Depolymerization of Kraft Lignin Using a Metal Chloride-Based Deep Eutectic Solvent: Pathways to Sustainable Lignin Valorization
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. DES Preparation
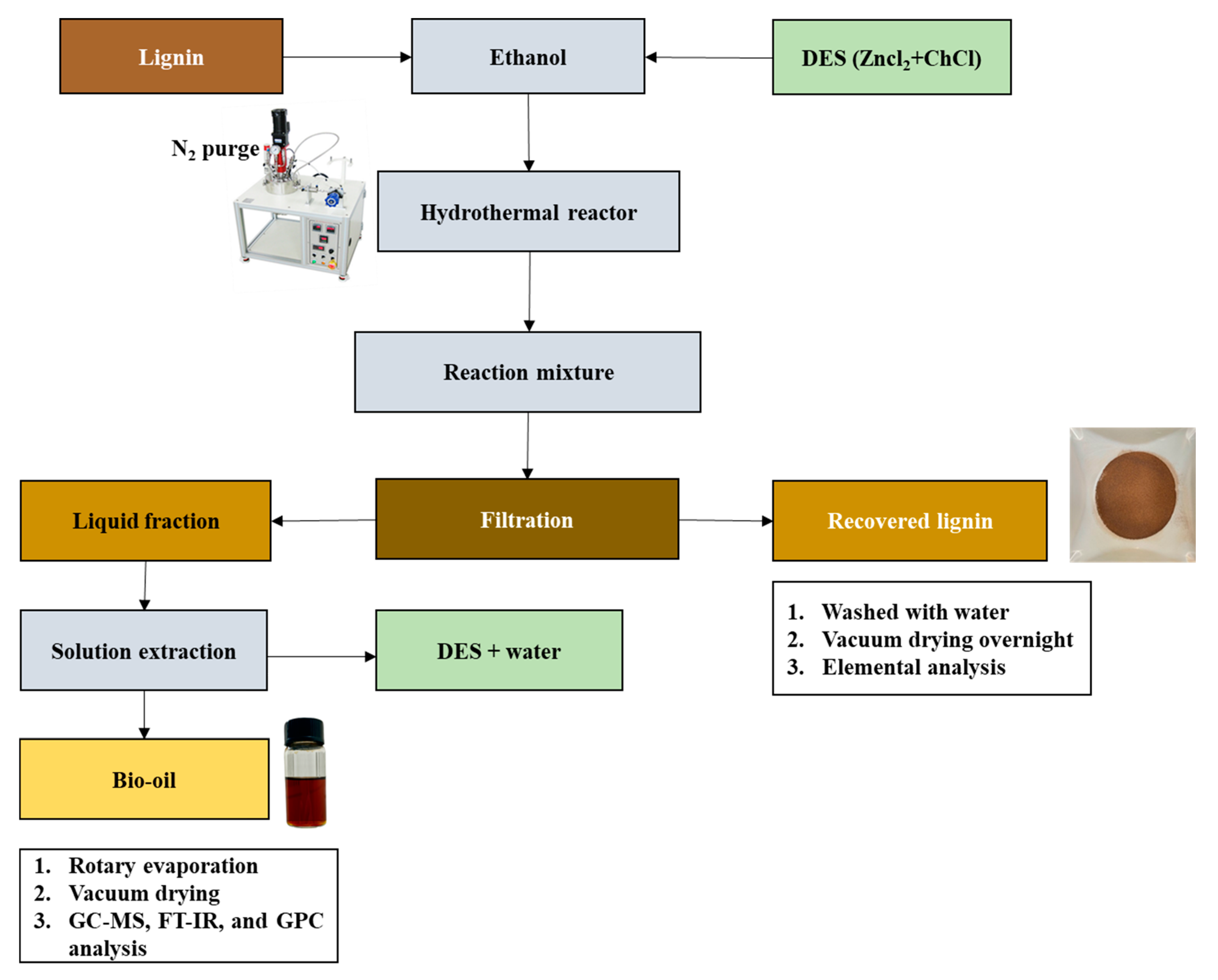
2.3. Lignin Depolymerization
2.4. Product Separation
2.5. Product Analyses
3. Results and Discussion
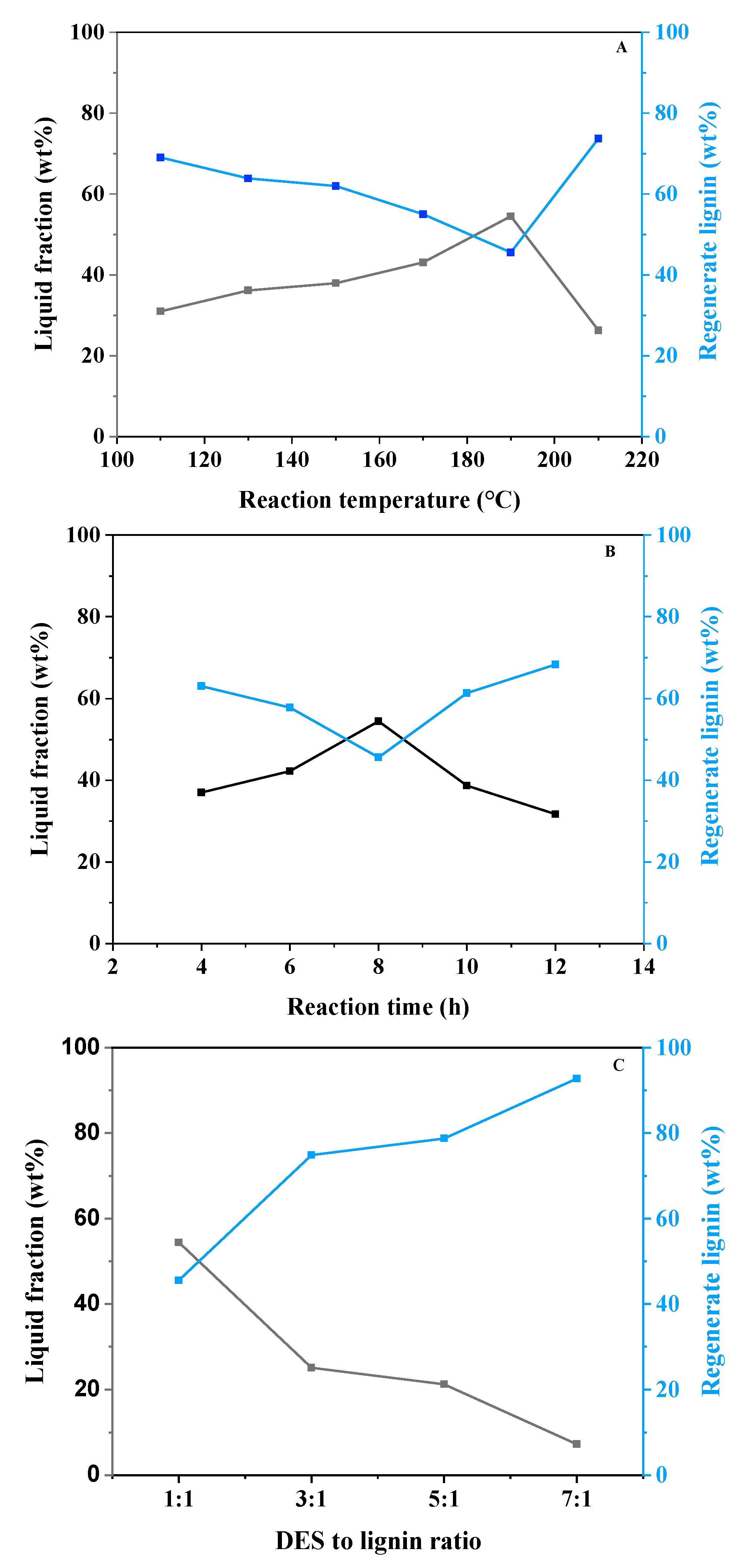
3.1. Effect of Reaction Temperature and Time
Effect of DES Ratio
3.2. Characterization of the RL
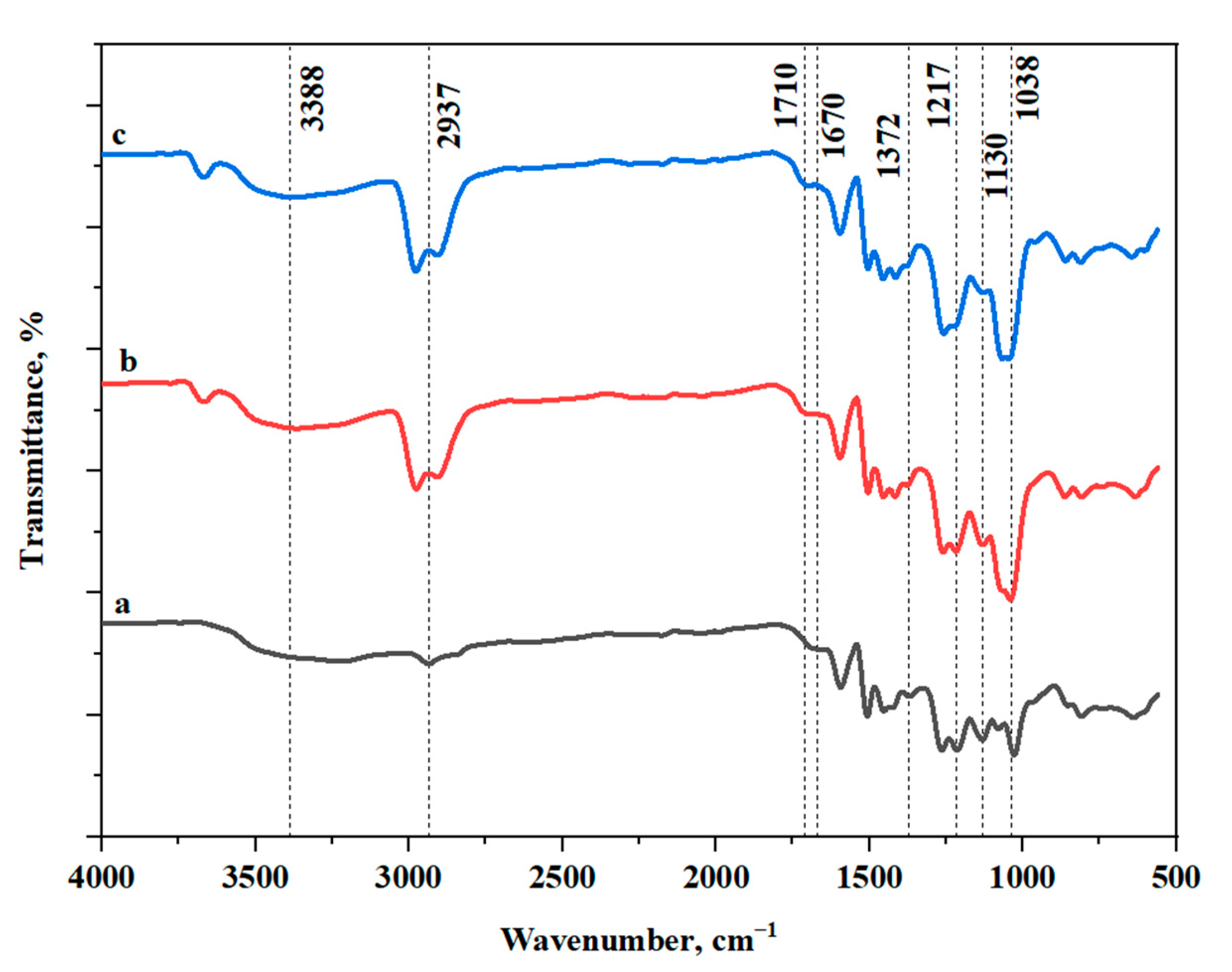
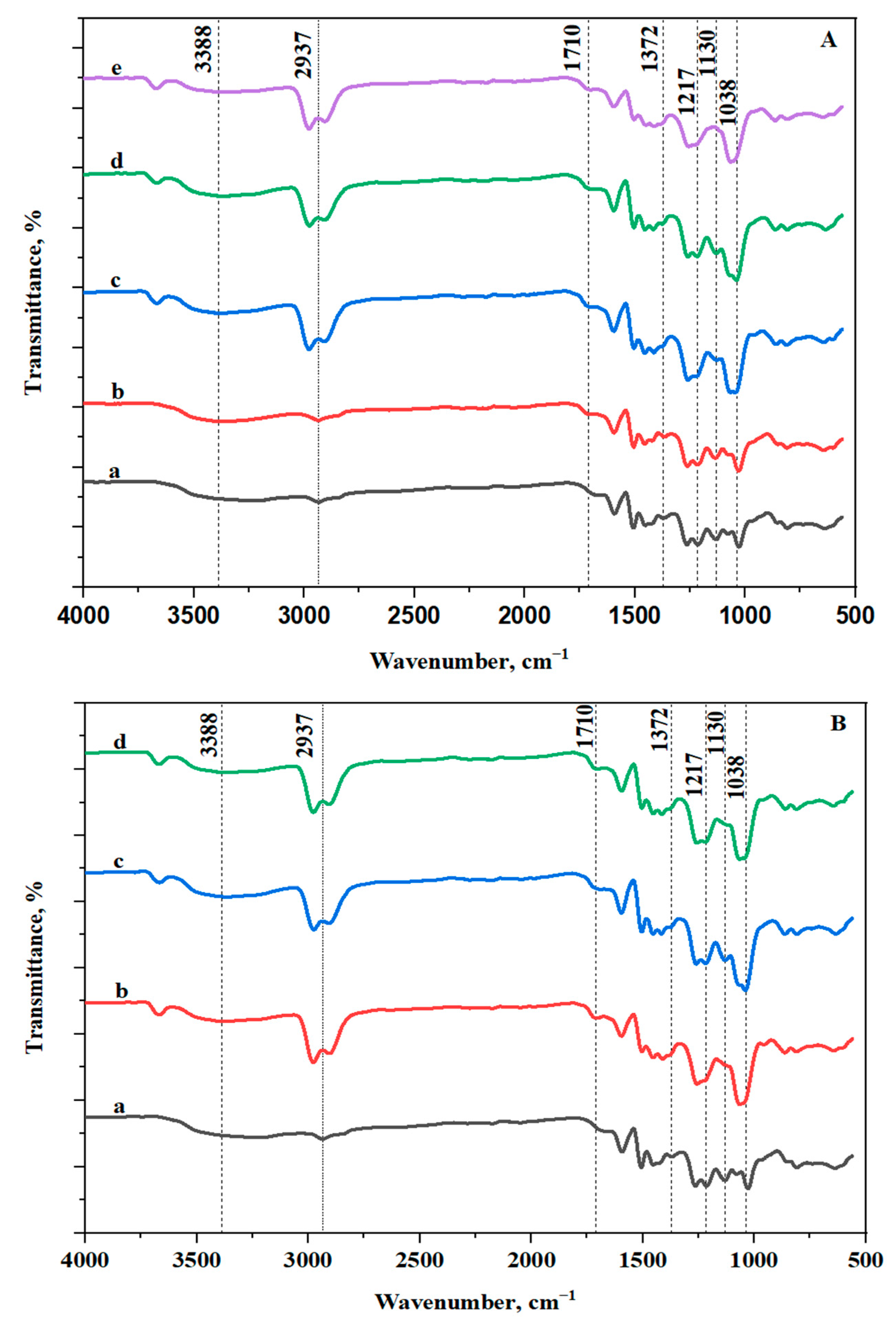
3.2.1. FT-IR Analysis of the RL
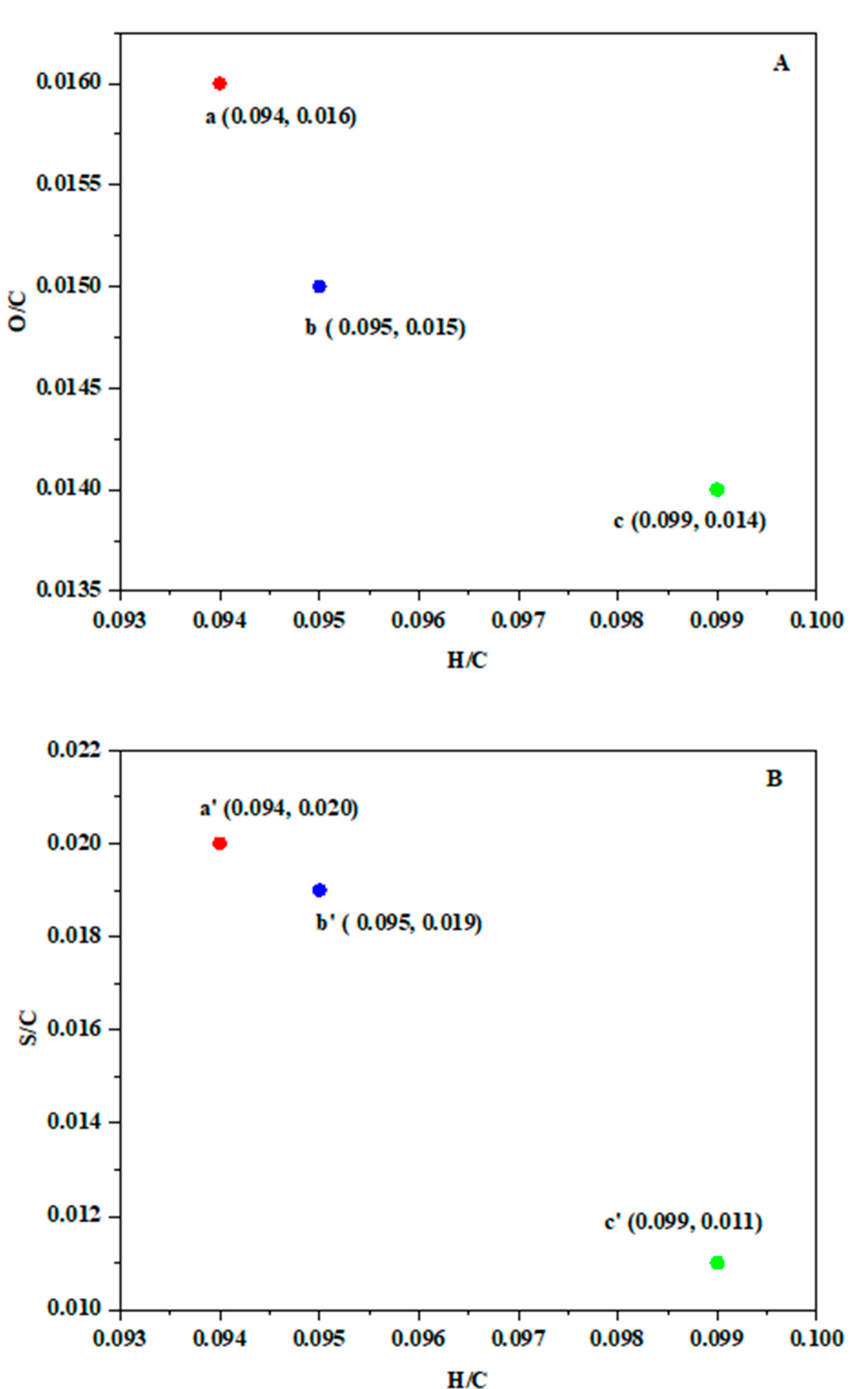
3.2.2. Elemental Analysis of the RL
3.3. Characterization of the Liquid Product
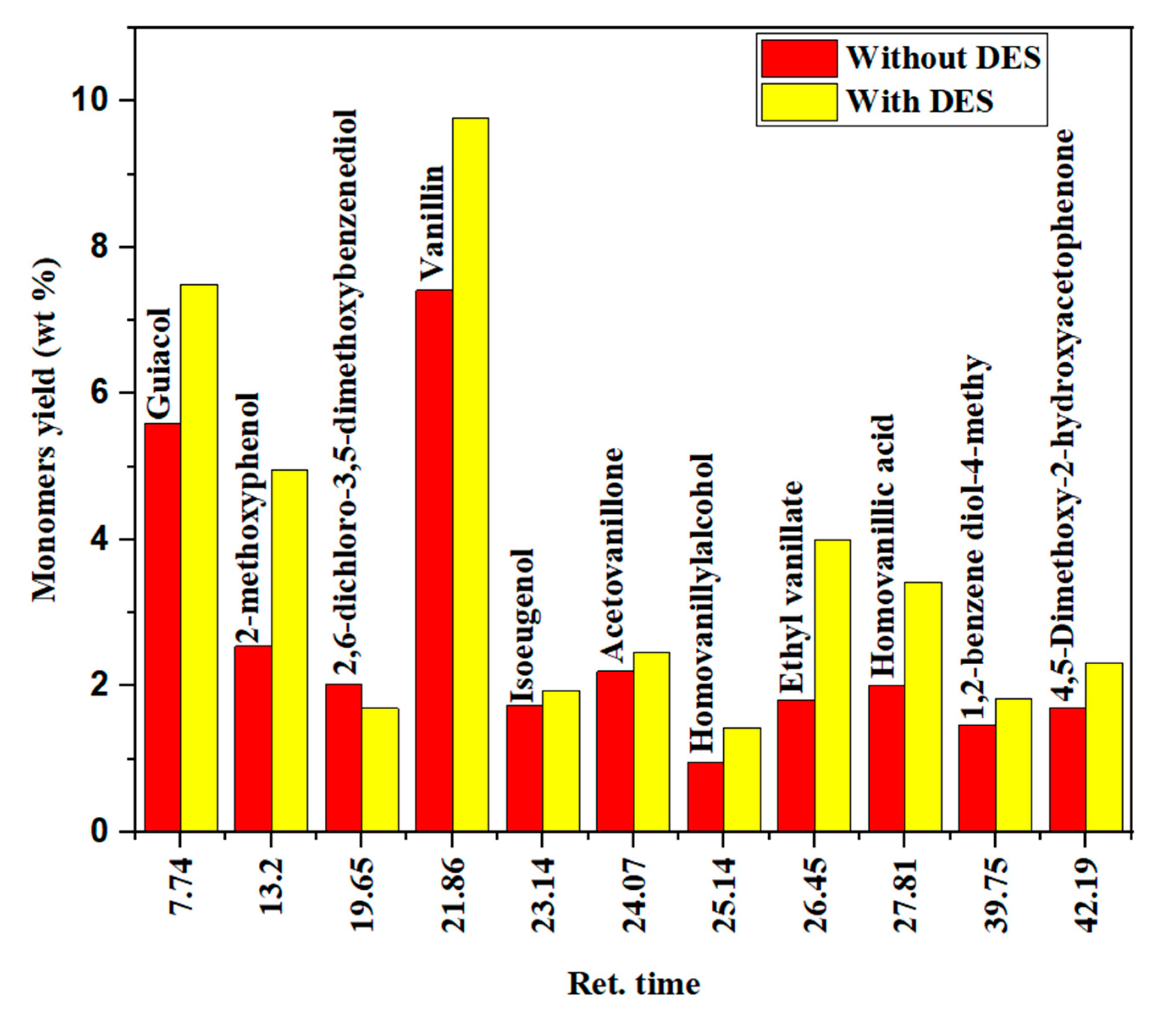
3.3.1. GC–MS Analysis of the Liquid Product
3.3.2. GPC Analysis of the Raw KL and Depolymerized Liquid Product
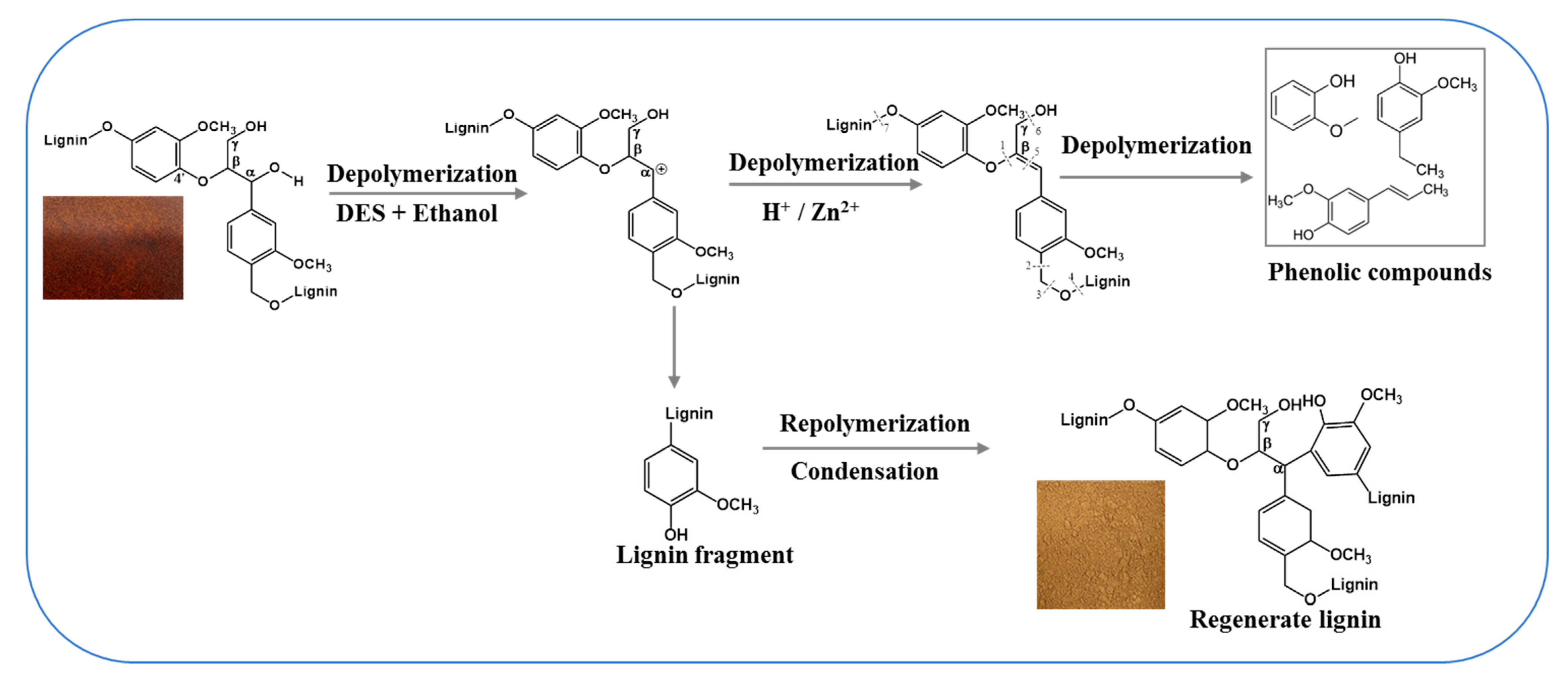
3.4. Reaction Routes and Mechanisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, V. Energy resources. In Textbook of Environment and Ecology; Springer Nature: Singapore, 2024; pp. 185–206. [Google Scholar]
- Laobuthee, A.; Khankhuean, A.; Panith, P.; Veranitisagul, C.; Laosiripojana, N. Ni–Fe Cocatalysts on Magnesium Silicate Supports for the Depolymerization of Kraft Lignin. ACS Omega 2023, 8, 8675–8682. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Taki, G.; Islam, M.N.; Agarwal, A.; Jo, Y.T.; Park, J.H. Effects of temperature and salt catalysts on depolymerization of kraft lignin to aromatic phenolic compounds. Energy Fuels 2019, 33, 6390–6404. [Google Scholar] [CrossRef]
- Holladay, J.E.; Bozell, J.J.; White, J.F.; Johnson, D. Top Value-Added Chemicals from Biomass; DOE Report PNNL.; U.S. Department of Energy: Oak Ridge, TN, USA, 2007; p. 16983. [Google Scholar]
