Abstract
(1) Background: Sea fish with a high level of omega-3 very long-chain (VLC) PUFAs makes them a valuable component of a healthy diet. One of the most popular fish products is fish fingers, for which the market is still growing. The content of omega-3 VLC PUFAs in fish fingers may differ across price categories, such as premium or budget, despite being manufactured from the same fish species. Furthermore, the labelling of fish fingers typically fails to indicate the quantity of omega-3 VLC PUFAs present. It is unclear whether fish fingers can increase the amount of omega-3 VLC PUFAs in the diet. Hence, this study aimed to examine the content of omega-3 VLC PUFAs in pollock fish fingers from premium and budget price categories manufactured by the same producer and sold in supermarkets. (2) Methods: The premium fish fingers were made with pollock fillet and the budget ones with minced pollock meat. Fat content and fatty acids profile were analysed. (3) Results: The primary fatty acid found in fish fingers was oleic acid, followed by linoleic acid. Saturated fatty acids were less than 7%. This reflected the composition of frying fat, which was rapeseed oil. Fish fingers contained a dietary noticeable amount of omega-3 VLC PUFAs; for premium and budget fish fingers it was 283.01 mg and 123.44 mg per 100 g of product, respectively. (4) Conclusions: Not all fish fingers have the same nutritional value, despite being manufactured by the same producer and from the same fish species. Even though both were made from pollock, premium fish fingers were a better dietary source omega-3 EPA and DHA than a low-cost alternative. Eating three sticks (100 g) of examined pollock fish fingers, either premium or budget, can meet the recommended daily intake level of omega-3 VLC PUFAs at 100% or 50%, respectively.
1. Introduction
Sea fish has been used for hundreds of years for food preparation; from simple salted, smoked, or dried fish to highly processed, multi-component fish products, preserved by various methods, such as freezing. One of the most commonly consumed fish products is fish fingers, which are called fish sticks in the US [1,2]. The fish finger market dynamically grew from USD 12.79 billion in 2023 to USD 13.81 billion in 2024. It is expected to continue growing at a growth rate of 8.11%, reaching USD 22.09 billion by 2030 [3]. Fish finger production began and increased significantly during the 1950s in times of dynamic development of predatory sea fishing and became a popular choice among frozen fish products. Usually they are produced from whitefish, such as cod, haddock, or pollock [1,4]. Fish are caught, beheaded, skinned, gutted, filleted, and frozen in blocks or plates and kept in huge freezing units, usually onboard a factory ship. After reaching land, fish fingers are formed at the processing plant [5]. They come from fish blocks being band-sawed into rectangles roughly 7.5 cm long and 2.5 cm wide, then breaded and pre-fried. Fish fingers are often found in the frozen food section of supermarkets, where they are commonly available. For marketing purposes, fish fingers are divided into two price categories: premium and budget. Premium fish fingers are made from fish fillets, whereas budget fish fingers are made from minced fish meat, which is mixed with other ingredients. Both types of fish fingers are coated with breadcrumbs, pre-fried and frozen [6]. Usually, the weight of a single fish finger does not exceed 35 g. Fish meat makes up about 35–65% of a fish finger’s weight. Breading consists of grain flour or other products of grain milling, possibly modified by processing with the addition of salt and spices. Breading increases the crispiness, juiciness, taste, smell, and colour of fish fingers. Additionally, it protects the meat from air during frying and storage, thus reducing meat drying and lipid oxidation. This is important because fish fingers are characterized by a large surface area compared to their mass. The colour of fish fingers is affected by the temperature of the frying fat, its quality, and frying time. After frying, the products are immediately sent to freezing [7,8]. Fish fingers are usually packaged in polyethylene-coated cardboard with low water evaporation and gas permeability [9].
Sea fish and fish products are valuable element of human diet [10]. They contain important nutrients, especially full-value proteins, valuable lipids, micro- and macro-elements and B vitamins, and oily fish contain a significant amount of vitamin D. But their most important feature is omega-3 very long-chain polyunsaturated fatty acids (VLC PUFAs) [11]. There are several types of omega-3 fatty acids, but the majority of research studies focus on alpha-linolenic acid (C18:3 ALA), eicosapentaenoic acid (C20:5 EPA), docosapentaenoic acid (C22:5 DPA), and docosahexaenoic acid (C22:6 DHA). Sea fish have the highest amounts of EPA and DHA, which depends on the fat content in fish meat and ranges from approximately 200.0 mg in lean fish to 2500.0 mg in oily fish per 100 g of meat [11,12]. Plant foods such as flaxseeds, chia seeds, and walnuts are good sources of ALA [12]. The main activity in the human body is demonstrated by omega-3 VLC PUFAs, while ALA must be bioconverted to VLC forms in the cycle of enzymatic transformations of elongation and desaturation. However, the conversion of ALA to EPA is typically reported as 5%, and the conversion to DHA is even lower [13,14]. Consuming ALA alone may not confer the health benefits of consuming omega-3. Therefore, neither plants nor flesh from terrestrial animals can substitute for marine fish in the diet [15]. The exception to this rule is microalgae, which can synthesize omega-3 VLC UFAs and which currently started to be cultured and used for oil recovery [16]. Other example may be food fortification with fish or microalgal or krill oils, as well as dietary supplements containing those oils. However, still, the main sources of EPA and DHA in the diet remain fish and fish products [17].
