Quantitative Immunofluorescence Mapping of HSP70’s Neuroprotective Effects in FUS-ALS Mouse Models
Abstract
1. Introduction
2. Materials and Methods
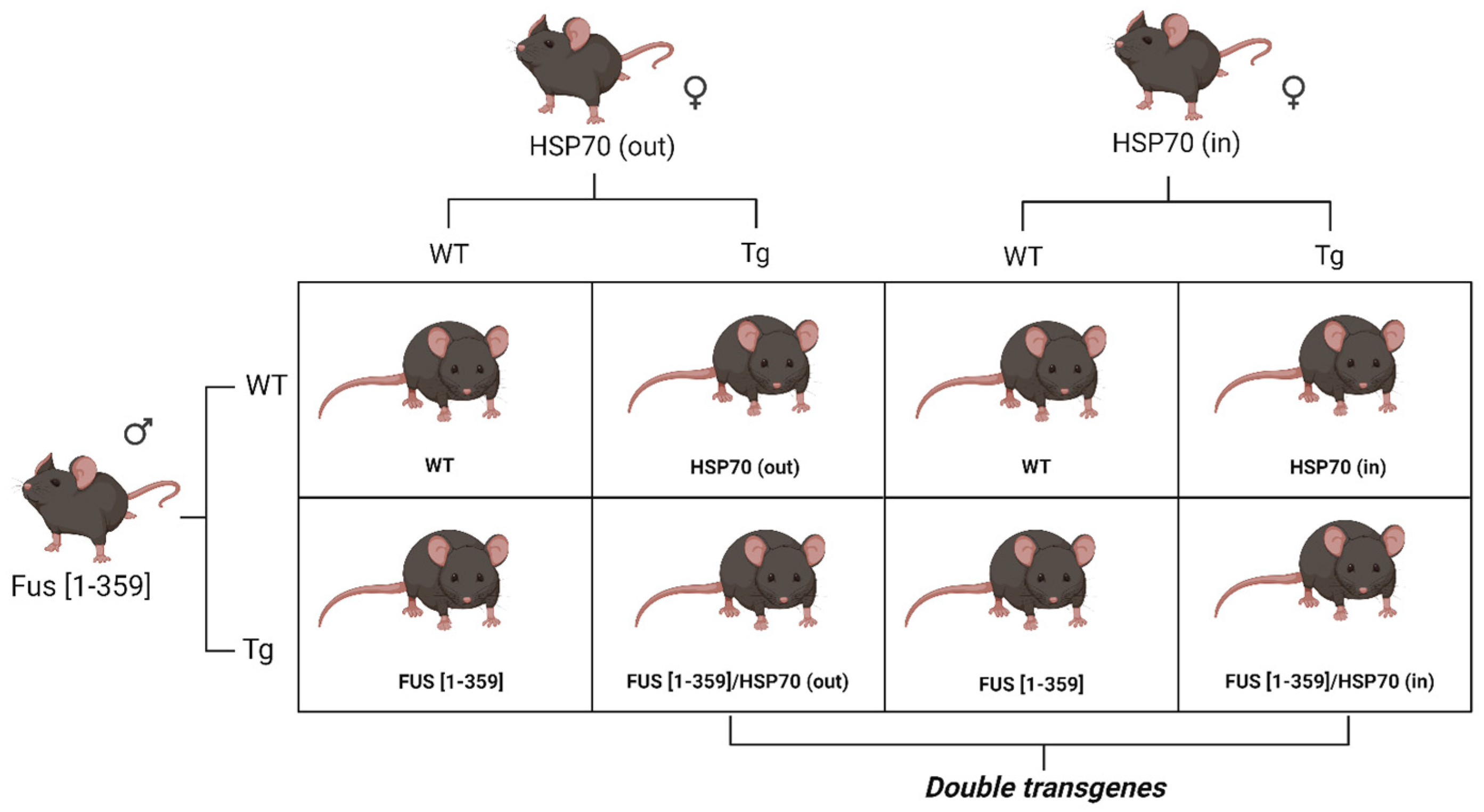
2.1. Animal Experiments
2.2. Immunofluorescent Study
2.3. Cell Detection
2.4. Data Curation
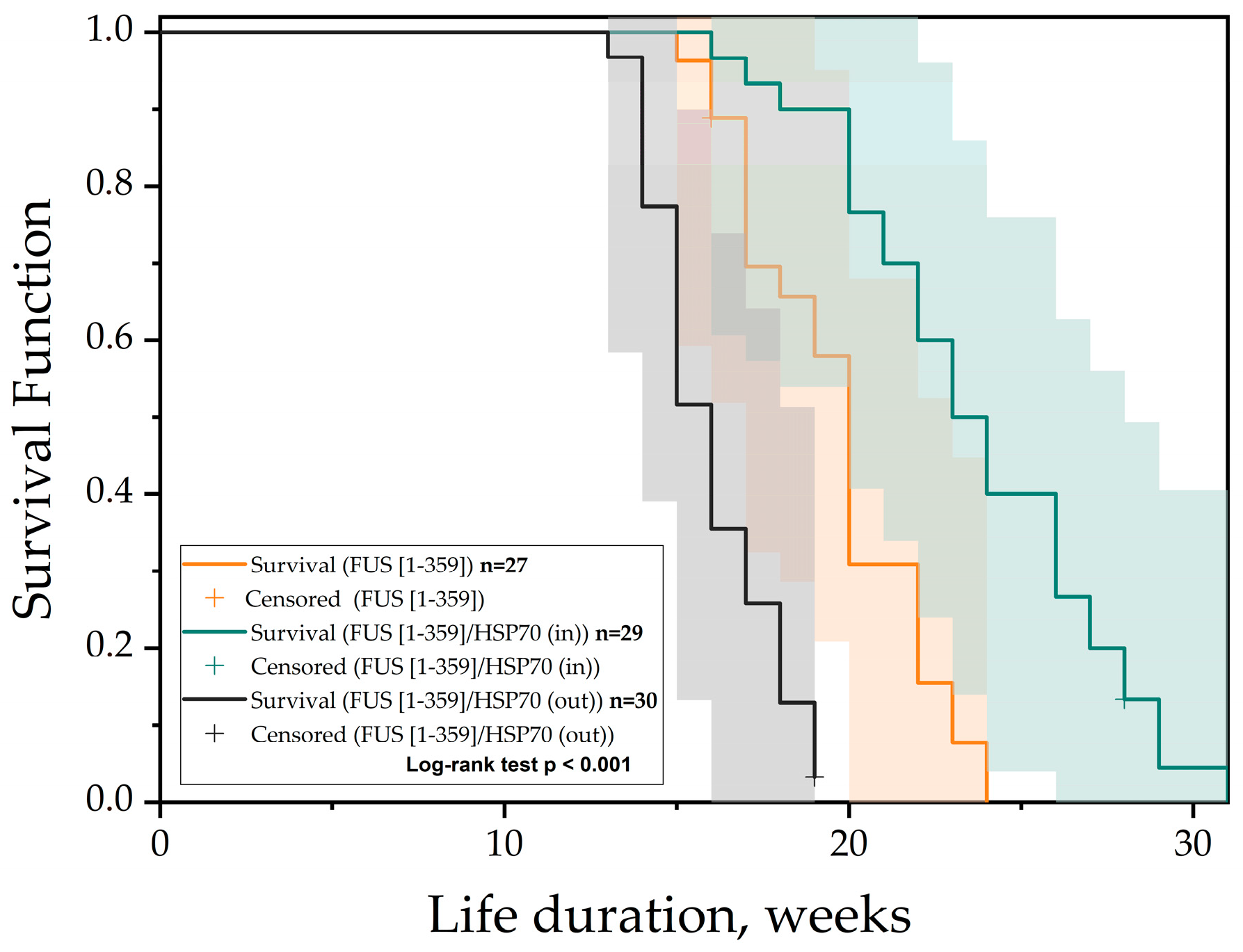
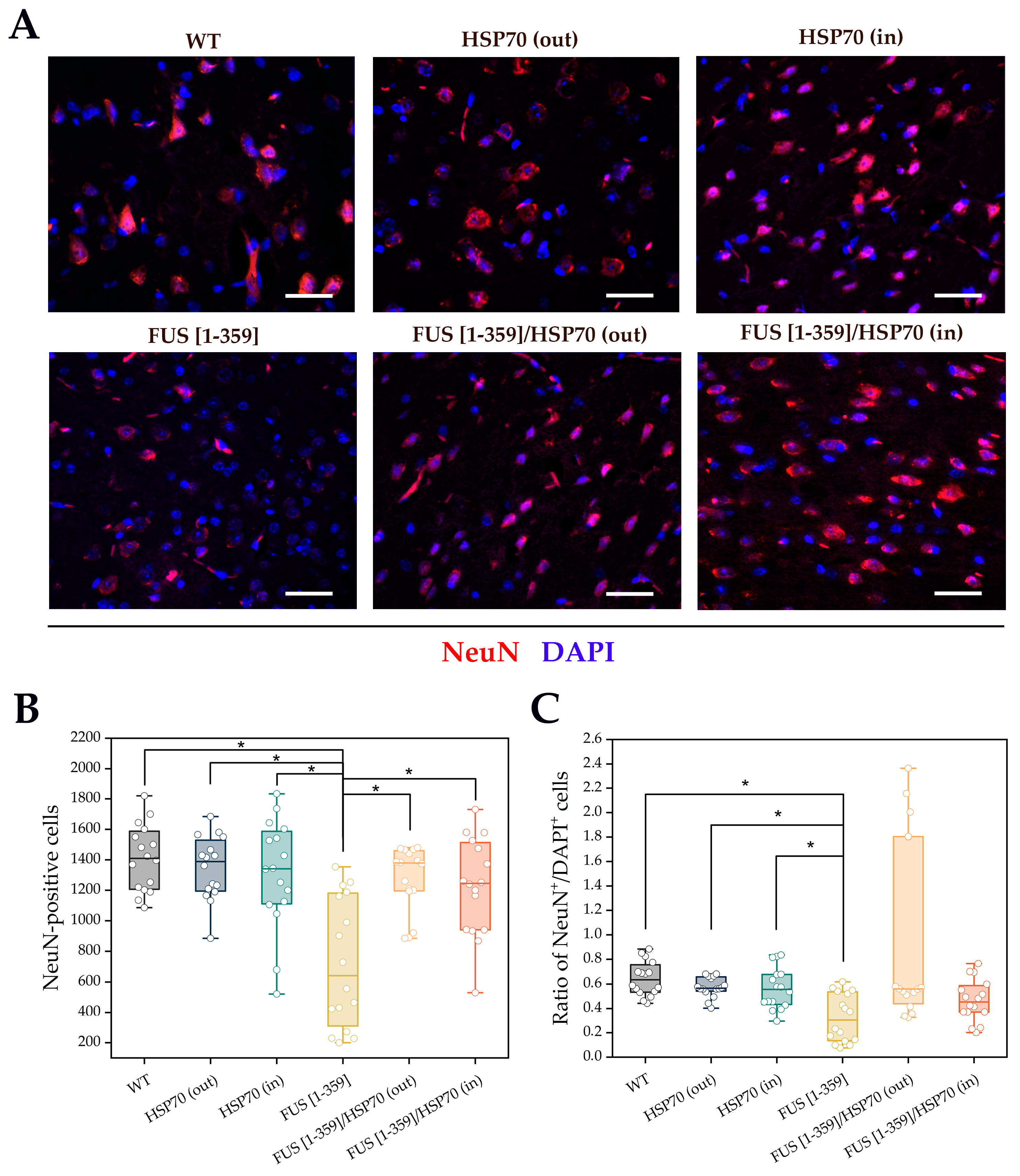
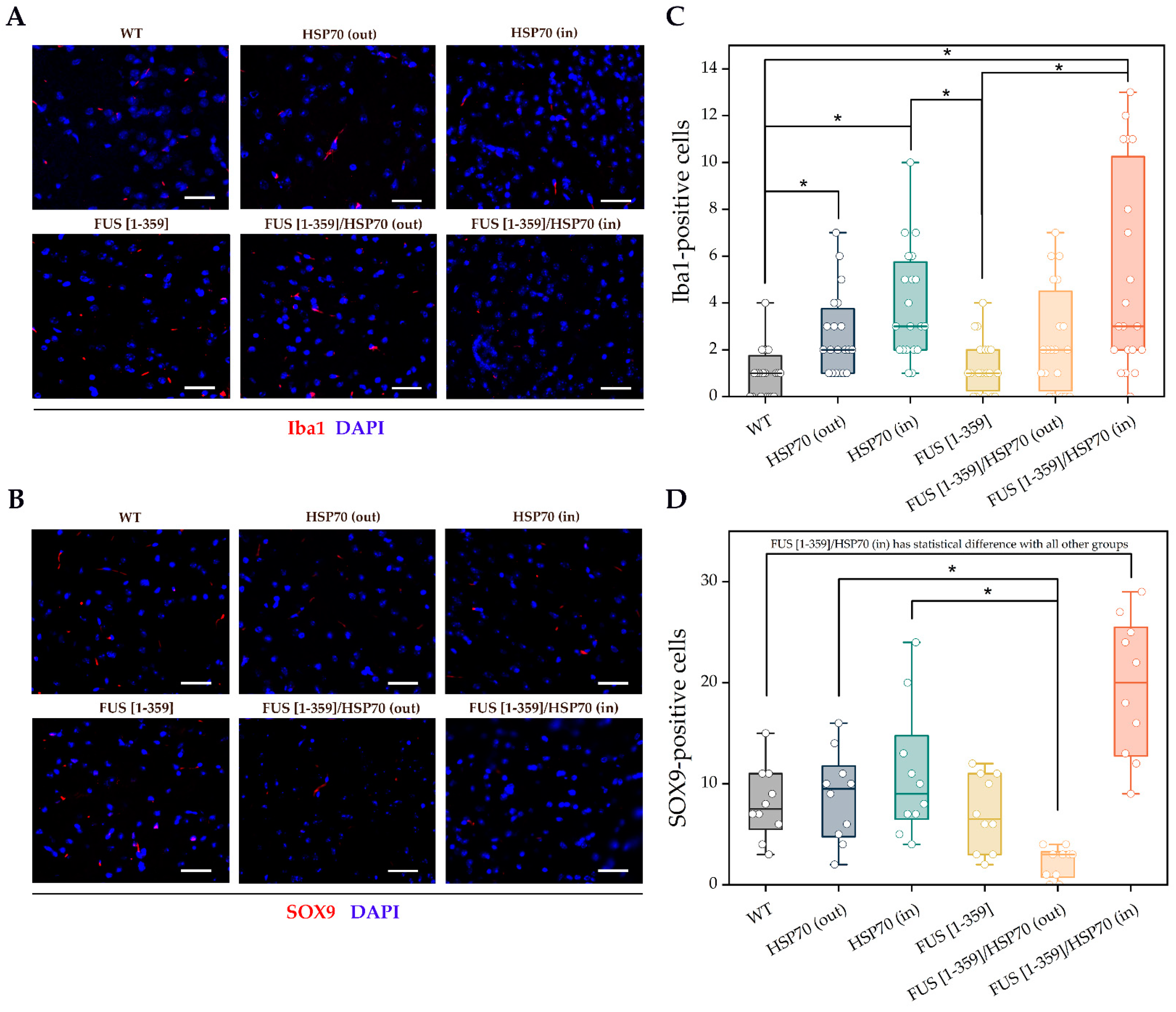
3. Results
4. Discussion
4.1. Limitations of This Study
- Murine models should be extrapolated to humans with caution, especially considering studies of this type to serve mainly for further pharmacological investigations.
- Body mass assessment has not been provided. We outline measurements of body mass dynamics for our double transgenic animals further to observe a greater number of animals for this biometric parameter.
4.2. Perspectives
- Study of neuroinflammation process with proinflammatory cytokines and enzymes of apoptotic cascades will potentially disclose machinery of HSP70 action in FUS-related changes in the primary motor cortex.
- Molecular and cellular approaches to assess disassembling of FUS aggregates and changes in aberrant FUS expression are promising to elucidate if there is a direct or indirect impact of HSP70 presence on FUS localization and fusopathy.
- Spinal cord and brainstem study is relevant for a comprehensive complex assessment of FUS expression, aggregation, and disassembling under HSP70 impact.
- HSP70 role in slowing ALS pathogenesis down can be shown in other in vivo models related to mutations in SOD1 and C9orf72 genes, as well as the roles of HSP70 co-chaperones and counterparts (i.e., HSP40 and HSP90).
- Physiological motor tests and electroencephalography of the motor cortex and striatum in our model of double FUS [1-359]/HSP70 transgenic mice are beneficial in future works to reveal the level of motor disorder in experimental animals.
- Brain proteome and transcriptome analysis are additional methods that would be appropriate at double transgenic mice with FUS [1-359]/HSP70 expression to assess the overall neuroprotection degree.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Chalabi, A.; Hardiman, O. The epidemiology of ALS: A conspiracy of genes, environment and time. Nat. Rev. Neurol. 