Abstract
The effect of the spatial arrangement in the physical vapor deposition (PVD) chamber on the composition and properties of coatings is considered using the example of the deposition of the (Ti,Al)N coating. The proposed method is one of the ways (along with varying the arc current of the cathodes and the bias voltage, as well as using alloy cathodes) to change the ratio of elements in the coating, and achieves this across a wide range of values. The three samples were located, respectively, opposite the evaporator with a titanium cathode, opposite the evaporator with an aluminum cathode and in an intermediate position between the two evaporators. The coating was deposited without rotating the turntable. The aluminum content in the coatings decreases from 94.2 at.% for the sample located directly opposite the evaporator with an Al cathode to 10.3 at.% for the sample located opposite the evaporator with a Ti cathode. In the coating deposited on the sample located opposite the aluminum cathode, the formation of a nitrided layer with a thickness of about 250 nm was observed in the substrate. The maximum hardness (32.3 ± 1.7 GPa) belongs to a coating on the sample occupying an intermediate position. The coating on the sample located opposite the aluminum cathode has a hardness of 16.7 ± 0.8 GPa. The coating hardness on the sample located opposite the titanium cathode is 28.5 ± 1.1 GPa. The best fracture strength in the scratch test was observed for the coating on the sample occupying an intermediate position. The nature of the coating fracture in the scratch test was studied. A sufficiently high-quality coating can be obtained without rotating the turntable, and the coating composition can be controlled by changing the position of the sample relative to the evaporators.
1. Introduction
Physical vapor deposition (PVD) methods are widely used to synthesize coatings of various compositions and applications. The very scheme of this method (the presence of plasma flows from different evaporators) predetermines the possible non-uniformity of the coating in composition and structure, which is one of the key problems of this method [1,2,3,4,5]. The main means of solving this problem is the rotation (circular, planetary or double planetary) of samples in the chamber. This ensures the relative identity of the coating properties in the horizontal plane, but such uniformity is not observed in the vertical plane. Obviously, when depositing a coating including two or more metal components, the composition of the coating will depend on its location relative to the plasma sources. At the same time, if we abandon the rotational movement of the samples, it is possible to vary the ratio of elements in the coating by changing the spatial location of the sample relative to the evaporators. Although such a deposition scheme is not rational for mass production (due to a significant reduction in the working area), its use may be justified in the process of research and in the manufacture of small batches of products.
Previously, a number of studies have been conducted examining the effect of the spatial arrangement of the sample on the properties of the coating. It was found that the arrangement of samples during the deposition of a CrN coating affects the coating thickness and surface roughness, but does not affect its hardness [6]. A significant effect of the arrangement of the substrate surface relative to the plasma flows on the elemental and phase composition, as well as the tribological properties, of a W-S-C coating was found [7].
There are results of studies of the peculiarities of the deposition of metallic and nitride coatings based on Ti and Al. The simultaneous deposition of metallic Ti and Al on a substrate with a non-central orientation allows for synthesizing the intermetallic compounds TiAl, TiAl2 and TiAl3, while the ratio of elements and the composition of intermetallic compounds depend on the angle between the plasma flow axis and the substrate surface [8]. Based on the analysis of the research results, a mathematical model was developed that allows for predicting the formation of Ti-Al intermetallic compounds depending on the spatial arrangement of the sample relative to the plasma flows [9]. The stable fcc phase (Ti,Al)N is formed as a result of a solid-phase reaction at a high nitrogen concentration, while aluminum atoms are replaced in large quantities by titanium atoms in the (Ti,Al)N solid solution [10]. A high hardness of the (Ti,Al)N coating (31.4 GPa/3200 HV) is observed at an Al/(Ti + Al) ratio of 60% [11]. The maximum hardness of the (Ti,Al)N coating is observed at the Al/Ti atomic ratio of 11:10 [12]. In this study, the ratio of the elements was changed by increasing the cathode arc current ratio IAl/ITi from 0.31 to 0.73, while the Al/Ti atomic ratio linearly increased from 0.49 to 1.29. When comparing (Ti,Al)N coatings with the Ti/Al ratios of 50/50 and 72/28, it was found that the maximum hardness of the coating was achieved at the Ti/Al ratio of 50/50 [13]. The properties of Ti1−xAlxN coatings were compared, where x = 0, 0.1, 0.3, 0.5, 0.6, 0.7, 0.8, 0.9 and 1.0 [14]. It was found that with an increase in the Al content to x = 0.6, the coating hardness increases (up to ~3200 HV), but with a further increase in the Al content, the hardness begins to decrease. With an increase in the Al content to x = 0.6, the lattice parameter decreases, but with a further increase in the Al content (x ≥ 0.7), the active formation of the hexagonal phase AlN is observed [15,16]. Depending on the deposition conditions and the type of equipment, the active formation of the hexagonal phase can begin at x > 0.52 [17] and at x > 0.7 [15,16]. With an increase in the Al content to x > 0.6, a columnar crystalline structure is not formed, while a decrease in the grain size is observed [15,18]. At an Al content of 0.5 ≤ x ≤ 0.66, the AlN phase is formed along the grain boundaries of (Ti,Al)N [18]. In this case, with a coating composition of 20 at.% Ti, 25 at.% Al and 55 at.% N, a superhard structure (hardness up to 47 GPa) can be formed, consisting of (Ti,Al)N grains (a size of about 30 nm) oriented in one direction, which are surrounded by an amorphous or nanocomposite c-AlN phase. Nitrogen pressure has an insignificant effect on the Al content in the coating [19]. An increase in nitrogen pressure from 0.66 to 6.60 Pa (5–50 mTorr) led to a slight change in the Al content within 3 wt.%.
The corrosion resistance of CVD TiN and TiAlN coatings in 3.5 wt.% NaCl solution was studied [20]. (Ti,Al)N coatings were deposited using TiCl4 precursors and AlCl3 precursors. The Al/Ti ratio was changed by changing the precursor ratio. It was found that with an increase in the Al content, the corrosion resistance first increases and then begins to decrease. The coating obtained with the AlCl3/TiCl4 ratio = 24/12 had the highest hardness in combination with the best corrosion resistance.
