Effect of the Concentration of Bioethanol Mixed with Gasoline on the Energy and Environmental Performance of a Hybrid Vehicle in the Worldwide Harmonized Light Vehicles Test Cycle (WLTC)
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Equipment
2.2. Fuels
2.3. Description of the WLTC Test
2.4. Methodology for Calculating Energy Performance and Emissions in the WLTC Cycle
3. WLTC-Based Analysis of Study Results
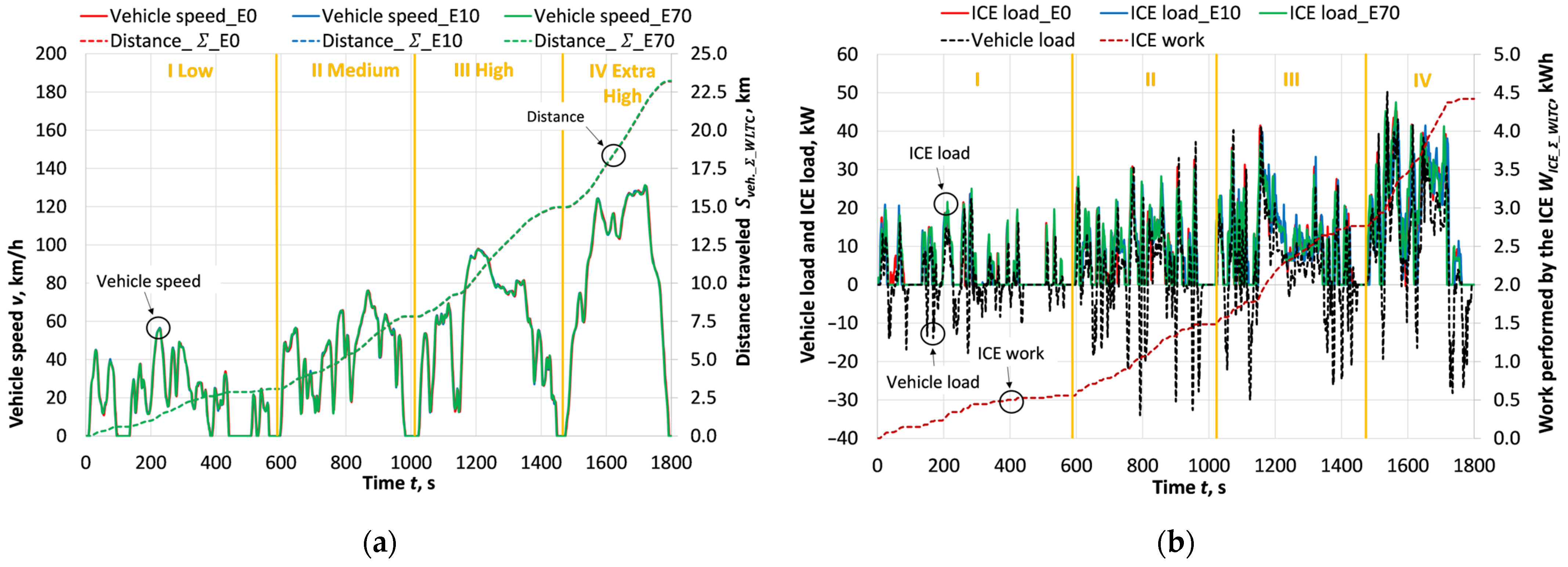
3.1. Energy Performance
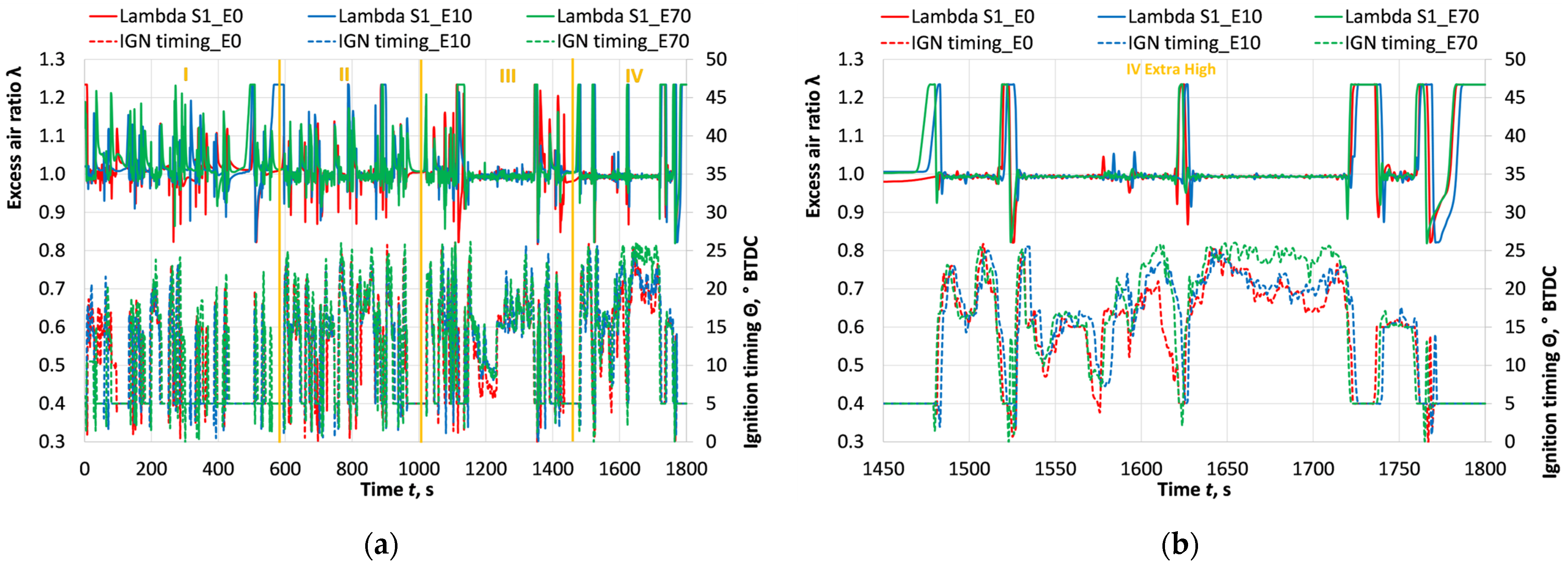
3.2. Ecological Parameters
4. Conclusions
- The fuel E0 was replaced by E10 and E70, resulting in an increase in fuel mass consumption of ~0.1% and ~23.9%, respectively, due to the decrease in the lower calorific value of the fuel. The higher ethanol density of E70 resulted in a smaller increase in volumetric fuel consumption (~19.1%) compared to mass consumption. The ICE of the hybrid car using E0 is quite efficient, with a ≈ 0.346 compared to a conventional spark ignition engine operating at low loads, as the hybrid system ensures engine operation in efficient modes. Increasing the bioethanol concentration to 10% and 70% resulted in an additional significant efficiency increase (~2.8% and ~9.0%), attributed to the more efficient combustion of bioethanol and the advanced ignition timing enabled by the higher knock resistance.
- Upstream of the catalytic converter, CO emissions with fuel E0 were reduced by 19% when E0 was substituted with E10 and E70. The use of the catalytic converter reduced CO emissions with E0 by approximately 170 times. Similarly, the substitution of E0 with E10 and E70 reduced unburned hydrocarbons by approximately 17% and 27%, while downstream of the catalytic converter, HC emissions with E0 were reduced by approximately 80 times. The addition of 70% bioethanol further reduced CO and HC emissions