Featured Application
Controlling evaporation temperature could be a key factor in determining the volatile component composition, retronasal aroma release, and sensory properties of non-centrifugal cane sugar (NCS). NCS with enhanced aroma traits is typically produced at higher final heating temperatures.
Abstract
Non-centrifugal cane sugar (NCS) is prepared by evaporating sugarcane syrup to form a solidified, dehydrated brown sugar with a distinct flavor. This study investigated the effect of final evaporation temperatures (120–140 °C) on the volatile components, retronasal aroma profile, and sensory characteristics of NCS. Solid-phase microextraction–gas chromatography–mass spectrometry showed that the concentration of most volatiles, including pyrazines, furans, and furanones, in the NCS significantly increased as the evaporation temperature increased (p < 0.05). The evaporation temperature affected the aroma release from NCS, as shown in proton transfer reaction time-of-flight-mass spectrometry, with the intensity of volatile compounds detected from panelists’ noses or mouths significantly increasing after consuming NCS obtained at higher temperatures. Moreover, the intensity of aroma release in the mouth was greater than that in the nose; the most prevalent released substance, m/z 87.10, which could be derived from dihydro-2(3H)-furanone and 2,3-butanedione, rapidly decreased over seven breath cycles compared to other ions, suggesting its importance as a top-note aroma substance in NCS. In addition, the perceived roasted aroma and bitterness of the NCS obtained at higher temperatures were intensified. These findings underscore the importance of modifying the evaporation temperature on the volatile component composition, aroma release, and sensory characteristics of NCS.
1. Introduction
Non-centrifuged cane sugar (NCS) is an unrefined sweetener prepared by evaporating moisture from sugarcane juice without centrifugation. This process produces a brown-colored solidified sugar product that retains various nutrients and bioactive substances from concentrated molasses [1,2]. The heating temperature during evaporation in NCS production is an important factor that alters the color and aroma properties of NCS, and thus, its flavor quality [2]. Distinct flavors and promising biofunctions contribute to the reputation of NCS as a healthier alternative to refined sugar, and it is widely used in many regions, demonstrating its cultural and economic importance [1,3].
Volatile organic components are essential for the flavor quality of foods because most of them are responsible for the aroma and taste that define our sensory experience [4,5]. Solid-phase microextraction (SPME) coupled with gas chromatography–mass spectrometry (GC-MS) is a widely used solvent-free analytical method for analyzing volatile compounds in foods, including NCS [3,4,5]. The method can identify a broad range of volatile component groups with good sensitivity, making it useful for NCS research [3]. Volatile components, particularly those produced via nonenzymatic browning reactions (Maillard reactions), including pyrazines, furans, and furanones, represent a key focus of NCS research [2,3]. Consequently, these volatile compounds should be studied to identify the substances contributing to the improvement in the overall flavor quality and sensory attributes of NCS.
Retronasal sensation is a sensory phenomenon in which the perception of the aroma released during consumption considerably influences flavor perception [6]. It occurs when aroma substances from food or drinks travel from the mouth to olfactory receptors in the nasal cavity via the nasopharynx during chewing and swallowing [6,7]. This process enables the combination of taste and aroma sensations, resulting in a multifaceted flavor experience that can be enjoyed while consuming foods [7]. In contrast to orthonasal odor, which is directly sensed through the nostrils, retronasal aroma is perceived internally, augmenting the overall sensory experience of eating or drinking [6,7,8]. The aroma release of foods can be monitored in real-time using analytical techniques such as the proton transfer reaction (PTR), coupled with a time-of-flight mass spectrometry (TOF-MS) [9]. This procedure involves ionizing volatile compounds in the nasal or oral cavities as they are emitted during food consumption, thus identifying dynamic alterations in the contribution of the retronasal aroma to the overall flavor experience [10]. This real-time analytical method can enhance our understanding of the complex interactions between food flavor and sensory perception, making it a valuable complementary analysis to GC-MS results [9,10].
To date, several reports have focused on the volatile components of NCS [1,2,3,11], but little is known about its retronasal aroma release profile. Therefore, this study aimed to evaluate the volatile composition, retronasal aroma release profile, and sensory characteristics of NCS produced at various final evaporation temperatures. The volatile components of the NCS were analyzed using SPME-GC-MS, whereas the retronasal aroma profile was evaluated using PTR-TOF-MS. To our knowledge, this is the first study to elucidate the effects of evaporation temperature in NCS production on the volatile component composition, aroma release profiles, and sensory attributes of the produced NCS.
2. Materials and Methods
2.1. NCS Production
The NCS was produced using tabletop manufacturing equipment (Nishikawa Keisoku, Tokyo, Japan) as described previously [2,12]. Briefly, 800 g of “Nourin No. 15” cultivar sugarcane syrup (50% Brix) was heated from 25 °C to reach a final heating temperature of 120, 130, or 140 °C. The heating times were recorded to have ended 89, 91, and 95 min, respectively. The mixing blade rotation speeds during the heating and solidification–evaporation stages were 100 and 200 rpm, respectively. The final torque of the rotor during evaporation was 2.08 N·m. After completing the evaporation process, approximately 300 g of solidified NCS was produced, which was immediately crushed into a powder. All experiments were performed in triplicates. The NCS was stored at −30 °C prior to analysis.
2.2. Volatile Components Analysis Using SPME-GC-MS
The volatile components of the NCS were analyzed using SPME-GC-MS [3]. Briefly, 3 g of NCS and 20 µL of 2.5 µg/mL internal standard 1,2-dichlorobenzene-D4 (Sigma-Aldrich, St. Louis, MO, USA) were placed into a 20 mL vial and heated at 60 °C for 5 min using a CombiPAL autosampler (CTC Analytics, Zwingen, Switzerland) to extract the volatiles in the headspace using a preconditioned SPME fiber (divinylbenzene/carboxen/polydimethylsiloxane 50/30 µm; Supelco, Bellefonte, PA, USA) at 60 °C for 20 min. Analysis was performed using a 7890B GC-5977A MSD (Agilent Technologies, Santa Clara, CA, USA) with helium as the carrier gas at a linear velocity of 21 cm/s. The volatiles were desorbed from the SPME fiber into the GC inlet at 250 °C for 2 min with a split ratio of 10:1. A DB-Wax column (30 m × 0.25 mm, 0.25 μm; Agilent Technologies) was used; the oven temperature was initially set at 40 °C for 1 min, then increased to 200 °C at 3 °C/min, and finally held at 200 °C for 17 min. MS acquisition was performed for m/z 33–450 in electron ionization mode (70 eV). The ion source and transfer line temperatures were both maintained at 230 °C. Volatile compounds were identified based on their MS similarities with the MS data obtained from the NIST/EPA/NIH Library Version 17 (> 80%) and linear retention index (RI) comparisons (<|20|). The weight intensity of the peaks was calibrated to the peak area response of internal standard, and concentrations were expressed in µg/100 g. All assays were performed in triplicates.
