Individualized, 3D Printed Matrices for the Reconstruction of Severely Destructed Teeth with Subgingival Margin—Case Series and Proof of Concept
Abstract
1. Introduction
2. Case Presentation
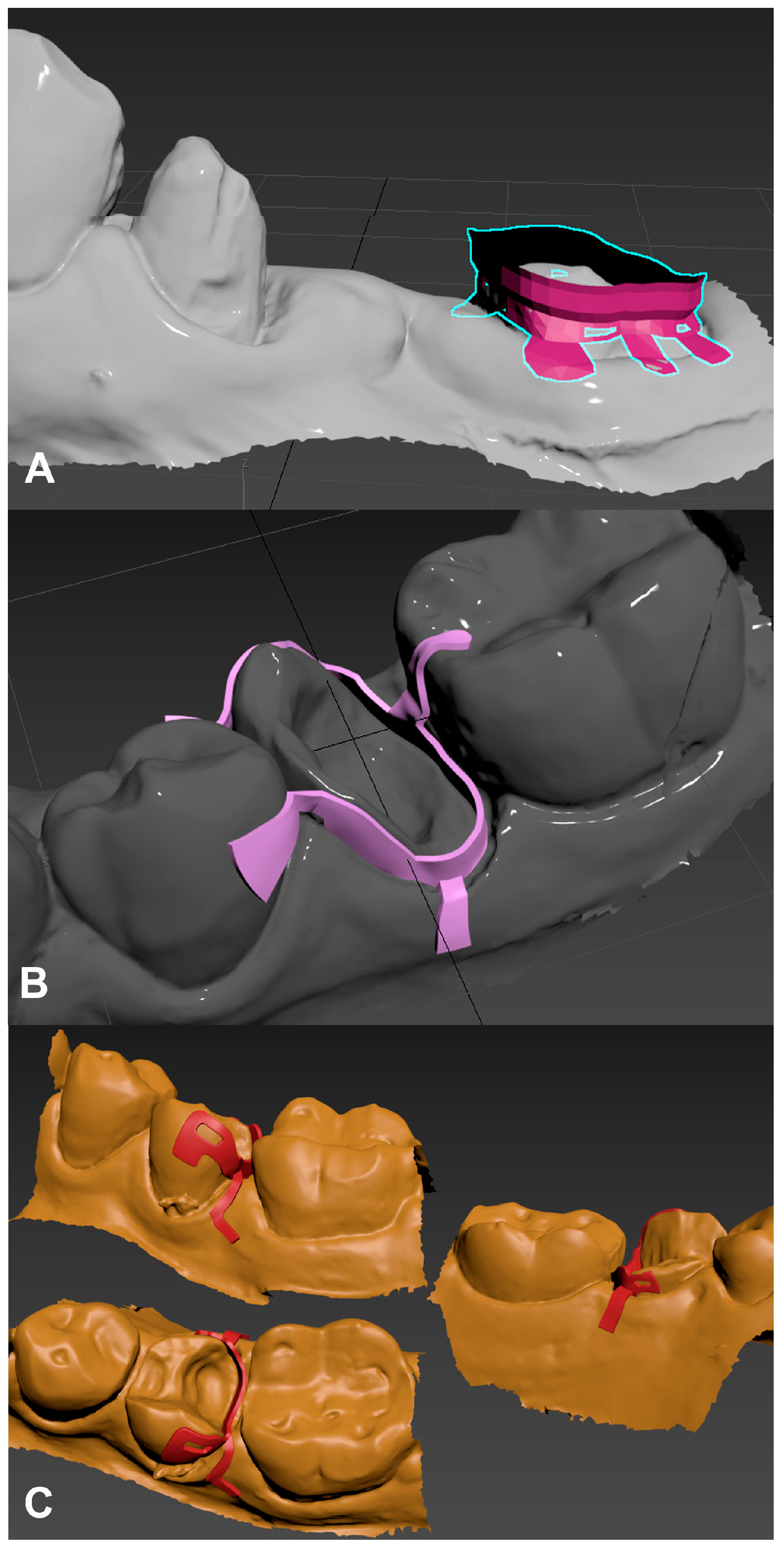
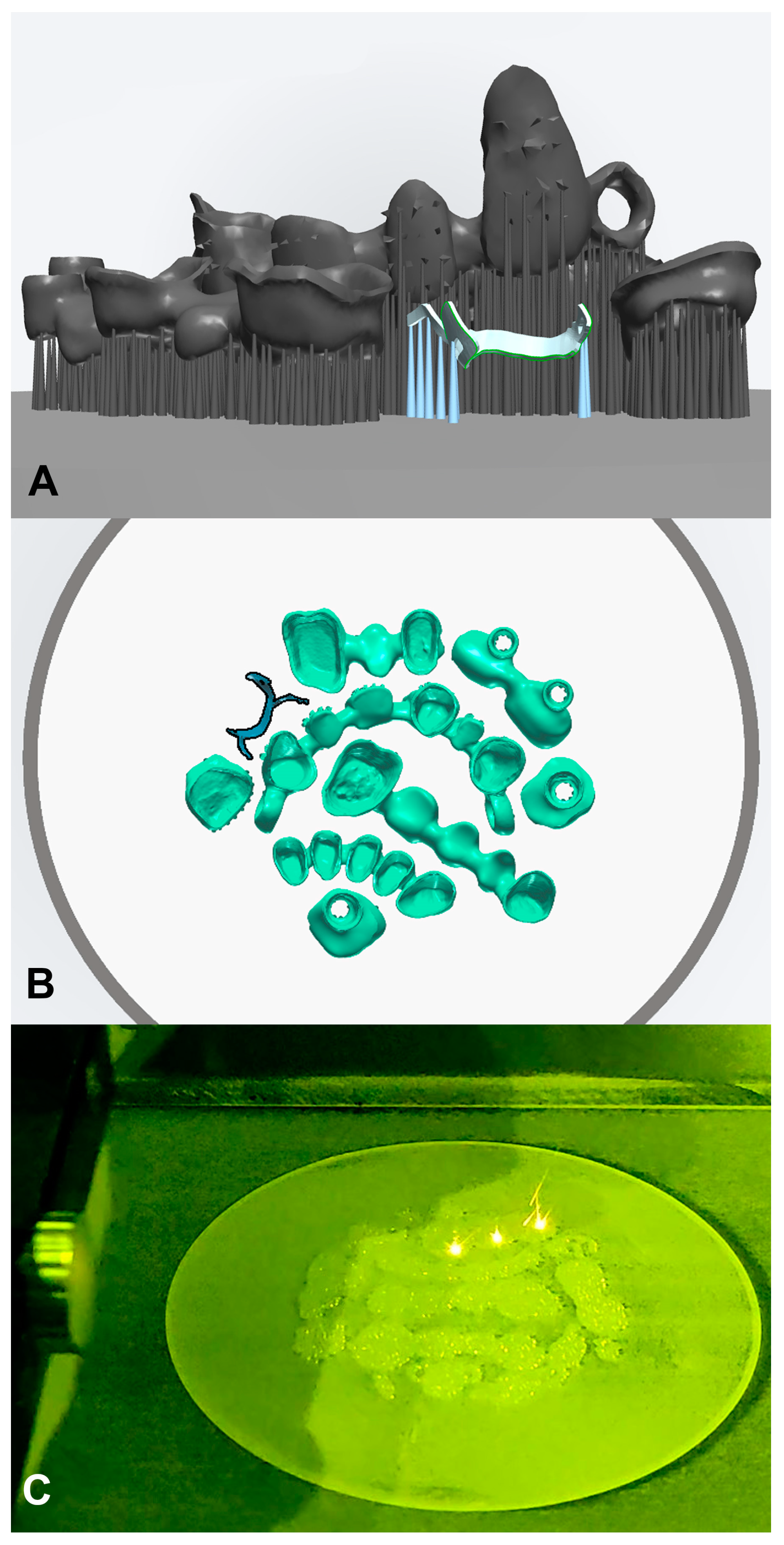
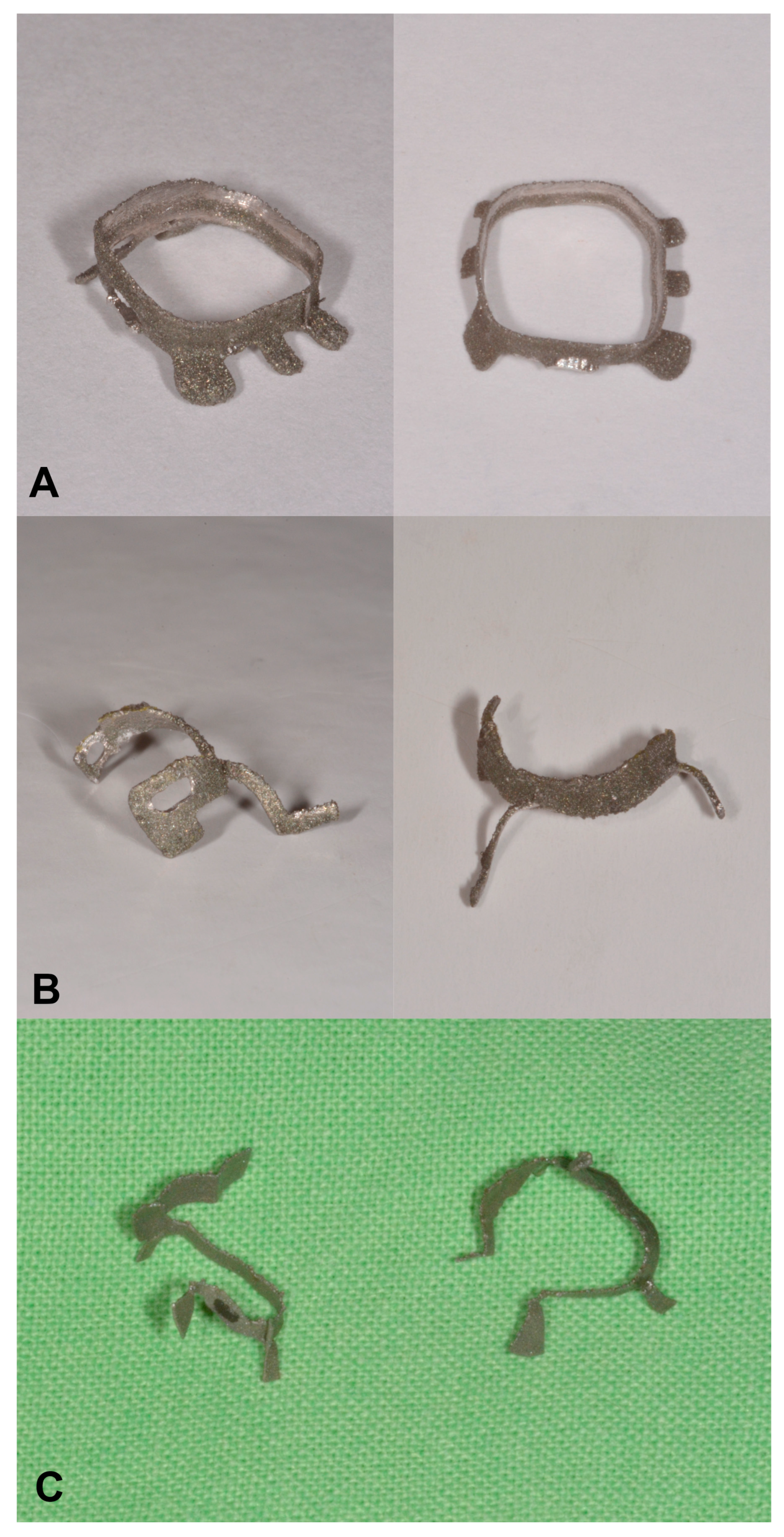
2.1. Design and Manufacturing of the Custom Matrices
2.1.1. D Modeling
2.1.2. Additive Manufacturing
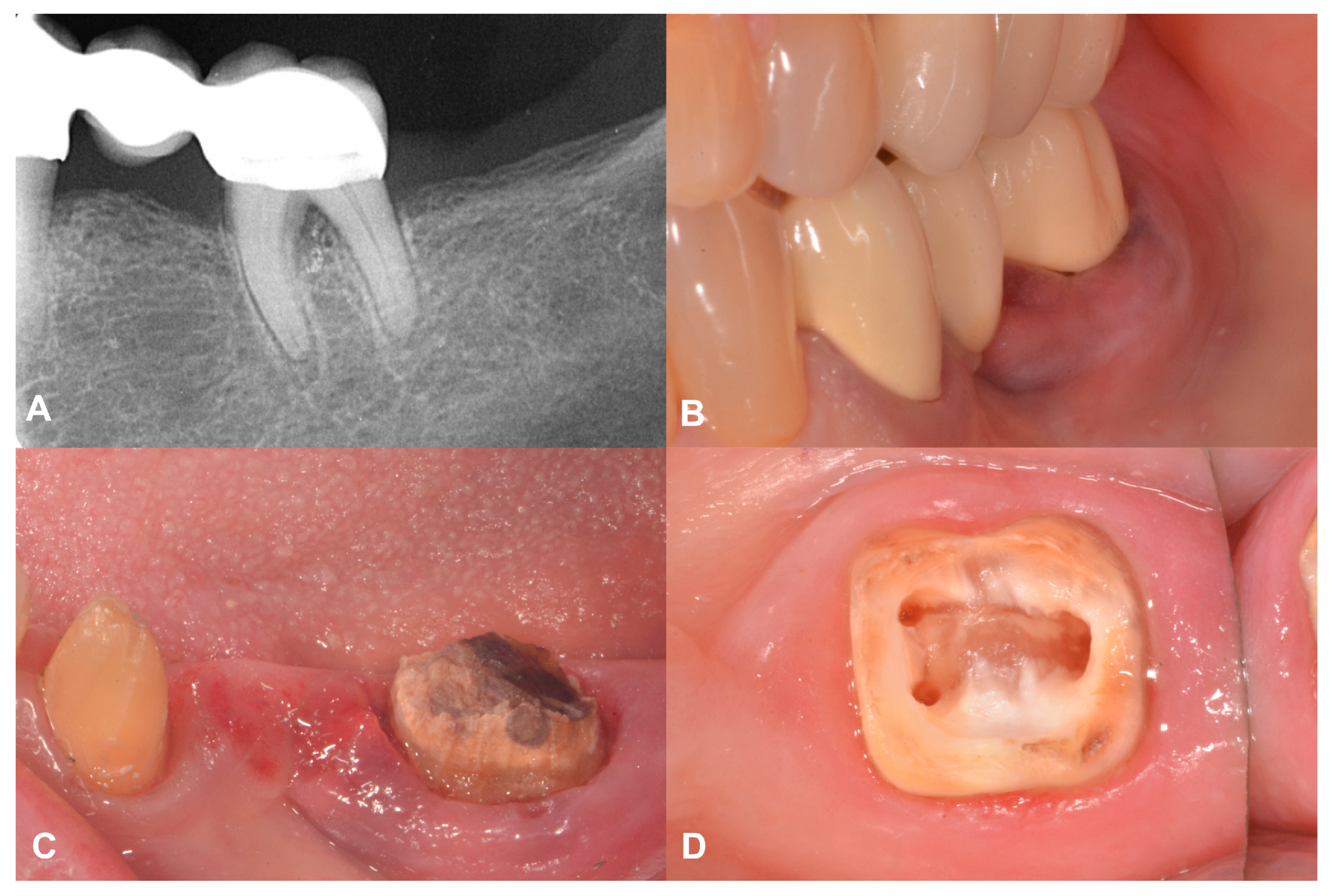
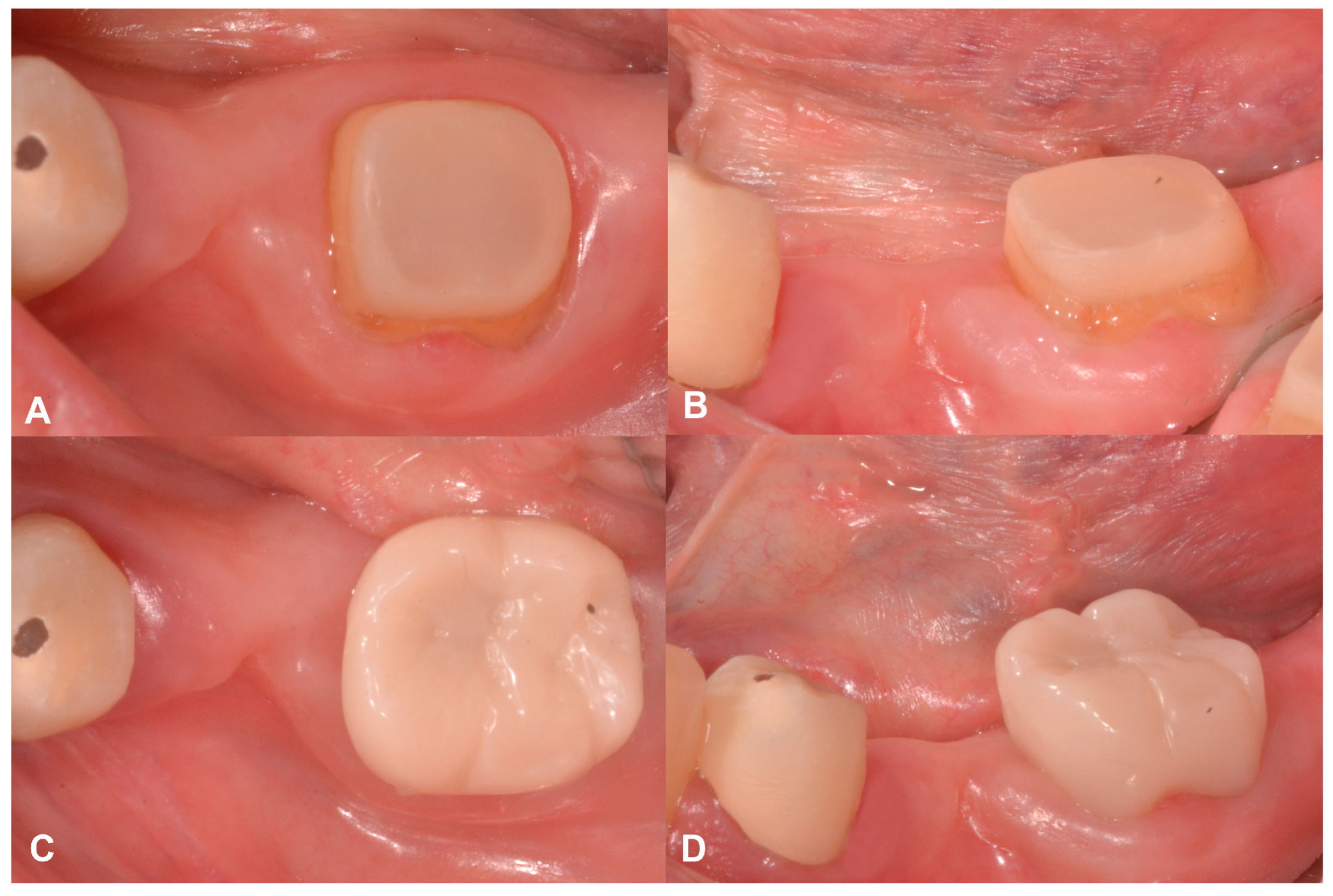
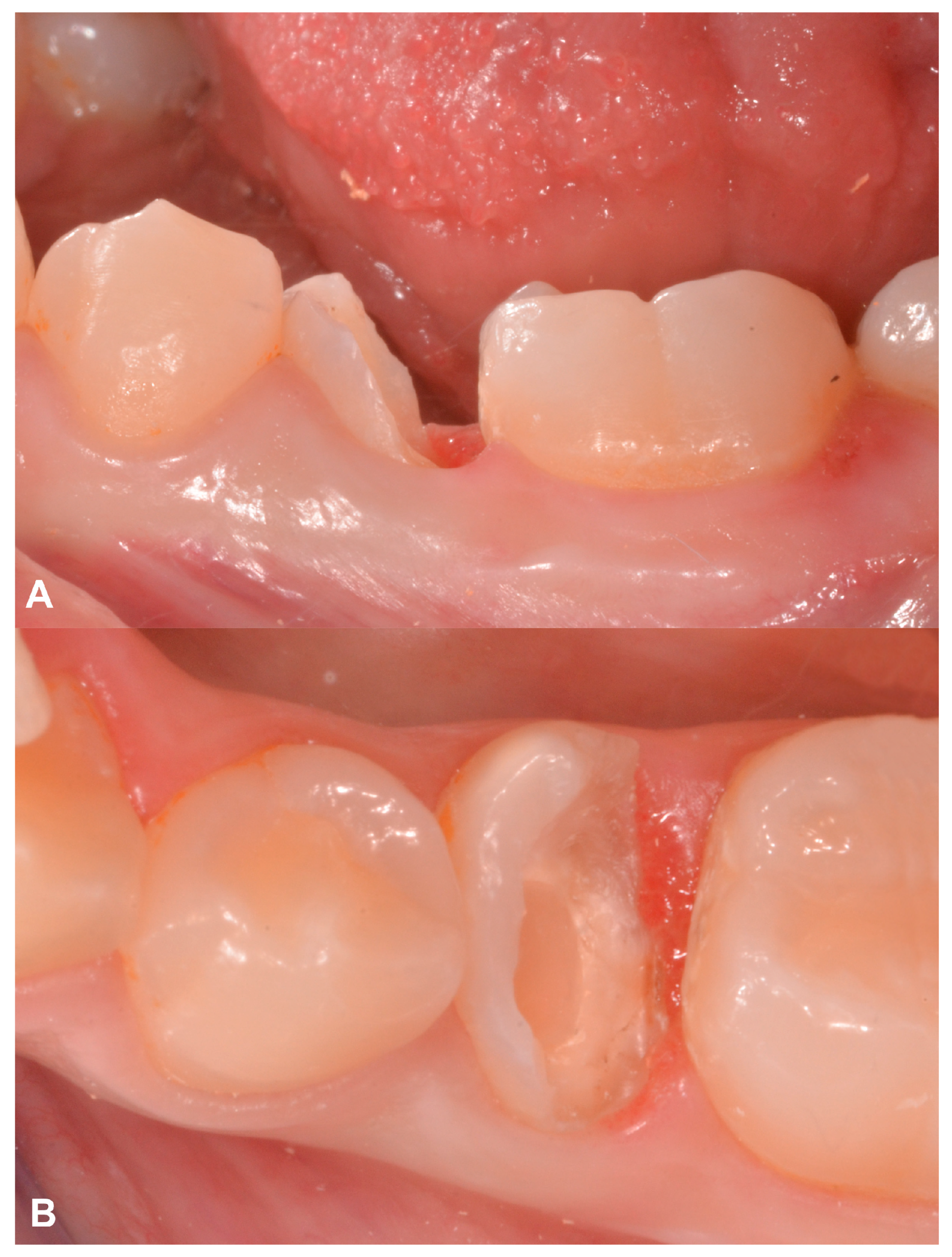
2.2. Case Presentation #1
2.2.1. Anamnesis, Physical Examination
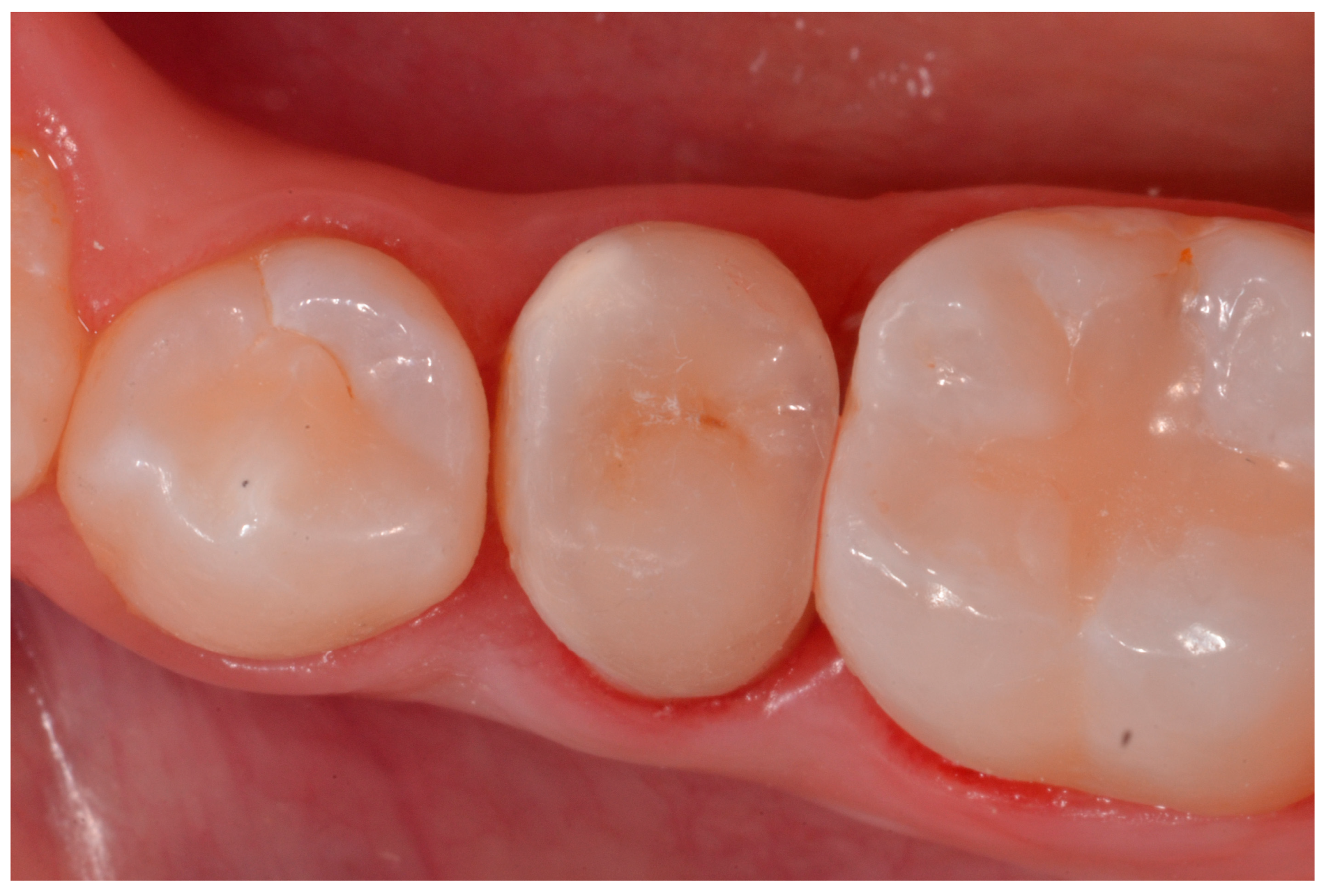
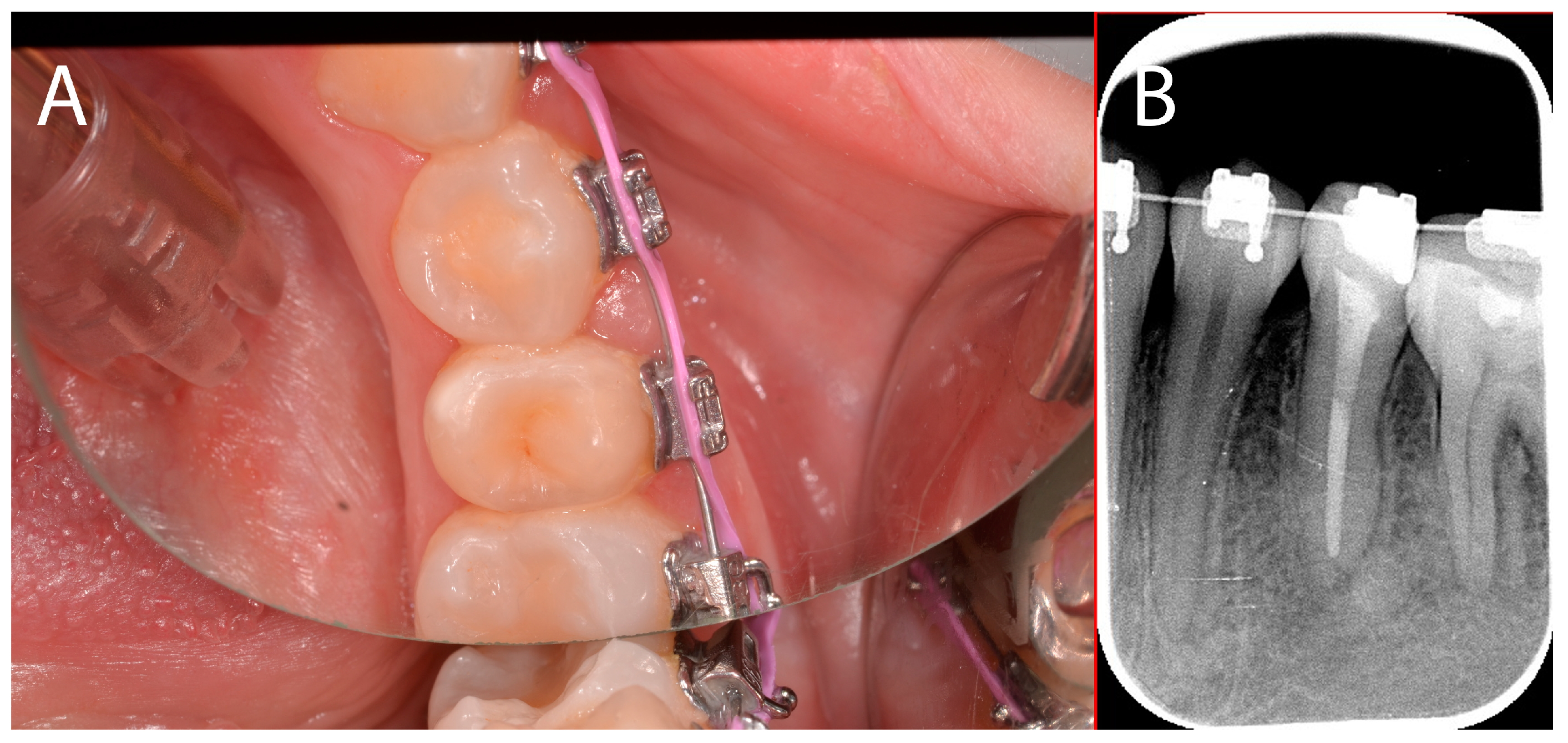
2.2.2. Treatment
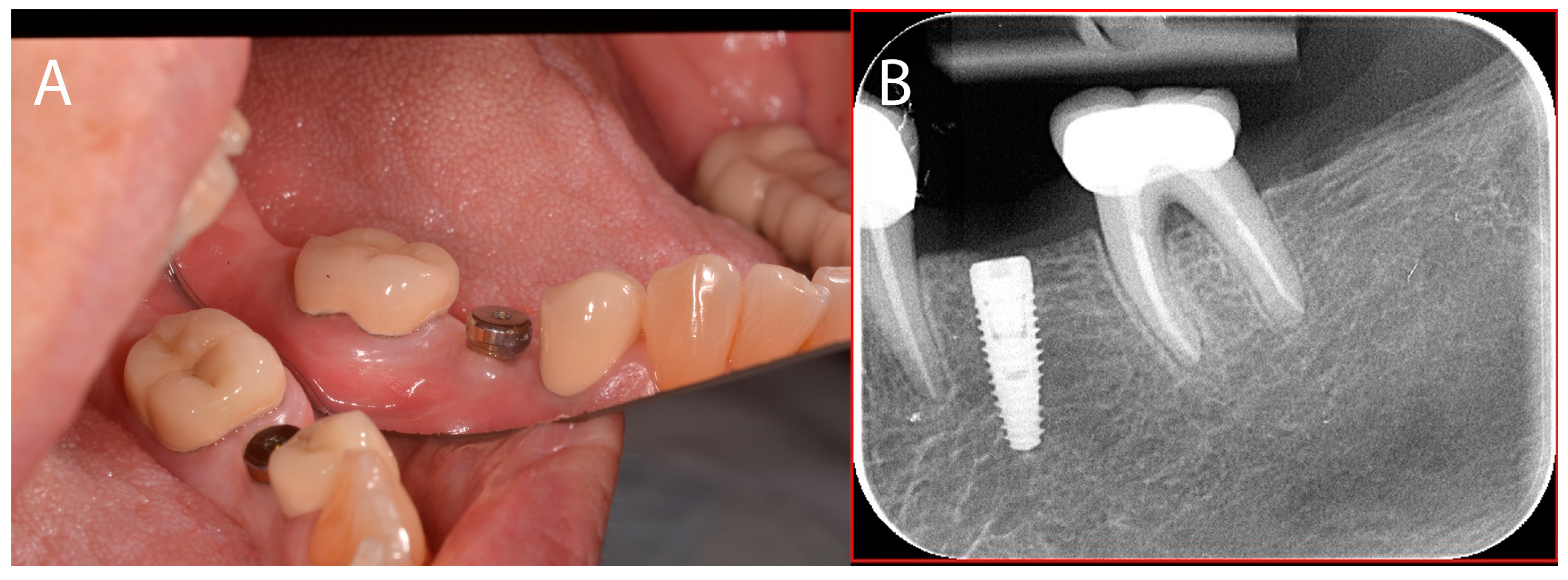
2.3. Case Presentation #2
2.3.1. Anamnesis, Physical Examination
2.3.2. Treatment
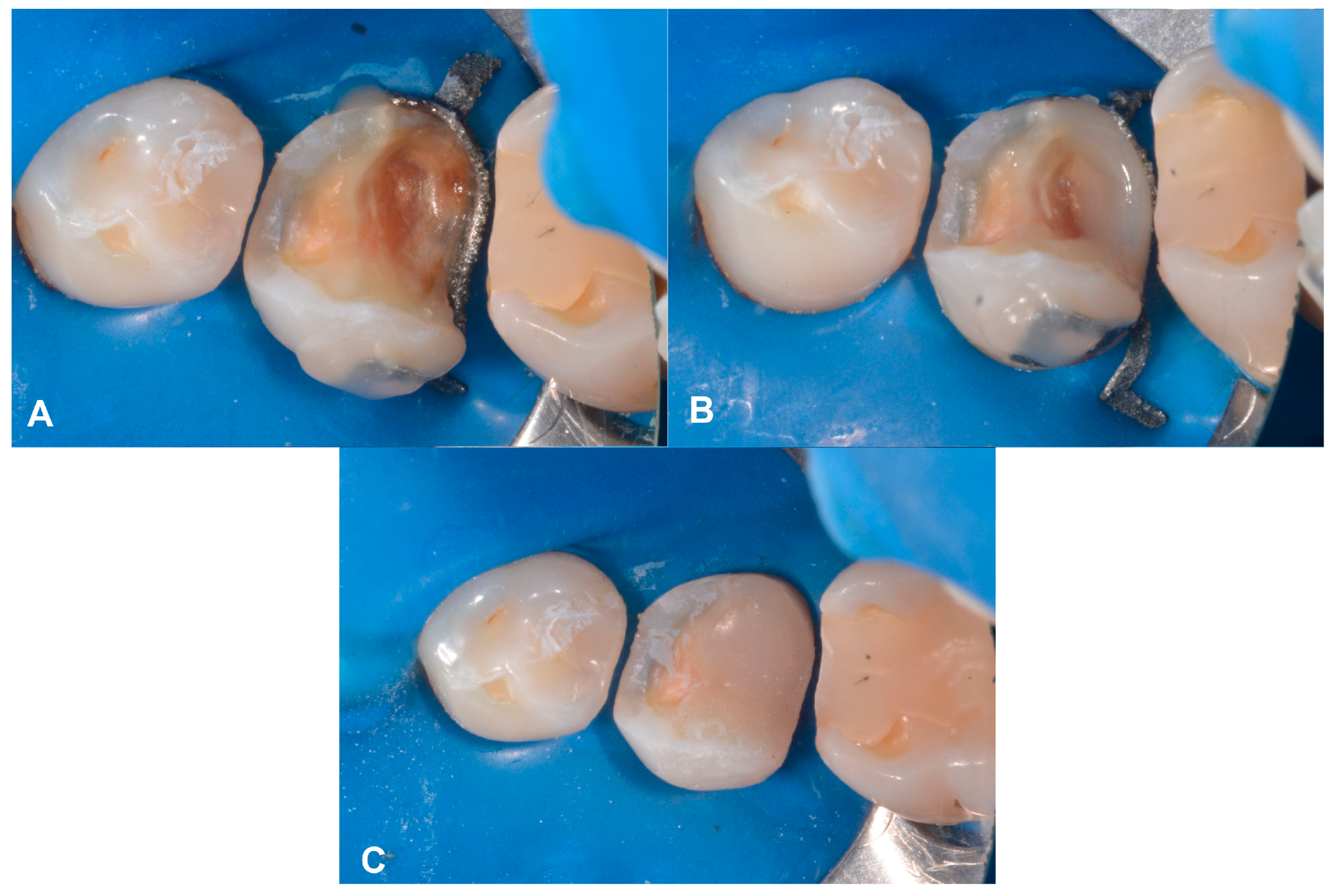
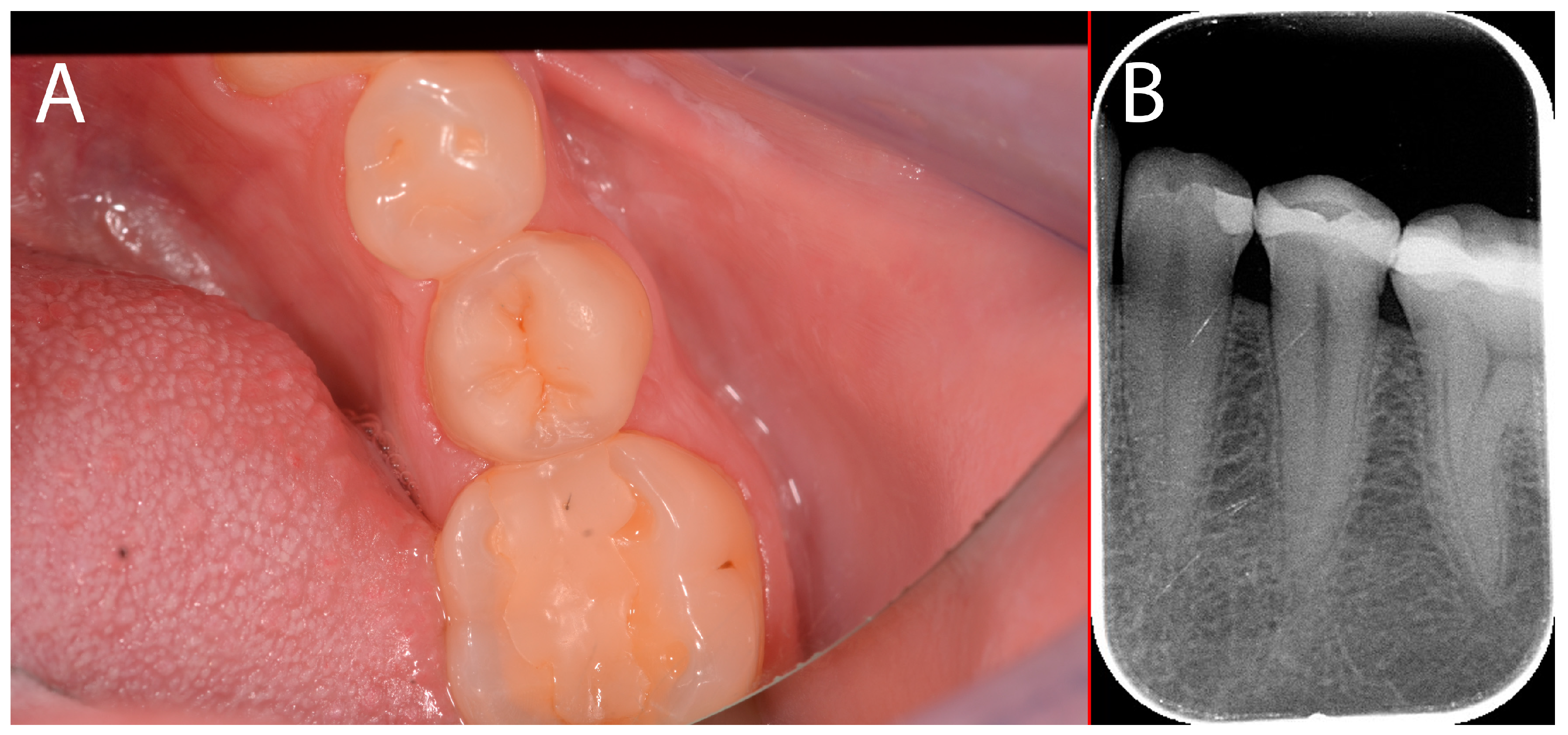
2.4. Case Presentation #3
2.4.1. Anamnesis, Physical Examination
2.4.2. Treatment
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haak, R.; Näke, T.; Park, K.-J.; Ziebolz, D.; Krause, F.; Schneider, H. Internal and Marginal Adaptation of High-Viscosity Bulk-Fill Composites in Class II Cavities Placed with Different Adhesive Strategies. Odontology 2019, 107, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Demarco, F.F.; Corrêa, M.B.; Cenci, M.S.; Moraes, R.R.; Opdam, N.J.M. Longevity of Posterior Composite Restorations: Not Only a Matter of Materials. Dent. Mater. 2012, 28, 87–101. [Google Scholar] [CrossRef]
