Influence of Thermomechanical Treatments and Chemical Composition on the Phase Transformation of Cu-Al-Mn Shape Memory Alloy Thin Sheets
Abstract
1. Introduction
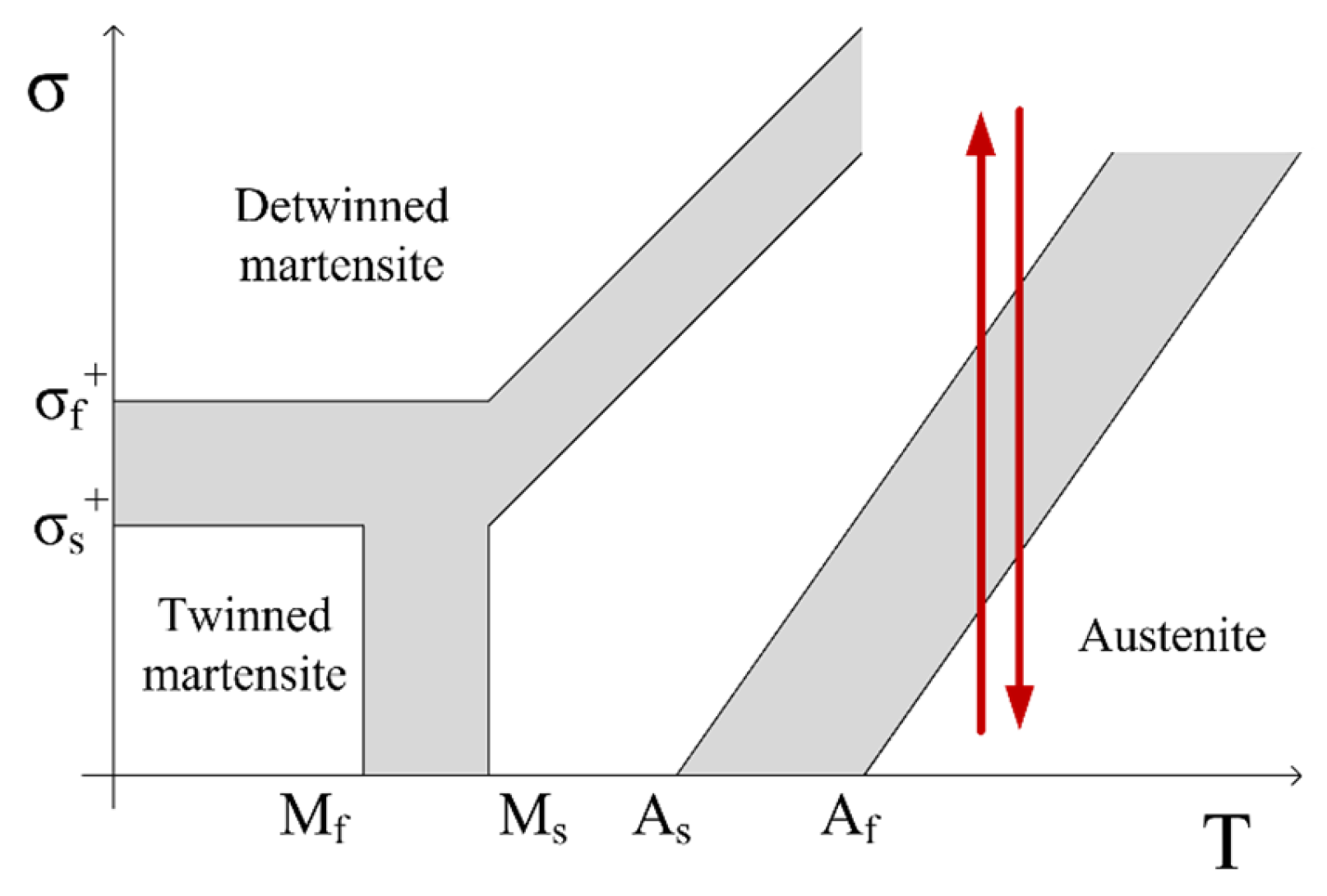
2. Materials and Methods
2.1. Experimental Setup
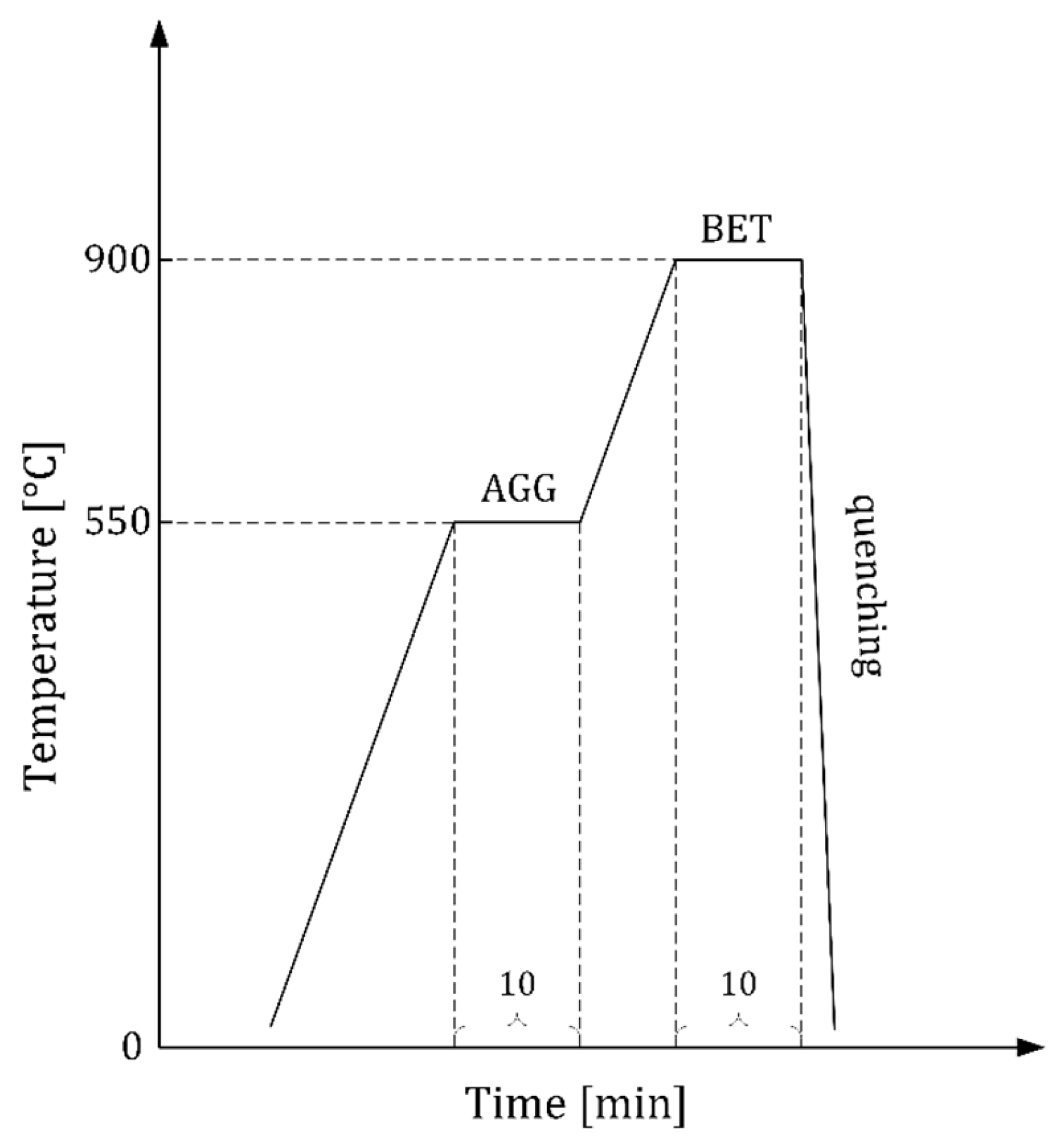
2.2. Alloy Fabrication and Thermomechanical Treatment to Obtain Thin Cu-Al-Mn SMA Sheets
2.3. Experimental Design
- Hot-rolled (0.4 mm);
- Hot-rolled (2 mm);
- Hot-rolled (2 mm), cold-rolled (0.4 mm);
- Hot-rolled (2 mm), cold-rolled (0.4 mm), grain growth (AGG);
- Hot-rolled (2 mm), cold-rolled (0.4 mm), grain growth (AGG), tensile cycling.