The effects of omega-3 VLC PUFAs on the human body are beneficial, especially in reducing inflammation [18]. They are essential in the prevention and treatment of inflammatory diseases, such as Crohn’s disease, rheumatic arthritis, ulcerative colitis, lupus, psoriasis, as well as cystic fibrosis or the development of atherosclerosis and cancer [19,20]. Polyunsaturated fatty acids from the omega-3 and omega-6 groups are a component of phospholipids of cell membranes. Their mutual proportion (omega-6/omega-3 ratio) in tissues is greatly dependent on their proportion in the diet [21,22,23]. After being released from phospholipids, they become a substrate for the synthesis of eicosanoids, which affect the regulation of cardiovascular function, blood pressure, plasma triglyceride concentration, inflammatory processes and immune response, cancer development, gene expression, kidney function, and pain sensation [24,25]. In addition, omega-3 acids are essential for the proper functioning of the brain and vision. This is very essential both in childhood and in old age. It has also been shown that the consumption of foods rich in omega-3 VLC PUFAs reduces the risk of depression and autism [26,27,28].
Unfortunately, average Western diet is deficient in omega-3 PUFAs, especially omega-3 VLC PUFAs, which promotes the pathogenesis of many diseases, including cardiovascular disease, cancer, and inflammatory and autoimmune diseases [29,30,31]. However, increased levels of omega-3 PUFA in the diet exert suppressive effects. An adequate intake of EPA and DHA was established at 250 mg per day for adults, which correspond to 2 to 3 servings of sea fish per week. This recommendation is the same in the EU and the US and is considered a primary prevention of cardiovascular diseases [27,32,33]. Due to the numerous health benefits of omega-3 VLC PUFA consumption of fish and fish products can help reduce the risk of many serious non communicable diseases [34].
The amount of omega-3 VLC PUFA in fish and fish products may differ because of numerous factors [35]. One is fish species [36]. Different species of fish have different amounts of omega-3 VLC PUFAs [37]. In the case of farmed fish, the composition of the feed plays a crucial role in the content of omega-3 VLC PUFA [38]. In the case of wild fish, the catch water, the type of feed available, the age of the fish, and the season are important [39,40]. As previously mentioned, fish fingers are typically produced from lean wild white fish, such as cod, haddock, or pollock. These fish are not very high in fat, which makes them not very rich in EPA and DHA. Nevertheless, in the composition of their fat, a significant quantity of omega-3 VLC PUFAs is present (Table 1). In this regard, pollock deserves great attention. The fat composition of pollock is often overlooked, yet it possesses much higher concentration of omega-3 VLC PUFAs than all other fish species commonly utilized for dietary purposes, as well as microalgae cultivated for the recovery of oil containing high level of omega-3 VLC PUFAs. Currently wild pollock market size was valued at USD 2.1 billion in 2023 and is expected to reach USD 3.4 billion by the end of 2030 with a growth rate of 4.9% during the forecast period 2024–2030 [41]. Despite the extensive overfishing of sea fish, pollock stocks remain among the most extensive and are not under threat of extinction [42].

Table 1.
Average contents of omega-3 VLC PUFAs in meat and fat of some wild fish species, %. Based on USDA [43].
The omega-3 VLC PUFA content in highly processed fish products, such as fish fingers or fish burgers, also depends on the technological process. The frying process is the most important factor in determining the fat content and fatty acid profile of fish fingers or burgers [44,45]. This is attributed to the significant absorption of fat by breadcrumbs during frying. Other factors to consider are the percentage of fish meat in the recipe and the amount of other ingredients added to the meat when it is minced. The final content of omega-3 VLC PUFAs in fish fingers may differ across price categories such as premium or budget, despite being manufactured from the same fish species. Furthermore, the labelling of fish fingers typically fails to indicate the quantity of omega-3 VLC PUFAs present. Nonetheless, this information is important to health-conscious consumers. However, it is unclear whether fish fingers can increase the amount of omega-3 VLC PUFAs in the diet. Hence, the aim of this study was to examine the content of omega-3 VLC PUFAs in pollock fish fingers from premium and budget price categories manufactured by the same producer and sold in the supermarkets. The evaluation focused on only one producer of fish finger, as it was a top market leader in Poland and the EU. This study sought to show that fish fingers, despite originating from the same fish species and producer, could have differing omega-3 VLC PUFA amounts. The study aimed to ascertain the omega-3 VLC PUFA content of fish fingers, also because the labelling of these products lacks such information. Furthermore, it was aimed to evaluate the nutritional value of the examined fish fingers in terms of their fat content and composition.
2. Materials and Methods
2.1. Materials
The material used in this work was two types of frozen fish fingers produced by the leader in the Polish frozen fish market and one of the largest companies of this type in the EU. This company makes frozen fish that is available in most European countries. Wild pollock fish fingers from two price ranges, premium and budget, were chosen for the study. The premium fish fingers were made from pollock fillet meat, while the budget ones were made from minced pollock meat. Both types of fish fingers were breaded, pre-fried, and deeply frozen.
Premium and budget fish fingers differed in terms of composition declared by the producer. The composition of budget fish fingers was as follows: minced pollock meat (36%), wheat flour, rapeseed oil, water, potato starch, onion, rice grits, potato flakes, wheat fibre, salt, spices, yeast. Composition of premium fish fingers: pollock fillet (65%), wheat flour, rapeseed oil, potato starch, water, salt, spices, yeast. The recipe for both products contained 15% dry breading. Declared nutritional value of 100 g of budget fish fingers: energy 260 kcal, protein 9.2 g, carbohydrates 30.7 g, fat 10.5 g, saturated fatty acids 1.0 g. Declared nutritional value of 100 g of premium fish fingers: energy 185 kcal, protein 13.0 g, carbohydrates 14.0 g, fat 8.9 g, saturated fatty acids 1.0 g. Both types of fish fingers differed significantly in the content of pollock meat. Budget fish fingers had almost half as much fish meat as premium ones. The weight of a single premium fish finger was greater than budget one (30 and 25 g, respectively). The fish fingers were bought in the three supermarkets, one package of both types from each supermarket.