- Opdam, N.J.M.; Van De Sande, F.H.; Bronkhorst, E.; Cenci, M.S.; Bottenberg, P.; Pallesen, U.; Gaengler, P.; Lindberg, A.; Huysmans, M.C.D.N.J.M.; Van Dijken, J.W. Longevity of Posterior Composite Restorations: A Systematic Review and Meta-Analysis. J. Dent. Res. 2014, 93, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Peumans, M.; Politano, G.; Van Meerbeek, B. Effective Protocol for Daily High-Quality Direct Posterior Composite Restorations. Cavity Preparation and Design. J. Adhes. Dent. 2020, 22, 581–596. [Google Scholar] [CrossRef]
- Peumans, M.; Venuti, P.; Politano, G.; Van Meerbeek, B. Effective Protocol for Daily High-Quality Direct Posterior Composite Restorations. The Interdental Anatomy of the Class-2 Composite Restoration. J. Adhes. Dent. 2021, 23, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Heintze, S.D.; Rousson, V. Clinical Effectiveness of Direct Class II Restorations—A Meta-Analysis. J. Adhes. Dent. 2012, 14, 407–431. [Google Scholar] [CrossRef] [PubMed]
- Koppolu, M.; Gogala, D.; Mathew, V.; Thangala, V.; Deepthi, M.; Sasidhar, N. Effect of Saliva and Blood Contamination on the Bond Strength of Self-Etching Adhesive System- An in Vitro Study. J. Conserv. Dent. 2012, 15, 270. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.J.; Lolato, A.L.; de Freitas, C.R.B.; Dinelli, W. SEM Analysis of Internal Adaptation of Adhesive Restorations after Contamination with Saliva. J. Adhes. Dent. 2005, 7, 51–56. [Google Scholar]