downstream of the catalytic converter by ~28% and ~15%, respectively. The reduction in CO and HC emissions can primarily be attributed to the increased oxygen content in the fuel mixture due to bioethanol, the lower C/H ratio, and the advanced ignition timing. However, it was observed that in low- and medium-speed driving modes, the higher frequency and duration of braking caused a reduction in exhaust and catalytic converter temperatures. This effect was further amplified with an increasing bioethanol concentration, due to the higher specific evaporation temperature and earlier ignition timing. As a result, CO and HC emissions were higher in these driving modes, and pre-catalytic converter and catalytic converter efficiencies were lower.
- Direct NOx emissions from the ICE were reduced by approximately 24% and 30% when E0 was replaced by E10 and E70, respectively. The catalytic converter reduced the NOx emissions of E0 by approximately 74 times. The addition of bioethanol further reduced NOx emissions by approximately 16% and 21%. However, in the low- and medium-speed vehicle modes, increasing the bioethanol concentration in the fuel led to an increase in NOx emissions. This was because the advanced ignition timing at lower engine speeds resulted in a greater rise in combustion temperatures, promoting NOx formation, while the lower exhaust gas temperatures reduced the catalytic converter’s efficiency. The most significant positive effect of bioethanol was observed in the high- and very-high-speed vehicle modes, where the hybrid system’s electric motor assistance is relatively lower and the ICE bears a higher load. In these conditions, the higher bioethanol content enhanced cylinder cooling, reducing high-temperature NOx emissions, while the catalytic converter temperatures were similar to those with E0 and effectively reduced NOx emissions.
