Less Is More: Influence of Cross-Linking Agent Concentration on PFOS Adsorption in Chitosan
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Preparation of Chitosan
2.3. PFAS Analysis
2.4. Adsorption Tests
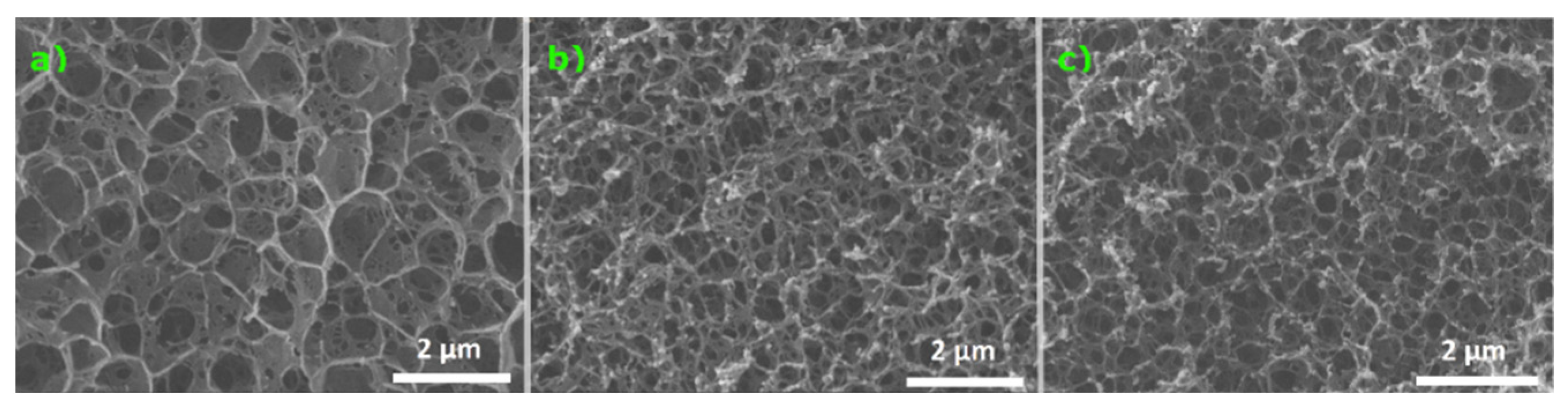
2.5. Scanning Electron Microscopy (SEM)
2.6. Rapid Small-Scale Column Test
3. Results and Discussion
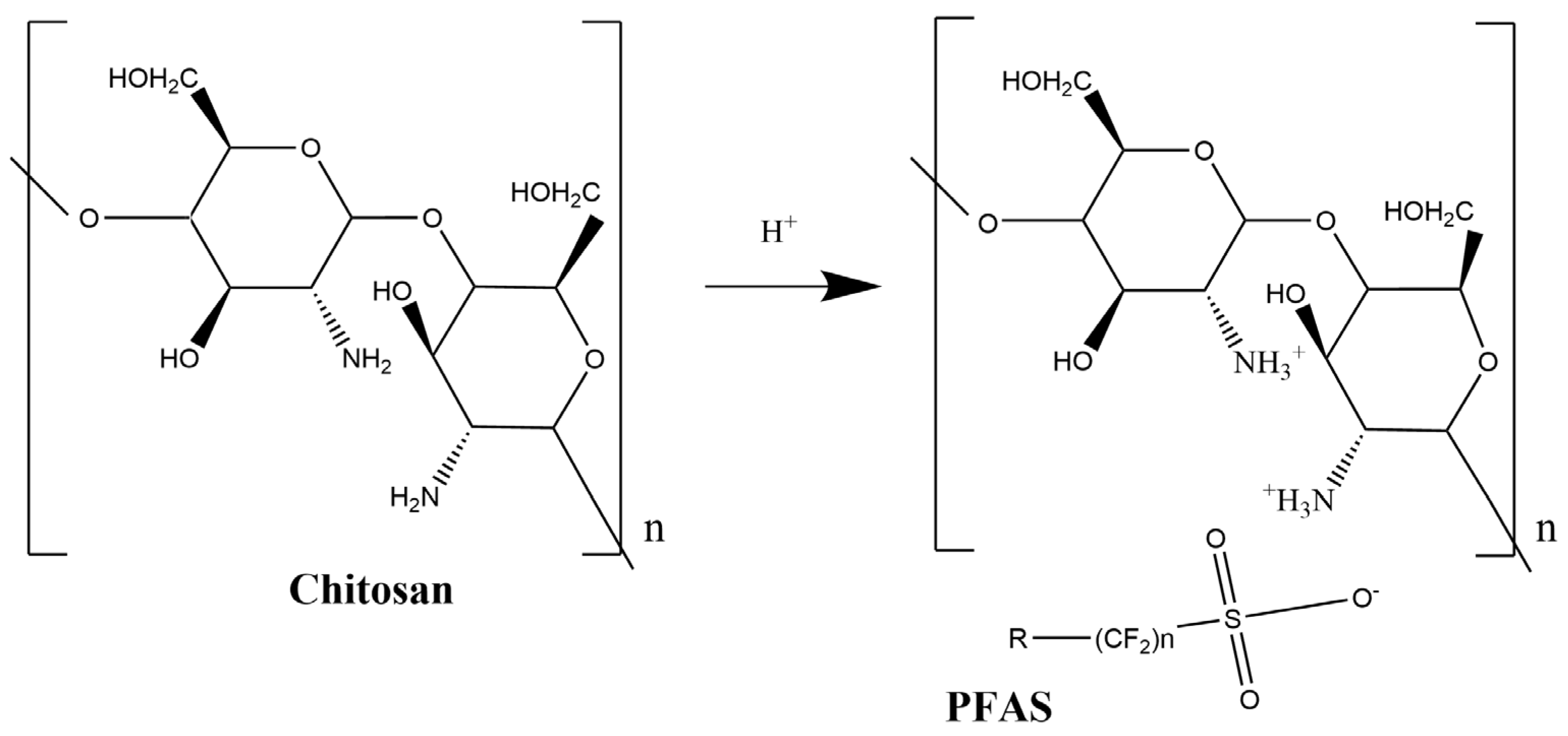
3.1. Molecular Imprinting on Chitosan
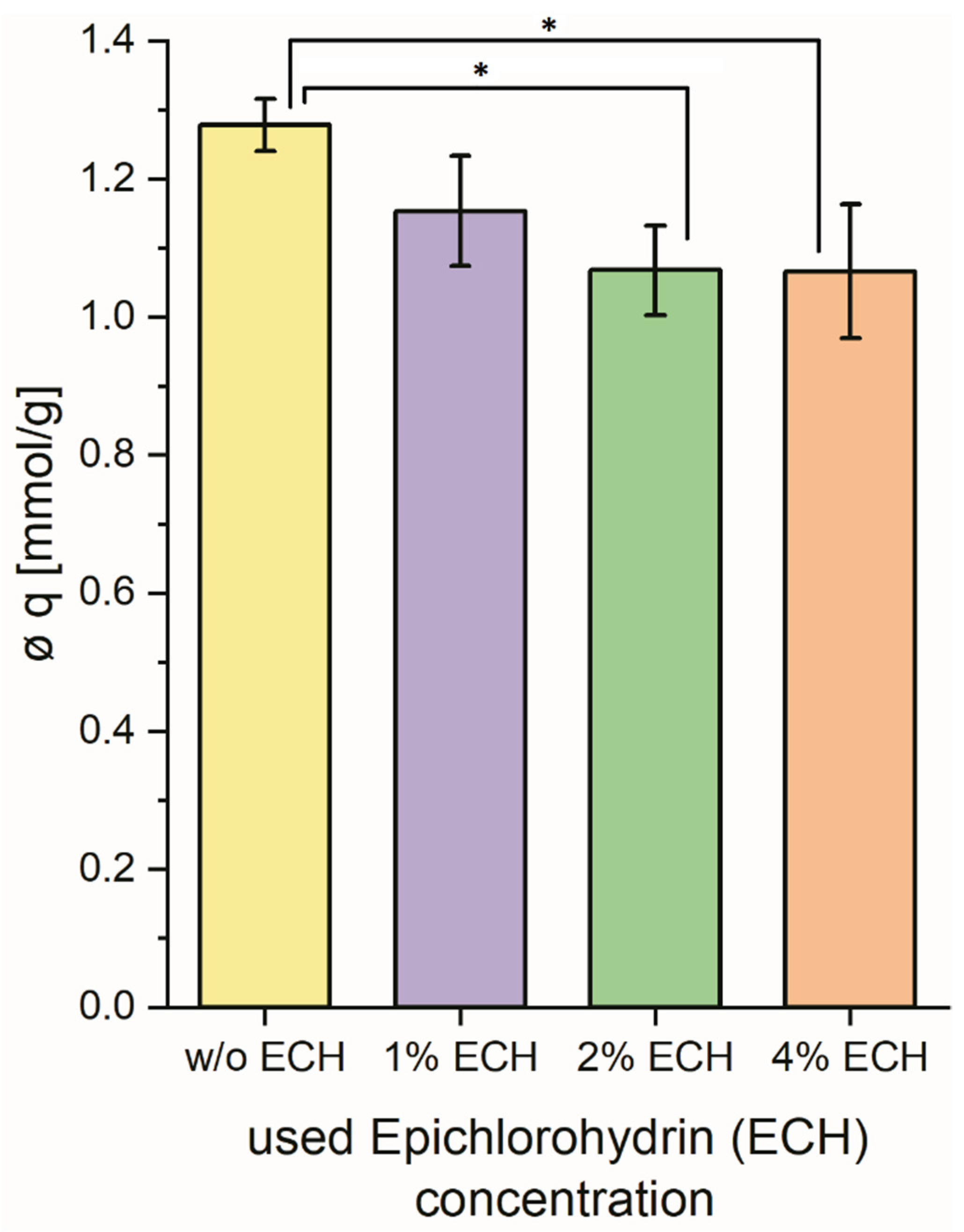
3.2. Varying the ECH Concentration During Synthesis
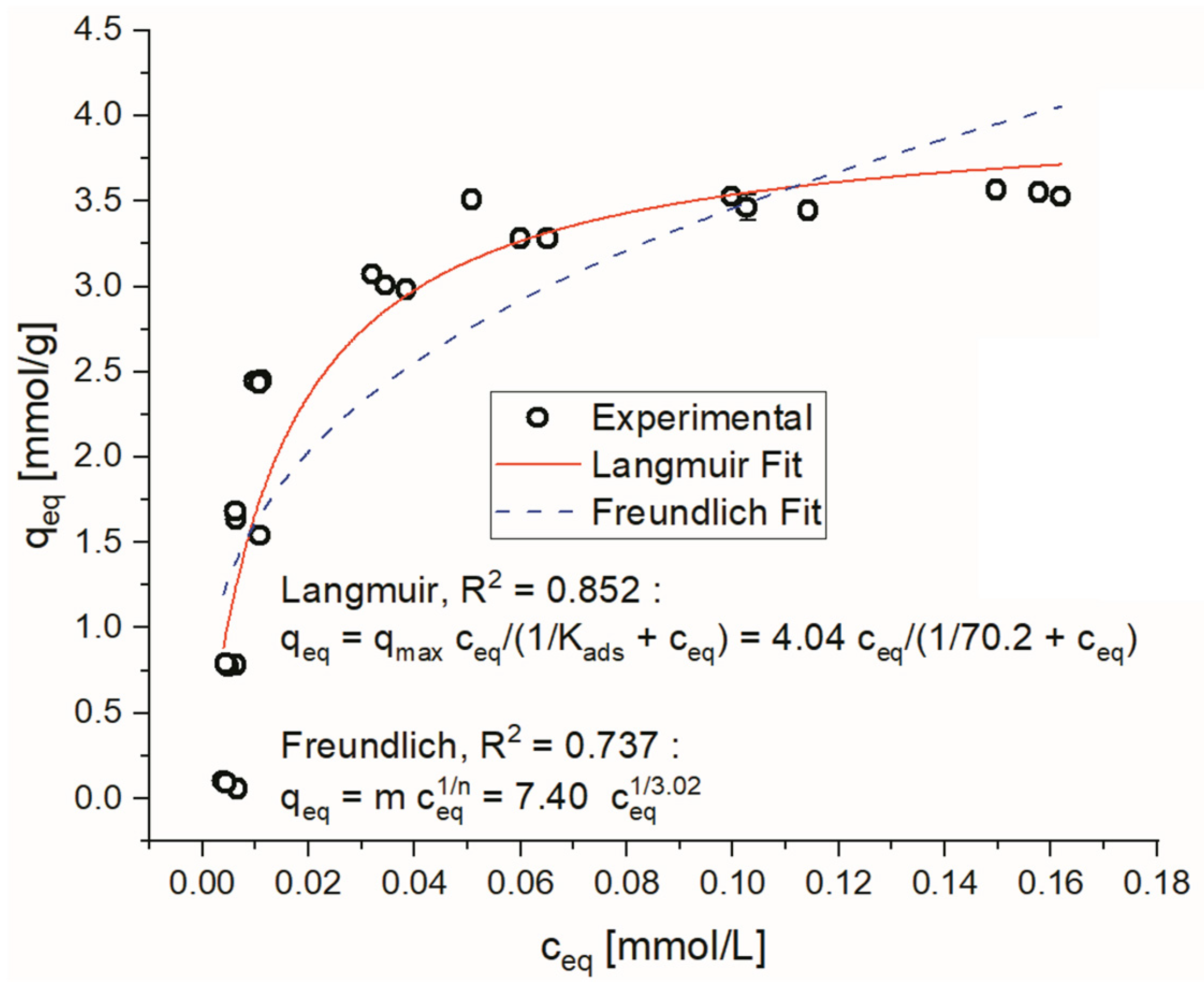
3.3. Adsorption Isotherms
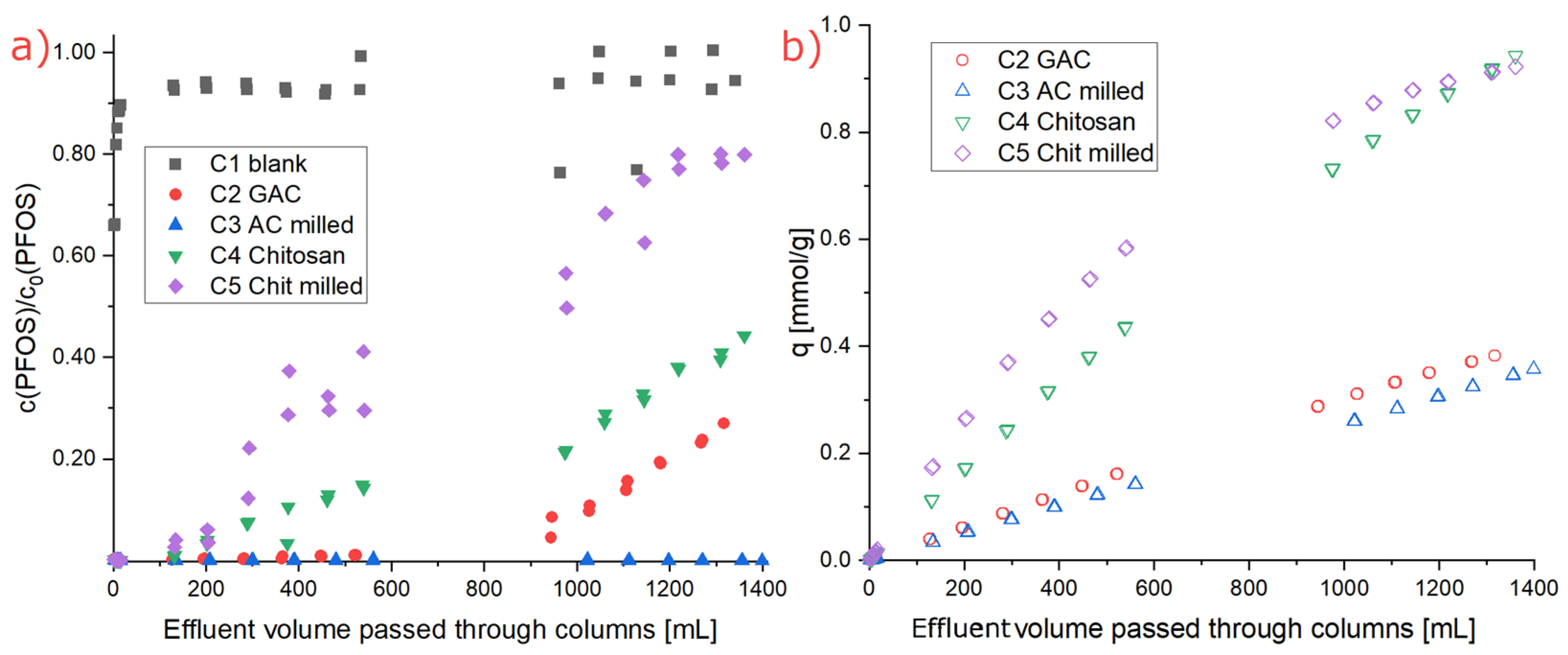
3.4. Rapid Small-Scale Column (RSSC) Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grabda, M.; Oleszek, S.; Matsumoto, M. Per- and polyfluoroalkyl substances: Problematic emerging pollutants of aquatic environment. Arch. Environ. Prot. 2020, 46, 3–21. [Google Scholar] [CrossRef]
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef] [PubMed]
- Stoiber, T.; Evans, S.; Naidenko, O.V. Disposal of products and materials containing per- and polyfluoroalkyl substances (PFAS): A cyclical problem. Chemosphere 2020, 260, 127659. [Google Scholar] [CrossRef]
- Vo, H.N.P.; Ngo, H.H.; Guo, W.; Nguyen, T.M.H.; Li, J.; Liang, H.; Deng, L.; Chen, Z.; Nguyen, T.A.H. Poly-and perfluoroalkyl substances in water and wastewater: A comprehensive review from sources to remediation. J. Water Process Eng. 2020, 36, 101393. [Google Scholar] [CrossRef]