3. Results and Discussion
4. Conclusions
- Hot-rolling thermomechanical treatment for Cu-Al-Mn sheets introduces manganese depletion if it is applied at low sheet thicknesses (below 2 mm) and is caused by oxidation.
- The increase in manganese oxidation with thickness reduction is attributed to an increase in the surface area of the alloy placed into contact with the open air during the hot-rolling process as the sheet thickness decreases.
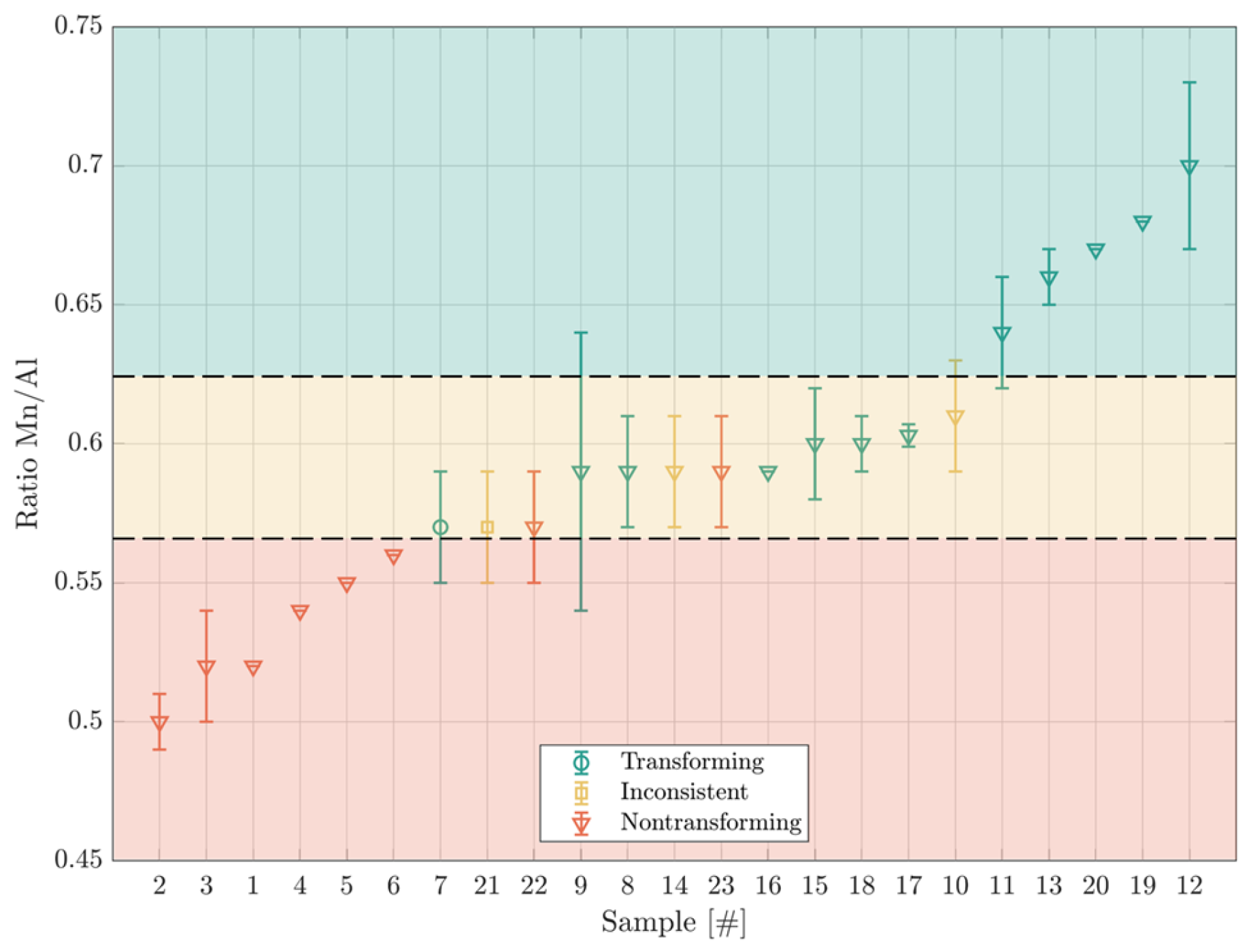
- Changes in the Mn/Al ratio in the alloy composition due to Mn oxidation result in the loss of the SME once the ratio drops below approximately 0.57.