- The specific CO2 greenhouse gas emissions upstream of the catalytic converter when running on E0 fuel were 138.8 g/km. The oxidation of CO and HC compounds in the catalytic converter resulted in an increase of approximately 5.9% in CO2 emissions to 153.8 g/km. Increasing the bioethanol concentration to 10% and 70% reduced the C/H ratio of the fuel blends by approximately 4% and 28%, respectively, and this led to a reduction in specific CO2 emissions by approximately 3.5% and 5.0%, respectively. An important aspect of a life cycle assessment is that bioethanol is a renewable fuel, and using E100 instead of pure gasoline can reduce CO2 emissions by approximately 60%. In addition, increasing the concentration of bioethanol in gasoline blends reduces not only CO2, but also NOx, which also contributes to the greenhouse effect.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| air mass consumption | |
| fuel mass consumption | |
| fuel mass consumption per WLTC | |
| BSFC | Brake Specific Fuel Consumption |
| Brake Specific Fuel Consumption per WLTC | |
| BTDC | Before Top Dead Centre |
| BTE | Brake Thermal Efficiency |
| ICE Brake Thermal Efficiency per WLTC | |
| CO | carbon monoxide |
| CO2 | carbon dioxide |
| E0 | pure gasoline |
| E10 | blend of gasoline 90% with bioethanol 100% by volume |
| E100 | pure bioethanol |
| E70 | blend of gasoline 30% with bioethanol 70% by volume |
| ECU | Engine Control Unit |
| pollutant emissions | |
| specific emissions of pollutant during the WLTC test cycle | |
| total mass emissions of pollutant during the WLTC cycle | |
| HC | unburnt hydrocarbon |
| HEV | Hybrid Electric Vehicles |
| ICE | Internal Combustion Engine |
| LHV | Lower Heating Value |
| molar mass of the engine exhaust gas | |
| mass emissions of engine exhaust | |
| NEDC | New European Driving Cycle |
| NOx | nitrogen oxides |
| PHEV | Plug-In Hybrid Electric Vehicles |
| SI | Spark Ignition |
| distance travelled by the vehicle during the WLTC | |
| work of the ICE during the WLTC cycle | |
| WLTC | Worldwide harmonized Light vehicles Test Cycle |
References
- Jeong, J.W.; In Lee, D.; Woo, S.; Lim, Y.; Lee, K. Analysis of Energy Consumption Efficiency and Emissions According to Urban Driving of Hybrid Electric Vehicles in Korea. Appl. Energy 2024, 371, 123686. [Google Scholar] [CrossRef]
- Yang, N.; Li, J.; He, C.; Wang, J.; Chen, Y.; Yao, Y. CO2 Emission Characteristics of China VI Hybrid Vehicles. Transp. Res. Part D Transp. Environ. 2024, 135, 104377. [Google Scholar] [CrossRef]
- Bai, S.; Liu, C. Overview of Energy Harvesting and Emission Reduction Technologies in Hybrid Electric Vehicles. Renew. Sustain. Energy Rev. 2021, 147, 111188. [Google Scholar] [CrossRef]
- Hu, R.; Chen, X.; Li, L.; Kong, F.; Liu, Y. Exhaust Emissions and Energy Conversion of Hybrid and Conventional CNG Buses. Transp. Res. Part D Transp. Environ. 2024, 135, 104405. [Google Scholar] [CrossRef]
- Bagheri, S.; Huang, Y.; Walker, P.D.; Zhou, J.L.; Surawski, N.C. Strategies for Improving the Emission Performance of Hybrid Electric Vehicles. Sci. Total Environ. 2021, 771, 144901. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, C.; Ge, Y.; Hao, L.; Tan, J.; Wang, X.; Zhang, P.; Wang, Y.; Tian, W.; Lin, Z.; et al. Fuel Consumption and Emission Performance from Light-Duty Conventional/Hybrid-Electric Vehicles over Different Cycles and Real Driving Tests. Fuel 2020, 278, 118340. [Google Scholar] [CrossRef]
- Allen, A. Hybrid Vehicle CO2 Emissions 20% Higher than Claimed, Research Reveals; AWE International: London, UK, 2024. [Google Scholar]
- Dornoff, J. Plug-in Hybrid Vehicle CO2 Emissions: How They Are Affected by Ambient Conditions and Driver Mode Selection; The International Council on Clean Transportation: Berlin/Heidelberg, Germany, 2021; p. 57. [Google Scholar]
- Transport & Environment Plug-in Hybrids to Lose ‘Low-CO2’ Status as EU Reassesses How Green They Really Are. Transp. Environ. 2022.