- Dietschi, D.; Spreafico, R. Current Clinical Concepts for Adhesive Cementation of Tooth-Colored Posterior Restorations. Pract. Periodontics Aesthet. Dent. 1998, 10, 47–54, quiz 56. [Google Scholar]
- Magne, P.; Spreafico, R. Deep Margin Elevation: A Paradigm Shift. Am. J. Esthet. Dent. 2012, 2, 86–96. [Google Scholar]
- Ferrari, M.; Koken, S.; Grandini, S.; Ferrari Cagidiaco, E.; Joda, T.; Discepoli, N. Influence of Cervical Margin Relocation (CMR) on Periodontal Health: 12-Month Results of a Controlled Trial. J. Dent. 2018, 69, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kielbassa, A.M.; Philipp, F. Restoring Proximal Cavities of Molars Using the Proximal Box Elevation Technique: Systematic Review and Report of a Case. Quintessence Int. 2015, 46, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Magne, P. M-i-M for DME: Matrix-in-a-Matrix Technique for Deep Margin Elevation. J. Prosthet. Dent. 2023, 130, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, C.; Brambilla, G.; Conti, A.; Dosoli, R.; Ceroni, F.; Ferrantino, L. Cervical Margin Relocation: Case Series and New Classification System. Int. J. Esthet. Dent. 2019, 14, 272–284. [Google Scholar]
- Veneziani, M. Adhesive Restorations in the Posterior Area with Subgingival Cervical Margins: New Classification and Differentiated Treatment Approach. Eur. J. Esthet. Dent. 2010, 5, 50–76. [Google Scholar]
- Romano, G.; Modoni, M.; Ferraris, F.; Zakaraya, A.; Rasperini, G. Supracrestal Tissue Esthetic Management (STEM) Technique and Current Approaches in Restorative and Surgical Treatment of Deep Margins. Int. J. Esthet. Dent. 2022, 17, 162–184. [Google Scholar]