- Otromke, M.; White, R.J.; Sauer, J. Hydrothermal base catalyzed depolymerization and conversion of technical lignin–An introductory review. Carbon Resour. Convers. 2019, 2, 59–71. [Google Scholar] [CrossRef]
- Rana, M.; Nshizirungu, T.; Park, J.H. Effect of simultaneous use of microwave and ultrasound irradiation on the sulfuric acid hydrolysis lignin (SAHL) depolymerization. Sustain. Energy Fuels 2022, 6, 861–878. [Google Scholar] [CrossRef]
- Guan, W.; Chen, X.; Zhang, J.; Hu, H.; Liang, C. Catalytic transfer hydrogenolysis of lignin α-O-4 model compound 4-(benzyloxy) phenol and lignin over Pt/HNbWO6/CNTs catalyst. Renew. Energy 2020, 156, 249–259. [Google Scholar] [CrossRef]
- Nde, D.B.; Muley, P.D.; Sabliov, C.M.; Nokes, S.E.; Boldor, D. Microwave assisted pyrolysis of Kraft lignin in single mode high-Q resonant cavities: Degradation kinetics, product chemical composition, and numerical modeling. Energy Convers. Manag. 2021, 230, 113754. [Google Scholar] [CrossRef]
- Peng, M.; Muraishi, T.; Hou, X.; Zhao, M.; Kamiya, K.; Qian, E.W. Oxidative depolymerization of lignin to vanillin and lactic acid in an aqueous solution. Fuel 2023, 348, 128486. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Wang, J.; Zhang, B.; Guo, G.; Shen, C.; Jiang, Y. Depolymerization of Kraft lignin into liquid fuels over a WO3 modified acid-base coupled hydrogenation catalyst. Fuel 2022, 323, 124428. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, P.; Reddy, S.N. Catalytic (copper) hydrothermal liquefaction for lignin to produce high quality bio-oil and nano Cu carbon hybrids material. Chem. Eng. Sci. 2023, 270, 118548. [Google Scholar] [CrossRef]