Therefore, when using the PVD method, there are three ways to change the ratio of elements in the coating:
- Using alloy cathodes with different ratios of elements. This method is very labor-intensive and requires significant financial costs. Suitable only for mass production when a large number of cathodes of a standard composition are used.
- Changing the arc currents of the cathodes. This method is quite effective, but allows for varying the ratio of elements in the coating only in a limited range due to the fact that at an arc current below the limit value, it is impossible to form a plasma flow, and at too high arc current values, a significant phase of microparticles is formed [1,2,3,21].
- Changing the spatial position of the sample relative to the plasma flows. This method assumes a static position of the samples during the coating deposition process. Theoretically, in this case, it is possible to vary the content of metal elements in the coating from 0 to 100%; however, given the dispersion of the plasma flow, when using two or more evaporators with cathodes of different compositions, it is impossible to obtain a “clean” coating that would not contain some amount of the second (third, etc.) element.
In this paper, the change in the composition and properties of the (Ti,Al)N coating depending on the spatial arrangement of the sample in the installation chamber was investigated. The objective of this study was to determine the limits of variation in composition using this method, as well as to study the quality of the coatings obtained.
2. Materials and Methods
The coating was deposited on ERTi-1 titanium alloy plates (99.58–99.90% Ti; ASME SFA-5.16/SFA-5.16M [22]). The plate size was 16 × 8 × 2 mm (Figure 1a). Before the deposition of the coatings, the samples were prepared, including washing in a special solution with ultrasonic stimulation, rinsing in purified water, drying with a stream of purified heated air and final wiping with highly purified medical alcohol.
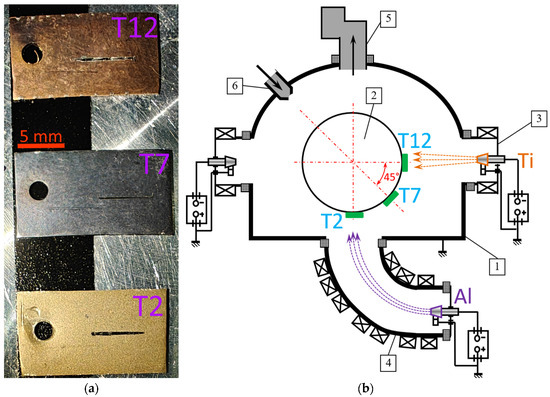
Figure 1.
General view of samples with deposited coatings (scratch test grooves are visible) (a) and general setup diagram and sample arrangement (b): 1—chamber; 2—rotary table (not rotated during coating deposition); 3—Controlled Accelerated Arc (CAA-PVD) system evaporator (with titanium cathode); 4—filtered cathodic vacuum arc deposition (FCVAD) system evaporator (with aluminum cathode); 5—vacuum system; 6—gas leak.
For coating deposition, a modernized VIT-2 PVD installation (IDTI RAS—MSUT «STANKIN», Moscow, Russia) [23,24,25,26,27] (Figure 1b) was used, in which two types of innovative evaporators are used:
- An evaporator of the filtered cathodic vacuum arc deposition (FCVAD) system [28], allowing for the effective (up to 98%) separation of microparticles. This evaporator was used for aluminum (99.80% Al) cathodes due to the high generation of microparticles during aluminum evaporation.
- Evaporators of the Controlled Accelerated Arc (CAA-PVD) system were used to install a titanium (99.95% Ti) cathode [5,29,30].
The arrangement of the samples in the chamber is shown in Figure 1a. The sample designated as T2 was located opposite the FCVAD evaporator with an aluminum cathode, the sample designated as T7 was installed in an intermediate position between the evaporators with aluminum and titanium cathodes (at an angle of 45° to the central axes of these evaporators) and the sample designated as T12 was installed opposite the titanium cathode. The samples were installed in the chamber according to the described scheme, then evacuation and preliminary etching in an inert gas environment (Ar) at a pressure of 0.20 Pa and an arc current of 100–150 A were carried out. Then, argon was removed and nitrogen (N) was supplied, final etching was carried out with a gas plasma flow (N) and then the coating was deposited for 30 min at a nitrogen pressure of 0.42 Pa, a bias voltage on the substrate of −150 V and arc currents by the cathodes of 65 A (for Ti) and 160 A (for Al).
To study the internal structure of the samples, a JEM 2100 transmission electron microscope (TEM) (JEOL, Tokyo, Japan) was used; to study the composition of the samples, an INCA energy-dispersive X-ray system (EDX) (OXFORD Instruments, Oxford, UK) was used.
Hardness was measured with a Nanovea SV-500 tester (Nanovea, Irvine, CA, USA) using a Berkovich pyramidal indenter at a load of 20 mN. Hardness was determined as the average value of the results of 40 measurements.
Scratch testing was performed on a Nanovea SV-500 tester (Nanovea, Irvine, CA, USA) according to the ASTM “Standard Test Method for Adhesion Strength and Mechanical Failure Modes of Ceramic Coatings by Quantitative Single Point Scratch Testing” [31], in the load range of 0–40 N, using a conical diamond indenter with an angle of 120° and a radius of 100 μm.
Since measuring the hardness of coatings (superhard film on a significantly softer substrate) presents certain difficulties (associated, among other things, with the heterogeneity and anisotropy of the coating), 30 measurements were taken for each coating, obviously unreliable (too high or too low) values were discarded (no more than 5), then the average value and the spread of values were determined.
3. Results
The nitrogen content in the coating is 51.4 ± 2.1 at.% and is practically identical for all three coatings. In what follows, we will consider only the metal content in nitride coatings; the total content of the metal component of the coating is taken as 100 at.%. The aluminum content in the coatings decreases from 94.2 at.% for sample T2, located directly opposite the evaporator with an Al cathode, to 10.3 at.% for sample T12, located opposite the evaporator with a Ti cathode (Figure 2a). Simultaneously with this, the titanium content increases from 7.6 at.% to 89.7 at.%. The slightly higher aluminum content in sample T12 compared to the titanium content in sample T2 can be explained by the significantly lower weight of aluminum atoms and, accordingly, a greater dispersion of these atoms in the chamber space. Sample T7, which occupies an intermediate position between the aluminum and titanium plasma flows, has a dominant titanium content (69.5 at.%) and a lower aluminum content (30.5 at.%). This disproportion can be explained by a wider angle of the titanium plasma flow compared to the more focused aluminum plasma flow in the FCVAD system [23]. An approximation of the obtained data shows that both elements are characterized by a polynomial (second-order) function, which allows for achieving an approximation accuracy of R2 = one (Figure 2b).