2.2. Sample Preparations
The fish fingers were stored in the laboratory freezer after purchase. Three fish fingers (approximately 100 g) from each package were taken for each analysis. Before analysis, they were defrosted at ambient temperature. The breaded fish fingers, separate fish meat, and breading were examined separately. Samples were ground in a meat grinder and homogenized before being processed. For analytical purposes, homogeneous mass samples were taken. The results were the average value of three parallel determinations.
2.3. Determination of Fat Content
A standard Soxhlet extraction was performed to determine the oil content in samples. The extraction took 4 h. Petroleum ether was used as a solvent. The solvent was removed under vacuum at 50 °C, and the flask containing the extracted fat was stored under a hood for 15 min. The determination of the fat content was conducted through gravimetric methods.
2.4. Fatty Acid Analysis
Gas chromatography (GC) was used to determine the omega-3 VLC PUFA content in fish fingers. To prepare the samples for methylation, the lipid fraction was extracted using the Folch method [46]. Folch extraction avoids high temperature and long duration, typical for Soxhlet extraction. One gram of the sample that had been homogenized was further homogenized using a cold mixture of chloroform and methanol in a ratio of 2:1. The mixture was filtered, and the solid residue was washed with a mixture of chloroform and methanol, before being filtered again. Both solutions were mixed together and put in the measuring cylinder. A quarter of the filtrate mixture of deionized water was added. Subsequently, the mixture was shaken and left to separate the two phases. The lower phase of chloroform was drained and the rest of the water was removed by passing it through anhydrous Na2SO4. The chloroform was then roto-evaporated at 40 °C under vacuum. The remaining lipid fraction was covered with nitrogen, weighed, and washed out using hexane, dried again using anhydrous Na2SO4, and sealed in a vial under nitrogen.
The AOCS method Ce 1b-89 was used to prepare fatty acid methyl esters (FAMEs) with some modifications [47]. Hexane was evaporated from previously prepared samples by nitrogen flow. The obtained dry lipid fraction was saponified using a 0.5 M NaOH solution with methanol, covered with nitrogen, and heated at boiling point for 40 min. The saponified sample was transmethylated using 14% BF3 in methanol under nitrogen for 3 min. After cooling, 3 mL of hexane was added and covered with nitrogen for 30 s. Then, 40 mL of a saturated NaCl water solution was added and shaken to separate the two phases. The top layer of hexane was removed with a syringe and transferred to a thin glass tube, dried with anhydrous Na2SO4, and decanted to a small vial and capped under nitrogen.
2.5. Chromatography
For the GC analysis, 1 L of prepared FAME was injected into the chromatograph under appropriate conditions, under appropriate conditions. The internal standard was tricosanoic acid methyl ester (C23:0) from Sigma-Aldrich, Steinheim, Germany. The Agilent 6890 N chromatograph from Agilent in Böblingen, Germany, was used with a silica column called Rtx 2330 that is 100 m long and has a diameter of 0.25 mm inside. It was made by Restek Corp in Bellefonte, PA, USA. As a carrier gas, hydrogen was used at a flow rate of 0.9 mL/s at a flow rate of 0.9 mL/s. A split-splitless (50:1) injector at 235 °C and a flame-ionization detector (FID) at 250 C were used. The initial temperature of the column was 155 °C for 55 min, followed by an increase of 1.5 °C/min to a maximum temperature of 210 °C. The outcomes were further elaborated using the software Agilent ChemStation ver. G1701AA (Agilent Technologies Inc., Santa Clarao, CA, USA). Peaks reflecting specific fatty acids were identified by comparison with menhaden oil standard and FAME Mix Supelco 37 standard (both Sigma-Aldrich, Burlington, MS, USA). The composition of the fatty acids in the samples was determined by analysing the area of the peaks corresponding to distinct fatty acids using the AOCS Ce 1b-89 method [47]. Three samples of each type were analysed. Each sample was tested in triplicate.
2.6. Statistics
Statistical analysis of the results was performed using Statistica ver. 13.3 software (Stat Soft, Krakow, Poland). It was assumed that the level of statistical significance of < 0.05, where p < 0.05, indicated significant differences, while values of p > 0.05 were interpreted as insignificant. The normal distribution of the results was examined using the Shapiro–Wilk test. Levene’s test was used to check the homogeneity of variance. Based on the results of the calculations, the Student’s t-test was used to compare the fat content and fatty acids composition values of premium and budget fish fingers.
3. Results
3.1. Fat Content
The analysed samples had different levels of fat content. Premium and budget fish fingers with breading contained 7.95 g vs. 9.40 g (p = 0.0117) of fat per 100 g of product, respectively. There were 1.64 g vs. 2.43 g (p = 0.0207) of fat per 100 g of meat, and 17.85 g vs. 19.78 g (p = 0.0219) per 100 g of breading, respectively, whereas the meat contained 1.64 g vs. 2.43 g (p = 0.0105) of fat per 100 g, respectively. The breading contained more than ¾ of the total fat of the fish fingers, where it was 85% in premium and 75% in budget (p = 0.0203). In the meat it was 15% and 25% (p = 0.0194), respectively. The minced meat of budget fish fingers contained more fat than the premium, probably because of the addition of fat-binding ingredients.