- Rana, M.; Nshizirungu, T.; Park, J.H. Synergistic effect of water-ethanol-formic acid for the depolymerization of industrial waste (black liquor) lignin to phenolic monomers. Biomass Bioenergy 2021, 153, 106204. [Google Scholar] [CrossRef]
- Rana, M.; Islam, M.N.; Agarwal, A.; Taki, G.; Park, S.J.; Dong, S.; Jo, Y.T.; Park, J.H. Production of phenol-rich monomers from Kraft lignin hydrothermolysates in basic-subcritical water over MoO3/SBA-15 catalyst. Energy Fuels 2018, 32, 11564–11575. [Google Scholar] [CrossRef]
- Islam, M.N.; Taki, G.; Rana, M.; Park, J.H. Yield of phenolic monomers from lignin hydrothermolysis in subcritical water system. Ind. Eng. Chem. Res. 2018, 57, 4779–4784. [Google Scholar] [CrossRef]
- Agarwal, A.; Rana, M.; Park, J.H. Advancement in technologies for the depolymerization of lignin. Fuel Process. Technol. 2018, 181, 115–132. [Google Scholar] [CrossRef]
- Wang, M.; Li, L.H.; Lu, J.M.; Li, H.J.; Zhang, X.C.; Liu, H.F.; Luo, N.C.; Wang, F. Acid promoted C–C bond oxidative cleavage of β-O-4 and β-1 lignin models to esters over a copper catalyst. Green Chem. 2017, 19, 702–706. [Google Scholar] [CrossRef]
- Mukundan, S.; Atanda, L.; Beltramini, J. Thermocatalytic cleavage of C–C and C–O bonds in model compounds and kraft lignin by NiMoS 2/C nanocatalysts. Sustain. Energy Fuels 2019, 3, 1317–1328. [Google Scholar] [CrossRef]
- Fasolini, A.; Martelli, G.; Piazzi, A.; Curcio, M.; De Maron, J.; Basile, F.; Mazzoni, R. Advances in the Homogeneously Catalyzed Hydrogen Production from Biomass Derived Feedstocks: A Review. ChemCatChem 2024, 16, e202400393. [Google Scholar] [CrossRef]