- The application of cold rolling after sheets reach lower levels of thickness ensures preservation of the chemical composition, thus preserving the SME.
- The presence of phosphorus impurities, even in low amounts of 0.04–0.06 wt.%, suggests a possibility of significantly altering the expected alloy performance by binding large amounts of manganese and thus preventing them from being in the solid solution.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Humbeeck, J.; Liu, Y. The High Damping Capacity of Shape Memory Alloys. In Shape Memory Implants; Yahia, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 46–60. ISBN 978-3-642-64118-3. [Google Scholar]
- Lagoudas, D.C. Shape Memory Alloys: Modeling and Engineering Applications; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-0-387-47684-1. [Google Scholar]
- Van Humbeeck, J. Damping Capacity of Thermoelastic Martensite in Shape Memory Alloys. J. Alloys Compd. 2003, 355, 58–64. [Google Scholar] [CrossRef]
- Elmay, W.; Peltier, L.; Gabrion, X.; Kubler, R.; Piotrowski, B.; Laheurte, P.; Berveiller, S. Damping Capacity of Ti–Nb Shape Memory Alloys Evaluated Through DMA and Single-Impact Tests. Shape Mem. Superelasticity 2022, 8, 349–355. [Google Scholar] [CrossRef]
- San Juan, J.; Nó, M.L. Damping Behavior during Martensitic Transformation in Shape Memory Alloys. J. Alloys Compd. 2003, 355, 65–71. [Google Scholar] [CrossRef]
- Koeda, N.; Omori, T.; Sutou, Y.; Suzuki, H.; Wakita, M.; Kainuma, R.; Ishida, K. Damping Properties of Ductile Cu-Al-Mn-Based Shape Memory Alloys. Mater. Trans. 2005, 46, 118–122. [Google Scholar]
- Chang, S.-H.; Chien, C.; Wu, S.-K. Damping Characteristics of the Inherent and Intrinsic Internal Friction of Ti50Ni50−xFex (x = 2, 3, and 4) Shape Memory Alloys. Mater. Trans. 2016, 57, 351–356. [Google Scholar] [CrossRef]