3.2. Fatty Acid Composition
The fatty acids profile varied significantly depending on the sample analysed: whole fish finger, meat, or breading. The level of saturated, monounsaturated, and polyunsaturated fatty acids in the fat fraction of both premium and budget breaded fish fingers was similar. The predominant fatty acid in the whole fish fingers samples was oleic acid, then linoleic acid, and linolenic acid (Table 2). Fat located in breading was frying fat. The predominant fatty acids in breading fat sample were oleic (62.04–63.14%), then linoleic (22.44–23.36%), and some linolenic and palmitic acids. Saturated fatty acids were less than 7%. The total trans fatty acids were at least below 1%. This reflected the composition of rapeseed oil as frying fat. However, omega-3 VLC PUFA levels were different. It was 3.56% vs. 1.26% (p = 0.0120) of total fatty acids in premium and budget fish fingers with breading, respectively. In fish finger meat (samples with breading removed) the predominant fatty acids in fat samples of premium fish fingers were EPA and DHA omega-3 VLC PUFA, whereas in budget samples it was oleic acid (C18:1) and linoleic acid (C18:2n-6). The omega-3 VLC PUFA content in the fat extracted from meat of premium and budget fish fingers was 43.08% vs. 9.47% (p = 0.0010), respectively. Figure 1 and Figure 2 show examples of chromatograms of premium and budget fish finger meat samples.

Table 2.
Fatty acid composition of fat extracted from fish fingers; % of total fatty acids.
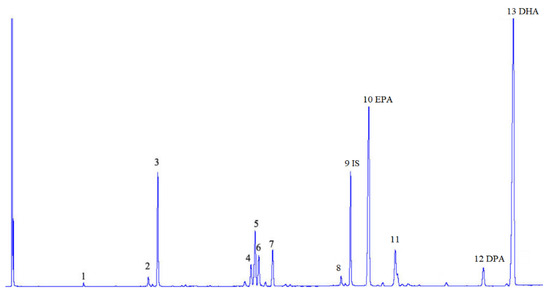
Figure 1.
Chromatogram of fatty acid methyl ester (FAME)—a sample of lipids extracted from meat of premium fish fingers (with breading removed) analysis: 1—C14:0, 2—C16:1, 3—C16:0, 4—C18:2n-6, 5—C18:1, 6—C18:3n-3, 7—C18:0, 8—20:4n-6, 9—IS internal standard C23:0, 10—EPA C20:5n-3, 11—C24:1, 12—DPA C22:5n-3, 13—DHA C22:6n-3.
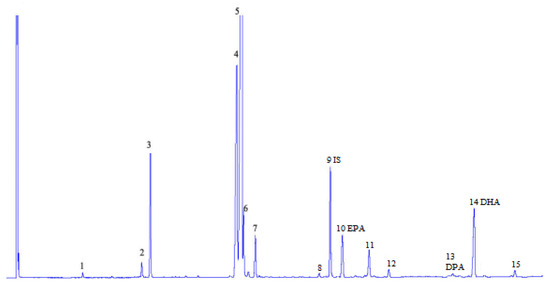
Figure 2.
Chromatogram of fatty acid methyl ester (FAME)—a sample of lipids extracted from meat of budget fish fingers (with breading removed) analysis: 1—C14:0, 2—C16:1, 3—C16:0, 4—C18:2n-6, 5—C18:1, 6—C18:3n-3, 7—C18:0, 8—20:4n-6, 9—IS internal standard C23:0, 10—EPA C20:5n-3, 11—C24:1, 12—C22:0, 13 DPA C22:5n-3, 14—DHA C22:6n3, 15—C24:1.
In the entire product, 100 g of premium and budget fish fingers with breading contained approximately 283.01 mg vs. 123.44 mg (p = 0.0011) of omega-3 VLC PUFA (Figure 3). In premium fish fingers, EPA, DPA, and DHA were 93.01 mg, 32.59 mg, and 157.41 mg per 100 g of product, respectively. In budget ones, it was 41.36 mg, 5.00 mg, and 77.08 mg, respectively, and these values were significantly different in comparison to premium fish fingers (p = 0.0021, p = 0.0018, p = 0.0012, respectively). Total omega-3 PUFAs, including ALA, in premium and budget fish fingers were 570.01 mg vs. 523.58 mg (p = 0.2992) per 100 mg of product, respectively, whereas the total omega-6 PUFAs were 1859.50 mg vs. 2019.12 mg (p = 0.2644), respectively. The ratio of polyunsaturated to saturated fatty acids PUFA/SFA (P/S) in premium and budget fish fingers was approximately 4.3 and 4.2 (p = 0.2111), respectively. It is worth noting that P/S values ≥ 2 and so the evaluated fish fingers may be considered hypocholesterolemic [31]. Due to the lower proportion of fish fillet meat in 100 g of budget fish fingers than in premium (36% vs. 65%, respectively), the omega-3 VLC PUFA concentration was two times less in the budget than premium products.
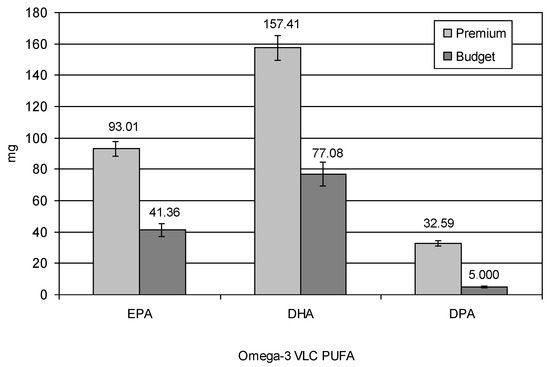
Figure 3.
Omega-3 VLC PUFAs in 100 g of fish fingers with breading, mg (all values indicated significant differences at p < 0.05).
