through Microbially Induced Calcite Precipitation (MICP) by Immersion. J. Vis. Exp. 2019, 151, e60059. [Google Scholar] [CrossRef]
- Zheng, X.; Lu, X.; Zhou, M.; Huang, W.; Zhong, Z.; Wu, X.; Zhao, B. Experimental Study on Mechanical Properties of Root–Soil Composite Reinforced by MICP. Materials 2022, 15, 3586. [Google Scholar] [CrossRef]
- Sharaky, A.M.; Mohamed, N.S.; Elmashad, M.E.; Shredah, N.M. Application of Microbial Biocementation to Improve the Physico-Mechanical Properties of Sandy Soil. Constr. Build. Mater. 2018, 190, 861–869. [Google Scholar] [CrossRef]
- Li, J.; Achal, V. Self-assembled Silk Fibroin Cross-linked with Genipin Supplements Microbial Carbonate Precipitation in Building Material. Environ. Microbiol. Rep. 2023, 15, 797–808. [Google Scholar] [CrossRef]
- Zhu, T.; Dittrich, M. Carbonate Precipitation through Microbial Activities in Natural Environment, and Their Potential in Biotechnology: A Review. Front. Bioeng. Biotechnol. 2016, 4, 4. [Google Scholar] [CrossRef]
- Chuo, S.C.; Mohamed, S.F.; Mohd Setapar, S.H.; Ahmad, A.; Jawaid, M.; Wani, W.A.; Yaqoob, A.A.; Mohamad Ibrahim, M.N. Insights into the Current Trends in the Utilization of Bacteria for Microbially Induced Calcium Carbonate Precipitation. Materials 2020, 13, 4993. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, X.; Miao, L.; Wang, H.; Wu, L.; Shi, W.; Kawasaki, S. State-of-the-Art Review of Soil Erosion Control by MICP and EICP Techniques: Problems, Applications, and Prospects. Sci. Total Environ. 2024, 912, 169016. [Google Scholar] [CrossRef]
- Ezzat, S.M. A Critical Review of Microbially Induced Carbonate Precipitation for Soil Stabilization: The Global Experiences and Future Prospective. Pedosphere 2023, 33, 717–730. [Google Scholar] [CrossRef]
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial Carbonate Precipitation in Construction Materials: A Review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- Oualha, M.; Bibi, S.; Sulaiman, M.; Zouari, N. Microbially Induced Calcite Precipitation in Calcareous Soils by Endogenous Bacillus Cereus, at High PH and Harsh Weather. J. Environ. Manag. 2020, 257, 109965. [Google Scholar] [CrossRef]
- Bindschedler, S.; Cailleau, G.; Verrecchia, E. Role of Fungi in the Biomineralization of Calcite. Minerals 2016, 6, 41. [Google Scholar] [CrossRef]
- Ahmad, A.; Rautaray, D.; Sastry, M. Biogenic Calcium Carbonate: Calcite Crystals of Variable Morphology by the Reaction of Aqueous Ca2+ Ions with Fungi. Adv. Funct. Mater. 2004, 14, 1075–1080. [Google Scholar] [CrossRef]
- Menon, R.R.; Luo, J.; Chen, X.; Zhou, H.; Liu, Z.; Zhou, G.; Zhang, N.; Jin, C. Screening of Fungi for Potential Application of Self-Healing Concrete. Sci. Rep. 2019, 9, 2075. [Google Scholar] [CrossRef]
- Takey, M.; Shaikh, T.; Mane, N.; Majumder, D.R. Bioremediation of Xenobiotics: Use of Dead Fungal Biomass as Biosorbent. Int. J. Res. Eng. Technol. 2014, 3, 565–570. [Google Scholar] [CrossRef]
- El Mountassir, G.; Minto, J.M.; van Paassen, L.A.; Salifu, E.; Lunn, R.J. Applications of Microbial Processes in Geotechnical Engineering. Adv. Appl. Microbiol. 2018, 104, 39–91. [Google Scholar] [CrossRef] [PubMed]
- Amobonye, A.; Bhagwat, P.; Singh, S.; Pillai, S. Plastic Biodegradation: Frontline Microbes and Their Enzymes. Sci. Total Environ. 2021, 759, 143536. [Google Scholar] [CrossRef]
- Sukumaran, R.K.; Christopher, M.; Kooloth-Valappil, P.; Sreeja-Raju, A.; Mathew, R.M.; Sankar, M.; Puthiyamadam, A.; Adarsh, V.-P.; Aswathi, A.; Rebinro, V.; et al. Addressing Challenges in Production of Cellulases for Biomass Hydrolysis: Targeted Interventions into the Genetics of Cellulase Producing Fungi. Bioresour. Technol. 2021, 329, 124746. [Google Scholar] [CrossRef]
