Speciation Characteristics and Risk Assessment of Heavy Metals in Cultivated Soil in Pingshui Village, Zhaoping County, Hezhou City, Guangxi
Abstract
1. Introduction
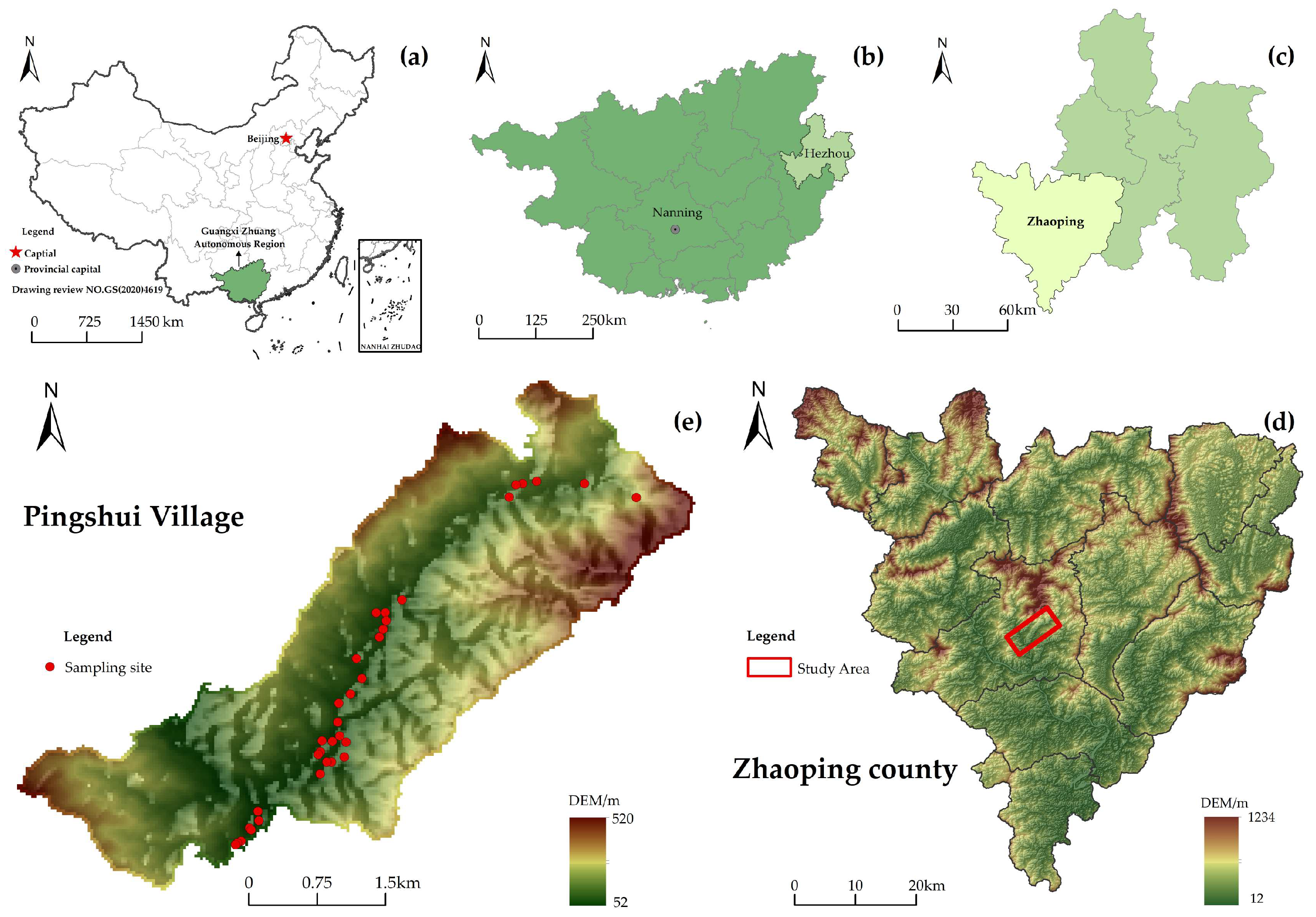
2. Overview of the Study Area
3. Materials and Methods
3.1. Sample Collection and Preservation
3.2. Experimental Methods
3.3. Evaluation Methods
3.3.1. The Geo-Accumulation Index Method
3.3.2. The Potential Ecological Risk Index Method
3.3.3. The Risk Assessment Code Method
3.4. Data Processing Methods
4. Results and Discussion
4.1. Analysis of Total Heavy Metals
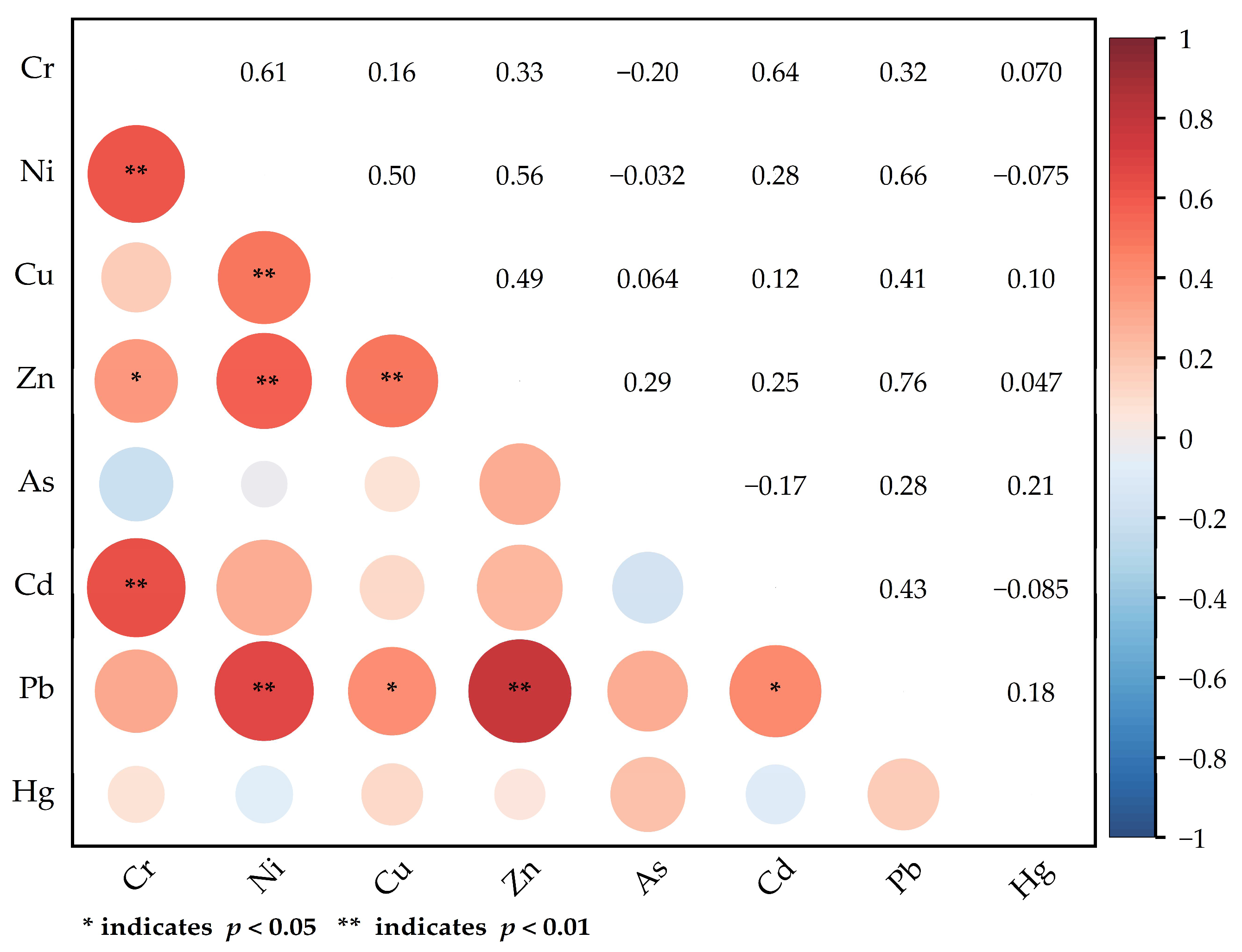
4.2. Analysis of Heavy Metal Sources
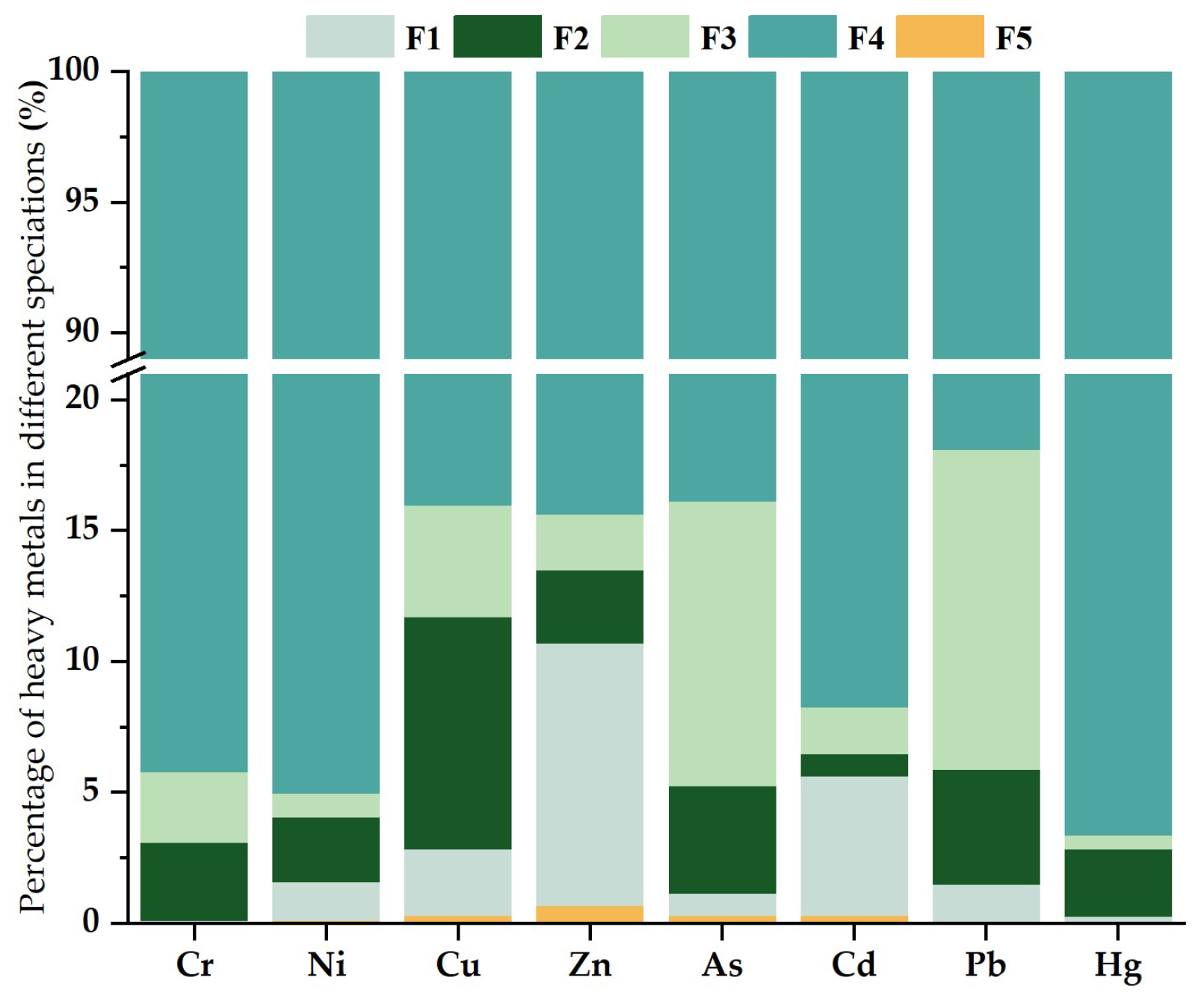
4.3. Speciation Distribution Characteristics of Heavy Metals
4.4. Risk Assessment of Soil’s Heavy Metals
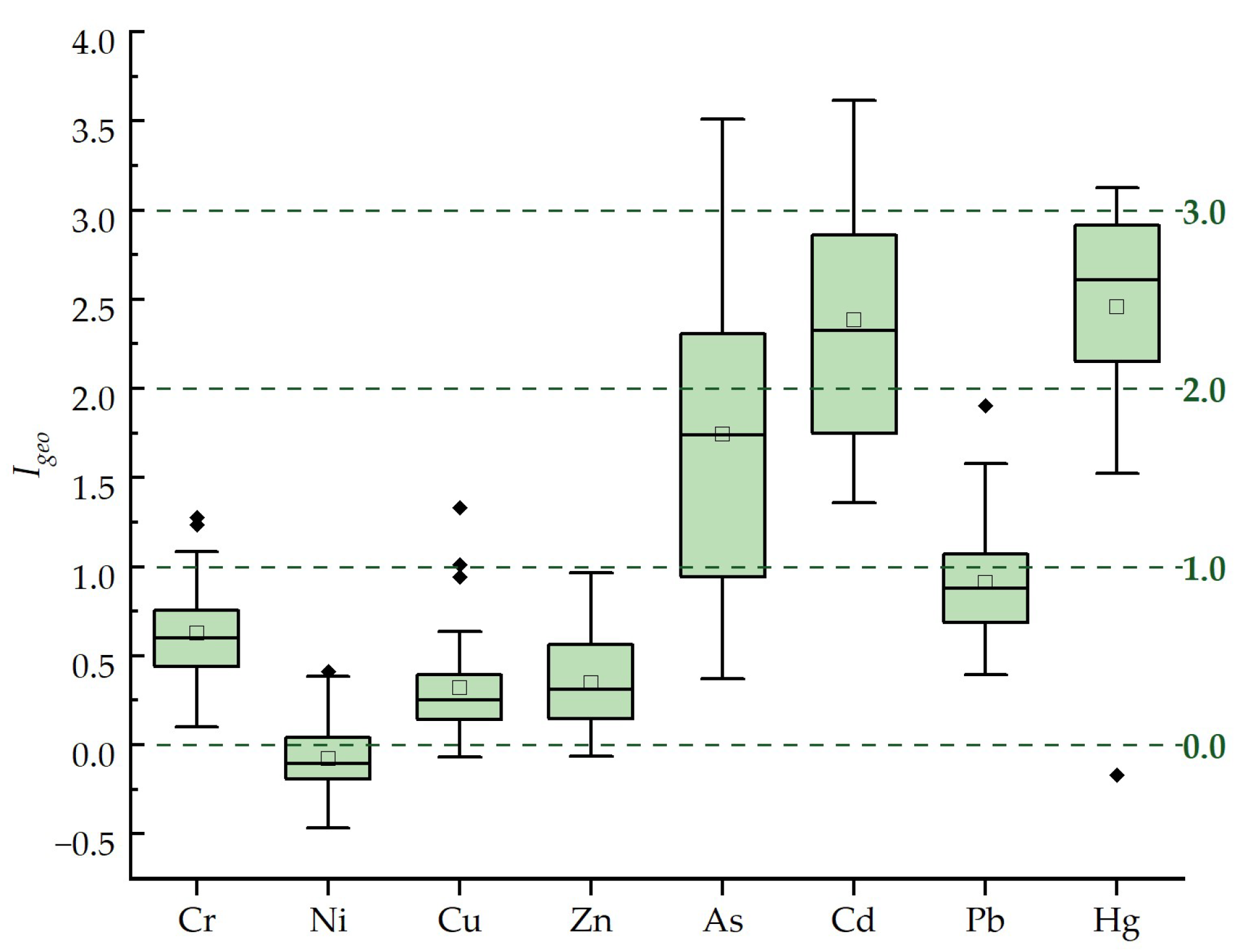
4.4.1. Evaluation Results of the Geo-Accumulation Index Method
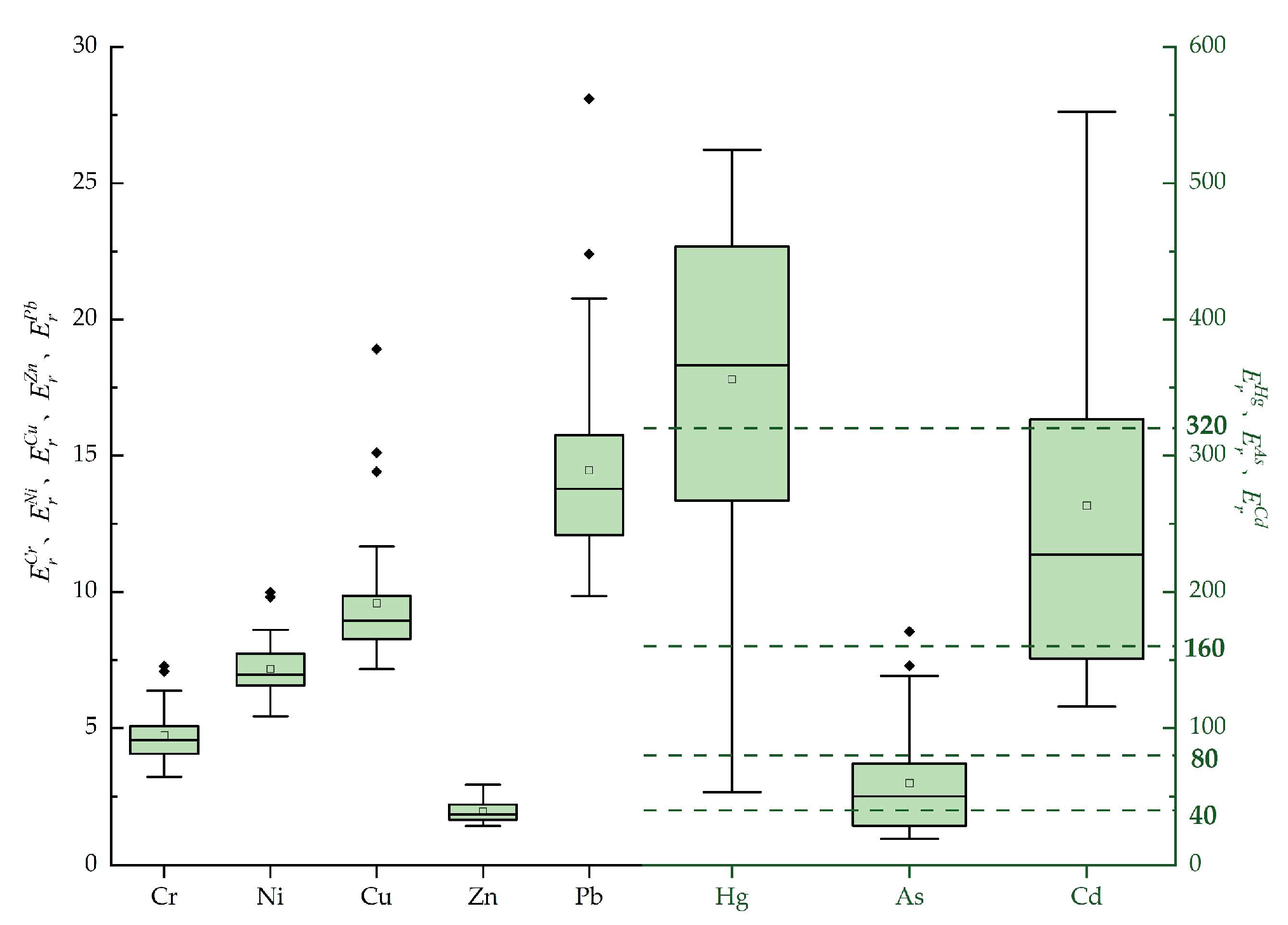
4.4.2. Evaluation Results of the Potential Ecological Risk Index Method
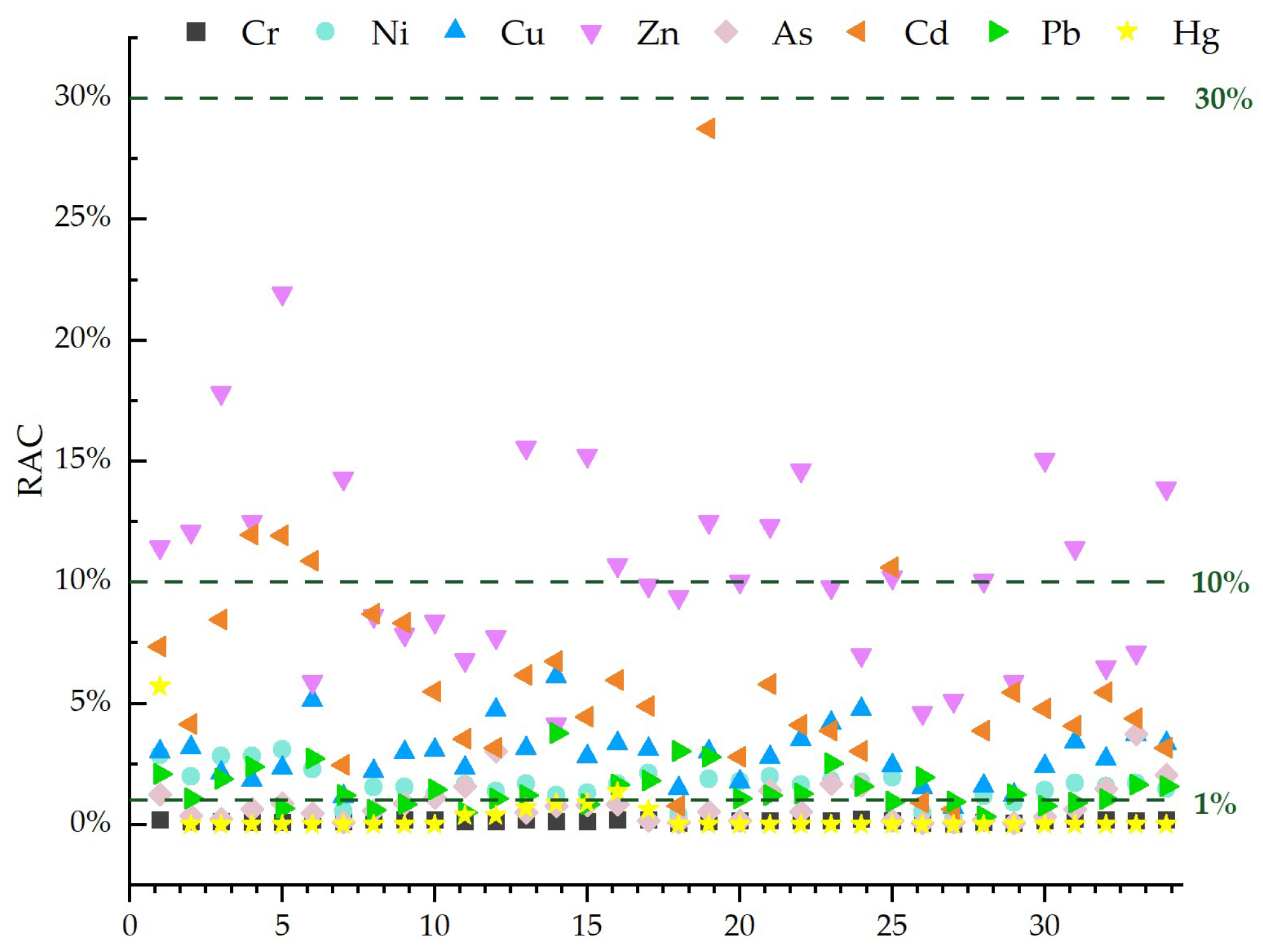
4.4.3. Evaluation Results of the Risk Assessment Code Method
4.5. Comparative Analysis of Three Risk Assessment Methods
5. Conclusions
- (1)