- Kokkinos, N.C.; Emmanouilidou, E.; Babouki, D.G.; Manousaki, S.C.; Mitkidou, S. Homogeneous Catalysis: Characterization and Spectroscopy. In Homogeneous Catalysis Concepts and Basics; Elsevier: Amsterdam, The Netherlands, 2024; pp. 159–179. [Google Scholar]
- Clarke, C.J.; Tu, W.C.; Levers, O.; Brohl, A.; Hallett, J.P. Green and sustainable solvents in chemical processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Carneiro, A.P.; Rodriguez, O.; Macedo, E.A. Dissolution and fractionation of nut shells in ionic liquids. Bioresour. Technol. 2017, 227, 188–196. [Google Scholar] [CrossRef]
- Cox, B.J.; Ekerdt, J.G. Depolymerization of oak wood lignin under mild conditions using the acidic ionic liquid 1-H-3-methylimidazolium chloride as both solvent and catalyst. Bioresour. Technol. 2012, 118, 584–588. [Google Scholar] [CrossRef]
- Jia, S.; Cox, B.J.; Guo, X.; Zhang, Z.C.; Ekerdt, J.G. Cleaving the β-O-4 Bonds of Lignin Model Compounds in an Acidic Ionic Liquid, 1-H-3-Methylimidazolium Chloride: An Optional Strategy for the Degradation of Lignin. ChemSusChem 2010, 3, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, H.; Brennecke, J.F. Temperature and composition dependence of the density and viscosity of binary mixtures of water+ ionic liquid. J. Chem. Eng. Data 2006, 51, 2145–2155. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2020, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.A.; Chavda, V.; Hirpara, D.; Sharma, V.S.; Shrivastav, P.S.; Kumar, S. Exploring the potential of deep eutectic solvents in pharmaceuticals: Challenges and opportunities. J. Mol. Liq. 2023, 390, 123171. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Malolan, R.; Gopinath, K.P.; Vo, D.V.N.; Jayaraman, R.S.; Adithya, S.; Ajay, P.S.; Arun, J. Green ionic liquids and deep eutectic solvents for desulphurization, denitrification, biomass, biodiesel, bioethanol and hydrogen fuels: A review. Environ. Chem. Lett. 2021, 19, 1001–1023. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Rana, M.; Ghosh, S.; Nshizirungu, T.; Park, J.H. Catalytic depolymerization of Kraft lignin to high yield alkylated-phenols over CoMo/SBA-15 catalyst in supercritical ethanol. RSC Adv. 2023, 13, 30022–30039. [Google Scholar] [CrossRef]
- DIN 51900-1; Determining the Gross Calorific Value of Solid and Liquid Fuels Using the Bomb Calorimeter, and Calculation of Net Calorific Value—Part 1: General Information. Deutsches Institut für Normung e.V.: Berlin, Germany, 2000.
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Microwave mediated enhanced production of 5-hydroxymethylfurfural using choline chloride-based eutectic mixture as sustainable catalyst. Renew. Energy 2021, 177, 643–651. [Google Scholar] [CrossRef]
- Shu, R.; Zhang, Q.; Ma, L.; Xu, Y.; Chen, P.; Wang, C.; Wang, T. Insight into the solvent, temperature and time effects on the hydrogenolysis of hydrolyzed lignin. Bioresour. Technol. 2016, 221, 568–575. [Google Scholar] [CrossRef]
- Riaz, A.; Kim, C.S.; Kim, Y.; Kim, J. High-yield and high-calorific bio-oil production from concentrated sulfuric acid hydrolysis lignin in supercritical ethanol. Fuel 2016, 172, 238–247. [Google Scholar] [CrossRef]
- Zhou, M.; Fakayode, O.A.; Yagoub, A.E.A.; Ji, Q.; Zhou, C. Lignin fractionation from lignocellulosic biomass using deep eutectic solvents and its valorization. Renew. Sustain. Energy Rev. 2022, 156, 111986. [Google Scholar] [CrossRef]
- Casas, A.; Omar, S.; Palomar, J.; Oliet, M.; Alonso, M.V.; Rodriguez, F. Relation between differential solubility of cellulose and lignin in ionic liquids and activity coefficients. RSC Adv. 2013, 3, 3453–3460. [Google Scholar] [CrossRef]