- Reinhart, D.R.; Bolyard, S.C.; Chen, J. Fate of Per- and Polyfluoroalkyl Substances in Postconsumer Products during Waste Management. J. Environ. Eng. 2023, 149, 03123002. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Stasinakis, A.S. Review on the occurrence, fate and removal of perfluorinated compounds during wastewater treatment. Sci. Total Environ. 2015, 524–525, 81–92. [Google Scholar] [CrossRef]
- Wanninayake, D.M. Comparison of currently available PFAS remediation technologies in water: A review. J. Environ. Manage. 2021, 283, 111977. [Google Scholar] [CrossRef]
- Longendyke, G.K.; Katel, S.; Wang, Y. PFAS fate and destruction mechanisms during thermal treatment: A comprehensive review. Environ. Sci. Process. Impacts 2022, 24, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, Z.; He, X.; Song, M.; Westerhoff, P.; Doudrick, K.; Hanigan, D. Critical Review of Thermal Decomposition of Per- and Polyfluoroalkyl Substances: Mechanisms and Implications for Thermal Treatment Processes. Environ. Sci. Technol. 2022, 56, 5355–5370. [Google Scholar] [CrossRef]
- Bolan, N.; Sarkar, B.; Yan, Y.; Li, Q.; Wijesekara, H.; Kannan, K.; Tsang, D.C.W.; Schauerte, M.; Bosch, J.; Noll, H.; et al. Remediation of poly- and perfluoroalkyl substances (PFAS) contaminated soils—To mobilize or to immobilize or to degrade? J. Hazard. Mater. 2021, 401, 123892. [Google Scholar] [CrossRef]
- Council of the European Union; European Parliament. Directive (EU) 2020/2184 on the quality of water intended for human consumption (recast). Off. J. Eur. Union 2020, 63, L 435/1. [Google Scholar]
- Boyer, T.H.; Fang, Y.; Ellis, A.; Dietz, R.; Choi, Y.J.; Schaefer, C.E.; Higgins, C.P.; Strathmann, T.J. Anion exchange resin removal of per- and polyfluoroalkyl substances (PFAS) from impacted water: A critical review. Water Res. 2021, 200, 117244. [Google Scholar] [CrossRef] [PubMed]