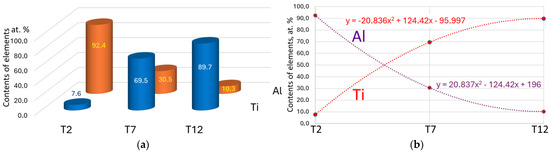
Figure 2.
Elemental composition of the coatings (the nitrogen content in the coating is 51.4 ± 2.1 at.%); the content of the metallic component of the coating is taken as 100 at.% (a). Construction of approximating polynomial curves describing the change in the content of elements in the coating (b).
The results of coating thickness measurements are presented in Table 1. The obtained data show a significant difference in the coating thicknesses depending on the sample location in the chamber. The T2 coating thickness is in the range of 0.8–1.5 µm, which is significantly less than the T12 coating thickness (2.8–3.4 µm), given that the duration of the deposition process for all samples was identical and was 30 min. Such a difference in thicknesses is associated with the features of the PVD process in general and FCVAD technology in particular. It is known that the PVD method, unlike CVD methods, does not allow for a completely unified coating thickness, since the coating thickness depends on the sample location in the chamber [5]. This is because aluminum forms a high amount of the microparticle phase during evaporation, and FCVAD technology allows for filtering the plasma flow from these microparticles, but this reduces the flow density and, accordingly, the coating deposition rate [5,30]. Accordingly, with identical parameters of the deposition process, the growth rate of the coating for the sample located opposite the CAA-PVD evaporator with a titanium cathode is significantly higher compared to the sample located opposite the FCVAD evaporator with an aluminum cathode.

Table 1.
Results of coating thickness measurements.
When measuring the hardness of coatings, it is usually assumed that the indentation depth should not exceed 10% of the total coating thickness [32,33]. This condition allows one to obtain an adequate value for the hardness of a coating deposited on a substrate with significantly different properties (in this case, a significantly softer and more plastic one). When working with nanoindentation data, the relative indentation depth should be determined by the contact depth normalized by the coating thickness [34]. Available data show that the adequate measurement of coating hardness with nanoindentation is possible with a coating thickness of more than 100 nm and an indenter indentation depth of more than 20 nm [34]. In general, the results of the nanoindentation of hard coatings on a soft substrate are completely adequate with coating thicknesses of about 1 μm, even if the indentation depth exceeds 10% of the coating thickness [35].
Other works contain data showing that in the case of a sufficiently thick (more than 1 μm) and hard coating on a relatively soft and plastic substrate, the plastic deformation of the substrate, affecting the accuracy of measuring the hardness of the coating, is observed at an indentation depth of about 20% of the coating thickness [36]. Given that the difficulties in measuring the hardness of coatings are associated not only with the possible plastic deformation of the substrate, but also with the noticeable heterogeneity and anisotropy of the properties of the coating itself (the presence of a crystalline and cluster structure, the possible inclusion of microparticles, including those embedded in the structure and therefore invisible, the presence of dislocations, vacancies and other defects in the crystalline structure, etc.). Therefore, only a large number of measurements with a subsequent rejection of extreme values and statistical processing allows us to obtain a sufficiently adequate hardness value. Results obtained in this way allow for at least a qualitative comparison of the hardness of various coatings with a high degree of reliability, as well as obtaining quantitative results close to real ones.
The results of changing the hardness of the coatings show that the T7 coating has the maximum hardness (32.3 ± 1.7 GPa) (Figure 3a). This result correlates well with the data [10,11,12,13,14,15,16] showing that the hardness of the (Ti,Al)N coating increases with an increase in the aluminum content to 60–70 at.%, but with a further increase in the Al content, the hardness begins to decrease, which is confirmed by the results of measuring the hardness of the T2 coating (16.7 ± 0.8 GPa). The hardness of the T12 coating with a dominant titanium content is 28.5 ± 1.1 GPa. The results of the study of fracture resistance in the scratch test correlate sufficiently with the hardness data (Figure 3b). The T7 coating has the best resistance to fracture (complete destruction is not observed under a load of 40 N). The T2 coating has the lowest fracture strength (it breaks under a load of LC2 = 14 N). The T12 coating breaks under a load of 28 N.
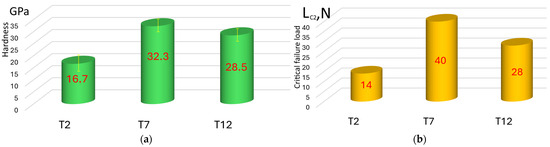
Figure 3.
Hardness (a) and resistance to destruction in the scratch test of the studied coatings (b).
Analysis of the scribing groove of the T2 coating (Figure 4) shows the absence of noticeable delaminations from the substrate. Since the coating is quite soft, there is a denting of the coating, accompanied by partial chipping along the boundaries of the groove (Figure 4b–d).
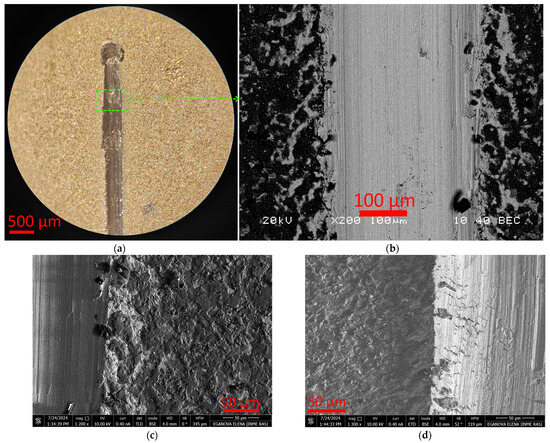
Figure 4.
Nature of T2 coating destruction during scratch test: end of scribing groove (a), nature of coating destruction (b–d).