- Chen, Z.; Bai, X.; Wan, C. High-solid lignocellulose processing enabled by natural deep eutectic solvent for lignin extraction and industrially relevant production of renewable chemicals. ACS Sustain. Chem. Eng. 2018, 6, 12205–12216. [Google Scholar] [CrossRef]
- Tabasso, S.; Grillo, G.; Carnaroglio, D.; Calcio Gaudino, E.; Cravotto, G. Microwave-assisted γ-valerolactone production for biomass lignin extraction: A cascade protocol. Molecules 2016, 21, 413. [Google Scholar] [CrossRef]
- Yoo, C.G.; Li, M.; Meng, X.; Pu, Y.; Ragauskas, A.J. Effects of organosolv and ammonia pretreatments on lignin properties and its inhibition for enzymatic hydrolysis. Green Chem. 2017, 19, 2006–2016. [Google Scholar] [CrossRef]
- Casas, A.; Alonso, M.V.; Oliet, M.; Rojo, E.; Rodríguez, F. FTIR analysis of lignin regenerated from Pinus radiata and Eucalyptus globulus woods dissolved in imidazolium-based ionic liquids. J. Chem. Technol. Biotechnol. 2012, 87, 472–480. [Google Scholar] [CrossRef]
- Santos, R.B.; Jameel, H.; Chang, H.M.; Hart, P.W. Impact of Lignin and Carbohydrate Chemical Structures on Degradation Reactions during Hardwood Kraft Pulping Processes. BioResources 2013, 8, 158–171. [Google Scholar] [CrossRef][Green Version]
- McClelland, D.J.; Motagamwala, A.H.; Li, Y.; Rover, M.R.; Wittrig, A.M.; Wu, C.; Buchanan, J.S.; Brown, R.C.; Ralph, J.; Dumesic, J.A.; et al. Functionality and molecular weight distribution of red oak lignin before and after pyrolysis and hydrogenation. Green Chem. 2017, 19, 1378–1389. [Google Scholar] [CrossRef]