- The results of the three risk evaluation methods concluded that the soil’s heavy metal contamination level in the study area is high, and the combined potential ecological risk is high, with As, Hg and Cd as the main contaminating elements. Zn has the highest proportion of directly available biological state, which is a medium pollution risk, but the toxicity coefficient of Zn is low, which makes it difficult to cause ecological risk. It mainly originates from the waste rock and tailings left behind by the mines, Hg mainly originates from the early mining areas and from the chaotic and unorganized mining and gold extraction methods, and Cd mainly originates from mining activities and agricultural activities. In the process of mining and smelting, the direct discharge of waste residue, wastewater and waste gas will cause serious pollution, destroy the surrounding ecological environment and pose a potential threat to human health. Therefore, the international advanced mining and sorting process should be adopted, and the tailings should be treated and utilized in a timely manner to progress in the direction of non-waste mining. At the same time, the generated flue gas is sprayed and purified to reduce the risk of dry and wet settlement.
- (2)
- The predominant components of Cr, Ni, Cu, Zn, As, Cd, Pb and Hg in the soil within the study area are residues, accounting for 94.20%, 95.01%, 84.00%, 84.35%, 83.85%, 91.80%, 81.99% and 96.63%, respectively. The average proportion of the speciation of heavy metals in the soil is not much different, and the fraction of Cd and Zn mild acid-soluble is relatively high in individual sampling points, which needs to be paid attention to. In general, the soil’s heavy metal forms in the study area are relatively stable, and the change of soil pH should be monitored and the ecological risk situation of individual sampling sites should be paid attention to. It is recommended that further testing and research on citrus, maize and other crops grown in the study area should be carried out as a next step.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Pan, Y.; Zhu, C. Priority areas identification for arable soil pollution prevention based on the accumulative risk of heavy metals. Sci. Total Environ. 2024, 954, 176440. [Google Scholar] [CrossRef]
- Zhou, W.T.; Li, Z. Soil type data provide new methods and insights for heavy metal pollution assessment and driving factors analysis. J. Hazard. Mater. 2024, 480, 135868. [Google Scholar] [CrossRef]