- Dixit, F.; Dutta, R.; Barbeau, B.; Berube, P.; Mohseni, M. PFAS removal by ion exchange resins: A review. Chemosphere 2021, 272, 129777. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Wang, J.; Kumar, P.; Mishra, V.; Arafat, H.; Sharma, R.S.; Dumée, L.F. Remediation of water from per-/poly-fluoroalkyl substances (PFAS)—Challenges and perspectives. J. Environ. Chem. Eng. 2021, 9, 105784. [Google Scholar] [CrossRef]
- Militao, I.M.; Roddick, F.A.; Bergamasco, R.; Fan, L. Removing PFAS from aquatic systems using natural and renewable material-based adsorbents: A review. J. Environ. Chem. Eng. 2021, 9, 105271. [Google Scholar] [CrossRef]
- Teymourian, T.; Teymoorian, T.; Kowsari, E.; Ramakrishna, S. A review of emerging PFAS contaminants: Sources, fate, health risks, and a comprehensive assortment of recent sorbents for PFAS treatment by evaluating their mechanism. Res. Chem. Intermed. 2021, 47, 4879–4914. [Google Scholar] [CrossRef]
- Lei, X.; Lian, Q.; Zhang, X.; Karsili, T.K.; Holmes, W.; Chen, Y.; Zappi, M.E.; Gang, D.D. A review of PFAS adsorption from aqueous solutions: Current approaches, engineering applications, challenges, and opportunities. Environ. Pollut. 2023, 321, 121138. [Google Scholar] [CrossRef]
- Parker, B.A.; Kanalos, C.A.; Radniecki, T.S.; Massey Simonich, S.L.; Field, J.A. Evaluation of sorbents and matrix effects for treating heavy metals and per- and polyfluoroalkyl substances as co-contaminants in stormwater. Environ. Sci. Water Res. Technol. 2023, 9, 3281–3289. [Google Scholar] [CrossRef]
- Wei, Z.; Xu, T.; Zhao, D. Treatment of per- and polyfluoroalkyl substances in landfill leachate: Status, chemistry and prospects. Environ. Sci. Water Res. Technol. 2019, 5, 1814–1835. [Google Scholar] [CrossRef]