4. Discussion
Fish fingers are very rarely mentioned in the scientific literature. There are also few reports regarding the importance of pollock in the diet, and only one report on fish fingers from pollock [37]. This makes it difficult to discuss the results. Even nutrient databases, like the USDA Nutrient Database, do not list it [43]. One example is the study of Strobel et al. [37], which analysed omega-3 VLC PUFAs in fish products, and pollock fish fingers as well. It analysed that pollock fish fingers only contained 65% of the fillet meat corresponding to premium fish fingers in our study. The amount of omega-3 VLC PUFAs in fish fingers with breading was 2.09% of total fatty acids and 164.06 mg per 100 g of product. In our study, those values were higher 3.46% of total fatty acids and 283.01 mg per 100 g of product. However, in fish fingers with breading removed, Strobel reported omega-3 VLC PUFAs of 32.08% of total fatty acids, but in our study it was 43.08%. In the case of total omega-6 PUFAs, it was 51.5% and 23.4%, respectively. Due to the lower proportion of fish fillet meat in 100 g of budget fish fingers than in premium (36% vs. 65%, respectively), the omega-3 VLC PUFA concentration was two times less in budget than premium products. Both studies showed noticeable dietary amounts of omega-3 VLC PUFAs in pollock fish fingers. The ratio of polyunsaturated to saturated fatty acids PUFA/SFA (P/S) in premium and budget fish fingers was approximately 4.3 and 4.2. P/S values were ≥2 making the evaluated fish fingers hypocholesterolemic [31]. Furthermore, a dose of 246.45 mg of EPA and DHA, established in 100 g of premium fish fingers, is considered a primary prevention of cardiovascular diseases [27,32,33]. This indicates that pollock fish fingers may serve as a cardioprotective food. Nevertheless, it is believed that fish fingers have a low nutritional value due to their high processing and soaking in frying fat, as well as their low content of omega-3 EPA and DHA [37].
Although fish fingers are low in omega-3 VLC PUFAs, it was shown that they can supplement the diet with certain amounts of EPA and DHA. The omission of fish fingers as a valuable component of a healthy diet is due to the fact that they are derived from lean fish with a low content of omega-3 VLC PUFAs, whereas dietary guidelines recommend the consumption of oily fish. Moreover, fish fingers contained a high amount of frying fat, which makes them an energy-dense product. The share of fish fillet flesh in fish fingers is reduced by breading and frying fat that it takes up. This is the case because the proportion of fish fillet meat in the sticks is between 35% and 65% [2]. However, it remains high enough to provide a substantial quantity of EPA and DHA. The obtained results add to the knowledge about the abundance of omega-3 VLC PUFAs in food and points to the importance of pollock fish fingers in the diet. Moreover, frying fats have a poor nutritional reputation because they contain saturated fatty acids and/or trans fatty acids [48]. However, nowadays very often trans-free fats are used for industrial frying [49]. For the purpose of industrial frying of the fish fingers examined in this study, rapeseed oil was employed. Rape seed oil possesses a beneficial fatty acid profile. Similar to olive oil, rapeseed oil is rich in oleic acid and ALA, while saturated fats are below 7% [50]. Consequently, the fatty acid profile of the examined fish fingers was similar to that of rapeseed oil, with the addition of omega-3 VLC PUFAs from pollock meat. Moreover, trans fatty acids were not detected in the fish fingers. Fish fingers are a highly processed product that is currently deemed a harmful dietary component. It is not true in many cases, such as pollock fish fingers, which have high-quality protein and may contain healthy fats that protect against NCD [51]. There was a high energy content in the fish fingers examined, but they also had a high nutritional density due to the presence of beneficial fatty acids such as monounsaturated and polyunsaturated fatty acids.
There are also scant reports of wild pollock as a source of omega-3 VLC PUFAs [37,52]. However, wild pollock are caught in large quantities and has a significant share of the fish market [41]. This study showed a noticeable amount of EPA and DHA in pollock fish fingers. Consuming a modest portion of 100 g (only three sticks) of premium and budget pollock fish fingers may provide 283.01 mg and 123.44 omega-3 VLC PUFAs, which corresponds to approximately 100% and 50% of the recommended daily intake of these fatty acids. A standard 200-g portion of fish fingers could provide approximately 500 mg and 250 mg. No plant or terrestrial animal food can provide such a substantial quantity of omega-3 VLC PUFAs, except for those that have been fortified with fish oil. Naturally, fish fingers prepared from pollock, which is a lean fish, contained a lower amount of EPA and DHA than dishes prepared with oily fish. However, it was still sufficient to consider them as a valuable source of these fatty acids.
It is important to emphasize the potential importance of pollock in one’s diet because the market for pollock is growing [41]. Pollock is described as a source of high-quality protein that has a beneficial effect on the functioning of the body. However, it is not considered as a source of omega-3 VLC PUFAs. Nonetheless, the outcomes obtained in this study suggest that pollock merits recognition in this regard as well. If fish fingers or other breaded fish products are fried in fat with a nutritionally beneficial fatty acid profile, they acquire additional nutritional value. The fish fingers examined in this study were pre-fried in rapeseed oil, rendering it the primary fat present in them. It contained between 70% and 85% of the product’s fat content. Rapeseed oil has a high content of oleic acid, which is similar to olive oil.
Another significant reason to recognize fish fingers as a source of omega-3 VLC PUFAs in the diet is the frequency of their consumption and the rapid increase of the fish fingers market [3]. Furthermore, fish fingers are generally acceptable to children, and in many cases, they are the only fish products that children are interested in consuming [1,53,54]. At the same time, omega-3 VLC PUFAs are particularly important for children’s health. It is not about preventing NCD, but rather about their beneficial impact on the proper development of the brain, learning ability, visual acuity, and counteracting allergy symptoms, such as atopic eczema [55,56]. This is why omega-3 VLC PUFAs should be provided regularly in children and adolescent diets. Pollock fish fingers are relatively common in the diet of children in kindergarten and schools in many countries [Eberle, 2021]. Usually, they occur several times a month. This is one of the cheapest fish dishes. Despite the modest amount of omega-3 VLC PUFAs present in pollock fish fingers, it suffices to meet 100% of the recommended daily intake when consuming three sticks (100 g).