- Wang, C.C.; Li, P.R. Spatial variability and risk assessment of heavy metals in the soil surrounding solid waste from coking plants in Shanxi, China. Environ. Monit. Assess. 2022, 195, 99. [Google Scholar] [CrossRef]
- Fei, X.F.; Lou, Z.H. Contamination and Health Risk Assessment of Heavy Metal Pollution in Soils Developed from Different Soil Parent Materials. Expo. Health 2023, 15, 395–408. [Google Scholar] [CrossRef]
- Renu, K.; Chakraborty, R. Molecular mechanism of heavy metals (Lead, Chromium, Arsenic, Mercury, Nickel and Cadmium)—induced hepatotoxicity—A review. Chemosphere 2021, 271, 129735. [Google Scholar] [CrossRef] [PubMed]
- Schlief, M.L.; West, T. Role of the Menkes copper-transporting ATPase in NMDA receptor-mediated neuronal toxicity. Proc. Natl. Acad. Sci. USA 2006, 103, 14919–14924. [Google Scholar] [CrossRef] [PubMed]
- Li, D.L.; Zhang, H.C. Environmental arsenic (As) and its potential relationship with endemic disease in southwestern China. J. Environ. Sci. 2024, 139, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Balza, J. Hidden toxins: Bathtubs as a potential source of lead exposure in children. J. Toxicol. Environ. Health Part A 2022, 85, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Song, Z. A critical review on bioremediation technologies for Cr(VI)-contaminated soils and wastewater. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1027–1078. [Google Scholar] [CrossRef]
- Iqbal, M. Vicia fababioassay for environmental toxicity monitoring: A review. Chemosphere 2016, 144, 785–802. [Google Scholar] [CrossRef]
- Chen, W.; Wei, Z.Y. Chemical evaluation methods for the phytoavailability of heavy metals in soils. J. Agro-Environ. Sci. 2024, 5, 1–19. [Google Scholar]
- Zhang, J.L.; Liu, Y.H. Speciation Analysis and Pollution Assessment of Heavy Metals in Farmland Soil of a Typical Mining Area: A Case Study of Dachang Tin Polymetallic Ore, Guangxi. Appl. Sci. 2023, 13, 708. [Google Scholar] [CrossRef]
- Sun, J.W.; Hu, G.R. Bioavailability of heavy metals in soil-tea plant system of Tieguanyin tea garden. Environ. Chem. 2020, 39, 2765–2776. [Google Scholar]
- Yang, E.L.; Li, H.P. Research on lightning protection design of gold mine. Technol. Innov. 2016, 118. [Google Scholar]
- GB/T 25282-2010; Soil and Sediment-Sequential Extraction Procedure of Speciation of 13 Trace Elements. Standardization Administration of China: Beijing, China, 2010.