- Wu, S.-K.; Chan, W.-J.; Chang, S.-H. Damping Characteristics of Inherent and Intrinsic Internal Friction of Cu-Zn-Al Shape Memory Alloys. Metals 2017, 7, 397. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Okotete, E.A. Reconciling Viability and Cost-Effective Shape Memory Alloy Options—A Review of Copper and Iron Based Shape Memory Metallic Systems. Eng. Sci. Technol. Int. J. 2016, 19, 1582–1592. [Google Scholar] [CrossRef]
- Sutou, Y.; Omori, T.; Kainuma, R.; Ishida, K. Ductile Cu-Al-Mn Based Shape Memory Alloys: General Properties and Applications. Mater. Sci. Technol. 2008, 24, 896–901. [Google Scholar] [CrossRef]
- Sutou, Y.; Omori, T.; Yamauchi, K.; Ono, N.; Kainuma, R.; Ishida, K. Effect of Grain Size and Texture on Pseudoelasticity in Cu-Al-Mn-Based Shape Memory Wire. Acta Mater. 2005, 53, 4121–4133. [Google Scholar] [CrossRef]
- Sutou, Y.; Omori, T.; Koeda, N.; Kainuma, R.; Ishida, K. Effects of Grain Size and Texture on Damping Properties of Cu-Al-Mn-Based Shape Memory Alloys. Mater. Sci. Eng. A 2006, 438–440, 743–746. [Google Scholar] [CrossRef]
- Sutou, Y.; Omori, T.; Kainuma, R.; Ishida, K. Grain Size Dependence of Pseudoelasticity in Polycrystalline Cu-Al-Mn-Based Shape Memory Sheets. Acta Mater. 2013, 61, 3842–3850. [Google Scholar] [CrossRef]
- Sutou, Y.; Kainuma, R.; Ishida, K. Effect of Alloying Elements on the Shape Memory Properties of Ductile Cu–Al–Mn Alloys. Mater. Sci. Eng. A 1999, 273–275, 375–379. [Google Scholar] [CrossRef]
- Sutou, Y.; Omori, T.; Furukawa, A.; Takahashi, Y.; Kainuma, R.; Yamauchi, K.; Yamashita, S.; Ishida, K. Development of Medical Guide Wire of Cu-Al-Mn-Base Superelastic Alloy with Functionally Graded Characteristics. J. Biomed. Mater. Res. 2004, 69B, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Sutou, Y.; Omori, T.; Wang, J.J.; Kainuma, R.; Ishida, K. Characteristics of Cu-Al-Mn-Based Shape Memory Alloys and Their Applications. Mater. Sci. Eng. A 2004, 378, 278–282. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, H.; Semperlotti, F. On the Nonlinear Analysis of Pre-Strained Shape Memory Alloy Beams. J. Sound Vib. 2023, 560, 117789. [Google Scholar] [CrossRef]