- Silva, C.; Ross, M.; Farias, T. Evaluation of Energy Consumption, Emissions and Cost of Plug-in Hybrid Vehicles. Energy Convers. Manag. 2009, 50, 1635–1643. [Google Scholar] [CrossRef]
- Dižo, J.; Blatnický, M.; Semenov, S.; Mikhailov, E.; Kostrzewski, M.; Droździel, P.; Šťastniak, P. Electric and Plug-in Hybrid Vehicles and Their Infrastructure in a Particular European Region. Transp. Res. Procedia 2021, 55, 629–636. [Google Scholar] [CrossRef]
- Mruzek, M.; Gajdáč, I.; Kučera, Ľ.; Barta, D. Analysis of Parameters Influencing Electric Vehicle Range. Procedia Eng. 2016, 134, 165–174. [Google Scholar] [CrossRef]
- Squalli, J. Greening the Roads: Assessing the Role of Electric and Hybrid Vehicles in Curbing CO2 Emissions. J. Clean. Prod. 2024, 434, 139908. [Google Scholar] [CrossRef]
- Vignesh, R.; Tanay, M.; Samay, G.; Kannan, C.; Ashok, B.; Senthil Kumar, M.; Szpica, D. Adoptability Assessment of HCDI and RCCI Modes in Plug-in Parallel Hybrid Electric Vehicles Using Sustainable Fuels and Model-Based Torque Structure Calibration Strategies. Results Eng. 2024, 23, 102859. [Google Scholar] [CrossRef]
- Dogdu, M.F.; Reyhancan, I.A. The Comparison of Gasoline Powered Vehicle and Serial Hybrid Vehicle on Emissions. Heliyon 2024, 10, e28532. [Google Scholar] [CrossRef] [PubMed]
- Sher, F.; Chen, S.; Raza, A.; Rasheed, T.; Razmkhah, O.; Rashid, T.; Rafi-ul-Shan, P.M.; Erten, B. Novel Strategies to Reduce Engine Emissions and Improve Energy Efficiency in Hybrid Vehicles. Clean. Eng. Technol. 2021, 2, 100074. [Google Scholar] [CrossRef]
- Si, S.; Yang, B.; Gao, B.; Zhang, Z.; Zhao, B.; Zhang, T.; Xu, H. A Real-Time Energy Management Strategy Combining Rule and Optimization for Minimizing Energy Consumption and Emissions of Flywheel Hybrid Electric Vehicle (FHEV). Appl. Therm. Eng. 2024, 255, 124013. [Google Scholar] [CrossRef]
- Rahman, M.M.; Zhou, Y.; Rogers, J.; Chen, V.; Sattler, M.; Hyun, K. A Comparative Assessment of CO2 Emission between Gasoline, Electric, and Hybrid Vehicles: A Well-To-Wheel Perspective Using Agent-Based Modeling. J. Clean. Prod. 2021, 321, 128931. [Google Scholar] [CrossRef]
- Kheir, N.A.; Salman, M.A.; Schouten, N.J. Emissions and Fuel Economy Trade-off for Hybrid Vehicles Using Fuzzy Logic. Math. Comput. Simul. 2004, 66, 155–172. [Google Scholar] [CrossRef]
- Mhaiti, N.; Radouani, M.; El Fahime, B. Multi-Physic Modeling and Simulation of a Hybrid Vehicle with Range Extender. IJESRT 2014, 3, 7058–7062. [Google Scholar]
- Huang, J.; Gao, J.; Yang, C.; Tian, G.; Ma, C. The Effect of Ignition Timing on the Emission and Combustion Characteristics for a Hydrogen-Fuelled ORP Engine at Lean-Burn Conditions. Processes 2022, 10, 1534. [Google Scholar] [CrossRef]
- Huang, Y.; Surawski, N.C.; Organ, B.; Zhou, J.L.; Tang, O.H.H.; Chan, E.F.C. Fuel Consumption and Emissions Performance under Real Driving: Comparison between Hybrid and Conventional Vehicles. Sci. Total Environ. 2019, 659, 275–282. [Google Scholar] [CrossRef]
- Taymaz, I.; Benli, M. Emissions and Fuel Economy for a Hybrid Vehicle. Fuel 2014, 115, 812–817. [Google Scholar] [CrossRef]
- Durand, T.; Dimopoulos Eggenschwiler, P.; Tang, Y.; Liao, Y.; Landmann, D. Potential of Energy Recuperation in the Exhaust Gas of State of the Art Light Duty Vehicles with Thermoelectric Elements. Fuel 2018, 224, 271–279. [Google Scholar] [CrossRef]