2013, 9, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Mázala, D.A.G.; Chen, D.; Chin, E.R. SERCA1 overexpression in skeletal muscle attenuates muscle atrophy and improves motor function in a mouse model of ALS. J. Neuromuscul. Dis. 2024, 11, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Yang, X.; He, D.; Zhang, K.; Liu, S.; Sun, X.; Li, J.; Cai, Z.; Liu, M.; Zhang, X.; et al. Clinical and genetic characteristics of 1672 cases of amyotrophic lateral sclerosis in China: A single-center retrospective study. J. Neurol. 2024, 271, 5541–5548. [Google Scholar] [CrossRef] [PubMed]
- Naumann, M.; Peikert, K.; Günther, R.; van der Kooi, A.J.; Aronica, E.; Hübers, A.; Danel, V.; Corcia, P.; Pan-Montojo, F.; Cirak, S.; et al. Phenotypes and malignancy risk of different FUS mutations in genetic amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 2019, 6, 2384–2394. [Google Scholar] [CrossRef] [PubMed]
- Farrugia Wismayer, M.; Farrugia Wismayer, A.; Borg, R.; Bonavia, K.; Abela, A.; Chircop, C.; Aquilina, J.; Soler, D.; Pace, A.; Vella, M.; et al. Genetic landscape of ALS in Malta based on a quinquennial analysis. Neurobiol. Aging 2023, 123, 200–207. [Google Scholar] [CrossRef]
- Leighton, D.J.; Ansari, M.; Newton, J.; Cleary, E.; Stephenson, L.; Beswick, E.; Artal, J.C.; Davenport, R.; Duncan, C.; Gorrie, G.H.; et al. Genotypes and phenotypes of motor neuron disease: An update of the genetic landscape in Scotland. J. Neurol. 2024, 271, 5256–5266. [Google Scholar] [CrossRef]
- Muchowski, P.J.; Wacker, J.L. Modulation of neurodegeneration by molecular chaperones. Nat. Rev. Neurosci. 2005, 6, 11–22. [Google Scholar] [CrossRef]
- Kinger, S.; Dubey, A.R.; Kumar, P.; Jagtap, Y.A.; Choudhary, A.; Kumar, A.; Prajapati, V.K.; Dhiman, R.; Mishra, A. Molecular chaperones’ potential against defective proteostasis of amyotrophic lateral sclerosis. Cells 2023, 12, 1302. [Google Scholar] [CrossRef]
- Sharp, F.R.; Zhan, X.; Liu, D.Z. Heat shock proteins in the brain: Role of Hsp70, Hsp 27, and HO-1 (Hsp32) and their therapeutic potential. Transl. Stroke Res. 2013, 4, 685–692. [Google Scholar] [CrossRef]