- Bresser, R.A.; Gerdolle, D.; Van Den Heijkant, I.A.; Sluiter-Pouwels, L.M.A.; Cune, M.S.; Gresnigt, M.M.M. Up to 12 Years Clinical Evaluation of 197 Partial Indirect Restorations with Deep Margin Elevation in the Posterior Region. J. Dent. 2019, 91, 103227. [Google Scholar] [CrossRef]
- Kihara, H.; Hatakeyama, W.; Komine, F.; Takafuji, K.; Takahashi, T.; Yokota, J.; Oriso, K.; Kondo, H. Accuracy and Practicality of Intraoral Scanner in Dentistry: A Literature Review. J. Prosthodont. Res. 2020, 64, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Laverty, D.P.; Thomas, M.B.; Clark, P.; Addy, L.D. The Use of 3D Metal Printing (Direct Metal Laser Sintering) in Removable Prosthodontics. Dent. Update 2016, 43, 826–835. [Google Scholar] [CrossRef]
- Mangano, F.; Gandolfi, A.; Luongo, G.; Logozzo, S. Intraoral Scanners in Dentistry: A Review of the Current Literature. BMC Oral Health 2017, 17, 149. [Google Scholar] [CrossRef]
- Kessler, A.; Hickel, R.; Reymus, M. 3D Printing in Dentistry—State of the Art. Oper. Dent. 2020, 45, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, E.J. Reconsidering the Declaration of Helsinki. Lancet 2013, 381, 1532–1533. [Google Scholar] [CrossRef] [PubMed]