- Lim, C.; Tanjore, D.; He, W.; Wong, J.; Gardner, J.L.; Sale, K.L.; Simmons, B.A.; Singh, S. Scale-up and evaluation of high solid ionic liquid pretreatment and enzymatic hydrolysis of switchgrass. Biotechnol. Biofuels 2013, 6, 154. [Google Scholar]
- Wen, J.L.; Xue, B.L.; Sun, S.L.; Sun, R.C. Quantitative structural characterization and thermal properties of birch lignins after auto-catalyzed organosolv pretreatment and enzymatic hydrolysis. J. Chem. Technol. Biotechnol. 2013, 88, 1663–1671. [Google Scholar] [CrossRef]
- Du, L.; Wang, Z.; Li, S.; Song, W.; Lin, W. A comparison of monomeric phenols produced from lignin by fast pyrolysis and hydrothermal conversions. Int. J. Chem. React. Eng. 2013, 11, 135–145. [Google Scholar] [CrossRef]
- Pielhop, T.; Larrazábal, G.O.; Studer, M.H.; Brethauer, S.; Seidel, C.M.; von Rohr, P.R. Lignin repolymerisation in spruce autohydrolysis pretreatment increases cellulase deactivation. Green Chem. 2015, 17, 3521–3532. [Google Scholar] [CrossRef]

| Sample | N (%) | C (%) | H (%) | S (%) | O (%) | HHV (MJ kg−1) * |
|---|---|---|---|---|---|---|
| Kraft lignin | 1.02 | 59.88 | 5.62 | 1.22 | 32.26 | 24.48 |
| RL at 110 °C/8 h | 0.46 | 60.65 | 5.93 | 1.06 | 31.9 | 25.10 |
| RL at 170 °C/8 h | 0.62 | 62.17 | 5.91 | 1.04 | 30.26 | 25.76 |
| RL at 190 °C/8 h | 0.71 | 62.89 | 6.24 | 0.72 | 29.44 | 26.44 |
| RL at 210 °C/8 h | 0.88 | 61.75 | 5.89 | 0.99 | 30.49 | 25.57 |
| RL at 190 °C/4 h | 0.93 | 60.51 | 5.85 | 1.01 | 31.70 | 24.99 |
| RL at 190 °C/8 h | 0.71 | 62.89 | 6.24 | 0.72 | 29.44 | 26.44 |
| RL at 190 °C/10 h | 0.83 | 62.03 | 6.14 | 0.85 | 30.15 | 25.98 |
| RL at 190 °C/8 h (no DES) | 0.61 | 60.39 | 5.77 | 1.13 | 32.10 | 24.82 |
| D:L of 1:1 at 190 °C/8 h | 0.71 | 62.89 | 6.24 | 0.72 | 29.44 | 26.44 |