- Venediktov, A.A.; Bushueva, O.Y.; Kudryavtseva, V.A.; Kuzmin, E.A.; Moiseeva, A.V.; Baldycheva, A.; Meglinski, I.; Piavchenko, G.A. Closest horizons of Hsp70 engagement to manage neurodegeneration. Front. Mol. Neurosci. 2023, 16, 1230436. [Google Scholar] [CrossRef]
- Gurskiy, Y.G.; Garbuz, D.G.; Soshnikova, N.V.; Krasnov, A.N.; Deikin, A.; Lazarev, V.F.; Sverchinskyi, D.; Margulis, B.A.; Zatsepina, O.G.; Karpov, V.L.; et al. The development of modified human Hsp70 (HSPA1A) and its production in the milk of transgenic mice. Cell Stress Chaperones 2016, 21, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; King, O.D.; Shorter, J.; Gitler, A.D. Stress granules as crucibles of ALS pathogenesis. J. Cell Biol. 2013, 201, 361–372. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Litscher, G.; Watanabe, N.; Wei, W.; Hashimoto, T.; Iwatsubo, T.; Buchman, V.L.; Shelkovnikova, T.A. ALS-linked cytoplasmic FUS assemblies are compositionally different from physiological stress granules and sequester hnRNPA3, a novel modifier of FUS toxicity. Neurobiol. Dis. 2022, 162, 105585. [Google Scholar] [CrossRef]
- Patel, A.; Lee, H.O.; Jawerth, L.; Maharana, S.; Jahnel, M.; Hein, M.Y.; Stoynov, S.; Mahamid, J.; Saha, S.; Franzmann, T.M.; et al. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 2015, 162, 1066–1077. [Google Scholar] [CrossRef]
- Li, Y.; Gu, J.; Wang, C.; Hu, J.; Zhang, S.; Liu, C.; Zhang, S.; Fang, Y.; Li, D. Hsp70 exhibits a liquid-liquid phase separation ability and chaperones condensed FUS against amyloid aggregation. iScience 2022, 25, 104356. [Google Scholar] [CrossRef]
- Chen, H.J.; Mitchell, J.C.; Novoselov, S.; Miller, J.; Nishimura, A.L.; Scotter, E.L.; Vance, C.A.; Cheetham, M.E.; Shaw, C.E. The heat shock response plays an important role in TDP-43 clearance: Evidence for dysfunction in amyotrophic lateral sclerosis. Brain 2016, 139, 1417–1432. [Google Scholar] [CrossRef]
- Lysikova, E.A.; Kukharsky, M.S.; Chaprov, K.D.; Vasilieva, N.A.; Roman, A.Y.; Ovchinnikov, R.K.; Deykin, A.V.; Ninkina, N.; Buchman, V.L. Behavioural impairments in mice of a novel FUS transgenic line recapitulate features of frontotemporal lobar degeneration. Genes Brain Behav. 2019, 18, e12607. [Google Scholar] [CrossRef]