- Riley, D.S.; Barber, M.S.; Kienle, G.S.; Aronson, J.K.; Von Schoen-Angerer, T.; Tugwell, P.; Kiene, H.; Helfand, M.; Altman, D.G.; Sox, H.; et al. CARE Guidelines for Case Reports: Explanation and Elaboration Document. J. Clin. Epidemiol. 2017, 89, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Fráter, M.; Sáry, T.; Braunitzer, G.; Balázs Szabó, P.; Lassila, L.; Vallittu, P.K.; Garoushi, S. Fatigue Failure of Anterior Teeth without Ferrule Restored with Individualized Fiber-Reinforced Post-Core Foundations. J. Mech. Behav. Biomed. Mater. 2021, 118, 104440. [Google Scholar] [CrossRef]
- Fráter, M.; Sáry, T.; Néma, V.; Braunitzer, G.; Vallittu, P.; Lassila, L.; Garoushi, S. Fatigue Failure Load of Immature Anterior Teeth: Influence of Different Fiber Post-Core Systems. Odontology 2021, 109, 222–230. [Google Scholar] [CrossRef]
- Fráter, M.; Lassila, L.; Braunitzer, G.; Vallittu, P.K.; Garoushi, S. Fracture Resistance and Marginal Gap Formation of Post-Core Restorations: Influence of Different Fiber-Reinforced Composites. Clin. Oral Investig. 2020, 24, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, F.; Paolone, G.; Di Domenico, G.L.; Pagani, N.; Gherlone, E.F. SEM Evaluation of the Marginal Accuracy of Zirconia, Lithium Disilicate, and Composite Single Crowns Created by CAD/CAM Method: Comparative Analysis of Different Materials. Materials 2023, 16, 2413. [Google Scholar] [CrossRef]
- Mattei, M.; Mattei, L.D. 3D-Guided Direct Composite Restorations: The Evolution of the Technique. Int. J. Esthet. Dent. 2022, 17, 266–279. [Google Scholar]