Regardless of the above discussion regarding the content of omega-3 VLC PUFAs in fish fingers, it should be emphasized that the consumption of fried breaded fish carries a certain health risk. The risk concerns toxic compounds developed during frying, including Maillard reaction products, acrylic amide, and lipid peroxidation products [57,58]. This risk increases with the frequency of consumption of food containing these toxic substances. Hence, it should be recommended not to consume fried products such as fish fingers on a frequent basis. With the above, they can only be treated as a dietary supplement for omega-3 VLC PUFAs. But they cannot be the primary source of these fatty acids.
5. Conclusions
Not all fish fingers have the same nutritional value, despite being manufactured from the same fish species and by the same producer. Pollock fish fingers contained a dietary noticeable amount of omega-3 VLC PUFAs. For premium and budget fish fingers, it was 283.01 mg and 123.44 mg per 100 g of product, respectively. Even though both were made from pollock, premium fish fingers were found to contain more EPA and DHA than a low-cost alternative. Consuming three sticks (100 g) of examined premium and budget pollock fish fingers can meet the recommended daily intake of omega-3 VLC PUFAs at 100% and 50%, respectively. Health-conscious consumers who care about the levels of omega-3 PUFAs in their diet should know this information, which will enable them to make a food choice.
Author Contributions
Conceptualization, W.K.; methodology, W.K.; software, J.T.; validation, A.C.; formal analysis, J.T.; investigation, W.K.; resources, J.T.; data curation, A.C.; writing—original draft preparation, W.K.; writing—review and editing, W.K.; visualization, J.T.; supervision, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Eberle, U. The Unlikely Success of Fish Sticks. Hakai Magazine, 23 April 2021. Available online: https://hakaimagazine.com/article-short/the-unlikely-prevailing-success-of-fish-sticks/ (accessed on 10 November 2024).
- Josephson, P.R. The Ocean’s Hot Dog: The Development of the Fish Stick. Technol. Culture 2007, 49, 41–61. [Google Scholar] [CrossRef]
- Research and Markets. Fish Fingers Market Outlook Report: Industry Size, Competition, Trends and Growth Opportunities by Region, YoY Forecasts from 2024 to 2031. Available online: https://www.researchandmarkets.com/report/fish-stick (accessed on 5 October 2024).
- Josephson, P.R. Fish Sticks, Sports Bras, and Aluminum Cans: The Politics of Everyday Technologies; Johns Hopkins University Press: Baltimore, MD, USA, 2015. [Google Scholar]
- Sen, D.P. Advances in Fish Processing Technology; Allied Publishers Private Limited: New Delhi, India, 2005. [Google Scholar]
- Mishra, R. Handbook on Fish Processing and Preservation; CRC Press: Bocca Ratton, FL, USA, 2022. [Google Scholar]
- Boziaris, I.S. Seafood Processing: Technology, Quality and Safety; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Borda, D.; Nicolau, A.I.; Raspor, P. Trends in Fish Processing Technologies; CRC Press: Bocca Ratton, FL, USA, 2017. [Google Scholar]
- Kontominas, M.G.; Badeka, A.V.; Kosma, I.S.; Nathanailides, C.I. Recent Developments in Seafood Packaging Technologies. Foods 2021, 10, 940. [Google Scholar] [CrossRef]
- Chen, J.; Jayachandran, M.; Bai, W.; Xu, B. A critical review on the health benefits of fish consumption and its bioactive constituents. Food Chem. 2022, 369, 130874. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Marine Omega-3 (N-3) Fatty Acids for Cardiovascular Health: An Update for 2020. Int. J. Mol. Sci. 2020, 21, 1362. [Google Scholar] [CrossRef]
- Curfman, G. Do Omega-3 Fatty Acids Benefit Health? JAMA. 2020, 324, 2280–2281. [Google Scholar] [CrossRef]
- Anderson, B.M.; Ma, D.W.L. Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis. 2009, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Rittenhouse, M.A.; Barringer, N.D.; Jaffe, D.A.; Morogiello, J.M.; Kegel, J.L.; McNally, B.A.; Deuster, P.A. Omega-3 Index improves after increased intake of foods with omega-3 polyunsaturated fatty acids among US service academy cadets. Nutr. Res. 2023, 117, 30–37. [Google Scholar] [CrossRef]
- Karlsson, T.; Rosendahl-Riise, H.; Dierkes, J.; Drevon, C.A.; Tell, G.S.; Nygård, O. Associations between fish intake and the metabolic syndrome and its components among middle-aged men and women: The Hordaland Health Study. Food Nutr. Res. 2017, 61, 1347479. [Google Scholar] [CrossRef]
- Perdana, B.A.; Chaidir, Z.; Kusnanda, A.J.; Dharma, A.; Syafrizayanti, I.J.Z.; Bayu, A.; Putra, M.Y. Omega-3 fatty acids of microalgae as a food supplement: A review of exogenous factors for production enhancement. Algal. Res. 2021, 60, 102542. [Google Scholar] [CrossRef]
- Kolanowski, W.; Stołyhwo, A.; Grabowski, M. Fatty acid composition of selected fresh water gammarids (Amphipoda, Crustacea)—A potentially innovative source of n-3 LC PUFA. J. Am. Oil Chem. Soc. 2007, 84, 827–833. [Google Scholar] [CrossRef]