2.3. Retronasal Aroma Release Analysis Using PTR-TOF-MS
The retronasal aroma release profile of the NCS was analyzed by ten panelists (three males and seven females, aged 22–61 years) using PTR-TOF-MS. The study adhered to the research ethics guidelines of Kyushu Sangyo University and verbal consent was obtained from all participants prior to the experiment. NCS in liquid form (15% w/v) was analyzed because the liquid form may less impede the physical flow of the sample in the panelists’ mouths compared to the powder form. Retronasal analysis was performed using a PTR-TOF 1000 ULTRA (Ionicon Analytik, Innsbruck, Austria) equipped with a capillary inlet hose and nose- and mouth-piece adapters. The MS detection limit was set at 10 ppt·V, with data recorded at one-second intervals. The ionization was performed at an E/N ratio of 122 Td (drift temperature, voltage, and pressure at 120 °C, 479.9 V, and 2.30 mbar, respectively). The panelists were asked to drink 20 mL water from a disposable paper cup, hold the water in their mouth for 5 s, and swallow it. After swallowing, they were required to put on a nose-piece adapter, immediately exhale for 2 s from the nose, and then inhale through the nose for 2 s. Exhalation–inhalation breathing was performed for seven consecutive cycles. After a 10 s break, the panelists were instructed to consume 20 mL of NCS solution and perform 2 exhalation–inhalation breathings through the nose (in-nose retronasal) for seven times. Afterward, they were asked to rest for 5 min and rinse their palates with water by gargling five times to prevent the NCS solution from remaining in their mouths. The same process was repeated for the in-mouth retronasal measurement, where the panelists were instructed to consume water or NCS solution and breathe through the mouth into a mouth-piece adapter. The room temperature during the experiment was maintained at 24 °C.
2.4. Sensory Characteristics Evaluation
The sensory characteristics of the NCS were analyzed using the unstructured line scale method [13] by 10 trained panelists as in the retronasal aroma release analysis. The panelists were asked to rate the 20 mL 15% (w/v) NCS solution supplied in disposable paper cups labeled with three-digit random numbers on an unstructured 10 cm scale anchored with the highest or lowest intensities of the sensory evaluation criteria, which were sweet aroma, roasted aroma, sweetness, bitterness, richness, and aftertaste. Water was available for the panelists to rinse their palates before each sample was evaluated.
2.5. Statistical Analysis
Statistical differences among the mean values of the groups were analyzed using Tukey’s multiple comparison test or an unpaired t-test (for two groups) (GraphPad Prism Version 9, GraphPad Software, Boston, MA, USA).
3. Results
3.1. Volatile Components of NCS
The volatile components of the NCS were greatly affected by the evaporation temperature (Table 1). Forty-seven compounds were identified in the NCS obtained using SPME-GC-MS analysis, and the total volatile component concentrations were significantly increased as the final heating temperature increased (10.37, 30.87, and 86.29 µg/100 g at 120, 130, and 140 °C, respectively; p < 0.05). The increases were generally observed in 10 volatile component groups, including Maillard reaction products such as pyrazine, furan, furanone, pyridine, pyrrole, and pyranone. The most noticeable increases were observed for 2-acetylfuran, 5-methyl-2-furanaldehyde, 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one, furfural, dihydro-2(3H)-furanone, 2-acetylpyrrole, dihydro-2-methyl-3(2H)-furanone, 2,3,5-trimethylpyrazine, 5-methyl-2-furanmethanol, and 2-furanmethanol (>90% increase from 120 to 140 °C evaporation temperature). Among them, the predominant compounds in NCS obtained at 140 °C were 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one, furfural, and 2-furanmethanol (21.05, 14.92, and 5.98 µg/100 g, respectively). Other major compounds included 2-methylpryzine, acetic acid, 2,5-dimethylpyrazine, and 2,6-dimethylpyrazine, although the content increases from 120 to 140 °C were lower than 90%. On the contrary, the content of two aldehydes (hexanal and heptanal), 2-pentylfuran, [R-(R*,R*)]-2,3-butanediol, and benzeneacetaldehyde significantly decreased as the evaporation temperature rose, whereas the chromatographic peaks of hexanal, heptanal, and 2-pentylfuran in NCS obtained at 140 °C were detected at trace levels. In contrast, 2-ethenylpyrazine, 2-furanmethyl acetate, 2-acetylpyridine, 2-acetyl-5-methylpyrazine, pyrazinamide, 2-ethenylbenzofuran, 3-phenylfuran, 2-phenyl-2-butenal, 2,5-dimethylfuran-3,4(2H,5H)-dione, and 5-hydroxymethylfurfural were not detected or found at trace levels in the NCS obtained at 120 °C.

Table 1.
Volatile components of NCS obtained at different evaporation temperatures (µg/100 g).