- Kabiri, S.; Monaghan, C.L.; Navarro, D.; McLaughlin, M.J. Hydrophobic interaction is the dominant mechanism of zwitterionic PFAS adsorption to carbon-based sorptive materials in water and soil. Environ. Sci. Water Res. Technol. 2024, 10, 420–430. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Removal of various pollutants from water and wastewater by modified chitosan adsorbents. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2331–2386. [Google Scholar] [CrossRef]
- Yu, Q.; Deng, S.; Yu, G. Selective removal of perfluorooctane sulfonate from aqueous solution using chitosan-based molecularly imprinted polymer adsorbents. Water Res. 2008, 42, 3089–3097. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, S.; Yu, G.; Huang, J. Removal of perfluorooctane sulfonate from aqueous solution by crosslinked chitosan beads: Sorption kinetics and uptake mechanism. Bioresour. Technol. 2011, 102, 2265–2271. [Google Scholar] [CrossRef]
- Jiao, Z.; Li, J.; Mo, L.; Liang, J.; Fan, H. A molecularly imprinted chitosan doped with carbon quantum dots for fluorometric determination of perfluorooctane sulfonate. Mikrochim. Acta 2018, 185, 473. [Google Scholar] [CrossRef]
- Sörengård, M.; Östblom, E.; Köhler, S.; Ahrens, L. Adsorption behavior of per- and polyfluoralkyl substances (PFASs) to 44 inorganic and organic sorbents and use of dyes as proxies for PFAS sorption. J. Environ. Chem. Eng. 2020, 8, 103744. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Zhu, X.; Zhu, D.; Wang, W.; Wang, B.; Deng, S.; Yu, G. Efficient removal of per/polyfluoroalkyl substances from water using recyclable chitosan-coated covalent organic frameworks: Experimental and theoretical methods. Chemosphere 2024, 356, 141942. [Google Scholar] [CrossRef] [PubMed]