- Muller, G. Index of Geoaccumulation in Sediments of the Rhine River. Acta Geol. Pol. 1969, 2, 109–118. [Google Scholar]
- Li, X.Y.; Zhang, J.Y. Status of chromium accumulation in agricultural soils across China (1989–2016). Chemosphere 2020, 256, 127036. [Google Scholar] [CrossRef] [PubMed]
- Environment Monitoring Total Station of China. Chinese Soil Element Background Values; China Environment Science Press: Beijing, China, 1990. (In Chinese) [Google Scholar]
- Chai, S.W.; Wen, Y. Application of index of geoaccumulation (Igeo) to pollution evaluation of heavy metals in soil. J. Tongji Univ. Nat. Sci. 2006, 34, 1657–1661. [Google Scholar]
- Hakanson, L. An ecological risk index for aquatic pollution control a sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Ni, S.J. Calculation of Heavy Metals Toxicity Coefficient in the Evaluation of Potential Ecological Risk Index. Environ. Sci. Technol. 2008, 31, 112–115. [Google Scholar]
- Zhang, J.; Peng, W. Environmental geochemical baseline determination and pollution assessment of heavy metals in farmland soil of typical coal-based cities: A case study of Suzhou City in Anhui Province, China. Heliyon 2023, 9, e14841. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.K. Metal fractionation study on bed sediments of River Yamuna, India. Water Res. 2004, 38, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.M.; Zhuang, T. Fraction distribution and ecological risk assessment of soil heavy metals in the farmland soil from the sewage irrigated area of Xiaoqing River. Environ. Chem. 2019, 38, 644–652. [Google Scholar]
- Sundaray, S.K.; Nayak, B.B. Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments-A case study: Mahanadi basin, India. J. Hazard. Mater. 2011, 186, 1837–1846. [Google Scholar] [CrossRef]
- GB 15618-2018; Soil Environmental Quality Risk Control Standard for Soil Contamination of Agricultural Land. China Environment Publishing Group: Beijing, China, 2018.
- Liu, P.F.; Wu, Z.Q. Pollution assessment and source analysis of heavy metals in acidic farmland of the karst region in southern China—A case study of Quanzhou County. Appl. Geochem. 2020, 123, 104764. [Google Scholar] [CrossRef]
- Hu, Z.X.; Wu, Z.Y. Spatial distribution, risk assessment, and source apportionment of soil heavy metals in a karst county based on grid survey. Sci. Total Environ. 2024, 953, 176049. [Google Scholar] [CrossRef]
- Chen, L.Y.; Ren, B.Z. Black shale bedrock control of soil heavy metal typical high geological background in China Loushao Basin: Pollution characteristics, source and Influence assessment based on spatial analysis. J. Hazard. Mater. 2024, 477, 135072. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Zhang, Y. Trace element geochemistry of pyrite from the Jinzhuzhou gold deposit in the Dayaoshan district, Qin-Hang Metallogenic Belt (South China): Implications for the metallogenesis. Geol. Bull. China 2024, 3, 1–23. [Google Scholar]
- Feng, Q.W.; Wang, B. Pollution Characteristics and Source Analysis of Heavy Metals in Soils of Typical Lead-zinc Mining Areas in Northwest Guizhou, China. Bull. Mineral. Petrol. Geochem. 2020, 39, 863–870. [Google Scholar]
- Zhao, L.Y.; Kong, L.H. Pollution characteristics, source analysis and risk assessment of heavy metals in soil around a gold mine in Jiaodong Peninsula. Geol. China 2024, 51, 1485–1500. [Google Scholar]
- Zhang, J.Y.; Zhang, X. Assessment and Source Apportionment of Heavy Metal Pollution in Soil of a Gold Ore Slag Muck Historical Remains Point. Shandong Chem. Ind. 2024, 53, 218–222. [Google Scholar]
- Cheng, X.D.; Sun, J.W. Pollution and source apportionment of heavy metals in the farmland soils of the gold mining area in Tantou Basin, western Henan metallogenic belt. East China Geol. 2022, 43, 313–323. [Google Scholar]
- Lian, H.B.; Li, P.F. Analysis of the sources of soil heavy metal pollution and remediation proposals in Tongguan gold mining area of the Little Qinling Mountains. South China Agriculture. 2018, 12, 184–185. [Google Scholar]
- Long, H.Y.; Wang, W.S. Speciation analysis and pollution risk assessment of heavy metals in the soils surrounding mine area. J. Guangxi Univ. Nat. Sci. Ed. 2016, 41, 1676–1682. [Google Scholar]
- Cong, Y.; Chen, Y.L. Chemical Forms of Heavy Metals in Soils and Potential Hazards to Ecosystem in Beijing Farmlands. Soils 2009, 41, 37–41. [Google Scholar]
- Cao, Q.Y.; Huang, Z.H. Review on speciation analysis of heavy metals in polluted soils and its influencing factors. Ecol. Sci. 2017, 36, 222–232. [Google Scholar]
- Jiang, S.H.; Hu, G.R. Evaluation of heavy metal accumulation and ecological risk in Anxi Tieguanyin tea plantation soils. Earth Environ. 2016, 44, 359–369. [Google Scholar]
