Abstract
Betulin and α-lipoic acid are naturally occurring substances with different biological properties. Combining two phytochemical units into a conjugate is a frequently used method to obtain new compounds with better pharmacokinetic parameters. This research concerned the preparation of lipoate derivatives of betulin using the Steglich method. Experimental lipophilicity values were determined for target compounds 6–10 by reversed-phase thin-layer chromatography. In silico methods were used to calculate the physicochemical parameters and lipophilicity of new derivatives and to determine the probable directions of biological activity. α-Lipoic acid, betulin, and lipoate derivatives 6–10 were tested for antiproliferative activity against MV4-11, A549, MCF-7, PC-3, HCT116, MiaPaca-2, and Hs294T cancer cells. 3-(5-(1,2-Dithiolan-3-yl)pentanoyl))betulin 10 showed moderate anticancer activity against MV4-11, PC-3, and HCT116, with IC50 values in the range of 39.8–76.7 µM. The introduction of a dithiolate substituent at the C3 position in 28-acetylbetulin gave compound 9 the highest activity (IC50 = 37.9 µM), in the ratio of biphenotypic B myelomonocytic leukemia cells (MV4-11). All lipoate derivatives were inactive towards normal cells.
1. Introduction
Since ancient times, plants have been a source of bioactive substances used in the treatment of many diseases [1,2]. Among the organic compounds produced by plants, phytochemicals constitute a large group. Most of them are secondary plant metabolites characterized by diverse chemical structures. Secondary plant metabolites include, among others terpenoids, alkaloids, polyphenols, flavonoids, lignans, steroids, saponins, curcumins, coumarins, and glucosides. Phytochemicals can be located in various parts of plants, usually in the bark, stem, root, leaves, flowers, fruit, or seeds. These compounds play an important role in the physiological processes of plants and protect them against threats such as drought, stress, environmental pollution, UV radiation, and pathogens [3,4,5].
The structural diversity of natural compounds of plant origin determines their wide range of biological effects. Phytochemicals include substances with antioxidant, antimicrobial, anthelmintic, antidiabetic, antiallergic, antiviral, and anticancer activity [6,7,8,9,10,11,12,13].
These compounds, in their native form, are often characterized by poor solubility in an aqueous environment, which limits their bioavailability. On the other hand, they have low toxicity, which allows for the administration of higher doses. Compounds of natural origin may be an alternative in the treatment of resistant cancers and may also be used in combination therapy. Efficacy and the probability of complications and side effects are the basic parameters in therapy selection [14].