- Alvarez, R.; Weilenmann, M. Effect of Low Ambient Temperature on Fuel Consumption and Pollutant and CO2 Emissions of Hybrid Electric Vehicles in Real-World Conditions. Fuel 2012, 97, 119–124. [Google Scholar] [CrossRef]
- Ji, C.; Wang, S.; Zhang, B.; Liu, X. Emissions Performance of a Hybrid Hydrogen–Gasoline Engine-Powered Passenger Car under the New European Driving Cycle. Fuel 2013, 106, 873–875. [Google Scholar] [CrossRef]
- Pavlovic, J.; Ciuffo, B.; Fontaras, G.; Valverde, V.; Marotta, A. How Much Difference in Type-Approval CO2 Emissions from Passenger Cars in Europe Can Be Expected from Changing to the New Test Procedure (NEDC vs. WLTP)? Transp. Res. Part A Policy Pract. 2018, 111, 136–147. [Google Scholar] [CrossRef]
- Fontaras, G.; Ciuffo, B.; Zacharof, N.; Tsiakmakis, S.; Marotta, A.; Pavlovic, J.; Anagnostopoulos, K. The Difference between Reported and Real-World CO 2 Emissions: How Much Improvement Can Be Expected by WLTP Introduction? Transp. Res. Procedia 2017, 25, 3933–3943. [Google Scholar] [CrossRef]
- Yang, Z.; Ge, Y.; Thomas, D.; Wang, X.; Su, S.; Li, H.; He, H. Real Driving Particle Number (PN) Emissions from China-6 Compliant PFI and GDI Hybrid Electrical Vehicles. Atmos. Environ. 2019, 199, 70–79. [Google Scholar] [CrossRef]
- Di Blasio, G.; Viscardi, M.; Alfè, M.; Gargiulo, V.; Ciajolo, A.; Beatrice, C. Analysis of the Impact of the Dual-Fuel Ethanol-Diesel System on the Size, Morphology, and Chemical Characteristics of the Soot Particles Emitted from a LD Diesel Engine. In SAE Technical Paper; Istituto Motori CNR, Istituto Ricerche sulla Combustione CNR: Napoli, Italy, 2014; p. 2014–01–1613. [Google Scholar]
- Thanapornsin, T.; Phongsri, R.; Laopaiboon, L.; Laopaiboon, P. Use of Spent Yeasts from Bioethanol Production Plant as Low-Cost Nitrogen Sources for Ethanol Fermentation from Sweet Sorghum Stem Juice in Low-Cost Bioreactors. Carbon Resour. Convers. 2024, 100269. [Google Scholar] [CrossRef]
- Xiang, H.; Xin, R.; Prasongthum, N.; Natewong, P.; Sooknoi, T.; Wang, J.; Reubroycharoen, P.; Fan, X. Catalytic Conversion of Bioethanol to Value-Added Chemicals and Fuels: A Review. Resour. Chem. Mater. 2022, 1, 47–68. [Google Scholar] [CrossRef]
- Puricelli, S.; Cardellini, G.; Casadei, S.; Faedo, D.; Van Den Oever, A.E.M.; Grosso, M. A Review on Biofuels for Light-Duty Vehicles in Europe. Renew. Sustain. Energy Rev. 2021, 137, 110398. [Google Scholar] [CrossRef]
- Biofuels Barometer; EurObserv’ER: Maastricht, The Netherlands, 2020; p. 16.
- Puricelli, S.; Casadei, S.; Bellin, T.; Cardellini, G.; Cernuschi, S.; Costa, D.; Faedo, D.; Lonati, G.; Rigamonti, L.; Rossi, T.; et al. Reducing the Environmental Impacts of Passenger Cars: A Comparison between Electricity and Biofuels. Transp. Res. Procedia 2023, 72, 1388–1395. [Google Scholar] [CrossRef]
- Hsieh, W.-D.; Chen, R.-H.; Wu, T.-L.; Lin, T.-H. Engine Performance and Pollutant Emission of an SI Engine Using Ethanol–Gasoline Blended Fuels. Atmos. Environ. 2002, 36, 403–410. [Google Scholar] [CrossRef]
- Karavalakis, G.; Durbin, T.D.; Shrivastava, M.; Zheng, Z.; Villela, M.; Jung, H. Impacts of Ethanol Fuel Level on Emissions of Regulated and Unregulated Pollutants from a Fleet of Gasoline Light-Duty Vehicles. Fuel 2012, 93, 549–558. [Google Scholar] [CrossRef]
- Melo, T.C.C.D.; Machado, G.B.; Belchior, C.R.P.; Colaço, M.J.; Barros, J.E.M.; De Oliveira, E.J.; De Oliveira, D.G. Hydrous Ethanol–Gasoline Blends—Combustion and Emission Investigations on a Flex-Fuel Engine. Fuel 2012, 97, 796–804. [Google Scholar] [CrossRef]