- Kainuma, R.; Takahashi, S.; Ishida, K. Ductile Shape Memory Alloys of the Cu-Al-Mn System. J. De Phys. IV 1995, 5, C8-961–C8-966. [Google Scholar] [CrossRef]
- Kainuma, R.; Takahashi, S.; Ishida, K. Thermoelastic Martensite and Shape Memory Effect in Ductile Cu-Al-Mn Alloys. Metall. Mater. Trans. A 1996, 27, 2187–2195. [Google Scholar] [CrossRef]
- Omori, T.; Sutou, Y.; Kainuma, R.; Ishida, K. Development of Cu–Al–Mn-Based Shape Memory Alloys. In Copper; Welter, J.-M., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 194–202. ISBN 978-3-527-61032-7. [Google Scholar]
- Moseneder-Frajria, M. Copper Based Shape Memory Alloys for Damping Applications. Master’s Thesis, Politecnico di Milano, Milan, Italy, 2021. [Google Scholar]
- Milosavljevic, D.; Bassani, P.; Cinquemani, S.; Lecis, N. Effects of Thermomechanical Treatments on Cu–Al–Mn Shape Memory Alloys. Mater. Chem. Phys. 2023, 302, 127756. [Google Scholar] [CrossRef]
- Kusama, T.; Omori, T.; Saito, T.; Ohnuma, I.; Ishida, K.; Kainuma, R. Two- and Three-Dimensional Grain Growth in the Cu–Al–Mn Shape Memory Alloy. Mater. Trans. 2013, 54, 2044–2048. [Google Scholar] [CrossRef]
- Omori, T.; Kusama, T.; Kawata, S.; Ohnuma, I.; Sutou, Y.; Araki, Y.; Ishida, K.; Kainuma, R. Abnormal Grain Growth Induced by Cyclic Heat Treatment. Science 2013, 341, 1500–1502. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.H.; Huang, H.; Xie, J. Microstructure and Superelasticity Control by Rolling and Heat Treatment in Columnar-Grained Cu-Al-Mn Shape Memory Alloy. Mater. Sci. Eng. A 2017, 696, 315–322. [Google Scholar] [CrossRef]
- Del Castillo, C.L.; Blázquez, M.L.; Gómez, C.; Mellor, B.G.; De Diego, N.; Del Rio, J. The Stabilization of Martensite in Cu-Al-Mn Alloys. J. Mater. Sci. 1988, 23, 3379–3382. [Google Scholar] [CrossRef]
- Brinson, L.C. One-Dimensional Constitutive Behavior of Shape Memory Alloys: Thermomechanical Derivation with Non-Constant Material Functions and Redefined Martensite Internal Variable. J. Intell. Mater. Syst. Struct. 1993, 4, 229–242. [Google Scholar] [CrossRef]