3.2. Retronasal Aroma Release Profiles of NCS
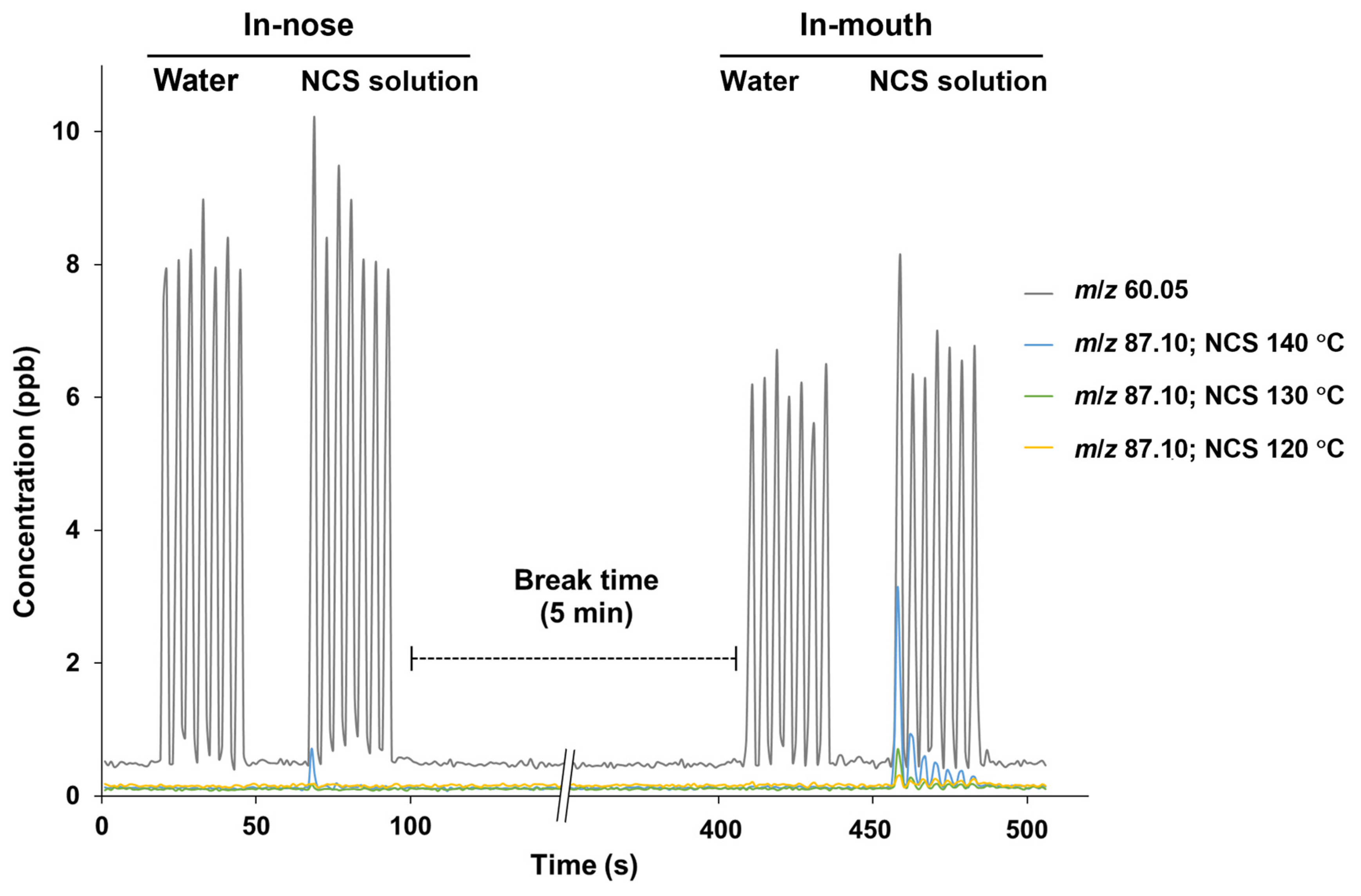
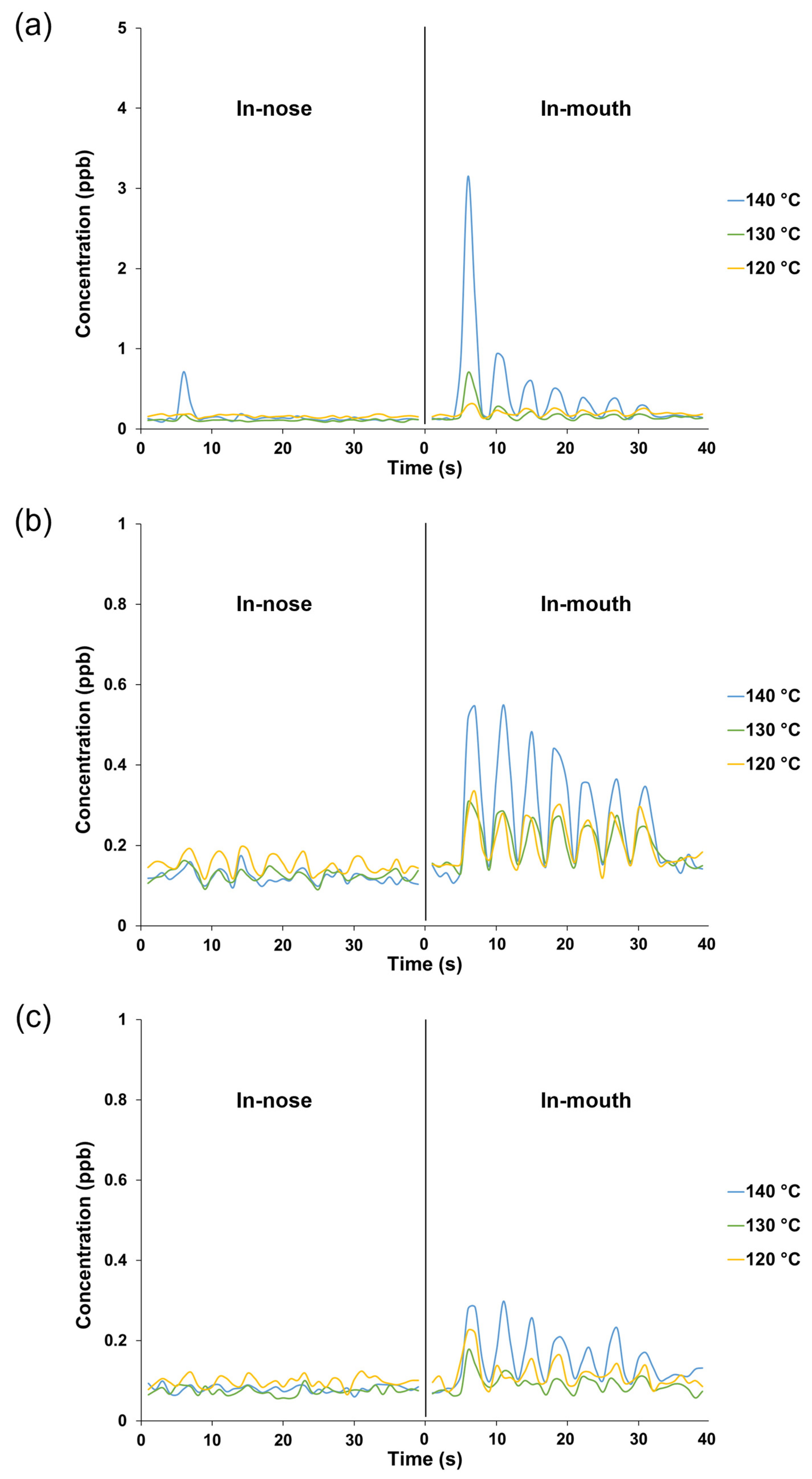
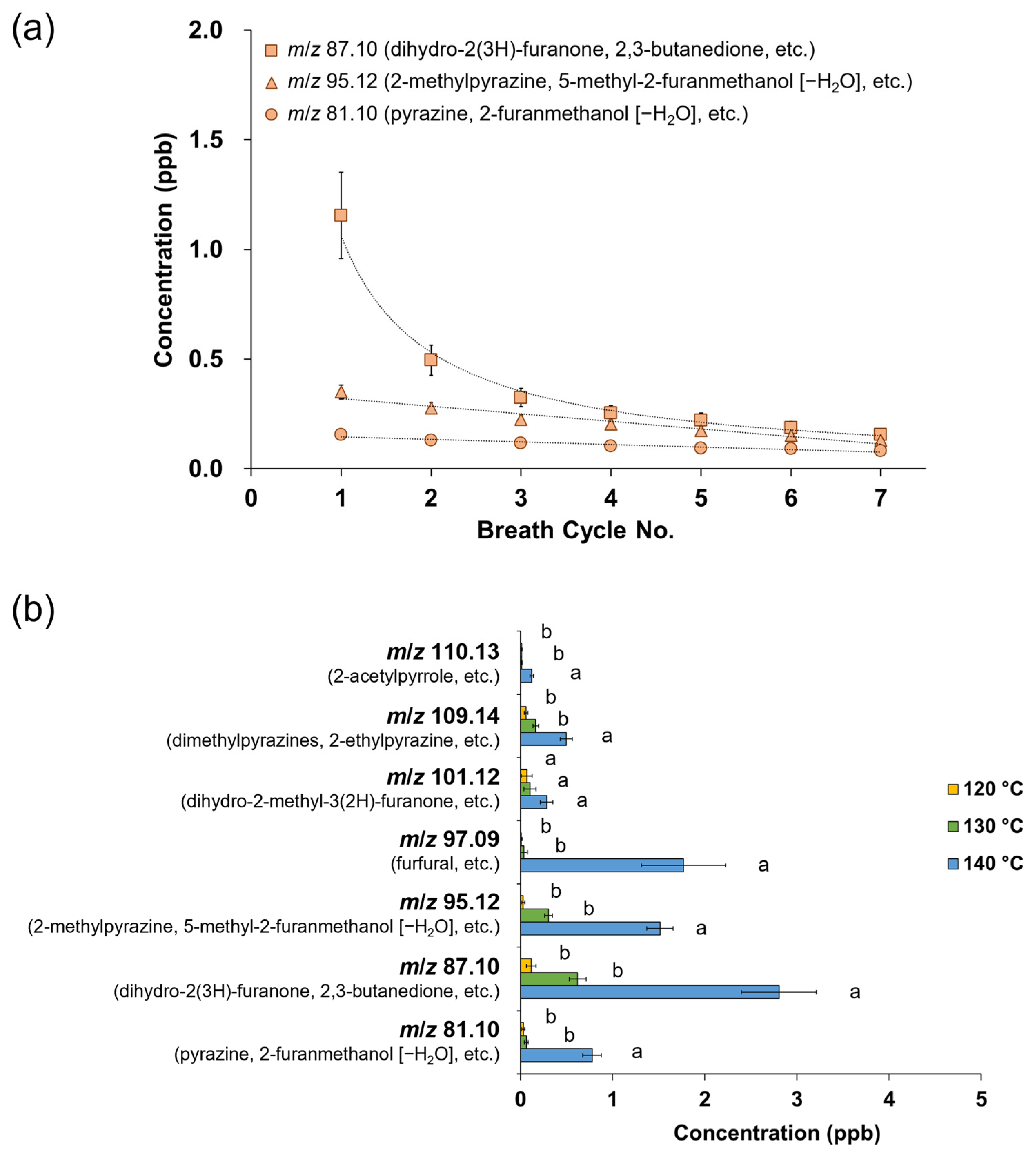
Different evaporation temperatures during NCS production affected the retronasal aroma release profiles obtained via PTR-TOF-MS analysis, wherein the respective ions were monitored from the seven breath cycles of water and NCS solution intake (Figure 1). In this analysis, the release plot of m/z 60.05 (acetone-13C) was used as a marker to indicate the progression of other detected ions from volatiles released at lower intensities. In addition to the acetone-13C ion, no additional ions of considerable intensity were detected after water intake, establishing a suitable baseline for monitoring volatile release from NCS consumption in the following stage. The NCS obtained at higher heating tended to be perceived more intensely in both the nasal cavity (in-nose) and oral cavity (in-mouth) retronasal pathways, as shown by a typical plot of the ion m/z 87.10 (detected after the released molecules collided with hydronium ions), which was monitored alongside the breath indicator ion m/z 60.05 (Figure 1 and Figure 2a). Moreover, the intensity of the released ion m/z 87.10, which could be derived from dihydro-2(3H)-furanone and 2,3-butanedione, was greater in the mouth plot