- Imran, M.A.; Gowthaman, S.; Nakashima, K.; Kawasaki, S. The Influence of the Addition of Plant-Based Natural Fibers (Jute) on Biocemented Sand Using MICP Method. Materials 2020, 13, 4198. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Wu, J.; Wang, G.; Wang, P.; Zheng, J.-J.; Yan, J.; Xu, L.; Yan, Y. Effect of Wool Fiber Addition on the Reinforcement of Loose Sands by Microbially Induced Carbonate Precipitation (MICP): Mechanical Property and Underlying Mechanism. Acta Geotech. 2021, 16, 1401–1416. [Google Scholar] [CrossRef]
- Wen, K.; Bu, C.; Liu, S.; Li, Y.; Li, L. Experimental Investigation of Flexure Resistance Performance of Bio-Beams Reinforced with Discrete Randomly Distributed Fiber and Bamboo. Constr. Build. Mater. 2018, 176, 241–249. [Google Scholar] [CrossRef]
- Chen, M.; Gowthaman, S.; Nakashima, K.; Komatsu, S.; Kawasaki, S. Experimental Study on Sand Stabilization Using Bio-Cementation with Wastepaper Fiber Integration. Materials 2021, 14, 5164. [Google Scholar] [CrossRef] [PubMed]
- Avramenko, M.; Nakashima, K.; Takano, C.; Kawasaki, S. Eco-Friendly Soil Stabilization Method Using Fish Bone as Cement Material. Sci. Total Environ. 2023, 900, 165823. [Google Scholar] [CrossRef] [PubMed]
- Golovkina, D.A.; Zhurishkina, E.V.; Filippova, A.D.; Baranchikov, A.E.; Lapina, I.M.; Kulminskaya, A.A. Integration of Organic Waste for Soil Stabilization through MICP. Appl. Sci. 2023, 14, 62. [Google Scholar] [CrossRef]
- Pavlov, I.Y.; Bobrov, K.S.; Sumacheva, A.D.; Masharsky, A.E.; Polev, D.E.; Zhurishkina, E.V.; Kulminskaya, A.A. Scytalidium candidum 3C Is a New Name for the Geotrichum candidum Link 3C Strain. J. Basic Microbiol. 2018, 58, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Golovkina, D.A.; Zhurishkina, E.V.; Ivanova, L.A.; Baranchikov, A.E.; Sokolov, A.Y.; Bobrov, K.S.; Masharsky, A.E.; Tsvigun, N.V.; Kopitsa, G.P.; Kulminskaya, A.A. Calcifying Bacteria Flexibility in Induction of CaCO3 Mineralization. Life 2020, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Shoba, S.A.; Rozhkov, V.A.; Alyabina, I.O.; Kolesnikova, V.M.; Urusevskaya, I.S.; Molchanov, E.N.; Stolbovoy, V.S.; Sheremet, B.V.; Konyushkov, D.E. Soil Geographic Database of Russia, 2nd ed.; Huang, P.M., Li, Y., Sumner, M.E., Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- ASTM D2487-17; Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System). ASTM International: West Conshohocken, PA, USA, 2000.
- ASTM D7012-14e1; Standard Test Methods for Compressive Strength and Elastic Moduli of Intact Rock Core Specimens Under Varying States of Stress and Temperatures. ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM D 4373; Standard Test 5 Method for Calcium Carbonate Content in Soil. ASTM International: West Conshohocken, PA, USA, 2021.
- Ignatov, K.B.; Blagodatskikh, K.A.; Shcherbo, D.S.; Kramarova, T.V.; Monakhova, Y.A.; Kramarov, V.M. Fragmentation Through Polymerization (FTP): A New Method to Fragment DNA for next-Generation Sequencing. PLoS ONE 2019, 14, e0210374. [Google Scholar] [CrossRef]
- Lu, J.; Rincon, N.; Wood, D.E.; Breitwieser, F.P.; Pockrandt, C.; Langmead, B.; Salzberg, S.L.; Steinegger, M. Metagenome Analysis Using the Kraken Software Suite. Nat. Protoc. 2022, 17, 2815–2839. [Google Scholar] [CrossRef]
- Golovkina, D.A.; Zhurishkina, E.V.; Xu, J. Field Trials of Soil Improvement Technology with a Bacterial Mixture. In Biogenic—Abiogenic Interactions in Natural and Anthropogenic Systems 2022; Springer Nature: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Qiu, R.; Tong, H.; Fang, X.; Liao, Y.; Li, Y. Analysis of Strength Characteristics of Carbon Fiber–Reinforced Microbial Solidified Sand. Adv. Mech. Eng. 2019, 11, 168781401988442. [Google Scholar] [CrossRef]