- Brinson, L.C.; Huang, M.S. Simplifications and Comparisons of Shape Memory Alloy Constitutive Models. J. Intell. Mater. Syst. Struct. 1996, 7, 108–114. [Google Scholar] [CrossRef]
- Milosavljevic, D.; Zhang, Q.; Moseneder, M.; Zhu, H.; Lecis, N.; Cinquemani, S.; Semperlotti, F. Progress on the Development of Shape-Memory-Alloy Metacomposites. In Proceedings of the American Society for Composites 2021, Virtual, 20–22 September 2021. [Google Scholar]
- Milosavljevic, D.; Lecis, N.; Cinquemani, S. Progress on the Study of Grain Growth and Tensile Cycling Effects on the Damping Performance in Cu-Al-Mn Shape Memory Alloy Thin Sheets. In Proceedings of the Active and Passive Smart Structures and Integrated Systems XVII; Tol, S., Nouh, M.A., Shahab, S., Yang, J., Huang, G., Eds.; SPIE: Long Beach, CA, USA, 2023; p. 36. [Google Scholar]
- Milosavljevic, D.; Caprio, L.; Busatto, M.; Cinquemani, S.; Lecis, N.; Previtali, B. Effects of Laser Cutting on the Chemical Composition and Phase Transformation Capacity in Cu-Al-Mn Shape Memory Alloy Sheets. In Proceedings of the Active and Passive Smart Structures and Integrated Systems XVII; Tol, S., Nouh, M.A., Shahab, S., Yang, J., Huang, G., Eds.; SPIE: Long Beach, CA, USA, 2023; p. 43. [Google Scholar]


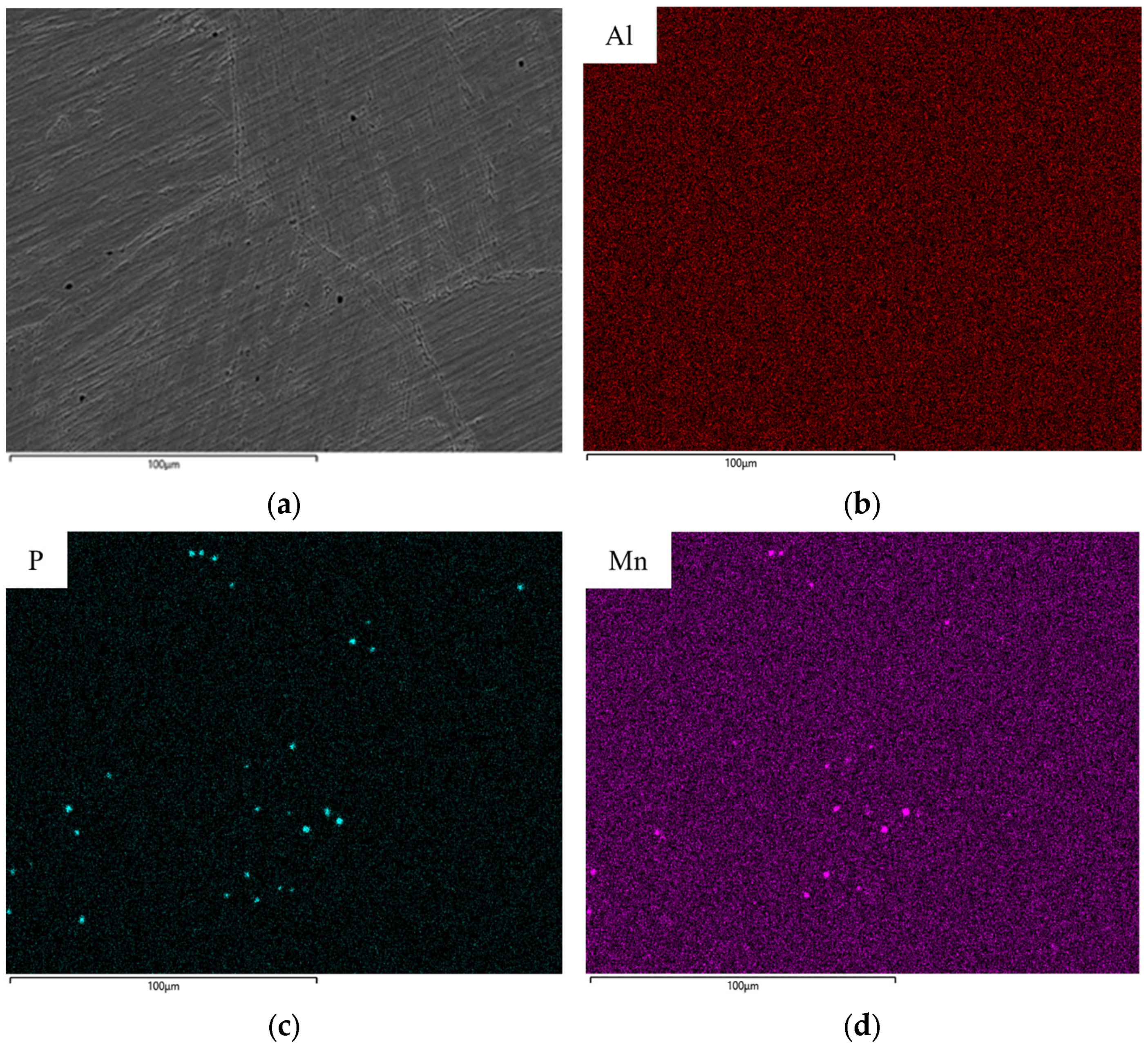
| Element | Purity [%] |
|---|---|
| Cu | 99.98 (P~0.04–0.06) |
| Al | 99.99 |
| Mn | 99.99 |
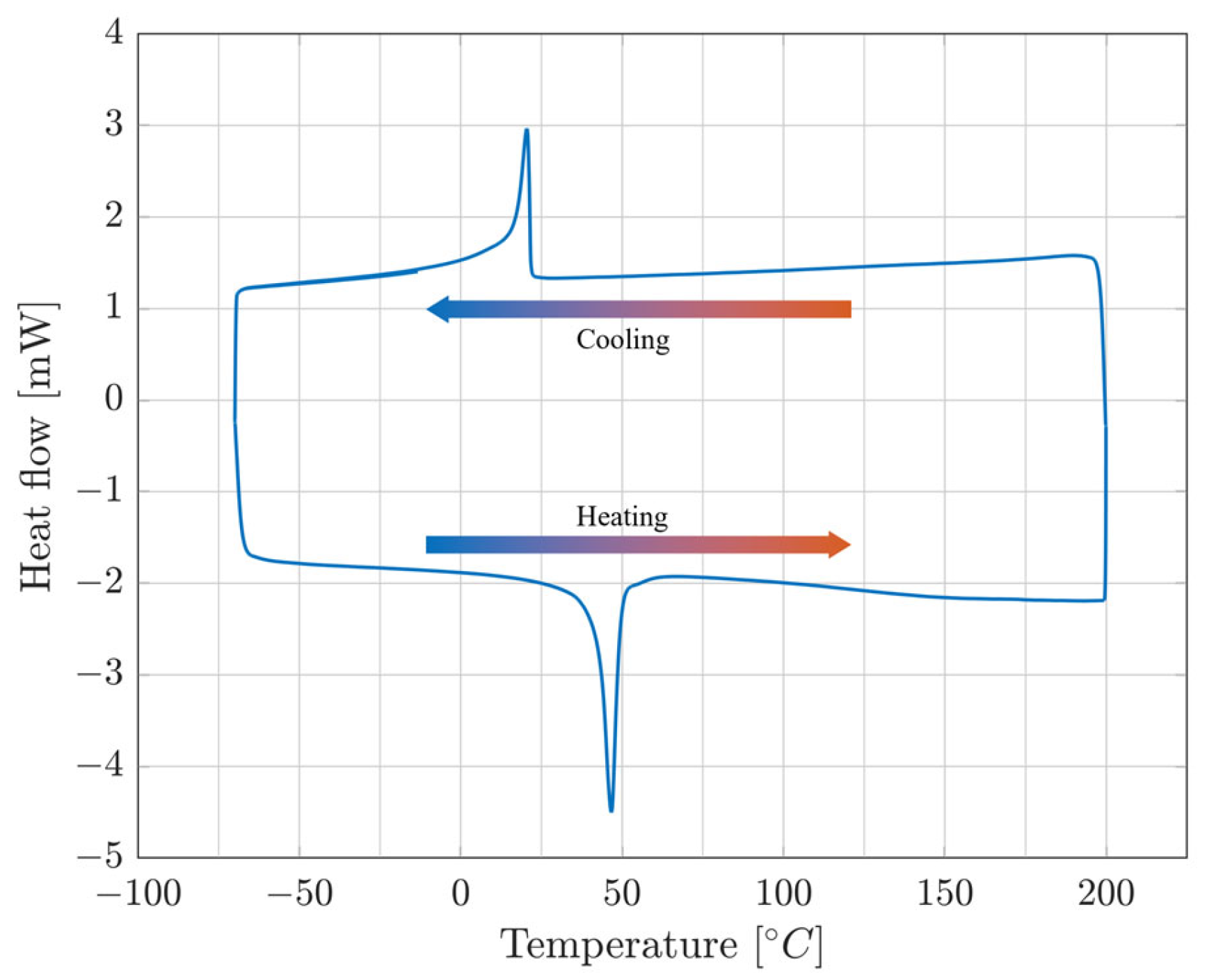
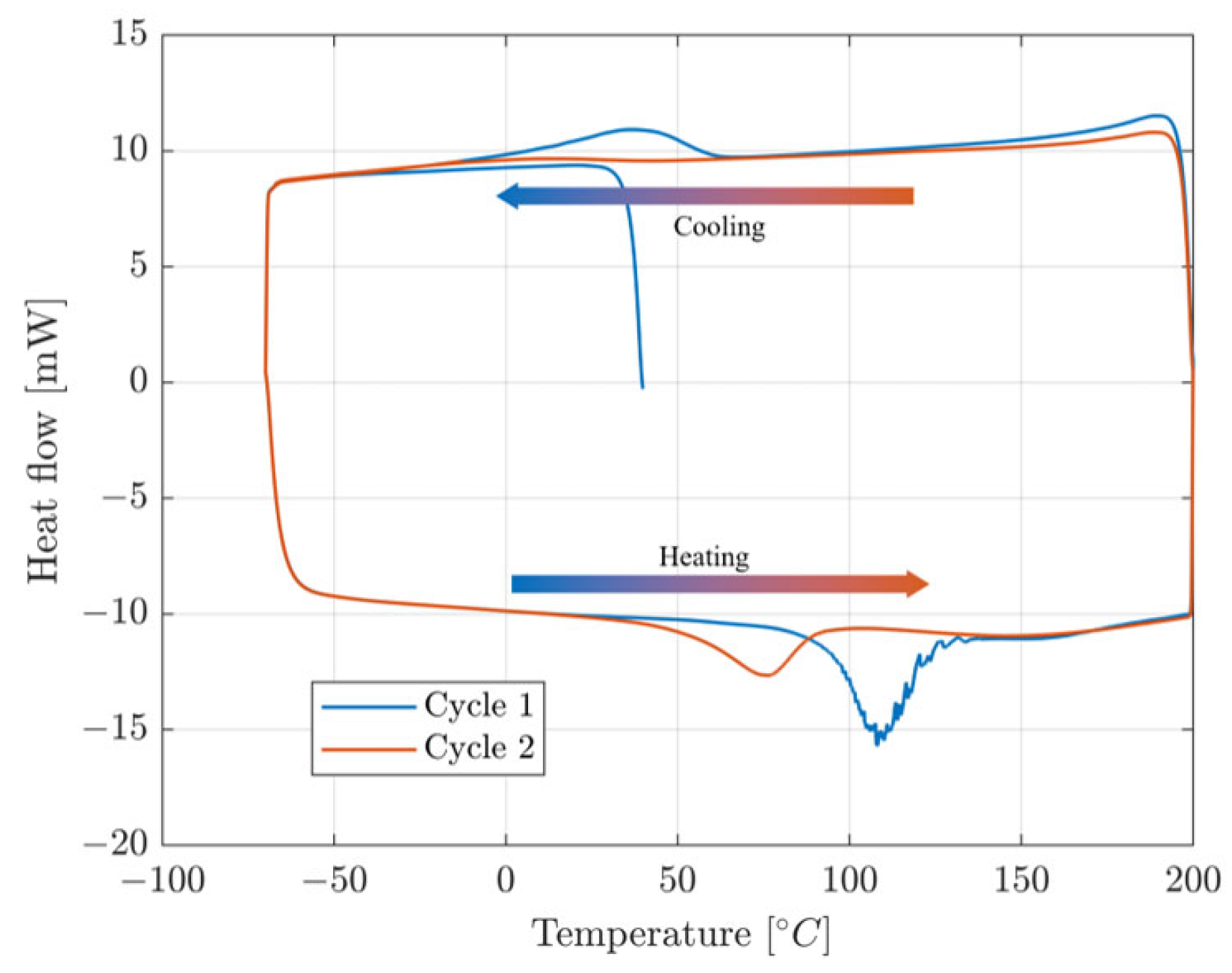
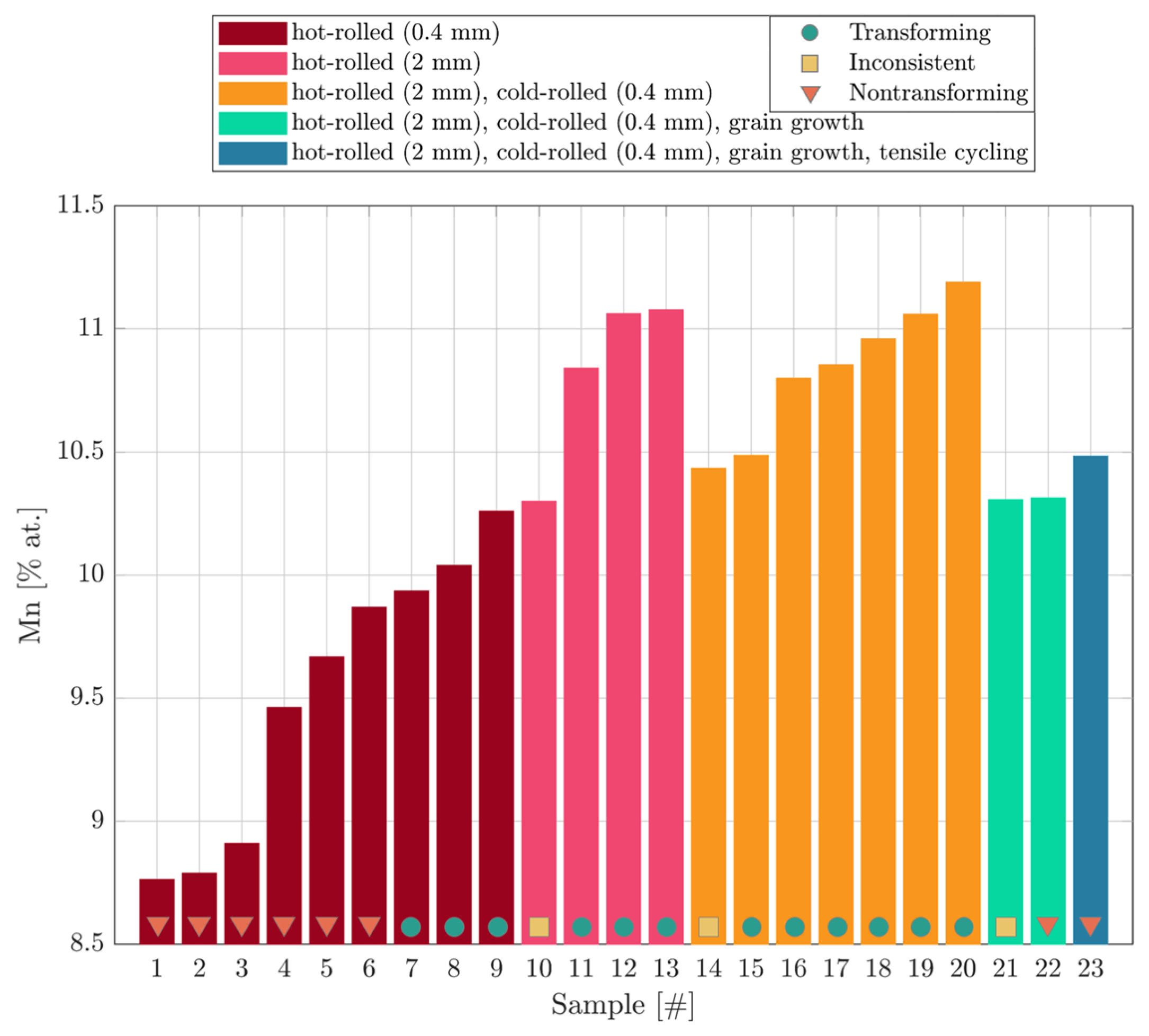
| Composition [% at.] | Transformation Temperatures [°C] | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample [%] | Mn | Al | Cu | As | Af | Ms | Mf | Treatment |
| 1 | 8.7 | 16.9 | 74.3 | nontransforming | HR1 1 | |||
| 2 | 8.8 | 17.1 | 74.1 | nontransforming | ||||
| 3 | 8.9 | 17.9 | 73.3 | nontransforming | ||||
| 4 | 9.5 | 17.8 | 72.8 | nontransforming | ||||
| 5 | 9.6 | 17.6 | 72.7 | nontransforming | ||||
| 6 | 9.9 | 17.65 | 72.5 | nontransforming | ||||