than in the nose plot. The released ion concentration in the mouth decreased over seven breath cycles, whereas the ion was only detectable in the in-nose plot during the first breath cycle. The intensity of other representative ions such as m/z 95.12 (derived from 2-methylpyrazine and 5-methyl-2-furanmethanol [–H2O]) and m/z 81.10 (derived from pyrazine and 2-furanmethanol [–H2O]) also reduced over the time-course of the in-mouth aroma release plots (Figure 2b and Figure 2c, respectively). However, the declining slope of ion m/z 87.10 was greater than that of the other two ions (Figure 3a). Notably, the ion m/z 87.10 was detected at 1.16 ppb during the first breath cycle, but its concentration had declined by 57 and 34% during the second and third breaths, respectively, and its concentration was 0.15 ppb during the seventh cycle. Conversely, the ions m/z 95.12 and 81.10 were detected at lower concentrations during the first breath cycle (0.35 and 0.16 ppb, respectively), but their decline rates were slower, 21 and 17%, respectively, during the second breath cycle.
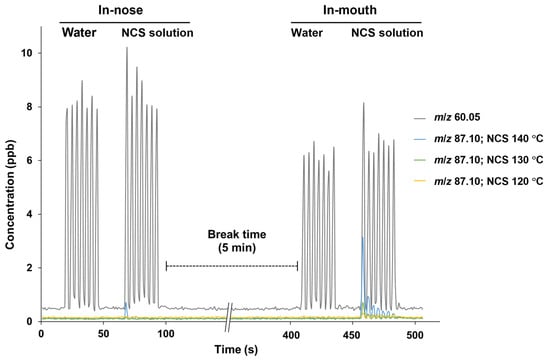
Figure 1.
Typical in-nose and in-mouth retronasal aroma release plots of m/z 60.05 (acetone-13C) and m/z 87.10 from 15% non-centrifugal cane sugar (NCS) solution intake; the NCS was produced at different evaporation temperatures.