- Sampaio, C.S.; Puppin-Rontani, J.; Tonolli, G.; Atria, P.J. Workflow of Digitally Guided Direct Composite Resin Restorations Using Open Source Software and 3D Printing: A Clinical Technique. Quintessence Int. 2021, 52, 104–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Fan, L.; Yu, H. Closing Post-Orthodontic Spaces between Anterior Teeth Using Sequential 3D-Printed Direct Composite Injection Guides. Oper. Dent. 2022, 47, 612–619. [Google Scholar] [CrossRef]
- D’haese, R.; Vrombaut, T.; Hommez, G.; De Bruyn, H.; Vandeweghe, S. Accuracy of Guided Implant Surgery Using an Intraoral Scanner and Desktop 3D-Printed Tooth-Supported Guides. Int. J. Oral Maxillofac. Implant. 2022, 37, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.; Kämmerer, P.W.; Hennig, M.; Schön, G.; Thiem, D.G.E.; Bschorer, R. Customized Virtual Surgical Planning in Bimaxillary Orthognathic Surgery: A Prospective Randomized Trial. Clin. Oral Investig. 2019, 23, 3115–3122. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Goil, P. Formulating an Easy, Affordable, and Reproducible Method for Virtual Planning and 3D Reconstruction: A State Institution’s Approach for Mandibular Reconstruction. Ann. Plast. Surg. 2021, 87, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, A.; Vignudelli, E.; Franceschi, D.; Randellini, E.; Lizio, G.; Fiorino, A.; Corinaldesi, G. Vertical and Horizontal Ridge Augmentation Using Customized CAD/CAM Titanium Mesh with versus without Resorbable Membranes. A Randomized Clinical Trial. Clin. Oral Implant. Res. 2021, 32, 1411–1424. [Google Scholar] [CrossRef] [PubMed]