- Ninkina, N. Stem cell therapy and FUS [1-359]-transgenic mice: A recent study highlighting a promising ALS model and a promising therapy. CNS Neurosci. Ther. 2020, 26, 502–503. [Google Scholar] [CrossRef]
- Serlidaki, D.; van Waarde, M.A.W.H.; Rohland, L.; Wentink, A.S.; Dekker, S.L.; Kamphuis, M.J.; Boertien, J.M.; Brunsting, J.F.; Nillegoda, N.B.; Bukau, B.; et al. Functional diversity between HSP70 paralogs caused by variable interactions with specific co-chaperones. J. Biol. Chem. 2020, 295, 7301–7316. [Google Scholar] [CrossRef]
- Shelkovnikova, T.A.; Peters, O.M.; Deykin, A.V.; Connor-Robson, N.; Robinson, H.; Ustyugov, A.A.; Bachurin, S.O.; Ermolkevich, T.G.; Goldman, I.L.; Sadchikova, E.R.; et al. Fused in sarcoma (FUS) protein lacking nuclear localization signal (NLS) and major RNA binding motifs triggers proteinopathy and severe motor phenotype in transgenic mice. J. Biol. Chem. 2013, 288, 25266–25274. [Google Scholar] [CrossRef]
- Rinwa, P.; Eriksson, M.; Cotgreave, I.; Bäckberg, M. 3R-Refinement principles: Elevating rodent well-being and research quality. Lab. Anim. Res. 2024, 40, 11. [Google Scholar] [CrossRef] [PubMed]
- Wijesekera, L.C.; Leigh, P.N. Amyotrophic lateral sclerosis. Orphanet J. Rare Dis. 2009, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.-W.; the Allen Institute for Brain Science. The Allen Reference Atlas: A Digital Color Brain Atlas of the C57BL/6J Male Mouse; Wiley: New York, NY, USA, 2008. [Google Scholar]
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates, 5th ed.; Academic Press: New York, NY, USA, 2019. [Google Scholar]
- Chon, U.; Vanselow, D.J.; Cheng, K.C.; Kim, Y. Enhanced and unified anatomical labeling for a common mouse brain atlas. Nat. Commun. 2019, 10, 5067. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open-source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Conroy, R.M. What hypotheses do “nonparametric” two-group tests actually test? Stata J. 2012, 12, 182–190. [Google Scholar] [CrossRef]
- Scekic-Zahirovic, J.; Sendscheid, O.; El Oussini, H.; Jambeau, M.; Sun, Y.; Mersmann, S.; Wagner, M.; Dieterlé, S.; Sinniger, J.; Dirrig-Grosch, S.; et al. Toxic gain of function from mutant FUS protein is crucial to trigger cell autonomous motor neuron loss. EMBO J. 2016, 35, 1077–1097. [Google Scholar] [CrossRef]