| 7 | 9.9 | 17.3 | 72.8 | 34.4 | 81.19 | / 6 | / | |
| 8 | 10.04 | 16.9 | 73.1 | 27.06 | 71.21 | / | / | |
| 9 | 10.2 | 16.9 | 73.1 | 91.69 | 109.03 | 83.23 | 66.4 | |
| 10 | 10.3 | 17 | 72.7 | 59.06 | 93.12 | 66.41 | −0.47 | HR2 2 |
| 11 | 10.8 | 16.9 | 72.2 | 35.83 | 67.19 | 59.06 | 17.85 | |
| 12 | 11.1 | 15.8 | 73.1 | 43.1 | 57.6 | 30.88 | 15.21 | |
| 13 | 11.1 | 16.9 | 72 | 43.10 7 | 57.6 | 30.88 | 15.21 | |
| 14 | 10.4 | 17.6 | 72 | 73.85 | 99.46 | 70.08 | 7.68 | HR/CR 3 |
| 15 | 10.5 | 17.5 | 72 | 56.64 | 89.97 | 65.44 | 22.1 | |
| 16 | 10.8 | 18.3 | 70.9 | 37.01 | 48.61 | 26.91 | 12.64 | |
| 17 | 10.85 | 18 | 71.1 | 41.76 | 58.82 | 21.88 | 2.82 | |
| 18 | 10.96 | 18.2 | 70.9 | 44.08 | 60.38 | 38.62 | 26.5 | |
| 19 | 11.1 | 16.4 | 72.5 | 42.15 | 49.4 | 21.74 | 16.18 | |
| 20 | 11.2 | 16.7 | 72.1 | 32.77 | 43.74 | 15.56 | 3.5 | |
| 21 | 10.3 | 18 | 71.7 | 11.01 | 28.53 | 15.41 | 2.71 | HR/CR/AGG 4 |
| 22 | 10.3 | 18.2 | 71.5 | nontransforming | ||||
| 23 | 10.5 | 17.8 | 71.8 | nontransforming | HR/CR/AGG/T 5 | |||
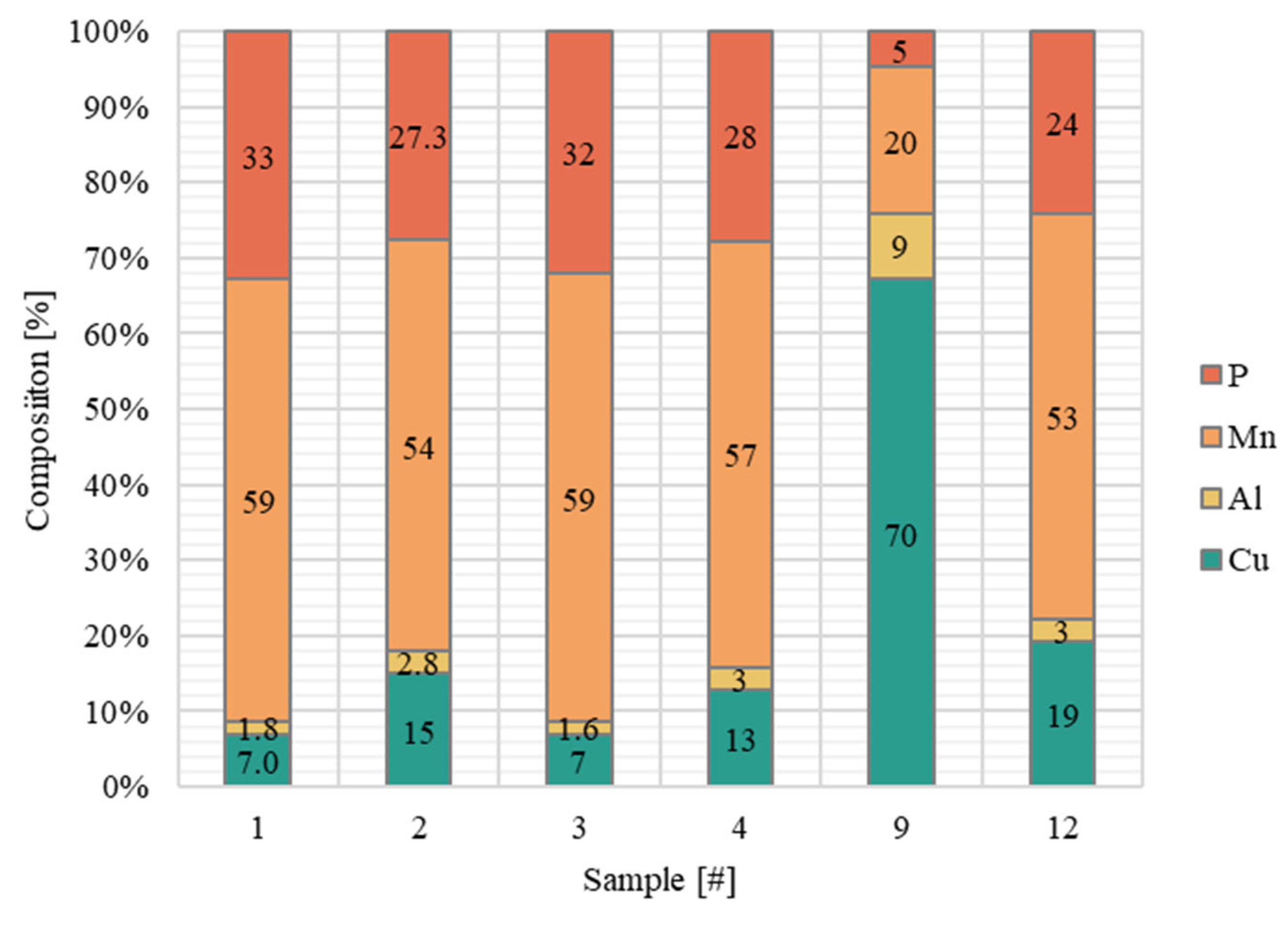
| Sample Compositions Considering Phosphorus | ||||
|---|---|---|---|---|
| Sample [#] | Mn [% at.] | Al [% at.] | Cu [% at.] | P [% at.] |
| 1 | 8.8 | 17.9 | 73.2 | 0.2 |
| 2 | 8.9 | 16.6 | 74.3 | 0.3 |
| 3 | 9.5 | 17.8 | 72.6 | 0.1 |
| 4 | 9.7 | 17.4 | 72.8 | 0.2 |
| 5 | 9.9 | 17.6 | 72.4 | 0.1 |
| 6 | 9.9 | 17.2 | 72.6 | 0.2 |
| 7 | 10.2 | 17.3 | 72.3 | 0.1 |
| 8 | 10.5 | 17.5 | 71.9 | 0.1 |
| 9 | 11.0 | 16.4 | 72.4 | 0.1 |
| 10 | 11.0 | 15.8 | 73.0 | 0.1 |
| 11 | 11.1 | 16.9 | 71.9 | 0.1 |
| 12 | 11.2 | 16.7 | 72.0 | 0.1 |
| Sample | Average Percentage Bound to P | Average Al, Mn, Cu in Solid Solution | Average Composition Change | ||||||
|---|---|---|---|---|---|---|---|---|---|
| [#] | Al | Mn | Cu | Al | Mn | Cu | Al | Mn | Cu |
| 1 | 0.011 | 0.4 | 0.04 | 18.0 | 8.4 | 74 | 0.1 | −0.4 | 1 |
| 2 | 0.031 | 0.59 | 0.165 | 16.735 | 8.39 | 74.88 | 0.135 | −0.51 | 0.38 |