- Jamioł-Milc, D.; Biernawska, J.; Liput, M.; Stachowska, L.; Domiszewski, Z. Seafood Intake as a Method of Non-Communicable Diseases (NCD) Prevention in Adults. Nutrients 2021, 13, 1422. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Richter, C.K.; Bowen, K.J.; Skulas-Ray, A.C.; Jackson, K.H.; Petersen, K.S.; Harris, W.S. Recent clinical trials shed new light on the cardiovascular benefits of omega-3 fatty acids. Method Debakey Cardiovasc. J. 2019, 15, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, D.; Lavie, C.J.; Elagizi, A.; Milani, R.V. Update on Omega-3 Polyunsaturated Fatty Acids on Cardiovascular Health. Nutrients 2022, 14, 5146. [Google Scholar] [CrossRef]
- Medoro, A.; Buonsenso, A.; Centorbi, M.; Calcagno, G.; Scapagnini, G.; Fiorilli, G.; Davinelli, S. Omega-3 Index as a Sport Biomarker: Implications for Cardiovascular Health, Injury Prevention, and Athletic Performance. J. Funct. Morphol. Kinesiol. 2024, 9, 91. [Google Scholar] [CrossRef]
- Elagizi, A.; Lavie, C.J.; Marshall, K.; DiNicolantonio, J.J.; O’Keefe, J.H.; Milani, R.V. Omega-3 polyunsaturated fatty acids and cardiovascular health: A comprehensive review. Prog. Cardiovasc. Dis. 2018, 61, 76–85. [Google Scholar] [CrossRef]
- Rimm, E.B.; Appel, L.J.; Chiuve, S.E.; Djousse, L.; Engler, M.B.; Kris-Etherton, P.M.; Mozaffarian, D.; Siscovick, D.S.; Lichtenstein, A.H. Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: A science advisory from the American Heart Association. Circulation 2018, 138, e35–e47. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Del Gobbo, L.; Tintle, N.L. The omega-3 index and relative risk for coronary heart disease mortality: Estimation from 10 cohort studies. Atherosclerosis 2017, 262, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Stanton, A.V.; James, K.; Brennan, M.M.; O’Donovan, F.; Buskandar, F.; Shortall, K.; El-Sayed, T.; Kennedy, J.; Hayes, H.; Fahey, A.G. Omega-3 index and blood pressure responses to eating foods naturally enriched with omega-3 polyunsaturated fatty acids: A randomized controlled trial. Sci. Rep. 2020, 10, 15444. [Google Scholar] [CrossRef] [PubMed]
- Dretsch, M.N.; Johnston, D.; Bradley, R.S.; MacRae, H.; Deuster, P.A.; Harris, W.S. Effects of omega-3 fatty acid supplementation on neurocognitive functioning and mood in deployed U.S. soldiers: A pilot study. Mil. Med. 2014, 179, 396–403. [Google Scholar] [CrossRef]
- Vannice, G.; Rasmussen, H. Position of the academy of nutrition and dietetics: Dietary fatty acids for healthy adults. J. Acad. Nutr. Diet. 2014, 114, 136–153. [Google Scholar] [CrossRef]
- Walker, R.E.; Jackson, K.H.; Tintle, N.L.; Shearer, G.C.; Bernasconi, A.; Masson, S.; Latini, R.; Heydari, B.; Kwong, R.Y.; Flock, M.; et al. Predicting the effects of supplemental EPA and DHA on the omega-3 index. Am. J. Clin. Nutr. 2019, 110, 1034–1040. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J. The Importance of Maintaining a Low Omega-6/Omega-3 Ratio for Reducing the Risk of Autoimmune Diseases, Asthma, and Allergies. Mo. Med. 2021, 118, 453–459. [Google Scholar] [PubMed]
- Camacho-Munoza, D.; Niven, J.; Kucuk, S.; Cucchi, D.; Certo, M.; Jones, S.W.; Fischer, D.P.; Mauro, C.; Nicolaou, A. Omega-3 polyunsaturated fatty acids reverse the impact of western diets on regulatory T cell responses through averting ceramide-mediated pathways. Biochem. Pharm. 2022, 204, 115211. [Google Scholar] [CrossRef] [PubMed]
- Willet, W.C. Dietary fats and coronary heart disease. J. Intern. Med. 2012, 272, 13–24. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th ed.; U.S. Government Printing Office: Washington, DC, USA, 2015. [Google Scholar]
- Laukkanen, J.A.; Bernasconi, A.A.; Lavie, C.L. Bringing the Potential Benefits of Omega-3 to a Higher Level. Mayo Clinic Proc. 2024, 99, 520–523. [Google Scholar] [CrossRef]
- Rodrigues, M.; Rosa, A.; Almeida, A.; Martins, R.; Ribeiro, T.; Pintado, M.; Gonçalves, R.F.S.; Pinheiro, A.C.; Fonseca, A.J.M.; Maia, M.R.G.; et al. Omega-3 fatty acids from fish by-products: Innovative extraction and application in food and feed. Food Bioprod. Process. 2024, 145, 32–41. [Google Scholar] [CrossRef]
- Ackman, R.G. Variability of fatty acids and lipids in seafoods. Omega-3 News 1990, 5, 1–4. [Google Scholar]
- Strobel, C.; Jahreis, G.; Kuhnt, K. Survey of n- 3 and n-6 polyunsaturated fatty acids in fish and fish products. Lipids Health Dis. 2012, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Stone, D.A.J.; Oliveira, A.C.M.; Ross, C.F.; Plante, S.; Smiley, S.; Bechtel, P.; Hardy, R.W. The effects of phase-feeding rainbow trout (Oncorhynchus mykiss) with canola oil and Alaskan pollock fish oil on fillet fatty acid composition and sensory attributes. Aquacult. Nutr. 2011, 17, 521–529. [Google Scholar] [CrossRef]
- Tufan, B.; Koral, S.; Kose, S. Changes during fishing season in the fat content and fatty acid profile of edible muscle, liver and gonads of anchovy (Engraulis encrasicolus) caught in the Turkish Black Sea. Int. J. Food Sci. Tech. 2011, 46, 800–810. [Google Scholar] [CrossRef]
- Olgunoglu, I.A. Review on Omega-3 (n-3) Fatty Acids in Fish and Seafood. J. Biol. Agricult. Healthcare 2017, 7, 37–45. [Google Scholar]
- Deore, N. Wild Pollock Market Report 2024 (Global Edition). Cognitive Market Research. Available online: https://www.cognitivemarketresearch.com/wild-pollock-market-report (accessed on 2 October 2024).