- Clairotte, M.; Adam, T.W.; Zardini, A.A.; Manfredi, U.; Martini, G.; Krasenbrink, A.; Vicet, A.; Tournié, E.; Astorga, C. Effects of Low Temperature on the Cold Start Gaseous Emissions from Light Duty Vehicles Fuelled by Ethanol-Blended Gasoline. Appl. Energy 2013, 102, 44–54. [Google Scholar] [CrossRef]
- Durbin, T.D.; Miller, J.W.; Younglove, T.; Huai, T.; Cocker, K. Effects of Fuel Ethanol Content and Volatility on Regulated and Unregulated Exhaust Emissions for the Latest Technology Gasoline Vehicles. Environ. Sci. Technol. 2007, 41, 4059–4064. [Google Scholar] [CrossRef]
- Graham, L.A.; Belisle, S.L.; Baas, C.-L. Emissions from Light Duty Gasoline Vehicles Operating on Low Blend Ethanol Gasoline and E85. Atmos. Environ. 2008, 42, 4498–4516. [Google Scholar] [CrossRef]
- Chao, H.-R.; Lin, T.-C.; Chao, M.-R.; Chang, F.-H.; Huang, C.-I.; Chen, C.-B. Effect of Methanol-Containing Additive on the Emission of Carbonyl Compounds from a Heavy-Duty Diesel Engine. J. Hazard. Mater. 2000, 73, 39–54. [Google Scholar] [CrossRef]
- Grosjean, D.; Grosjean, E.; Gertler, A.W. On-Road Emissions of Carbonyls from Light-Duty and Heavy-Duty Vehicles. Environ. Sci. Technol. 2001, 35, 45–53. [Google Scholar] [CrossRef]
- Colón, M.; Pleil, J.D.; Hartlage, T.A.; Lucia Guardani, M.; Helena Martins, M. Survey of Volatile Organic Compounds Associated with Automotive Emissions in the Urban Airshed of São Paulo, Brazil. Atmos. Environ. 2001, 35, 4017–4031. [Google Scholar] [CrossRef]
- Bakeas, E.B.; Argyris, D.I.; Siskos, P.A. Carbonyl Compounds in the Urban Environment of Athens, Greece. Chemosphere 2003, 52, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Branco, J.E.H.; Da Rocha, F.V.; Péra, T.G.; De Bastiani, F.P.; Bartholomeu, D.B.; Costa, E.L.; Grilo Junior, I. Assessing Greenhouse Gas Emissions and Costs of Brazilian Light-Duty Vehicles. Renew. Sustain. Energy Rev. 2024, 206, 114845. [Google Scholar] [CrossRef]
- Auto-Data.Net 2003 Toyota Prius II (NHW20) 1.5 VVT-i (113 Hp) Hybrid e-CVT. Available online: https://www.auto-data.net/en/toyota-prius-ii-nhw20-1.5-vvt-i-113hp-hybrid-e-cvt-3552 (accessed on 14 September 2024).
- HORIBA MEXA-ONE Motor Exhaust Gas Analyzer. Available online: https://www.horiba.com/int/automotive/products/detail/action/show/Product/mexa-one-41/ (accessed on 14 September 2024).
- EMERSON Micro MotionTM ELITE Coriolis Meter Consisting of CMF010M Sensor and 4700 Transmitter. Available online: https://www.emerson.com/en-us/catalog/micro-motion-pkg-cmf010m-sensor-and-4700-transmitter (accessed on 14 September 2024).
- DieselNet Worldwide Harmonized Light Vehicles Test Cycle (WLTC). Available online: https://dieselnet.com/standards/cycles/wltp.php (accessed on 14 September 2024).
- TransportPolicy.net International: Light-Duty: Worldwide Harmonized Light Vehicles Test Procedure (WLTP). Available online: https://www.transportpolicy.net/standard/international-light-duty-worldwide-harmonized-light-vehicles-test-procedure-wltp/ (accessed on 14 September 2024).
- DieselNet EU: Cars: Greenhouse Gas Emissions. Available online: https://dieselnet.com/standards/eu/ghg.php (accessed on 14 September 2024).
- DieselNet ECE 15 + EUDC/NEDC. Available online: https://dieselnet.com/standards/cycles/ece_eudc.php (accessed on 14 September 2024).