The nature of the destruction of the T7 coating during the scratch test differs significantly from that of the T2 coating. The very hard T7 coating is characterized by brittle destruction with the formation of a crack network in the depth of the scribing groove and fragmentary chips at the groove boundary up to 50 μm wide (Figure 5). Moreover, the complete destruction of the coating does not occur even at the maximum load of 40 N in this test. Despite the presence of areas of delamination in the substrate, these areas are local in nature and do not lead to the complete destruction of the coating. Thus, this coating can be characterized as hard and at the same time quite brittle, which correlates well with the data on (Ti,Al)N coatings with a high aluminum content [37,38,39,40].
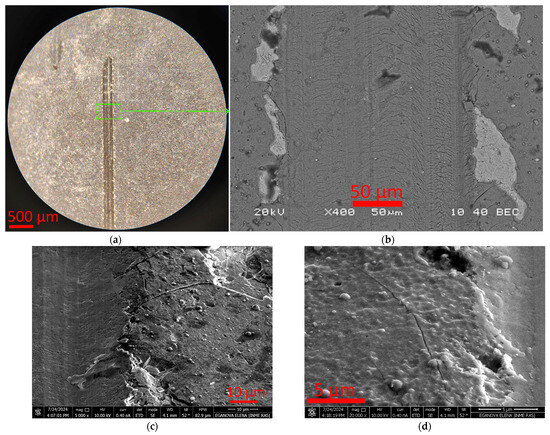
Figure 5.
Nature of T7 coating destruction during scratch test: end of scribing groove (a), nature of coating destruction (b–d).
The T12 coating is characterized by destruction as a result of extensive delamination (Figure 6a). Along the scribing groove, there is an extended continuous delamination (Figure 6b). The nature of the coating destruction is stepwise (Figure 6c,d), which may be a consequence of the coating structure formed as a result of periodic heating (activation) during the deposition process [41,42,43,44,45]. Figure 6c shows the location of the lamella cutout.
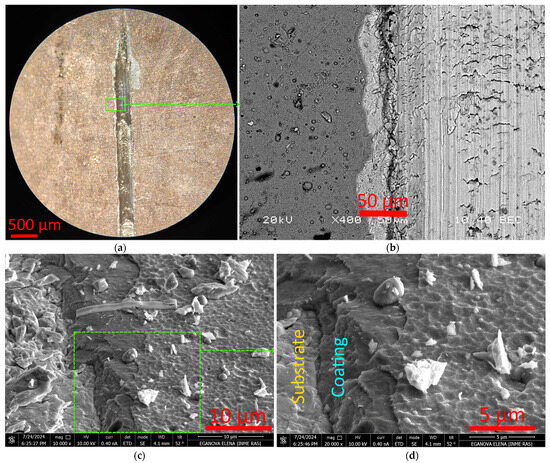
Figure 6.
Nature of T12 coating destruction during scratch test: end of scribing groove (a), nature of coating destruction (b–d).
For cutting out the lamellae, areas on the border of the scribing groove were selected (Figure 7). These areas allow us not only to study the structure of the coatings, but also provide an opportunity to study the nature of the destruction of the coating and adjacent layers of the substrate under the influence of the movement of the indenter during the scratch test.

Figure 7.
General view of lamellae for studying coatings and areas of their cutting for T2 (a), T7 (b) and T12 (c).
The study of the interface region of the T2 coating and the substrate reveals a complex, multilayered structure of this region (Figure 8a). As a result of the SAED phase analysis (Figure 8b) and the analysis of the change in the elemental composition (Figure 8c,d), three regions can be distinguished:
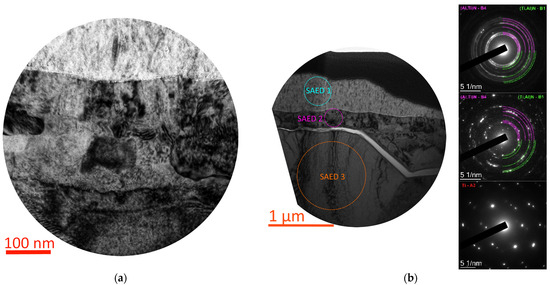
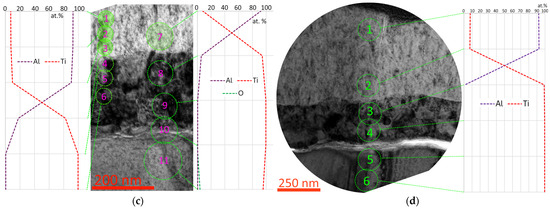
Figure 8.
Study of the structure and elemental and phase composition of the T2 coating: structure of the coating–substrate interface (a), phase composition of the coating and adjacent substrate layers (SAED) (b), change in elemental composition in the coating–substrate interface region (c,d).
A. The coating region in which the hexagonal phase based on aluminum nitride (Al,Ti)N B4 dominates, but a cubic phase based on titanium nitride (Ti,Al)N B1 is also present.
B. The nitrided layer [46,47,48,49,50] of the titanium substrate with a significant diffusion of aluminum, in which the phases (Al,Ti)N B4 and (Ti,Al)N B1 are also present. This layer differs significantly in crystal structure from the next layer of the titanium substrate. These changes in the elemental composition indicate that the diffusion of aluminum into this layer is uneven; the aluminum content is in the range from 0.5 to 17.0 at.%. The presence of reflections of the (Al,Ti)N B4 phase can be explained by the fact that since this layer is quite thin (about 250 nm), the SAED analysis also captures the (Al,Ti)N layer located above with the selector diaphragm, as well as the presence of a certain amount of diffused aluminum in the B layer itself. A distinctive feature of this layer is also a significant decrease in the grain size (down to the nanometer) compared to the titanium substrate. Of particular interest is the fact of the nitriding of this layer with a flow of gas (nitrogen) plasma during the preliminary etching of the samples.
C. An unmodified layer of the titanium substrate with the dominant Ti A3 phase.
Between layers A and B, a thin (10–20 nm) light-contrast layer can be seen, which may be formed by residual titanium oxide (precise identification is impossible due to the low thickness of this layer) [51,52,53]. A crack can also be seen that turns into a delamination between the titanium substrate (C) and the nitrided layer with aluminum diffusion (B). This crack was formed as a result of the action of the indenter during the scratch test, and it is worth noting that the delamination did not occur between the coating and the substrate, but between two layers of the substrate.