| 3 | 0.0050 | 0.184 | 0.022 | 17.8505 | 9.345 | 72.804 | 0.0505 | −0.155 | 0.104 |
| 4 | 0.021 | 0.41 | 0.09 | 17.487 | 10.84 | 73.16 | 0.087 | 1.14 | 0.26 |
| 5 | 0.18 | 0.4 | 1.4 | 16.6 | 10.8 | 72.6 | 0.2 | −0.2 | 0.0 |
| 6 | 0.01250 | 0.221 | 0.079 | 16.757 | 11.025 | 72.220 | 0.057 | −0.175 | 0.120 |
| Sample | St. dev. of Percentage Bound to P | St. dev. of Al, Mn, Cu in Solid Solution | St. dev. of the Composition Change | ||||||
| [#] | Al | Mn | Cu | Al | Mn | Cu | Al | Mn | Cu |
| 1 | 0.006 | 0.1 | 0.03 | 0.5 | 0.4 | 1 | 0.9 | 0.7 | 2 |
| 2 | 0.001 | 0.01 | 0.008 | 0.004 | 0.01 | 0.02 | 0.004 | 0.01 | 0.02 |
| 3 | 0.0003 | 0.002 | 0.001 | 0.0008 | 0.002 | 0.003 | 0.0008 | 0.002 | 0.003 |
| 4 | 0.002 | 0.01 | 0.01 | 0.006 | 0.01 | 0.02 | 0.006 | 0.01 | 0.02 |
| 5 | 0.04 | 0.1 | 0.3 | 0.1 | 0.1 | 0.6 | 0.1 | 0.1 | 0.6 |
| 6 | 0.0005 | 0.004 | 0.003 | 0.002 | 0.005 | 0.009 | 0.002 | 0.005 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milosavljevic, D.; Lecis, N.; Cinquemani, S. Influence of Thermomechanical Treatments and Chemical Composition on the Phase Transformation of Cu-Al-Mn Shape Memory Alloy Thin Sheets. Appl. Sci. 2024, 14, 10406. https://doi.org/10.3390/app142210406
Milosavljevic D, Lecis N, Cinquemani S. Influence of Thermomechanical Treatments and Chemical Composition on the Phase Transformation of Cu-Al-Mn Shape Memory Alloy Thin Sheets. Applied Sciences. 2024; 14(22):10406. https://doi.org/10.3390/app142210406
Chicago/Turabian StyleMilosavljevic, Dusan, Nora Lecis, and Simone Cinquemani. 2024. "Influence of Thermomechanical Treatments and Chemical Composition on the Phase Transformation of Cu-Al-Mn Shape Memory Alloy Thin Sheets" Applied Sciences 14, no. 22: 10406. https://doi.org/10.3390/app142210406
APA StyleMilosavljevic, D., Lecis, N., & Cinquemani, S. (2024). Influence of Thermomechanical Treatments and Chemical Composition on the Phase Transformation of Cu-Al-Mn Shape Memory Alloy Thin Sheets. Applied Sciences, 14(22), 10406. https://doi.org/10.3390/app142210406