- De Souza, F.A.; Blois, M.C.; Collares, K.; Dos Santos, M.B.F. 3D-Printed and Conventional Provisional Single Crown Fabrication on Anterior Implants: A Randomized Clinical Trial. Dent. Mater. 2024, 40, 340–347. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, Q.; Yuan, F.; Zhang, L.; Sun, Y.; Xie, Q. Accuracy of a Chairside, Fused Deposition Modeling Three-dimensional-printed, Single Tooth Surgical Guide for Implant Placement: A Randomized Controlled Clinical Trial. Clin. Oral Implant. Res. 2022, 33, 1000–1009. [Google Scholar] [CrossRef]





| Traditional Prefabricated Matrices | Comparison | Customized Matrices |
|---|---|---|
| Prefabricated matrices do not follow the cavity margin and in many cases, it is necessary to improve their fit with wedges and rings. | Fitting | Customized matrices follow the cavity margin better. |
| Traditional matrices are stable in smaller defects, where there is enough supporting tooth material. | Stability | Individualized matrices are stable even in large defects. |
| Conventional matrices are hard to apply with rubber dams, especially in cases of deep approximal lesions. | Rubber dam isolation | Customized matrices can significantly support the rubber dam isolation. |
| In many cases, traditional matrices need to be adjusted with matrix rings. | Matrix rings | 3D-printed matrices follow the remaining tooth structure without the use of matrix rings. |
| Conventional matrices need to be fixed with wedges or matrix rings. | Fixation | Customized matrices need to be fixed with composite materials. |