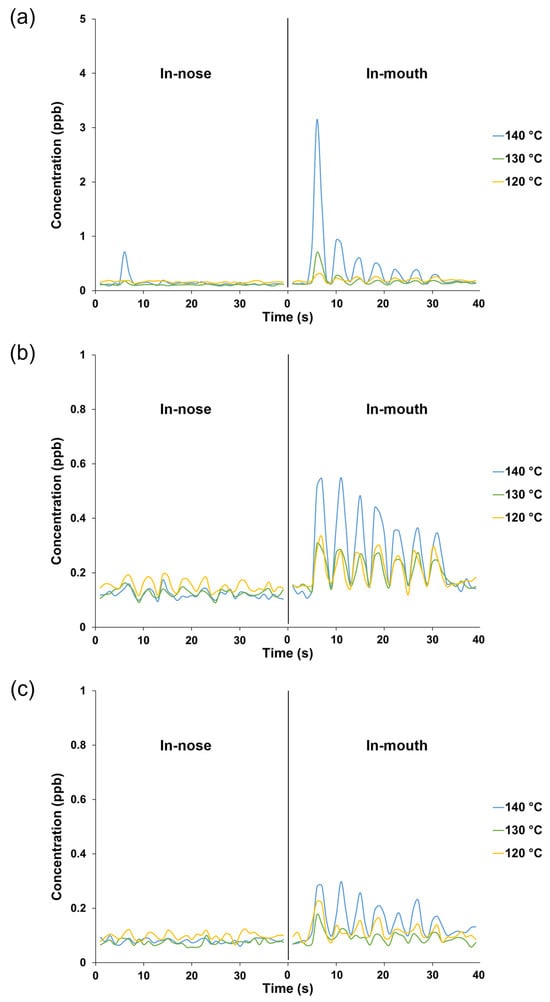
Figure 2.
Typical in-nose and in-mouth retronasal aroma release profiles of aroma compounds from 15% non-centrifugal cane sugar (NCS) solution intake; the NCS was produced at different evaporation temperatures: (a) m/z 87.10 (dihydro-2(3H)-furanone and 2,3-butanedione); (b) m/z 95.12 (2-methylpyrazine and 5-methyl-2-furanmethanol [–H2O]); (c) m/z 81.10 (pyrazine and 2-furanmethanol [–H2O]).
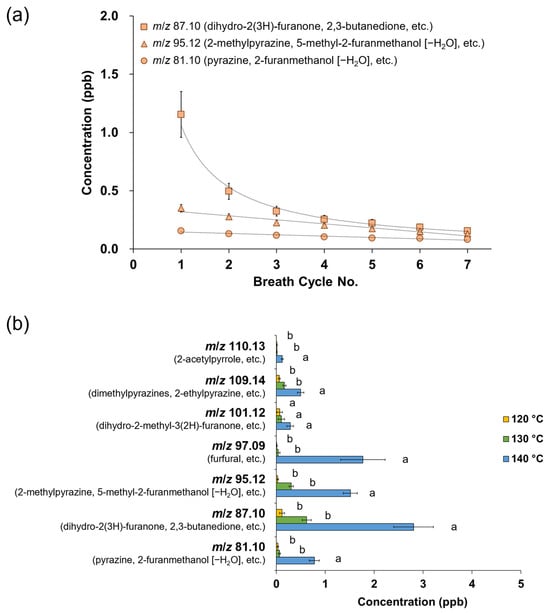
Figure 3.
(a) Time-course of the in-mouth retronasal release pattern of m/z 87.10 (dihydro-2(3H)-furanone and 2,3-butanedione), m/z 95.12 (2-methylpyrazine and 5-methyl-2-furanmethanol [–H2O]), and m/z 81.10 (pyrazine and 2-furanmethanol [–H2O]) after 15% non-centrifugal cane sugar (NCS) solution intake (a breath cycle hold for 4 s); the NCS was produced at 140 °C; (b) total intensity of in-mouth release of aroma compounds from 15% NCS solution intake over seven breath cycles (each value is expressed as the mean ± standard error of 10 panelists; different letters indicate significant differences at p < 0.05); the NCS was produced at different evaporation temperatures.
The total intensity of the in-mouth retronasal release of aroma compounds tended to increase with an increase in evaporation temperature during NCS production (Figure 3b). The volatiles were observed via detectable ions after they collided with hydronium ions. The concentration of ion m/z 87.10, which could be derived from dihydro-2(3H)-furanone (butyrolactone), 2,3-butanedione, and similar compounds, significantly increased from 0.62 to 2.80 ppb for NCS obtained at 130 and 140 °C, respectively (p < 0.05). Nonetheless, the released ions were not significantly different between the NCS obtained at 120 °C and that obtained at 130 °C. Other aroma compounds with the ions m/z 81.10, 95.12, 97.09, 109.14, and 110.13 also showed similar trends. However, the ion m/z 101.12, which could represent dihydro-2-methyl-3(2H)-furanone, was monitored at a steady level in all three types of NCS.
3.3. Sensory Characteristics of NCS
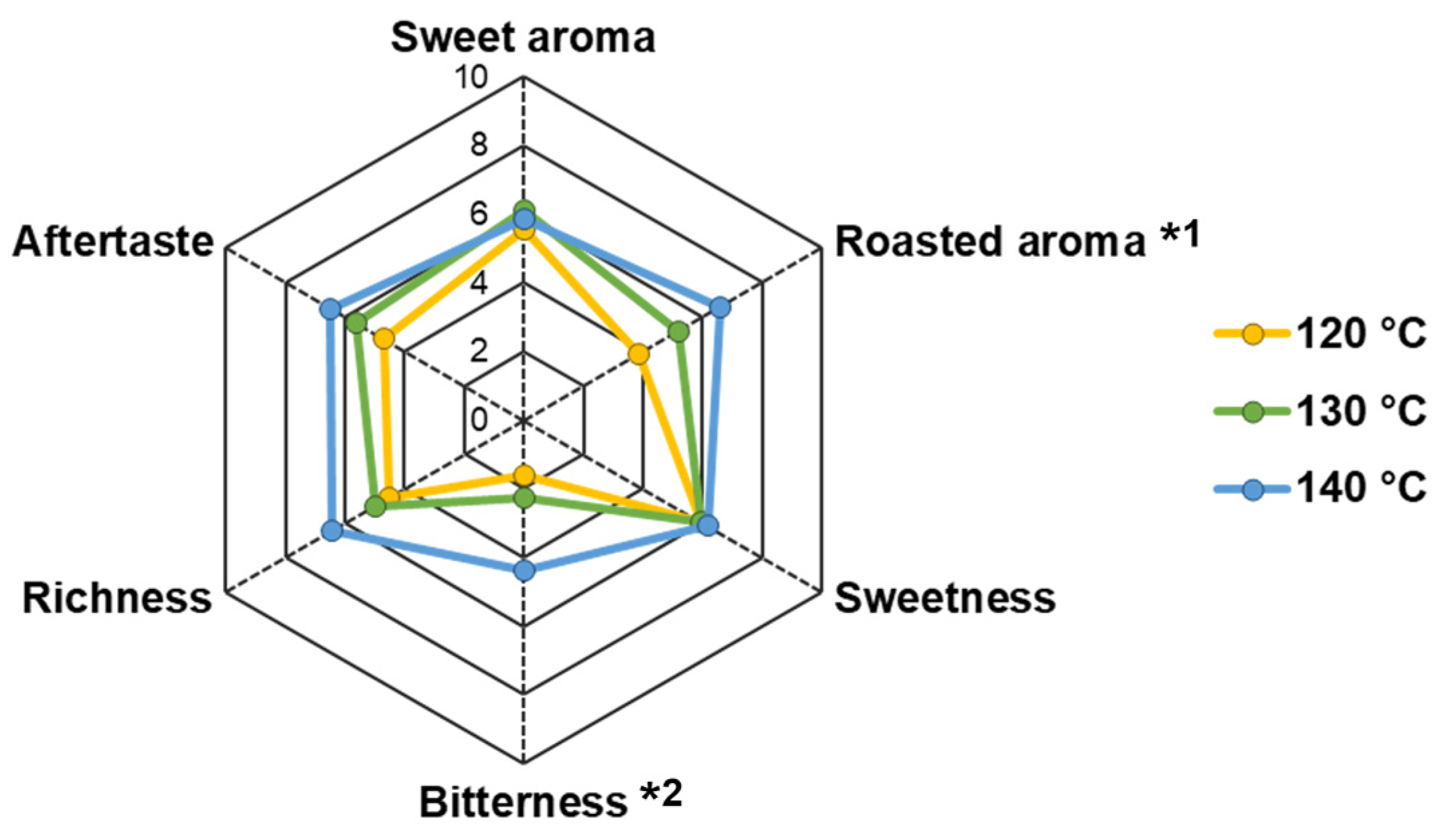
The NCS obtained at different evaporation temperatures were perceived to have varying sensory intensities, which tended to increase as the temperature increased (Figure 4). However, the sweet aroma and sweetness traits were perceived at similar levels of moderate strength (average values: 5.82 and 6.01 out of 10.00, respectively). Roasted aroma and bitterness significantly increased in NCS produced at higher temperatures (p < 0.05). The perceived roasted aroma intensity significantly increased from 3.83 to 6.57 in NCS obtained at 120 and 140 °C, respectively. Furthermore, bitterness was distinguished at lower intensities in the NCS obtained at 120 and 130 °C (1.60 and 2.26, respectively), whereas the bitter taste was significantly increased in NCS obtained at 140 °C (4.40).
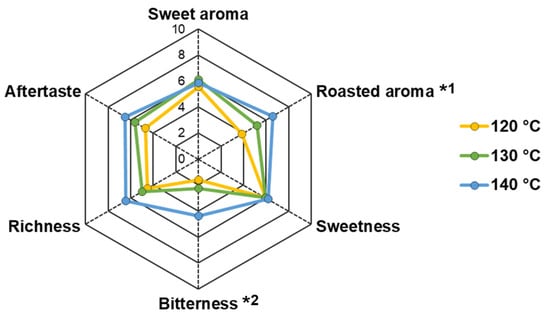
Figure 4.
Sensory characteristics of non-centrifugal cane sugar (NCS) obtained at different evaporation temperatures. Significant differences between groups were observed at p < 0.05 as represented with different letters as follows: *1 120 °C (b), 130 °C (ab), 140 °C (a); *2 120 °C (b), 130 °C (b), 140 °C (a).
4. Discussion