- Cagnetta, G.; Yin, Z.; Qiu, W.; Vakili, M. Mechanochemical Synthesis of Cross-Linked Chitosan and Its Application as Adsorbent for Removal of Per- and Polyfluoroalkyl Substances from Simulated Electroplating Wastewater. Materials 2024, 17, 3006. [Google Scholar] [CrossRef]
- Durner, W.; Iden, S.C.; von Unold, G. The integral suspension pressure method (ISP) for precise particle-size analysis by gravitational sedimentation. Water Resour. Res. 2017, 53, 33–48. [Google Scholar] [CrossRef]
- Kosaraju, S.L.; D’ath, L.; Lawrence, A. Preparation and characterisation of chitosan microspheres for antioxidant delivery. Carbohydr. Polym. 2006, 64, 163–167. [Google Scholar] [CrossRef]
- Paulino, A.T.; Simionato, J.I.; Garcia, J.C.; Nozaki, J. Characterization of chitosan and chitin produced from silkworm crysalides. Carbohydr. Polym. 2006, 64, 98–103. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Hanafiah, M.A.K.M.; Yong, S.S. Adsorption of humic acid from aqueous solutions on crosslinked chitosan-epichlorohydrin beads: Kinetics and isotherm studies. Colloids Surf. B 2008, 65, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Kamari, A.; Pulford, I.D.; Hargreaves, J.S. Chitosan as a potential amendment to remediate metal contaminated soil—A characterisation study. Colloids Surf. B 2011, 82, 71–80. [Google Scholar] [CrossRef]