- Shimshek, D.R.; Mueller, M.; Wiessner, C.; Schweizer, T.; van der Putten, P.H. The HSP70 molecular chaperone is not beneficial in a mouse model of alpha-synucleinopathy. PLoS ONE 2010, 5, e10014. [Google Scholar] [CrossRef][Green Version]
- Korzhevskii, D.E.; Gilerovich, E.G.; Zin’kova, N.N.; Grigor’ev, I.P.; Otellin, V.A. Immunocytochemical detection of brain neurons using the selective marker NeuN. Neurosci. Behav. Physiol. 2006, 36, 857–859. [Google Scholar] [CrossRef]
- Albakova, Z.; Armeev, G.A.; Kanevskiy, L.M.; Kovalenko, E.I.; Sapozhnikov, A.M. HSP70 multi-functionality in cancer. Cells 2020, 9, 587. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kadluczka, J.; Kuter, K.Z. Beyond the GFAP—Astrocyte protein markers in the brain. Biomolecules 2021, 11, 1361. [Google Scholar] [CrossRef]
- Asea, A.; Rehli, M.; Kabingu, E.; Boch, J.A.; Bare, O.; Auron, P.E.; Stevenson, M.A.; Calderwood, S.K. Novel signal transduction pathway utilized by extracellular HSP70: Role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002, 277, 15028–15034. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhang, C.; Guan, Y.; Chen, Y.; Lu, Q.; Jie, L.; Gao, H.; Du, H.; Zhang, H.; Liu, Y.; et al. Screening the expression characteristics of several miRNAs in G93A-SOD1 transgenic mouse: Altered expression of miRNA-124 is associated with astrocyte differentiation by targeting Sox2 and Sox9. J. Neurochem. 2018, 145, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Korzhevskiy, D.E.; Kirik, O.V. Cerebral microglia and microglial markers. Morfologiia 2015, 147, 37–44. [Google Scholar] [PubMed]
- Trofimov, A.; Pavlov, D.; Goswami, A.; Gorlova, A.; Chaprov, K.; Umriukhin, A.; Kalueff, A.; Deykin, A.; Lesch, K.-P.; Anthony, D.C.; et al. Lipopolysaccharide triggers exacerbated microglial activation, excessive cytokine release and behavioural disturbances in mice with truncated fused-in-sarcoma protein (FUS). Brain Behav. Immun. Health 2023, 33, 100686. [Google Scholar] [CrossRef]
- Robberecht, W.; Philips, T. The changing scene of amyotrophic lateral sclerosis. Nat. Rev. Neurosci. 2013, 14, 248–264. [Google Scholar] [CrossRef]