| Traditional matrices are relatively inexpensive. | Cost | The use of customized matrices incurs additional costs. |
| Prefabricated matrices can be used in a single visit. | Time management | In order to create an individualized matrix, an additional visit is required. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, B.; Néma, V.; Jakab, A.; Braunitzer, G.; Palkovics, D.; Fráter, M. Individualized, 3D Printed Matrices for the Reconstruction of Severely Destructed Teeth with Subgingival Margin—Case Series and Proof of Concept. Appl. Sci. 2024, 14, 10792. https://doi.org/10.3390/app142310792
Szabó B, Néma V, Jakab A, Braunitzer G, Palkovics D, Fráter M. Individualized, 3D Printed Matrices for the Reconstruction of Severely Destructed Teeth with Subgingival Margin—Case Series and Proof of Concept. Applied Sciences. 2024; 14(23):10792. https://doi.org/10.3390/app142310792
Chicago/Turabian StyleSzabó, Balázs, Viktória Néma, András Jakab, Gábor Braunitzer, Dániel Palkovics, and Márk Fráter. 2024. "Individualized, 3D Printed Matrices for the Reconstruction of Severely Destructed Teeth with Subgingival Margin—Case Series and Proof of Concept" Applied Sciences 14, no. 23: 10792. https://doi.org/10.3390/app142310792
APA StyleSzabó, B., Néma, V., Jakab, A., Braunitzer, G., Palkovics, D., & Fráter, M. (2024). Individualized, 3D Printed Matrices for the Reconstruction of Severely Destructed Teeth with Subgingival Margin—Case Series and Proof of Concept. Applied Sciences, 14(23), 10792. https://doi.org/10.3390/app142310792