- Rinki, K.; Dutta, P.K.; Hunt, A.J.; Macquarrie, D.J.; Clark, J.H. Chitosan Aerogels Exhibiting High Surface Area for Biomedical Application: Preparation, Characterization, and Antibacterial Study. Int. J. Polymer. Mater. 2011, 60, 988–999. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Ibrahim, M.H.; Abdullah, A.Z. Elimination of reactive blue 4 from aqueous solutions using 3-aminopropyl triethoxysilane modified chitosan beads. Carbohydr. Polym. 2015, 132, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Qu, L.; Tian, M.; Zhu, S.; Zhang, X.; Tang, X.; Sun, K. Chitosan/Graphene Oxide Composite as an Effective Adsorbent for Reactive Red Dye Removal. Water Environ. Res. 2016, 88, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Reyna-Urrutiaa, V.A.; Mata-Harob, V.; Cauich-Rodrigueza, J.V.; Herrera-Kaoa, W.A.; Cervantes-Uc, J.M. Effect of two crosslinking methods on the physicochemical and biological properties of the collagen-chitosan scaffolds. Eur. Polym. J. 2019, 117, 424–433. [Google Scholar] [CrossRef]
- Hsien, T.-Y.; Rorrer, G.L. Effects of Acylation and Crosslinking on the Material Properties and Cadmium Ion Adsorption Capacity of Porous Chitosan Beads. Sep. Sci. Technol. 1995, 30, 2455–2475. [Google Scholar] [CrossRef]
- Du, Z.; Deng, S.; Bei, Y.; Huang, Q.; Wang, B.; Huang, J.; Yu, G. Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents—A review. J. Hazard. Mater. 2014, 274, 443–454. [Google Scholar] [CrossRef]
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Vagliasindi, F.G.A.; Roccaro, P. Removal of poly- and perfluoroalkyl substances (PFAS) from water by adsorption: Role of PFAS chain length, effect of organic matter and challenges in adsorbent regeneration. Water Res. 2020, 171, 115381. [Google Scholar] [CrossRef]