- Shelkovnikova, T.A.; Robinson, H.K.; Southcombe, J.A.; Ninkina, N.; Buchman, V.L. Multistep process of FUS aggregation in the cell cytoplasm involves RNA-dependent and RNA-independent mechanisms. Hum. Mol. Genet. 2014, 23, 5211–5226. [Google Scholar] [CrossRef]
- Nomura, T.; Watanabe, S.; Kaneko, K.; Yamanaka, K.; Nukina, N.; Furukawa, Y. Intranuclear aggregation of mutant FUS/TLS as a molecular pathomechanism of amyotrophic lateral sclerosis. J. Biol. Chem. 2014, 289, 1192–1202. [Google Scholar] [CrossRef]
- Dilliott, A.A.; Andary, C.M.; Stoltz, M.; Petropavlovskiy, A.A.; Farhan, S.M.K.; Duennwald, M.L. DnaJC7 in amyotrophic lateral sclerosis. Int. J. Mol. Sci. 2022, 23, 4076. [Google Scholar] [CrossRef]
- Novoselov, S.S.; Mustill, W.J.; Gray, A.L.; Dick, J.R.; Kanuga, N.; Kalmar, B.; Greensmith, L.; Cheetham, M.E. Molecular chaperone mediated late-stage neuroprotection in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. PLoS ONE 2013, 8, e73944. [Google Scholar] [CrossRef]
- Coyne, A.N.; Lorenzini, I.; Chou, C.C.; Torvund, M.; Rogers, R.S.; Starr, A.; Zaepfel, B.L.; Levy, J.; Johannesmeyer, J.; Schwartz, J.C.; et al. Post-transcriptional inhibition of Hsc70-4/HSPA8 expression leads to synaptic vesicle cycling defects in multiple models of ALS. Cell Rep. 2017, 21, 110–125. [Google Scholar] [CrossRef]
- Liu, F.; Morderer, D.; Wren, M.C.; Vettleson-Trutza, S.A.; Wang, Y.; Rabichow, B.E.; Salemi, M.R.; Phinney, B.S.; Oskarsson, B.; Dickson, D.W.; et al. Proximity proteomics of C9orf72 dipeptide repeat proteins identifies molecular chaperones as modifiers of poly-GA aggregation. Acta Neuropathol. Commun. 2022, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Bascos, N.A.D.; Landry, S.J. A history of molecular chaperone structures in the protein data bank. Int. J. Mol. Sci. 2019, 20, 6195. [Google Scholar] [CrossRef] [PubMed]
- Rozales, K.; Younis, A.; Saida, N.; Meller, A.; Goldman, H.; Kellerman, L.; Heinrich, R.; Berlin, S.; Shalgi, R. Differential roles for DNAJ isoforms in HTT-polyQ and FUS aggregation modulation revealed by chaperone screens. Nat. Commun. 2022, 13, 516. [Google Scholar] [CrossRef] [PubMed]