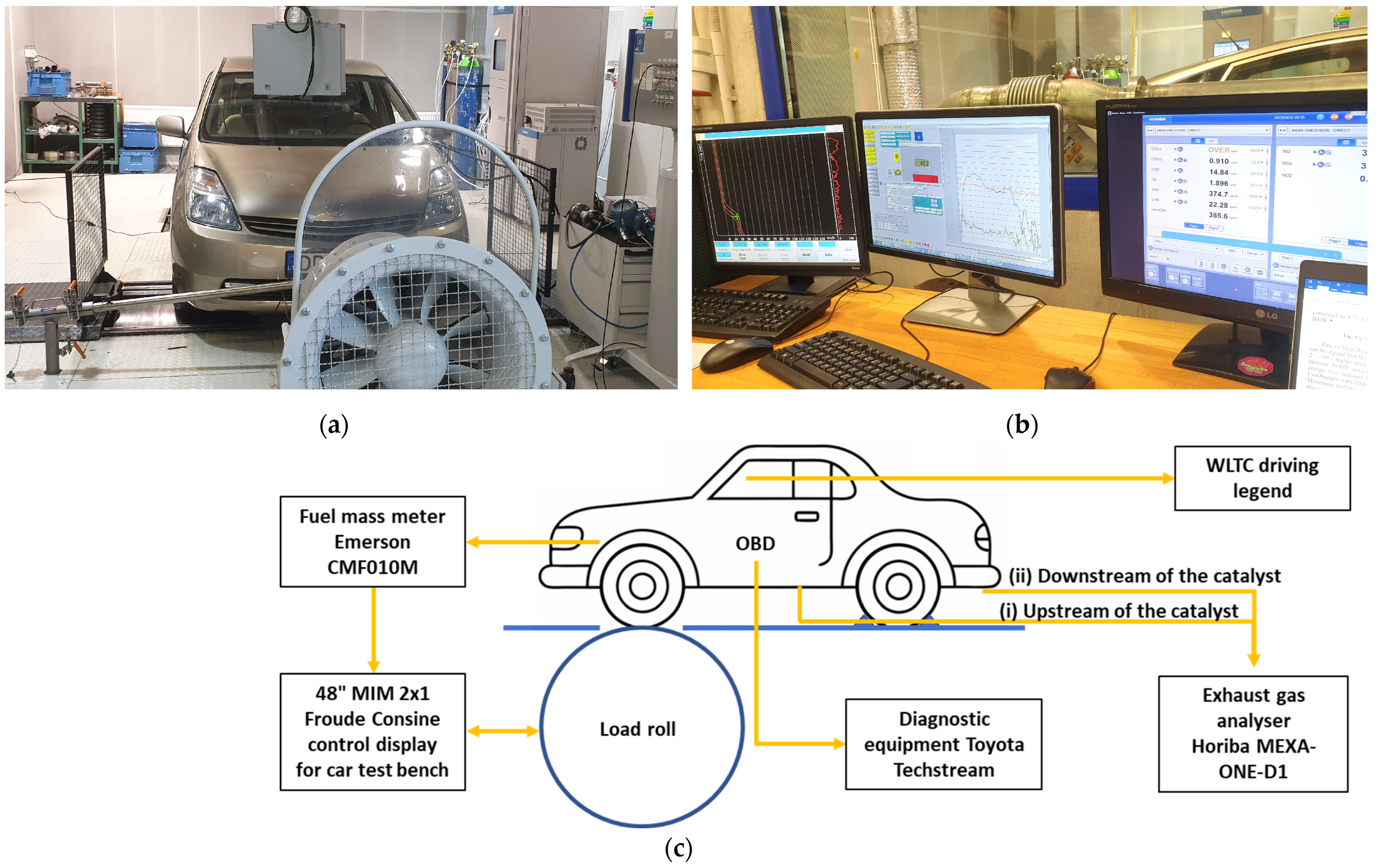






| Parameter | Value |
|---|---|
| Model | Toyota Prius II |
| Year of manufacture | 2005 |
| Engine type, model | Spark-ignition, 1NZ-FXE |
| Engine displacement | 1497 cm3 |
| Number and arrangement of cylinders | 4 in-line |
| Cylinder bore/stroke | 75 mm/84.7 mm |
| Compression ratio | 13 |
| Valve timing system | DOHC Atkinson Cycle |
| Fuel supply | Fuel injection before intake valves |
| Internal combustion engine torque | 111 Nm at 4200 rpm |
| Electric motor torque | 400 Nm |
| Internal combustion engine power | 57 kW at 5000 rpm |
| Electric motor power | 50 kW |
| Combined power | 82 kW |
| Hybrid system type | Fully Hybrid Electric Vehicle (FHEV) |
| Transmission | Electronic continuously variable transmission (e-CVT) |
| Battery type | Nickel-metal hydride (NiMH) |
| Battery capacity | 1.31 kWh |
| Fuel type | Gasoline A95 |
| Fuel tank capacity | 45 L |
| Fuel consumption (EPA) | 4.9 L/100 km city/5.2 L/100 km highway/5.1 L/100 km combined |
| Acceleration 0–100 km/h | ~10.5 s |
| Maximum speed | 170 km/h |
| Weight (empty) | 1310 kg |
| Dimensions (length × width × height) | 4.445 m × 1.725 m × 1.476 m |
| Wheelbase | 2.700 m |
| Driven wheels | Front |
| Brakes | Disc brakes (front), drum brakes (rear) |
| Tyre size | 185/65R15 |
| Aerodynamic drag coefficient | 0.26 |
| Device | Parameter | Value |
|---|---|---|
| Chassis dynamometer | Model | 48″ MIM 2 × 1 Froude Consine CD Modernized by AVL |
| Axles | 1 | |
| Rollers | 2 | |
| Roller diameter | 1219.2 mm | |
| Base inertia | 1354 kg | |
| Mass of the vehicle | 500–2700 kg | |
| Nominal power | 100 kW | |
| Maximum force | 3000 N, accuracy 0.2% (FS) | |
| Maximum speed | 200 km/h, accuracy 0.05% (FS) | |
| Fuel mass meter | Model | Emerson CMF010M302NACZEZZZ |
| Measurement method | Coriolis principle | |
| Flow range | 0.2–80 kg/h, accuracy ±0.1% of the flow | |
| Fuel input pressure | Lower limit > 2.0 bar, upper limit ≤ 125 bar | |
| Pressure drop | 0.7 bar at 30 kg/h fuel flow | |
| Gas analyser | Model | Horiba MEXA-ONE-D1 |
| CO | Range: 0–5000 ppm, up to 20% (vol.) | |
| HC | Range: 0–5000 ppm, up to 20,000 ppm (vol.) | |
| NOx | Range: 0–5000 ppm, up to 10,000 ppm (vol.) | |
| CO2 | 0–20% (vol.) | |
| O2 | 0–25% (vol.) | |
| Diagnostic equipment | Model | Toyota Techstream |
| Real-time parameters | Engine speed, rpm; | |
| Engine load, kW | ||
| Air intake, g/s; | ||
| Engine coolant temperature, °C | ||