The final heating temperature used during the evaporation process considerably impacts the flavor quality of NCS. Higher temperatures may accelerate Maillard reaction and other thermal degradation processes, resulting in higher volatile compound concentrations [2,11,14]. This outcome is consistent with that of a previous study on modifying the heating temperature, which affected not only the physical properties of NCS, such as color and moisture content, but also its volatile Maillard reaction products [2]. In this study, the relative increases in concentrations of furans (2-acetylfuran, 5-methyl-2-furanaldehyde, furfural, 5-methyl-2-furanmethanol, and 2-furanmethanol), furanones (dihydro-2-methyl-3(2H)-furanone), a pyranone (2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one), a pyrazine (2,3,5-trimethylpyrazine), and a pyrrole (2-acetylpyrrole) were greater than 90% in the NCS obtained at the evaporation temperature of 140 °C compared to that in the NCS obtained at the lowest applied evaporation temperature. Increased levels of furans in the NCS may enhance desirable aromas, such as the nutty-sweet odor (2-acetylfuran), brown caramel-sweet odor (5-methyl-2-furanaldehyde and furfural), fruity-sweet odor (5-methyl-2-furanmethanol), and nutty-roasted odor (2-furanmethanol) [15] (the aroma descriptions were also retrieved from http://www.thegoodscentscompany.com/, accessed on 20 October 2024). Augmented buttery-toasted, sweet-maple-like, nutty-earthy-roasted, and herbaceous-metallic scents in this NCS may also be attributed to dihydro-2-methyl-3(2H)-furanone, 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one, 2,3,5-trimethylpyrazine, and 2-acetylpyrrole, respectively [15]. Among the individual compounds, 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one, furfural, and 2-furanmethanol were predominant in the NCS produced at 140 °C, emphasizing the temperature dependence of their formations. These compounds provide distinct aroma qualities to various brown sugars of different origins that greatly contribute to their overall flavor quality [15,16]. Thus, manufacturers can enhance flavor substances in NCS, particularly Maillard reaction products, by optimizing the thermal processing conditions, which are also responsible for forming darker colors in NCS and other foods [15,17]. However, the decline in certain aldehydes, such as hexanal and heptanal, suggests thermal instability as they decompose or react further at higher temperatures [18]. Similarly, the decrease in 2-pentylfuran and [R-(R*,R*)]-2,3-butanediol indicates that some of these molecules may either volatilize or break down during heating.
PTR-TOF-MS retronasal monitoring analysis revealed the real-time detection of volatiles during tasting, providing detailed insight into how aroma compounds from NCS evolve in an in vivo consumption environment. The aroma release plots indicated that different evaporation temperatures during NCS production greatly affected the progression of volatile compounds toward the olfactory receptors in the nasal cavity, which is consistent with the SPME-GC-MS results (Figure 2 and Figure 3b vs. Table 1). Thus, the volatile compounds from NCS produced at higher temperatures were more prominently perceived in both the mouth and nose retronasal pathways. Similar studies have also reported that higher roasting temperatures enhance the release of desirable volatile compounds in food products, such as bread and tea, leading to more intense aroma perceptions [17,19]. However, the intensities of the NCS volatiles that could pass through the nasopharynx and be detected from the nose by the PTR-TOF-MS instrument were limited in both duration and strength, indicating that only a few aroma-active substances can be sensed after food consumption, thus affecting part of post-intake aroma enjoyment. The results revealed that volatile compounds were more prominently retained and perceived through retronasal pathways during in-mouth monitoring; this can be influenced by complex interactions during mastication, salivation, and temperature changes [20,21]. In contrast, in-nose aroma release exhibited lower volatile concentrations, suggesting that the pathway to the nasal cavity may result in the loss of certain compounds [21].