The study of the coating–substrate interface structure in sample T7 shows that a strong adhesive bond is maintained between them (Figure 9a). Directly at the boundary of the coating and the substrate, a layer of grains 3–10 nm in size is observed, possibly residual titanium oxide (an accurate determination of the composition is impossible due to the small grain sizes) (Figure 9b).
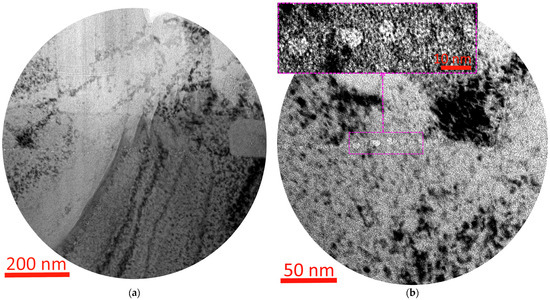
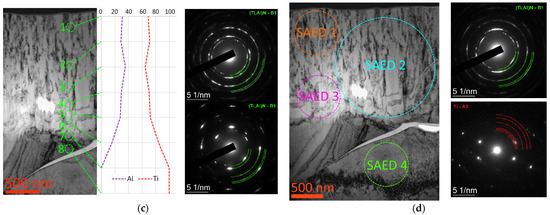
Figure 9.
Coating–substrate interface structure (a), grain layer at coating–substrate interface (b), study of elemental (c) and phase composition (d) of T7 coating.
In the T7 coating, the cubic phase (Ti,Al)N B1 is dominant (Figure 9d), and no hexagonal phase based on aluminum nitride is observed, which may indicate the complete dissolution of aluminum in the TiN structure. A crack is observed in the observed structure of the titanium substrate, apparently formed under the action of the scratch test, but in this case the crack propagated along the boundary of the titanium grains.
The study of the nature of the T7 coating destruction during the scratch test showed the tendency of this coating to crack formation. The nature of crack propagation in the coating under consideration is shown in Figure 10. The crack trajectory is directed at an angle close to 45° to the substrate surface. The coating layers associated with its periodic thermal activation do not have a significant effect on the crack development. Moreover, it can be seen that the crack development is influenced by the crystalline structure of the coating (Figure 10, upper right inset). When passing through stronger grains, the crack can change its trajectory or bypass (“flow around”) these grains. In this case, the crack does not completely cut through the coating structure. It is worth noting that this type of crack development differs significantly from the type of crack development in coatings with a nanolayer structure, where interlayer interfaces can slow down the crack development by changing its trajectory [54,55,56,57].
Figure 10.
Nature of crack formation in the T7 coating.
In the structure of the interface between the T12 coating and the substrate, pores (nanocavities) and light-contrasting grains (possibly also remnants of the oxide layer) up to 50 nm in size are observed (Figure 11a,b). The phase composition is dominated by (Ti,Al)N B1, but metallic titanium Ti A3 is also present at the boundary with the substrate (Figure 11d). In the structure of the substrate, at the boundary with the coating, there is a light-contrasting layer with an insignificant (up to 1 at.%) diffusion of aluminum (Figure 11c, point 5). The presence of pores and larger grains at the boundary of the coating and the substrate can cause the relatively low adhesion of the T12 coating.
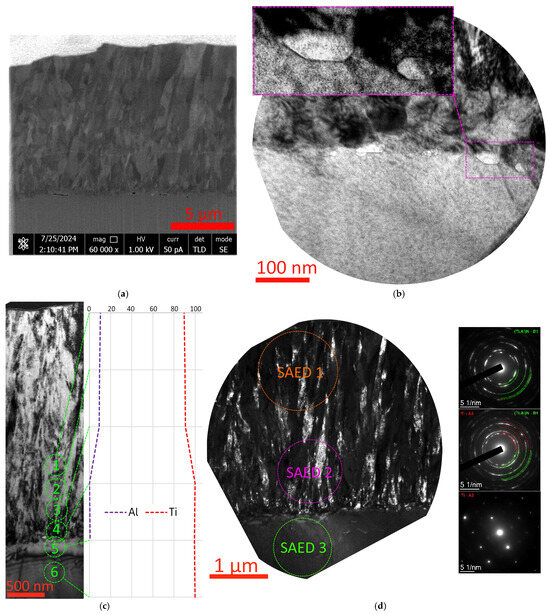
Figure 11.
Coating–substrate interface structure (a), crystal structure at the coating–substrate interface (b), elemental (c) and phase (d) composition study of the T12 coating.
4. Conclusions
The compositions, structures and properties of three (Ti,Al)N coatings deposited on samples with different spatial arrangements in the chamber were compared. The three samples were located, respectively, opposite the evaporator with a titanium cathode, opposite the evaporator with an aluminum cathode and in an intermediate position between the two evaporators. The coating was deposited without rotating the turntable. The study of the coatings showed the following:
- The aluminum content in the coatings decreases from 94.2 at.% for the sample located directly opposite the evaporator with an Al cathode to 10.3 at.% for the sample located opposite the evaporator with a Ti cathode. Moreover, the titanium content increases from 7.6 at.% to 89.7 at.%. The sample occupying an intermediate position between the aluminum and titanium plasma flows has a dominant titanium content (69.5 at.%) and a lower aluminum content (30.5 at.%).
- In the coating deposited on the sample located opposite the aluminum cathode, a nitrided layer of about 250 nm in thickness is formed in the substrate.
- The results of hardness changes show that the coating on the sample occupying an intermediate position has the maximum hardness (32.3 ± 1.7 GPa). The coating on the sample located opposite the aluminum cathode has a hardness of 16.7 ± 0.8 GPa. The hardness of the coating on the sample located opposite the titanium cathode is 28.5 ± 1.1 GPa.
- The results of the study of the resistance to destruction in the scratch test correlate sufficiently with the hardness data. The coating on the sample occupying an intermediate position has the best resistance to destruction (complete destruction is not observed under a load of 40 N). The coating on the sample located opposite the aluminum cathode has the lowest fracture strength (it is destroyed under a load of LC2 = 14 N). The destruction of the coating on the sample located opposite the titanium cathode occurs under a load of 28 N.