- Pham, C.V.; Wang, H.C.; Chen, S.H.; Lee, J.M. The Threshold Effect of Overfishing on Global Fishery Outputs: International Evidence from a Sustainable Fishery Perspective. Fishes 2023, 8, 71. [Google Scholar] [CrossRef]
- USDA National Nutrient Database for Standard Reference, Legacy Release. Available online: https://agdatacommons.nal.usda.gov/articles/dataset/USDA_National_Nutrient_Database_for_Standard_Reference_Legacy_Release/24661818 (accessed on 18 October 2024).
- Ansorena, D.; Guembe, A.; Mendizábal, T.; Astiasarán, I. Effect of fish and oil nature on frying process and nutritional product quality. J. Food Sci. 2010, 75, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Suryati, M.A.; Azrina, A.; Ismail, A.; Nor-Khaizura, M.A.R. Review on retention of long-chain omega-3 polyunsaturated fatty acids (EPA and DHA) in fish as affected by cooking methods. Int. Food Res. J. 2022, 29, 975–990. [Google Scholar]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- American Oil Chemists’ Society. Official Method Ce 1b-89 Fatty Acid Composition by GLC-Marine Oils (Modified); AOCS: Champaign, IL, USA, 1997. [Google Scholar]
- Song, J.; Park, J.; Jung, J.; Lee, C.; Gim, S.Y.; Ka, H.; Yi, B.; Kim, M.; Kim, C.; Lee, J. Analysis of Trans Fat in Edible Oils with Cooking Process. Toxicol. Res. 2015, 31, 307–312. [Google Scholar] [CrossRef]
- Bhat, S.; Maganja, D.; Huang, L.; Wu, J.H.Y.; Marklund, M. Influence of Heating during Cooking on Trans Fatty Acid Content of Edible Oils: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 1489. [Google Scholar] [CrossRef]
- Shen, J.; Liu, Y.; Wang, X.; Bai, J.; Lin, L.; Luo, F.; Zhong, H. A Comprehensive Review of Health-Benefiting Components in Rapeseed Oil. Nutrients 2023, 15, 999. [Google Scholar] [CrossRef]
- Takada, N.; Hosomi, R.; Fukunaga, K. Processing Alaska Pollock Protein (Theragra chalcogramma) into Kamaboko Protein Mitigates Elevated Serum Cholesterol and Postprandial Glucose Levels in Obese Zucker fa/fa Rats. Foods 2022, 11, 3434. [Google Scholar] [CrossRef]
- Williams, S. Determining the Net Growth Efficiency of Eicosapentaenoic Acid and Docosahexaenoic Acid in Atlantic Pollock (Pollachius virens) Using a Mass Balance Approach Dalhousie University Halifax: Halifax, NS, Canada. 2020. Available online: https://dalspace.library.dal.ca/items/01573d43-74bf-40a1-a8bf-a3b1c1828c16 (accessed on 23 October 2024).
- Laureati, M.; Cattaneo, C.; Bergamaschi, V.; Proserpio, C.; Pagliarini, E. School children preferences for fish formulations: The impact of child and parental food neophobia. J. Sensory Stud. 2016, 31, 408–415. [Google Scholar] [CrossRef]
- Moura, A.P.; Cunha, L.M.; Azeiteiro, U.M.; Lima, R.C. Acceptance of fish and fish products by Portuguese young consumers: An exploratory study based on mothers’ evaluation. In Culinary Arts and Sciences VII Global, National and Local Perspectives; Hartwell, H.H., Lugosi, P., Edwards, J.S.A., Eds.; International Centre for Tourism and Hospitality Research Bournemouth University: Poole, UK, 2011; pp. 98–103. [Google Scholar]
- Carwile, J.L.; Butler, L.J.; Janulewicz, P.A.; Winter, M.R.; Aschengrau, A. Childhood Fish Consumption and Learning and Behavioral Disorders. Int. J. Environ. Res. Public Health 2016, 13, 1069. [Google Scholar] [CrossRef] [PubMed]
- Borasio, F.; De Cosmi, V.; D’Oria, V.; Scaglioni, S.; Syren, M.-L.E.; Turolo, S.; Agostoni, C.; Coniglio, M.; Molteni, M.; Antonietti, A.; et al. Associations between Dietary Intake, Blood Levels of Omega-3 and Omega-6 Fatty Acids and Reading Abilities in Children. Biomolecules 2023, 13, 368. [Google Scholar] [CrossRef] [PubMed]
- NurSyahirah, S.; Rozzamri, A. Effects of frying on fish, fish products and frying oil—A review. Food Res. 2022, 6, 14–32. [Google Scholar]
- Xiong, K.; Li, M.; Chen, Y.; Hu, Y.; Jin, W. Formation and Reduction of Toxic Compounds Derived from the Maillard Reaction During the Thermal Processing of Different Food Matrices. J. Food Protect. 2024, 87, 100338. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).