| D:L of 3:3 at 190 °C/8 h | 0.74 | 61.94 | 5.86 | 1.09 | 30.37 | 25.62 |
| D:L of 5:5 at 190 °C/8 h | 1.12 | 60.01 | 5.70 | 1.18 | 31.99 | 24.65 |
| Sample | Reaction Conditions | Mw | Mn | PDI (Mw/Mn) |
|---|---|---|---|---|
| Kraft lignin | 2450 | 1493 | 1.64 | |
| DES-treated bio-oil | 110 °C for 8 h | 1856 | 1261 | 1.47 |
| 150 °C for 8 h | 1813 | 1235 | 1.46 | |
| 170 °C for 8 h | 1653 | 1155 | 1.43 | |
| 190 °C for 8 h | 1498 | 1061 | 1.41 | |
| 210 °C for 8 h | 1651 | 1146 | 1.44 | |
| 4 h at 190 °C | 1734 | 1142 | 1.52 | |
| 6 h at 190 °C | 1652 | 1112 | 1.48 | |
| 8 h at 190 °C | 1498 | 1061 | 1.41 | |
| 10 h at 190 °C | 1817 | 1254 | 1.45 | |
| Without DES | 190 °C for 8 h | 1872 | 1259 | 1.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, S.; Rana, M.; Park, J.-H. Depolymerization of Kraft Lignin Using a Metal Chloride-Based Deep Eutectic Solvent: Pathways to Sustainable Lignin Valorization. Appl. Sci. 2024, 14, 11571. https://doi.org/10.3390/app142411571
Ghosh S, Rana M, Park J-H. Depolymerization of Kraft Lignin Using a Metal Chloride-Based Deep Eutectic Solvent: Pathways to Sustainable Lignin Valorization. Applied Sciences. 2024; 14(24):11571. https://doi.org/10.3390/app142411571
Chicago/Turabian StyleGhosh, Shubho, Masud Rana, and Jeong-Hun Park. 2024. "Depolymerization of Kraft Lignin Using a Metal Chloride-Based Deep Eutectic Solvent: Pathways to Sustainable Lignin Valorization" Applied Sciences 14, no. 24: 11571. https://doi.org/10.3390/app142411571
APA StyleGhosh, S., Rana, M., & Park, J.-H. (2024). Depolymerization of Kraft Lignin Using a Metal Chloride-Based Deep Eutectic Solvent: Pathways to Sustainable Lignin Valorization. Applied Sciences, 14(24), 11571. https://doi.org/10.3390/app142411571