- The coating on the sample located opposite the aluminum cathode shows a dent in the coating, accompanied by partial chipping along the groove boundaries. The coating on the sample occupying an intermediate position is characterized by brittle fracture with the formation of a crack network in the depth of the scribing groove and fragmentary chips at the groove boundary up to 50 μm wide. The coating on the sample located opposite the titanium cathode is characterized by fracture as a result of extensive delamination.
Therefore, a sufficiently high-quality coating can be obtained without rotating the rotary table, while it is possible to control the coating composition by changing the positioning of the sample relative to the evaporators.
The purpose of this work is to change the ratio of elements in the coating and to vary and study the properties of coatings deposited under different conditions. In the absence of rotation, a coating with an individual composition and properties will be formed at each point in space. As a consequence, having carried out a full volume of research, it will be possible to create a three-dimensional scheme that allows for obtaining a coating with a clearly determined ratio of two (and in the future—three or more) elements.
Author Contributions
Conceptualization, A.V. and Y.B.; methodology, Y.B., A.V., C.S., M.V., A.S. and F.M.; resources, S.G.; data curation, C.S., M.V. and I.S.; investigation, C.S., Y.B., F.M. and I.S.; supervision, S.G. and A.V.; writing—original draft preparation, A.V.; writing—review and editing, A.V. and C.S.; project administration, A.V., M.V. and C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the state assignment of the Ministry of Science and Higher Education of the Russian Federation, Project No. FSFS-2023-0003.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The study used the equipment from the Center for Collective Use of Moscow State Technological University STANKIN (agreement no. 075-15-2021-695, 26 July 2021).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Daalder, J.E. Components of cathode erosion in vacuum arcs. J. Phys. D Appl. Phys. 1976, 9, 2379–2395. [Google Scholar] [CrossRef]
- Anders, A. Cathodic Arcs; Springer Science Business Media LLC: New York, NY, USA, 2008. [Google Scholar]
- Aksenov, I.I.; Konovalov, I.I.; Pershin, V.F.; Khoroshikh, V.M.; Shpilinskii, L.F. Low-pressure arc cathode erosion. High Temp. 1988, 26, 315–318. [Google Scholar]
- Baouchi, A.W.; Perry, A.J. A study of the macroparticle distribution in cathodic-arc- evaporated TiN films. Surf. Coat. Technol. 1991, 49, 253–257. [Google Scholar] [CrossRef]
- Grigoriev, S.; Vereschaka, A.; Zelenkov, V.; Sitnikov, N.; Bublikov, J.; Milovich, F.; Andreev, N.; Mustafaev, E. Specific features of the structure and properties of arc-PVD coatings depending on the spatial arrangement of the sample in the chamber. Vacuum 2022, 200, 111047. [Google Scholar] [CrossRef]
- Tuchinda, K.; Damkam, C.; Preechapongkij, W.; Ratanabunlang, R.; Wittayapairot, N. The effect of substrate location arrangement in the PVD deposition chamber on the mechanical properties of the coatings. In Proceedings of the Minerals, Metals and Materials Society—3rd International Conference on Processing Materials for Properties 2008, Bangkok, Thailand, 7–10 December 2008; Volume 2, pp. 1167–1174. [Google Scholar]
- Vuchkov, T.; Evaristo, M.; Yaqub, T.B.; Cavaleiro, A. The effect of substrate location on the composition, microstructure and mechano-tribological properties of W-S-C coatings deposited by magnetron sputtering. Surf. Coat. Technol. 2020, 386, 125481. [Google Scholar] [CrossRef]
- Vardanyan, E.L.; Kireev, R.M.; Budilov, V.V. Synthesis of coatings based on intermetallic titanium-aluminum by vacuum arc deposition. In Proceedings of the International Symposium on Discharges and Electrical Insulation in Vacuum, ISDEIV, Tomsk, Russia, 2–7 September 2012; pp. 549–551, Art. No 6412577. [Google Scholar]
- Vardanyan, E.L.; Kireev, R.M.; Budilov, V.V.; Ramaznov, K.N. Producing of coatings based on intermetallic Tix-Aly on the surface of the part by using vacuum arc plasma. In Proceedings of the International Symposium on Discharges and Electrical Insulation in Vacuum, ISDEIV, Mumbai, India, 28 September–3 October 2014; pp. 541–543, Art. No 6961739. [Google Scholar]
- Zhang, Z.; Zhang, L.; Yuan, H.; Qiu, M.; Zhang, X.; Liao, B.; Zhang, F.; Ouyang, X. Tribological Behaviors of Super-Hard TiAlN Coatings Deposited by Filtered Cathode Vacuum Arc Deposition. Materials 2022, 15, 2236. [Google Scholar] [CrossRef]
- PalDey, S.; Deevi, S.C. Single layer and multilayer wear resistant coatings of (Ti,Al)N: A review. Mater. Sci. Eng. A 2003, 342, 58–79. [Google Scholar] [CrossRef]
- Sveen, S.; Andersson, J.; M’Saoubi, R.; Olsson, M. Scratch adhesion characteristics of PVD TiAlN deposited on high speed steel, cemented carbide and PCBN substrates. Wear 2013, 308, 133–141. [Google Scholar] [CrossRef]
- Han, J.G.; Yoon, J.S.; Kim, H.J.; Song, K. High temperature wear resistance of (TiAl)N films synthesized by cathodic arc plasma deposition. Surf. Coat. Technol. 1996, 86–87, 82–87. [Google Scholar] [CrossRef]
- Lin, K.L.; Hwang, M.Y.; Wu, C.D. The deposition and wear properties of cathodic arc plasma deposition TiAlN deposits. Mater. Chem. Phys. 1996, 46, 77–83. [Google Scholar] [CrossRef]
- Kimura, A.; Hasegawa, H.; Yamada, K.; Suzuki, T. Metastable Ti1-xAlxN films with different Al content. J. Mater. Sci. Lett. 2000, 19, 601–602. [Google Scholar] [CrossRef]
- Tanaka, Y.; Gür, T.M.; Kelly, M.; Hagstrom, S.B.; Ikeda, T. Structure and properties of (Ti1-xAlx)N films prepared by reactive sputtering. Thin Solid Film. 1993, 228, 238–241. [Google Scholar] [CrossRef]
- Wahlström, U.; Hultman, L.; Sundgren, J.-E.; Adibi, F.; Petrov, I.; Greene, J.E. Crystal growth and microstructure of polycrystalline Ti1-xAlxN alloy films deposited by ultra-high-vacuum dual-target magnetron sputtering. Thin Solid Films 1993, 235, 62–70. [Google Scholar] [CrossRef]
- Musil, J.; Hrubý, H. Superhard nanocomposite Ti1−xAlxN films prepared by magnetron sputtering. Thin Solid Films 2000, 365, 104–109. [Google Scholar] [CrossRef]
- Coll, B.F.; Fontana, R.; Gates, A.; Sathrum, P. (Ti,Al)N advanced films prepared by arc process. Mater. Sci. Eng. A 1991, 140, 816–824. [Google Scholar] [CrossRef]
- Elmkhah, H.; Fattah-alhosseini, A.; Babaei, K.; Abdollah-Zadeh, A.; Mahboubi, F. Correlation between the Al content and corrosion resistance of TiAlN coatings applied using a PACVD technique. J. Asian Ceram. Soc. 2020, 8, 72–80. [Google Scholar] [CrossRef]
- Steffens, H.-D.; Mack, M.; Moehwald, K.; Reichel, K. Reduction of droplet emission in random arc technology. Surf. Coat. Technol. 1991, 46, 65–74. [Google Scholar] [CrossRef]
- Steel Grade ERTi-1 Chemical Information, Mechanical Properties. Available online: https://www.steel-grades.com/index.php?m=pdfmetal&c=index&a=pdf&catid=85&id=11519?ERTi-1-Datasheet.pdf (accessed on 14 August 2024).