- Ma, X.L.; Zuo, H. Assessment of heavy metals contamination in sediments from three adjacent regions of the Yellow River using metal chemical fractions and multivariate analysis techniques. Chemosphere 2016, 144, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Q.; Huang, H.B.; Lin, Y. Assessment of the Speciation and Pollution of Heavy Metals in Paddy Soils from the Jiulong River Basin. Environ. Sci. 2019, 40, 453–460. [Google Scholar]
- Yu, J.; Zhang, H.; Cai, Y.B. Distribution and ecological risk of heavy metals in water and sediments of a river polluted by gold mining. Environ. Pollut. Control 2015, 37, 1–9. [Google Scholar]
- Wang, J.; Han, Z.Y. Morphological distribution characteristics and ecological risk assessment of heavy metal in the green soil of industrial zone in Chengdu. Ecol. Environ. Sci. 2021, 30, 1923–1932. [Google Scholar]
- Ji, L.; Ma, K. Evaluation of Heavy Metal Distribution Characteristics and Ecological Risk of Soil at Vegetable-land for Hong Kong in Ningxia. Environ. Sci. 2024, 45, 1–17. [Google Scholar]
- Wei, C.M.; Zhang, Y.H. Speciation Analysis and Bioavailability Evaluation of Heav Metals in Soil of a Decommissioned Coking Plant in Jiangxi Province. J. Soil Sci. 2024, 55, 810–818. [Google Scholar]

| Step | Speciation | Pretreatment |
|---|---|---|
| 1 | mild acid-soluble fraction (F1) | The soil sample was weighed at 1.00 g and transferred into a 250 mL polyethylene centrifuge tube with a lid. In total, 40 mL of 0.1 mol/L HAC solution was added to the sample, then shaken on a reciprocating shaker at 22 ± 5 °C and at a speed of 200 r/min for a duration of 16 h. Subsequently, the mixture was centrifuged at 3000× g for 20 min to extract the solution. The resulting solution was filtered through a 0.22 µm filter membrane and the supernatant was transferred into a 10 mL polyethylene tube. Then, 1 mL of concentrated HNO3 was introduced and stored in a refrigerator until analysis was conducted. Additionally, 20 mL of ultrapure water was added to the centrifuge tube, agitated for an additional period of 15 min, followed by another round of centrifugation for 20 min in order to discard the supernatant. |
| 2 | reducible fraction (F2) | In total, 40 mL of 0.5 mol/L NH2OH·HCl solution was added to the centrifuge tube and shaken for 16 h at 22 ± 5 °C and 200 r/min. The mixture was then centrifuged for 20 min, and the supernatant was transferred to a 10 mL polyethylene tube. A total of 1 mL of concentrated HNO3 was added, and it was stored in the refrigerator for testing. After shaking the centrifuge tube with 20 mL of ultrapure water for 15 min, it was centrifuged for another 20 min before discarding the supernatant. |
| 3 | oxidizable fraction (F3) | A total of 10 mL of 8.8 mol/L H2O2 solution (pH = 2.0) was slowly added into the centrifuge tube, covered with a lid and disintegrated at room temperature for 1 h, shaking manually every 10 min during the disintegration process. Then it was placed in an adjustable electrothermal thermostatic water bath (85 ± 2 °C) to disintegrate for 1 h, the lid was removed, and heating continued until the volume was less than 3 mL. The centrifuge tube was then removed, allowed to cool down, and another 10 mL of 8.8 mol/L H2O2 solution (pH = 2.0) was added, dissolved in the water bath for 1 h. The lid was again removed and heating continued until the volume was about 1 mL. After removing the centrifuge tube, 50 mL of 1 mol/L NH4AC solution (pH = 2.0) was added again, sealed and shaken for 16 h (22 ± 5 °C, 200 r/min), centrifuged for 20 min, and the extracted solution was filtered through 0.22 µm filter membrane, and then the supernatant was transferred to a 10 mL polyethylene tube, and added with 1 mL of concentrated HNO3, and then kept in refrigerator to wait for the measurement. Add 20 mL of ultrapure water to the centrifuge tube, shake for 15 min and centrifuge for 20 min, then discard the supernatant. |
| 4 | residual fraction (F4) | The centrifuge tube containing the solid residue was placed in a water bath at 60 °C until evaporation, and after constant weighing at 60 °C, it was transferred to a sample bag and preserved by grinding. A total of 0.1 g of the solid residue was taken and eliminated by HCl-HNO3-HF-HClO4 method and left to be measured. |
| 5 | water-soluble fraction (F5) | In total, 1.00 g of sample was weighed into a 100 mL capped polyethylene centrifuge tube, 20 mL of boiled and cooled ultrapure water pH = 7.0 was added, sealed and shaken for 16 h and then centrifuged for 30 min at a force of 4000 g. The extract was filtered through a 0.45 µm membrane, and the supernatant was pipetted into a 10 mL polyethylene tube and added with 1 mL of concentrated HNO3, stored under refrigeration and stored for measurement. |