| Air-fuel ratio; | ||
| Ignition timing, °BTDC; | ||
| Temperature before catalytic converter, °C; Temperature after catalytic converter, °C |
| Parameter | Fuels | ||||
|---|---|---|---|---|---|
| E0 | E10 | E70 | E100 | ||
| Bioethanol volume concentration, % | 0 | 10 | 70 | 100 | |
| Bioethanol mass concentration, % | 0 | 10.5 | 71.1 | 100 | |
| Density (15 °C), kg/m3 | 748 | 752 | 778 | 790 | |
| Viscosity (40 °C) (mm2/s) | 0.6 | 1.13 | |||
| Specific heat of vaporisation, kJ/kg | 364 | 840 | |||
| Laminar flame speed, cm/s | 51 | 63 | |||
| Adiabatic combustion temperature, °C | 2307 | 2247 | |||
| Freezing point, °C | –40 | –114 | |||
| Octane number | 95 | 109 | |||
| Elemental composition, % | C | 86.42 | 82.82 | 62.02 | 52.10 |
| H | 13.58 | 13.66 | 14.11 | 14.32 | |
| O | 0.00 | 3.52 | 23.88 | 33.58 | |
| C/H ratio | 6.36 | 6.06 | 4.40 | 3.64 | |
| Stoichiometric air–fuel ratio, kg of air/1 kg of fuel | 14.70 | 14.10 | 10.65 | 9.00 | |
| Lower heating value of fuel, MJ/kg | 43.53 | 41.88 | 32.33 | 27.78 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rimkus, A.; Mejeras, G.; Dittrich, A.; Pukalskas, S.; Barta, D. Effect of the Concentration of Bioethanol Mixed with Gasoline on the Energy and Environmental Performance of a Hybrid Vehicle in the Worldwide Harmonized Light Vehicles Test Cycle (WLTC). Appl. Sci. 2024, 14, 10858. https://doi.org/10.3390/app142310858
Rimkus A, Mejeras G, Dittrich A, Pukalskas S, Barta D. Effect of the Concentration of Bioethanol Mixed with Gasoline on the Energy and Environmental Performance of a Hybrid Vehicle in the Worldwide Harmonized Light Vehicles Test Cycle (WLTC). Applied Sciences. 2024; 14(23):10858. https://doi.org/10.3390/app142310858
Chicago/Turabian StyleRimkus, Alfredas, Gabrielius Mejeras, Aleš Dittrich, Saugirdas Pukalskas, and Dalibor Barta. 2024. "Effect of the Concentration of Bioethanol Mixed with Gasoline on the Energy and Environmental Performance of a Hybrid Vehicle in the Worldwide Harmonized Light Vehicles Test Cycle (WLTC)" Applied Sciences 14, no. 23: 10858. https://doi.org/10.3390/app142310858
APA StyleRimkus, A., Mejeras, G., Dittrich, A., Pukalskas, S., & Barta, D. (2024). Effect of the Concentration of Bioethanol Mixed with Gasoline on the Energy and Environmental Performance of a Hybrid Vehicle in the Worldwide Harmonized Light Vehicles Test Cycle (WLTC). Applied Sciences, 14(23), 10858. https://doi.org/10.3390/app142310858