- Grigoriev, S.; Vereschaka, A.; Milovich, F.; Sitnikov, N.; Andreev, N.; Bublikov, J.; Kutina, N. Investigation of the properties of the Cr,Mo-(Cr,Mo,Zr,Nb)N-(Cr,Mo,Zr,Nb,Al)N multilayer composite multicomponent coating with nanostructured wear-resistant layer. Wear 2021, 468–469, 203597. [Google Scholar] [CrossRef]
- Vereschaka, A.A.; Bublikov, J.I.; Sitnikov, N.N.; Oganyan, G.V.; Sotova, C.S. Influence of nanolayer thickness on the performance properties of multilayer composite nano-structured modified coatings for metal-cutting tools. Int. J. Adv. Manuf. Technol. 2018, 95, 2625–2640. [Google Scholar] [CrossRef]
- Grigoriev, S.; Vereschaka, A.; Milovich, F.; Migranov, M.; Andreev, N.; Bublikov, J.; Sitnikov, N.; Oganyan, G. Investigation of the tribological properties of Ti-TiN-(Ti,Al,Nb,Zr)N composite coating and its efficiency in increasing wear resistance of metal cutting tools. Tribol. Int. 2021, 164, 107236. [Google Scholar] [CrossRef]
- Vereschaka, A.S.; Grigoriev, S.N.; Sotova, E.S.; Vereschaka, A.A. Improving the efficiency of the cutting tools made of mixed ceramics by applying modifying nano-scale multilayered coatings. Adv. Mat. Res. 2013, 712–715, 391–394. [Google Scholar]
- Volosova, M.; Grigoriev, S.; Metel, A.; Shein, A. The Role of Thin-Film Vacuum-Plasma Coatings and Their Influence on the Efficiency of Ceramic Cutting Inserts. Coatings 2018, 8, 287. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Volosova, M.A.; Vereschaka, A.A.; Sitnikov, N.N.; Milovich, F. Properties of (Cr,Al,Si)N-(DLC-Si) composite coatings deposited on a cutting ceramic substrate. Ceram. Int. 2020, 46, 18241–18255. [Google Scholar] [CrossRef]
- Grigoriev, S.; Vereschaka, A.; Milovich, F.; Sitnikov, N.; Seleznev, A.; Sotova, C.; Bublikov, J. Influence of the yttrium cathode arc current on the yttrium content in the (Ti, Y, Al) N coating and the coating properties. Vacuum 2024, 222, 113028. [Google Scholar] [CrossRef]
- Grigoriev, S.; Vereschaka, A.; Zelenkov, V.; Sitnikov, N.; Bublikov, J.; Milovich, F.; Andreev, N.; Sotova, C. Investigation of the influence of the features of the deposition process on the structural features of microparticles in PVD coatings. Vacuum 2022, 202, 111144. [Google Scholar] [CrossRef]
- ASTM C1624-05(2015); Standard Test Method for Adhesion Strength and Mechanical Failure Modes of Ceramic Coatings by Quantitative Single Point Scratch Testing. ASTM International: West Conshohocken, PA, USA, 2010.