- Kuta, R.; Larochelle, N.; Fernandez, M.; Pal, A.; Minotti, S.; Tibshirani, M.; St. Louis, K.; Gentil, B.J.; Nalbantoglu, J.N.; Hermann, A.; et al. Depending on the stress, histone deacetylase inhibitors act as heat shock protein co-inducers in motor neurons and potentiate arimoclomol, exerting neuroprotection through multiple mechanisms in ALS models. Cell Stress Chaperones 2020, 25, 173–191. [Google Scholar] [CrossRef]
- Comaduran, M.F.; Minotti, S.; Jacob-Tomas, S.; Rizwan, J.; Larochelle, N.; Robitaille, R.; Sephton, C.F.; Vera, M.; Nalbantoglu, J.N.; Durham, H.D. Impact of histone deacetylase inhibition and arimoclomol on heat shock protein expression and disease biomarkers in primary culture models of familial ALS. Cell Stress Chaperones 2024, 29, 359–380. [Google Scholar] [CrossRef]
- Pelaez, M.C.; Fiore, F.; Larochelle, N.; Dabbaghizadeh, A.; Comaduran, M.F.; Arbour, D.; Minotti, S.; Marcadet, L.; Semaan, M.; Robitaille, R.; et al. Reversal of cognitive deficits in FUSR521G amyotrophic lateral sclerosis mice by arimoclomol and a class I histone deacetylase inhibitor independent of heat shock protein induction. Neurotherapeutics 2024, 21, e00388. [Google Scholar] [CrossRef]
- Jackrel, M.E.; DeSantis, M.E.; Martinez, B.A.; Castellano, L.M.; Stewart, R.M.; Caldwell, K.A.; Caldwell, G.A.; Shorter, J. Potentiated Hsp104 variants antagonize diverse proteotoxic misfolding events. Cell 2014, 156, 170–182. [Google Scholar] [CrossRef]
- Shorter, J. Engineering therapeutic protein disaggregases. Mol. Biol. Cell 2016, 27, 1556–1560. [Google Scholar] [CrossRef]
- von Rüden, E.L.; Wolf, F.; Keck, M.; Gualtieri, F.; Nowakowska, M.; Oglesbee, M.; Potschka, H. Genetic modulation of HSPA1A accelerates kindling progression and exerts pro-convulsant effects. Neuroscience 2018, 386, 108–120. [Google Scholar] [CrossRef]
- Piavchenko, G.A.; Venediktov, A.A.; Kuzmin, E.A.; Kuznetsov, S.L. Morphofunctional features in mice treated by low and high Hsp70 doses. Sechenov Med. J. 2023, 14, 31–41. [Google Scholar] [CrossRef]
- Kuzmin, E.A.; Shamitko, Z.V.; Piavchenko, G.A.; Venediktov, A.A.; Ivanova, M.Y.; Kuznetsov, S.L. Biomarkers of neuroinflammation in the diagnosis of traumatic brain injury and neurodegenerative diseases: A literature review. Sechenov Med. J. 2024, 15, 20–35. [Google Scholar] [CrossRef]