- Vu, C.T.; Wu, T. Recent progress in adsorptive removal of per- and poly-fluoroalkyl substances (PFAS) from water/wastewater. Crit. Rev. Environ. Sci. Technol. 2020, 52, 90–129. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Zhang, W.L.; Liang, Y.N. Adsorption of perfluoroalkyl and polyfluoroalkyl substances (PFASs) from aqueous solution—A review. Sci. Total Environ. 2019, 694, 133606. [Google Scholar] [CrossRef] [PubMed]


| Material | GAC (C2) | GAC Milled (C3) | ECH1 String (C4) | ECH1 Milled (C5) |
|---|---|---|---|---|
| BET area [m2/g] | 1291 | 1272 | 94.1 | 3.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wittwer, P.; Roesch, P.; Vogel, C.; Simon, F.; Gehrenkemper, L.; Feldmann, I.; Simon, F.-G. Less Is More: Influence of Cross-Linking Agent Concentration on PFOS Adsorption in Chitosan. Appl. Sci. 2024, 14, 11145. https://doi.org/10.3390/app142311145
Wittwer P, Roesch P, Vogel C, Simon F, Gehrenkemper L, Feldmann I, Simon F-G. Less Is More: Influence of Cross-Linking Agent Concentration on PFOS Adsorption in Chitosan. Applied Sciences. 2024; 14(23):11145. https://doi.org/10.3390/app142311145
Chicago/Turabian StyleWittwer, Philipp, Philipp Roesch, Christian Vogel, Fabian Simon, Lennart Gehrenkemper, Ines Feldmann, and Franz-Georg Simon. 2024. "Less Is More: Influence of Cross-Linking Agent Concentration on PFOS Adsorption in Chitosan" Applied Sciences 14, no. 23: 11145. https://doi.org/10.3390/app142311145
APA StyleWittwer, P., Roesch, P., Vogel, C., Simon, F., Gehrenkemper, L., Feldmann, I., & Simon, F.-G. (2024). Less Is More: Influence of Cross-Linking Agent Concentration on PFOS Adsorption in Chitosan. Applied Sciences, 14(23), 11145. https://doi.org/10.3390/app142311145