- Imran, M.A.; Nakashima, K.; Evelpidou, N.; Kawasaki, S. Durability Improvement of Biocemented Sand by Fiber-Reinforced MICP for Coastal Erosion Protection. Materials 2022, 15, 2389. [Google Scholar] [CrossRef]
- Roeselers, G.; Van Loosdrecht, M.C.M. Microbial Phytase-Induced Calcium-Phosphate Precipitation—A Potential Soil Stabilization Method. Folia Microbiol. 2010, 55, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and Soil Structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Seifan, M.; Samani, A.K.; Berenjian, A. Bioconcrete: Next Generation of Self-Healing Concrete. Appl. Microbiol. Biotechnol. 2016, 100, 2591–2602. [Google Scholar] [CrossRef]
- Pavlov, I.Y.; Eneyskaya, E.V.; Bobrov, K.S.; Polev, D.E.; Ivanen, D.R.; Kopylov, A.T.; Naryzhny, S.N.; Kulminskaya, A.A. Comprehensive Analysis of Carbohydrate-Active Enzymes from the Filamentous Fungus Scytalidium candidum 3C. Biochemistry 2018, 83, 1399–1410. [Google Scholar] [CrossRef]
- Kimeklis, A.K.; Gladkov, G.V.; Orlova, O.V.; Afonin, A.M.; Gribchenko, E.S.; Aksenova, T.S.; Kichko, A.A.; Pinaev, A.G.; Andronov, E.E. The Succession of the Cellulolytic Microbial Community from the Soil during Oat Straw Decomposition. Int. J. Mol. Sci. 2023, 24, 6342. [Google Scholar] [CrossRef] [PubMed]

| >20 mm | 20–10 mm | 10–5 mm | 5–2 mm | 2–1 mm | 1–0.5 mm | 0.5–0.25 mm | 0.25–0.1 mm | 0.1–0.05 mm | 0.05–0.01 mm | 0.01–0.005 mm | <0.005 mm |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10.5 | 3.5 | 6.0 | 3.7 | 4.0 | 5.7 | 4.4 | 15.0 | 3.8 | 3.2 | 5.5 | 45.2 |
| Chemical Element | Wastepaper | Soil with Wastepaper | |||
|---|---|---|---|---|---|
| Content of Wastepaper, % | |||||
| 0 | 2 | 4 | 6 | ||
| C | 49.07 | 4.57 | 5.46 | 6.35 | 7.24 |
| O | 50.19 | 52.52 | 52.48 | 52.43 | 52.38 |
| Na | 0.28 | 0.84 | 0.83 | 0.82 | 0.81 |
| Mg | 0.03 | 0.86 | 0.84 | 0.83 | 0.81 |
| Al | 0.05 | 5.68 | 5.57 | 5.45 | 5.34 |
| Si | 0.03 | 27.92 | 27.36 | 26.80 | 26.25 |
| P | — * | 0.10 | 0.10 | 0.10 | 0.09 |
| S | 0.13 | 0.04 | 0.04 | 0.04 | 0.05 |
| Cl | 0.01 | — | 0.00 | 0.00 | 0.00 |
| K | 0.03 | 2.40 | 2.35 | 2.31 | 2.26 |
| Ca | 0.18 | 1.00 | 0.98 | 0.97 | 0.95 |
| Ti | — | 0.60 | 0.59 | 0.58 | 0.56 |
| Mn | — | 0.09 | 0.09 | 0.09 | 0.08 |
| Fe | — | 3.33 | 3.26 | 3.20 | 3.13 |
| Cu | — | 0.02 | 0.02 | 0.02 | 0.02 |
| Zn | — | 0.03 | 0.03 | 0.03 | 0.03 |
| Pb | — | — | — | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golovkina, D.A.; Zhurishkina, E.V.; Saitova, A.T.; Bezruchko, M.V.; Lapina, I.M.; Kulminskaya, A.A. From Waste to Strength: Applying Wastepaper, Fungi and Bacteria for Soil Stabilization. Appl. Sci. 2024, 14, 11678. https://doi.org/10.3390/app142411678
Golovkina DA, Zhurishkina EV, Saitova AT, Bezruchko MV, Lapina IM, Kulminskaya AA. From Waste to Strength: Applying Wastepaper, Fungi and Bacteria for Soil Stabilization. Applied Sciences. 2024; 14(24):11678. https://doi.org/10.3390/app142411678
Chicago/Turabian StyleGolovkina, Darya A., Elena V. Zhurishkina, Alina T. Saitova, Mikhail V. Bezruchko, Irina M. Lapina, and Anna A. Kulminskaya. 2024. "From Waste to Strength: Applying Wastepaper, Fungi and Bacteria for Soil Stabilization" Applied Sciences 14, no. 24: 11678. https://doi.org/10.3390/app142411678
APA StyleGolovkina, D. A., Zhurishkina, E. V., Saitova, A. T., Bezruchko, M. V., Lapina, I. M., & Kulminskaya, A. A. (2024). From Waste to Strength: Applying Wastepaper, Fungi and Bacteria for Soil Stabilization. Applied Sciences, 14(24), 11678. https://doi.org/10.3390/app142411678