- Kwon, H.J.; Kim, W.; Jung, H.Y.; Kang, M.S.; Kim, J.W.; Hahn, K.R.; Yoo, D.Y.; Yoon, Y.S.; Hwang, I.K.; Kim, D.W. Heat shock protein 70 increases cell proliferation, neuroblast differentiation, and the phosphorylation of CREB in the hippocampus. Lab. Anim. Res. 2019, 35, 21. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Kim, M.; Min, H.K.; Lee, Y.U.; Kwon, D.H.; Lee, M.; Lee, S.; Kook, T.; Joung, H.; Nam, K.-I.; et al. Inhibition of heat shock protein 70 blocks the development of cardiac hypertrophy by modulating the phosphorylation of histone deacetylase 2. Cardiovasc. Res. 2019, 115, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.C.; Brooke, S.M.; Sapolsky, R.M. GP120 neurotoxicity fails to induce heat shock defenses, while the over expression of HSP70 protects against GP120. Brain Res. Bull. 2003, 61, 183–188. [Google Scholar] [CrossRef]
- Giffard, R.G.; Xu, L.; Zhao, H.; Carrico, W.; Ouyang, Y.; Qiao, Y.; Sapolsky, R.; Steinberg, G.; Hu, B.; Yenari, M.A. Chaperones, protein aggregation, and brain protection from hypoxic/ischemic injury. J. Exp. Biol. 2004, 207, 3213–3220. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piavchenko, G.A.; Pokidova, K.S.; Kuzmin, E.A.; Venediktov, A.A.; Izmailov, I.Y.; Meglinski, I.V.; Kuznetsov, S.L. Quantitative Immunofluorescence Mapping of HSP70’s Neuroprotective Effects in FUS-ALS Mouse Models. Appl. Sci. 2024, 14, 11614. https://doi.org/10.3390/app142411614
Piavchenko GA, Pokidova KS, Kuzmin EA, Venediktov AA, Izmailov IY, Meglinski IV, Kuznetsov SL. Quantitative Immunofluorescence Mapping of HSP70’s Neuroprotective Effects in FUS-ALS Mouse Models. Applied Sciences. 2024; 14(24):11614. https://doi.org/10.3390/app142411614
Chicago/Turabian StylePiavchenko, Gennadii A., Ksenia S. Pokidova, Egor A. Kuzmin, Artem A. Venediktov, Ilya Y. Izmailov, Igor V. Meglinski, and Sergey L. Kuznetsov. 2024. "Quantitative Immunofluorescence Mapping of HSP70’s Neuroprotective Effects in FUS-ALS Mouse Models" Applied Sciences 14, no. 24: 11614. https://doi.org/10.3390/app142411614
APA StylePiavchenko, G. A., Pokidova, K. S., Kuzmin, E. A., Venediktov, A. A., Izmailov, I. Y., Meglinski, I. V., & Kuznetsov, S. L. (2024). Quantitative Immunofluorescence Mapping of HSP70’s Neuroprotective Effects in FUS-ALS Mouse Models. Applied Sciences, 14(24), 11614. https://doi.org/10.3390/app142411614