The observed differences in the aroma release profiles of various ions in the NCS, particularly the intensity and rapid decline in the ion m/z 87.10 (dihydro-2(3H)-furanone and 2,3-butanedione) over seven breath cycles, underscore the complexity of aroma perception. This complexity could be influenced by the interactions between volatile compounds and food matrices as well as the physiological mechanisms of aroma release during ingestion [22,23]. Accordingly, the volatile compounds that discharged ion m/z 87.10 could potently act as a buttery-caramel top-note to NCS, while other compounds, such as pyrazine, 2-methylpyrazine, 2-furanmethanol, and 5-methyl-2-furanmethanol, could provide nutty-roasted sweet base aromas [15,16] (the aroma descriptions were also retrieved from http://www.thegoodscentscompany.com/, accessed on 20 October 2024). The retronasal kinetics of volatile compounds, such as the top or base (body) aroma, are important in flavor and sensory studies, particularly when using NCS as a raw material in various processed foods with different matrices, necessitating compatibility with other aroma-emitting compounds possessing distinct flavor qualities [24,25]. The dynamic nature of aroma release and its dependence on the food matrix and processing conditions have also been observed in other food products, such as wine and coffee [23,26]. This phenomenon highlights the importance of understanding aroma release kinetics and the impact of various flavor substances in shaping the overall sensory experience.
The evaporation temperature influenced the sensory attributes of the NCS. As the temperature increased, the sensory intensities of the NCS tended to increase, and the most altered attributes were roasted aroma and bitterness, which could intensify its sensory perceptions. The increase in roasted aroma, which could be attributed to Maillard reaction products such as pyrazines [2,16], aligns with the enhanced concentration of these compounds and their retronasal aroma releases in NCS produced at higher temperatures, notably NCS produced at 140 °C (Figure 4 vs. Table 1, Figure 3). This pattern demonstrates a correlation between higher heating temperatures and enhanced aroma development, most likely due to more apparent Maillard reactions and caramelization [14,17]. On the other hand, the increase in the bitterness of the NCS obtained at higher temperatures could be attributed to sugar breakdown and bitter-tasting compound formation through thermal processing [14,27]. Nonetheless, the balance between the roasted aroma and bitterness increase in the NCS might be an important factor in determining its acceptance requirements. Therefore, this study provides an important milestone for further practical applications of NCS products with varying flavor qualities and sensory properties, either consumed as table sugars or used as raw materials in processed foods and beverages.
5. Conclusions
The final evaporation temperature in NCS production influenced the volatile component composition, retronasal aroma release profile, and sensory characteristics of NCS. The quantity of volatile components, particularly those categorized as Maillard reaction products such as pyrazine, furan, furanone, pyridine, pyrrole, and pyranone, as well as the retronasal aroma releases from NCS, significantly increased with increasing evaporation temperature. The in-mouth retronasal release of the NCS solution was stronger than the in-nose release. Moreover, PTR-TOF-MS analysis identified ion m/z 87.10 (derived from dihydro-2(3H)-furanone and 2,3-butanedione) as the primary top-note odor contributors to NCS, providing a buttery-caramel aroma, whereas other volatile compounds might be perceived as its base aroma. The variation in the concentration of these aroma compounds was possibly a key contributor to improving the acceptable sensory characteristics, particularly the roasted aroma, of the NCS produced at higher evaporation temperatures. Taken together, this study elucidated the dynamic changes in the volatile composition and retronasal aroma release of NCS produced at various evaporation temperatures. These insights are valuable for optimizing the NCS processing conditions to obtain the desired flavor quality. The considerable increase in the Maillard reaction products demonstrates the potential of controlled thermal processing to enhance the sensory attributes of NCS. These findings also serve as a solid basis for further research on how thermal processing affects flavor development during large-scale NCS production.
Author Contributions
Conceptualization, Y.A., H.K. and K.W.; methodology, Y.A., M.O., H.K., G.M. and K.W.; software, Y.A. and H.K.; validation, H.K., G.M., K.T. and K.W.; formal analysis, Y.N. and M.O.; investigation, Y.A., Y.N. and M.O.; resources, G.M. and K.W.; data curation, Y.A. and Y.N.; writing—original draft preparation, Y.A. and Y.N.; writing—review and editing, Y.A. and K.W.; visualization, Y.A. and Y.N.; supervision, Y.A., H.K., K.T. and K.W.; project administration, Y.A. and K.W.; funding acquisition, Y.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Urakami Foundation for Food and Food Culture Promotion.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Research Ethics Committee of Kyushu Sangyo University (approval number and date: No. 2022-0003 and 20 September 2022, respectively).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
We thank Eriko Arakaki (University of the Ryukyus) for technical assistance with GC–MS analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Azlan, A.; Khoo, H.E.; Sajak, A.A.B.; Aizan Abdul Kadir, N.A.A.A.; Yusof, B.N.M.; Mahmood, Z.; Sultana, S. Antioxidant activity, nutritional and physicochemical characteristics, and toxicity of minimally refined brown sugar and other sugars. Food Sci. Nutr. 2020, 8, 5048–5062. [Google Scholar] [CrossRef] [PubMed]
- Asikin, Y.; Nakaza, Y.; Maeda, G.; Kaneda, H.; Takara, K.; Wada, K. Evaporation temperature alters physicochemical characteristics and volatile Maillard reaction products of non-centrifugal cane sugar (NCS): Comparison of polyethylene membrane and retronasal aroma simulator techniques for the extraction of volatile organic compounds in NCS. Appl. Sci. 2023, 13, 6402. [Google Scholar] [CrossRef]