| Contamination Level | |
|---|---|
| ≤0 | Non-polluted |
| 0 < ≤ 1 | Slightly polluted |
| 1 < ≤ 2 | Moderately polluted |
| 2 < ≤ 3 | Moderately–highly polluted |
| 3 < ≤ 4 | Highly polluted |
| 4 < ≤ 5 | Highly–extremely polluted |
| >5 | Extremely polluted |
| Single Factor Ecological Risk Pollution Degree | RI | Total Potential Ecological Risk Degree | |
|---|---|---|---|
| < 40 | Slight | RI < 150 | Slight |
| 40 ≤ < 80 | Mediate | 150 ≤ RI < 300 | Mediate |
| 80 ≤ < 160 | High | 300 ≤ RI < 600 | High |
| 160 ≤ < 320 | Very high | 600 ≤ RI < 1200 | Very high |
| < 320 | Extremely high | RI > 1200 | Extremely high |
| RAC | <1% | 1–10% | 10–30% | 30–50% | >50% |
|---|---|---|---|---|---|
| Evaluation criteria | None | Slight | Moderate | High | Very High |
| Item | Maximum | Minimum | Average | Coefficient of Variation (%) | Background Value of the Soil Environment in Guangxi | Screening Value for Agricultural Land |
|---|---|---|---|---|---|---|
| Cr | 298.57 | 132.29 | 194.05 | 20.30 | 82.10 | 150.00 |
| Ni | 53.10 | 28.85 | 38.14 | 14.58 | 26.60 | 60.00 |
| Cu | 105.12 | 39.80 | 53.29 | 24.93 | 27.80 | 150.00 |
| Zn | 221.77 | 108.58 | 147.11 | 19.80 | 75.60 | 200.00 |
| As | 350.33 | 39.75 | 122.35 | 63.56 | 20.50 | 40.00 |
| Cd | 4.97 | 1.04 | 2.37 | 49.82 | 0.27 | 0.30 |
| Pb | 134.85 | 47.26 | 69.40 | 25.49 | 24.00 | 70.00 |
| Hg | 1.97 | 0.20 | 1.33 | 32.67 | 0.15 | 1.30 |
| pH | 5.16 | 3.42 | 4.02 | 8.23 |
| Item | Principal Components | ||
|---|---|---|---|
| PC1 | PC2 | PC3 | |
| Ni | 0.872 | −0.169 | −0.113 |
| Pb | 0.843 | 0.298 | −0.004 |
| Zn | 0.804 | 0.325 | −0.192 |
| Cd | 0.691 | −0.472 | 0.168 |
| Cr | 0.664 | −0.475 | 0.3620 |
| Cu | 0.577 | 0.272 | −0.285 |
| As | 0.103 | 0.797 | −0.019 |
| Hg | 0.079 | 0.471 | 0.826 |
| Eigenvalue | 3.387 | 1.602 | 0.972 |
| Variance contribution rate (%) | 42.335 | 20.019 | 12.154 |
| Cumulative variance contribution rate (%) | 42.335 | 62.354 | 74.509 |
| Item | Cr | Ni | Cu | Zn | As | Cd | Pb | Hg |
|---|---|---|---|---|---|---|---|---|
| Minimum | 0.10 | −0.47 | −0.07 | −0.06 | 0.37 | 1.36 | 0.39 | −0.17 |
| Maximum | 1.28 | 0.41 | 1.33 | 0.97 | 3.51 | 3.62 | 1.91 | 3.13 |
| Average | 0.63 | −0.08 | 0.32 | 0.35 | 1.74 | 2.38 | 0.91 | 2.46 |
| Standard deviation | 0.28 | 0.20 | 0.30 | 0.28 | 0.85 | 0.70 | 0.32 | 0.65 |
| contamination degree | Slight | None | Sligh | Slight | Moderate | Moderate –High | Slight | Moderate –High |
| Item | Eri | RI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cr | Ni | Cu | Zn | As | Cd | Pb | Hg | ||
| Minimum | 3.22 | 5.42 | 7.16 | 1.44 | 19.3 | 115.65 | 9.85 | 53.33 | 344.42 |
| Maximum | 7.27 | 9.98 | 18.91 | 2.93 | 170.89 | 552.31 | 28.01 | 524.21 | 1109.18 |
| Average | 4.73 | 7.17 | 9.58 | 1.95 | 59.68 | 263.18 | 14.46 | 355.94 | 716.69 |
| Hazard degree | Slight | Slight | Slight | Slight | Mediate | Very high | Slight | Extremely high | Very high |
| Item | Cr | Ni | Cu | Zn | As | Cd | Pb | Hg |
|---|---|---|---|---|---|---|---|---|
| Minimum | 0.02% | 0.22% | 0.69% | 4.14% | 0.06% | 0.06% | 0.34% | 0.00% |
| Maximum | 0.24% | 3.09% | 6.11% | 21.95% | 3.74% | 28.75% | 3.76% | 5.71% |
| Average | 0.15% | 1.64% | 2.84% | 10.46% | 0.85% | 6.08% | 1.49% | 0.32% |
| contamination degree | None | Slight | Slight | Mediate | None | Slight | Slight | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Wen, M.; Liu, P.; Jiang, Y.; Zhang, X. Speciation Characteristics and Risk Assessment of Heavy Metals in Cultivated Soil in Pingshui Village, Zhaoping County, Hezhou City, Guangxi. Appl. Sci. 2024, 14, 11361. https://doi.org/10.3390/app142311361
Ma Y, Wen M, Liu P, Jiang Y, Zhang X. Speciation Characteristics and Risk Assessment of Heavy Metals in Cultivated Soil in Pingshui Village, Zhaoping County, Hezhou City, Guangxi. Applied Sciences. 2024; 14(23):11361. https://doi.org/10.3390/app142311361
Chicago/Turabian StyleMa, Yunxue, Meilan Wen, Panfeng Liu, Yuxiong Jiang, and Xiaohan Zhang. 2024. "Speciation Characteristics and Risk Assessment of Heavy Metals in Cultivated Soil in Pingshui Village, Zhaoping County, Hezhou City, Guangxi" Applied Sciences 14, no. 23: 11361. https://doi.org/10.3390/app142311361
APA StyleMa, Y., Wen, M., Liu, P., Jiang, Y., & Zhang, X. (2024). Speciation Characteristics and Risk Assessment of Heavy Metals in Cultivated Soil in Pingshui Village, Zhaoping County, Hezhou City, Guangxi. Applied Sciences, 14(23), 11361. https://doi.org/10.3390/app142311361