- Buckle, H.; Westbrook, J.H.; Conrad, H. (Eds.) The Science of Microhardness Testing and its Research Applications; American Society for Metals: Russell Township, OH, USA, 1973; p. 453. [Google Scholar]
- Peggs, G.N.; Leigh, I.C. Recommended Procedure for Micro-Indentation Vickers Hardness Test, Report MOM62; National Physical Laboratory: Middlesex, UK, 1983. [Google Scholar]
- Chen, J.; Bull, S.J. On the factors affecting the critical indenter penetration for measurement of coating hardness. Vacuum 2009, 83, 911–920. [Google Scholar] [CrossRef]
- Vingsbo, O.; Hogmark, S.; Jönsson, B.; Ingemarsson, B.; Blau, P.J.; Lawn, B.R. (Eds.) Microindentation Techniques in Materials Science and Engineering; ASTM: Philadelphia, PA, USA, 1986. [Google Scholar]
- Burnett, P.J.; Rickerby, D.S. The mechanical properties of wear-resistant coatings I: Modelling of hardness behaviour. Thin Solid Films 1987, 148, 41–50. [Google Scholar] [CrossRef]
- Chen, L.; Du, Y.; Wang, A.J.; Wang, S.Q.; Zhou, S.Z. Effect of Al content on microstructure and mechanical properties of Ti-Al-Si-N nanocomposite coatings. Int. J. Refract. Met. Hard Mater. 2009, 27, 718–721. [Google Scholar] [CrossRef]
- Zou, C.W.; Zhang, J.; Xie, W.; Shao, L.X.; Fu, D.J. Structure and mechanical properties of Ti-Al-N coatings deposited by combined cathodic arc middle frequency magnetron sputtering. J. Alloys Compd. 2011, 509, 1989–1993. [Google Scholar] [CrossRef]
- Chen, L.; Paulitsch, J.; Du, Y.; Mayrhofer, P.H. Thermal stability and oxidation resistance of Ti-Al-N coatings. Surf. Coat. Technol. 2012, 206, 2954–2960. [Google Scholar] [CrossRef]
- Fan, G.; Zhang, J.; Zhang, P.; Du, J.; Xu, C.; Yi, M.; Zhang, G. Effect of Al Content on the Wear Evolution of Ti1-xAlxN-Coated Tools Milling Ti-6Al-4V Alloy. Micromachines 2023, 14, 1228. [Google Scholar] [CrossRef] [PubMed]
- Vereschaka, A.; Milovich, F.; Migranov, M.; Andreev, N.; Alexandrov, I.; Muranov, A.; Mikhailov, M.; Tatarkanov, A. Investigation of the tribological and operational properties of (Mex,Moy,Al1-(x+y))N (Me –Ti, Zr or Cr) coatings. Tribol. Int. 2022, 165, 107305. [Google Scholar] [CrossRef]
- Larijani, M.M.; Manouchehrian, M.; Seyedi, H.; Yari, M. Corrosion behavior of solid solution (Ti,Al)N as a function of Al concentration. Cryst. Res. Technol. 2012, 47, 834–840. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Pradeep, A.M.V.; Mohanty, B.S.; Jaswanth, A. Influence of inductive heating on coating geometry during deposition of AISI 316 stainless steel over EN8 carbon steel using friction surfacing process. Mater. Today 2020, 27, 1641–1650. [Google Scholar] [CrossRef]
- Garcia, J.; Pitonak, R.; Weissenbacher, R.; Köpf, A. Production and characterization of wear resistant Ti(C,N) coatings manufactured by modified chemical vapor deposition process. Surf. Coat. Technol. 2010, 205, 2322–2327. [Google Scholar] [CrossRef]
- Ji, X.; Wang, L.J.; Zhang, C.M.; Mi, Y.M. Heating and Time Processes on Growth and Adhesive Property of Ta-Mo Coatings. Mater. Manuf. Process. 2016, 31, 1269–1273. [Google Scholar] [CrossRef]
- Sun, F.; Liu, X.-L.; Luo, S.-Q.; Xiang, D.-D.; Ba, D.-C.; Lin, Z.; Song, G.-Q. Duplex treatment of arc plasma nitriding and PVD TiN coating applied to dental implant screws. Surf. Coat. Technol. 2022, 439, 128449. [Google Scholar] [CrossRef]
- Tan, C.; Zhou, K.; Kuang, T.; Li, Y.; Ma, W. Novel performances of in situ plasma nitriding-PVD duplex-treated nanocrystalline TiN coatings. Surf. Eng. 2018, 34, 520–526. [Google Scholar] [CrossRef]
- Nolan, D.; Huang, S.W.; Leskovsek, V.; Braun, S. Sliding wear of titanium nitride thin films deposited on Ti-6Al-4V alloy by PVD and plasma nitriding processes. Surf. Coat. Technol. 2006, 200, 5698–5705. [Google Scholar] [CrossRef]
- Durst, O.; Ellermeier, J.; Berger, C. Influence of plasma-nitriding and surface roughness on the wear and corrosion resistance of thin films (PVD/PECVD). Surf. Coat. Technol. 2008, 203, 848–854. [Google Scholar] [CrossRef]
- Alkan, S.; Gök, M.S. Influence of plasma nitriding pre-treatment on the corrosion and tribocorrosion behaviours of PVD CrN, TiN and AlTiN coated AISI 4140 steel in seawater. Lubr. Sci. 2022, 34, 67–83. [Google Scholar] [CrossRef]
- Liu, J.-K.; Liu, I.-H.; Liu, C.; Chang, C.-J.; Kung, K.-C.; Liu, Y.-T.; Lee, T.-M.; Jou, J.-L. Effect of titanium nitride/titanium coatings on the stress corrosion of nickel-titanium orthodontic archwires in artificial saliva. Appl. Surf. Sci. 2014, 317, 974–981. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Deng, C.; Huo, L.; Wang, L.; Cao, S. Feasibility study on preparation of coatings on Ti-6Al-4V by combined ultrasonic impact treatment and electrospark deposition. Mater. Des. 2014, 63, 488–492. [Google Scholar] [CrossRef]
- Zykova, A.; Safonov, V.; Walkowich, J.; Rogowska, R.; Yakovin, S. Corrosion properties of nitride, oxide and multilayer coatings on stainless steel and titanium-based substrates. J. Phys. Conf. Ser. 2010, 223, 012024. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, S.H.; Yang, L.; Jin, C.J.; Pan, L.Y. Investigation of Fracture Performance and Interface Stress Behavior of Zn-Zn-Al Multilayer Coating-304 Stainless Steel Substrate System. J. Mater. Civ. Eng. 2022, 34, 04021472. [Google Scholar] [CrossRef]
- Vereschaka, A.; Grigoriev, S.; Milovich, F.; Sitnikov, N.; Migranov, M.; Andreev, N.; Bublikov, J.; Sotova, C. Investigation of tribological and functional properties of Cr,Mo-(Cr,Mo)N-(Cr,Mo,Al)N multilayer composite coating. Tribol. Int. 2021, 155, 106804. [Google Scholar] [CrossRef]
- Long, R.; Dunn, M.L. Channel cracks in atomic-layer and molecular-layer deposited multilayer thin film coatings. J. Appl. Phys. 2014, 115, 233514. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Walck, S.D.; Zabinski, J.S. Architecture of multilayer nanocomposite coatings with super-hard diamond-like carbon layers for wear protection at high contact loads. Wear 1997, 203–204, 516–527. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).