- Ayustaningwarno, F.; Asikin, Y.; Amano, R.; Vu, N.T.; Hajar-Azhari, S.; Anjani, G.; Takara, K.; Wada, K. Composition of minerals and volatile organic components of non-centrifugal cane sugars from Japan and ASEAN countries. Foods 2023, 12, 1406. [Google Scholar] [CrossRef] [PubMed]
- Tahir, H.E.; Hashim, S.B.H.; Arslan, M.; Mahunu, G.K.; Shishir, M.R.I.; Zhihua, L.; Khan, S.; Mariod, A.A.; Abaker, H.A.M.; Ibrahim, H.E.; et al. Characterization and identification of the key volatile and non-volatile substances of Vangueria madagascariensis J.F. Gmel fruits (Kirkir) and exploration of their binding interactions with olfactory and taste receptors using computational chemistry methodology. Food Chem. 2024, 460, 140631. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Guo, D.; Wen, P.; Chen, H.; Hu, Y.; Li, J.; Tu, Z.; Wang, H. Insight into the aroma and taste enrichment pattern in Chinese traditional braised soup based on HS-SPME-GC-MS and HS-GC-IMS. Food Biosci. 2024, 60, 104345. [Google Scholar] [CrossRef]
- Sagawa, T.; Sakakura, M. A short-term time-series data analysis algorithm for flavor release during the start of eating. Mass Spectrom. 2023, 12, A0126. [Google Scholar] [CrossRef]
- Bojanowski, V.; Hummel, T. Retronasal perception of odors. Physiol. Behav. 2012, 107, 484–487. [Google Scholar] [CrossRef]
- Piornos, J.A.; Delgado, A.; de La Burgade, R.C.J.; Methven, L.; Balagiannis, D.P.; Koussissi, E.; Brouwer, E.; Parker, J.K. Orthonasal and retronasal detection thresholds of 26 aroma compounds in a model alcohol-free beer: Effect of threshold calculation method. Food Res. Int. 2019, 123, 317–326. [Google Scholar] [CrossRef]
- van Eck, A.; Pedrotti, M.; Brouwer, R.; Supapong, A.; Fogliano, V.; Scholten, E.; Biasioli, F.; Stieger, M. In vivo aroma release and dynamic sensory perception of composite foods. J. Agric. Food Chem. 2021, 69, 10260–10271. [Google Scholar] [CrossRef]
- Chen, L.; Yan, R.; Zhao, Y.; Sun, J.; Zhang, Y.; Li, H.; Zhao, D.; Wang, B.; Ye, X.; Sun, B. Characterization of the aroma release from retronasal cavity and flavor perception during baijiu consumption by Vocus-PTR-MS, GC×GC-MS, and TCATA analysis. LWT 2023, 174, 114430. [Google Scholar] [CrossRef]
- Ge, Y.; Li, K.; Xie, C.; Xu, Y.; Shi, C.; Hang, F.; Doherty, W.O.S. Formation of volatile and aroma compounds during the dehydration of membrane-clarified sugarcane juice to non-centrifugal sugar. Foods 2021, 10, 1561. [Google Scholar] [CrossRef] [PubMed]
- Hirose, N.; Ono, H.; Maeda, G.; Wada, K. Development of tabletop type manufacturing equipment for test production of non-centrifugal cane sugar “Kokuto” and the rise of syrup temperature during the cooling-agitation process of Kokuto production. Nippon Shokuhin Kagaku Kogaku Kaishi 2019, 66, 27–31. [Google Scholar] [CrossRef]
- Aranda-Gonzalez, I.; Perera-Pacheco, M.; Barbosa-Martín, E.; Betancur-Ancona, D. Replacing sugar with S. rebaudiana extracts on the physicochemical and sensory properties of strawberry ice cream. Cienc. Rural 2016, 46, 604–609. [Google Scholar] [CrossRef]
- Göncüoğlu Taş, N.G.; Gökmen, V. Maillard reaction and caramelization during hazelnut roasting: A multiresponse kinetic study. Food Chem. 2017, 221, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- Asikin, Y.; Hirose, N.; Tamaki, H.; Ito, S.; Oku, H.; Wada, K. Effects of different drying–solidification processes on physical properties, volatile fraction, and antioxidant activity of non-centrifugal cane brown sugar. LWT Food Sci. Technol. 2016, 66, 340–347. [Google Scholar] [CrossRef]
- Chen, E.; Zhao, S.; Song, H.; Zhang, Y.; Lu, W. Analysis and comparison of aroma compounds of brown sugar in Guangdong, Guangxi and Yunnan using GC-O-MS. Molecules 2022, 27, 5878. [Google Scholar] [CrossRef]
- Starowicz, M.; Zieliński, H. How Maillard reaction influences sensorial properties (color, flavor and texture) of food products? Food Rev. Int. 2019, 35, 707–725. [Google Scholar] [CrossRef]
- Ning, H.; Qiu, H.; Miao, J.; Qu, Y.; Lai, K. Effects of frying and baking processing conditions changes on biogenic amines and volatile components in Jumbo squid (Dosidicus gigas). Appl. Food Res. 2022, 2, 100114. [Google Scholar] [CrossRef]
- Sasaki, T.; Yuikawa, N.; Tanihiro, N.; Michihata, T.; Enomoto, T. The effects of roasting conditions on the physical appearance traits and aroma and taste components of roasted stem tea. Food Sci. Technol. Res. 2020, 26, 643–654. [Google Scholar] [CrossRef]
- Muñoz-González, C.; Canon, F.; Feron, G.; Guichard, E.; Pozo-Bayón, M.A. Assessment wine aroma persistence by using an in vivo PTR-ToF-MS approach and its relationship with salivary parameters. Molecules 2019, 24, 1277. [Google Scholar] [CrossRef]
- Itobe, T.; Nishimura, O.; Kumazawa, K. Influence of milk on aroma release and aroma perception during consumption of coffee beverages. Food Sci. Technol. Res. 2015, 21, 607–614. [Google Scholar] [CrossRef]
- Büttner, A.; Schieberle, P. Influence of mastication on the concentrations of aroma volatiles—Some aspects of flavour release and flavour perception. Food Chem. 2000, 71, 347–354. [Google Scholar] [CrossRef]
- Lyu, J.; Chen, S.; Nie, Y.; Xu, Y.; Tang, K. Aroma release during wine consumption: Factors and analytical approaches. Food Chem. 2021, 346, 128957. [Google Scholar] [CrossRef] [PubMed]
- Januszewska, R.; Giret, E.; Clement, F.; Van Leuven, I.; Goncalves, C.; Vladislavleva, E.; Pradal, P.; Nåbo, R.; Landuyt, A.; D’Heer, G.; et al. Impact of vanilla origins on sensory characteristics of chocolate. Food Res. Int. 2020, 137, 109313. [Google Scholar] [CrossRef] [PubMed]
- Schifferstein, H.N.J.; Kudrowitz, B.M.; Breuer, C. Food perception and aesthetics—Linking sensory science to culinary practice. J. Culin. Sci. Technol. 2020, 20, 293–335. [Google Scholar] [CrossRef]
- Romano, A.; Cappellin, L.; Bogialli, S.; Pastore, P.; Navarini, L.; Biasioli, F. Monitoring in vitro and in vivo aroma release of espresso coffees with proton-transfer-reaction time-of-flight mass spectrometry. Appl. Sci. 2022, 12, 10272. [Google Scholar] [CrossRef]
- Karangwa, E.; de D. Habimana, J.; JingYang, Y.; Murekatete, N.; Zhang, X.; Masamba, K.; Duhoranimana, E.; Muhoza, B. Sensory characteristics of Maillard reaction products obtained from sunflower protein hydrolysates and different sugar types. Int. J. Food Eng. 2017, 13, 20160006. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).