The increase in interest in phytochemicals and the decrease in the share of synthetic substances in anticancer agents results from the failure of completely synthetic products to achieve therapeutic effects and the occurrence of drug resistance [15].
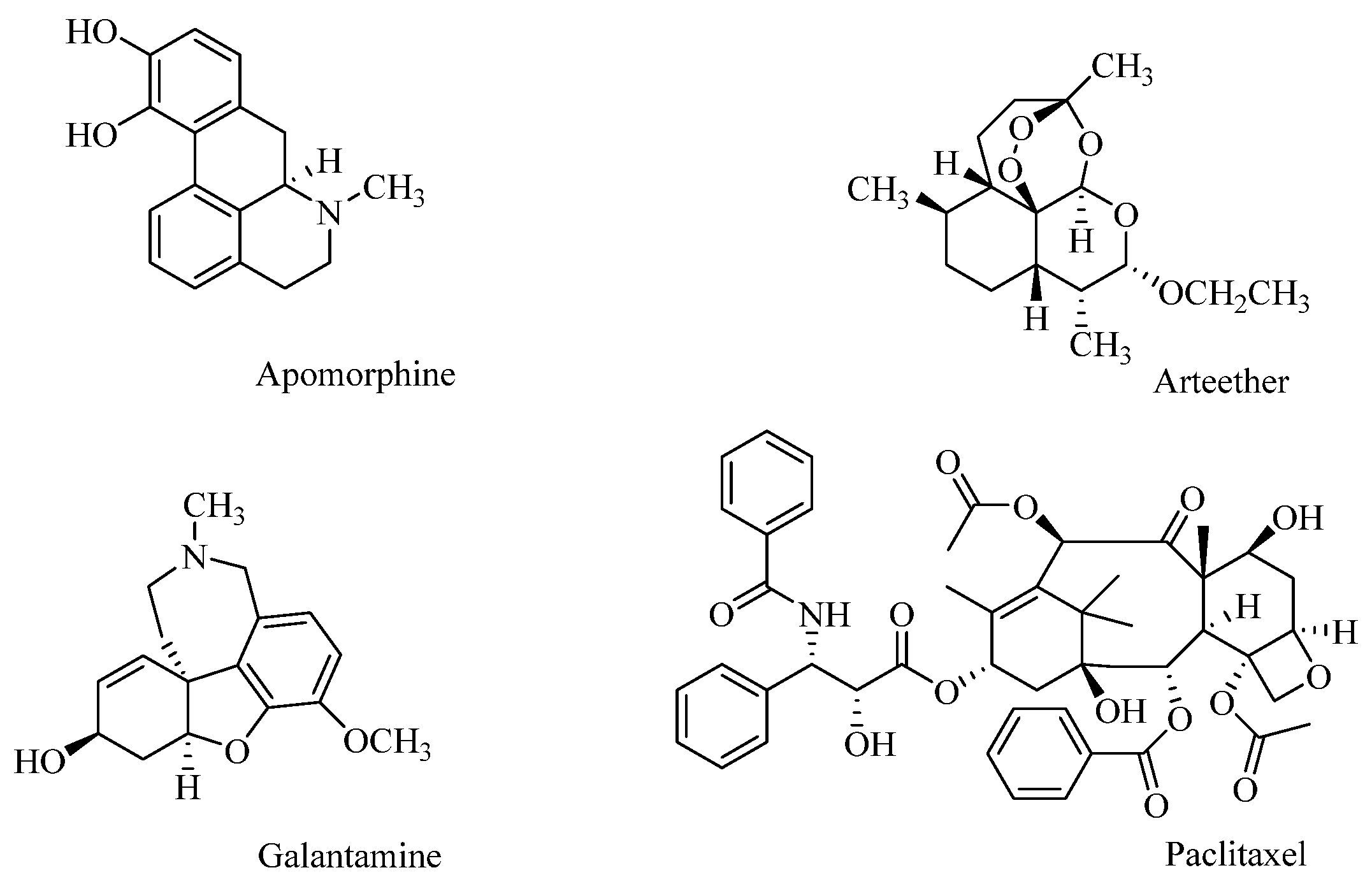
Examples of compounds of plant origin with high therapeutic potential include Apomorphine, used in Parkinson’s disease, isolated from Papaver somniferum L., and the antimalarial drug Arteether, a semi-synthetic derivative of artemisinin obtained from the species Artemisia annua. The alkaloid Galantamine isolated from Galanthus woronowii has been used to treat symptoms of Alzheimer’s disease. Research on phytochemicals for anticancer efficacy has led to the discovery of paclitaxel (isolated from Taxus brevifolia Nutt.), commonly used in the treatment of ovarian, breast, and non-small-cell lung cancer (Figure 1) [16,17].
Figure 1.
Plant-derived drugs.
Phytochemicals and derivatives obtained from them are currently a promising source of obtaining new anticancer drugs that can effectively improve the treatment of cancer patients and reduce side effects related to chemotherapy [16]. Much attention has been paid in recent years to the study of pentacyclic triterpenoids of the lupane type. Betulin, which belongs to this group of derivatives, was first isolated almost 250 years ago from birch bark [18]. Currently, numerous studies have confirmed that this compound has a wide spectrum of biological activity (anticancer, antiviral, antibacterial, and others). The structure of betulin, composed of four six-membered rings and one five-membered ring, also contains two hydroxyl groups at the C3 and C28 positions and an isopropenyl moiety at carbon 19. Structural changes at the mentioned positions obtained by chemical synthesis or biotransformation enable the transformation of betulin into derivatives with anticancer potential [19,20,21].
Isolated from liver by Reed in 1951, α-lipoic acid is an endogenous organosulfur compound synthesized de novo in mitochondria. The substrates for de novo synthesis of α-lipoic acid in the body are fatty acids and cysteine. This process takes place in the liver and other tissues, but it is not efficient enough to meet the energy needs of the cell, so this compound must also be supplied to the body from exogenous sources. A significant amount of α-lipoic acid is found in green vegetables such as spinach and broccoli. α-Lipoic acid is a cofactor of pyruvate dehydrogenase, the function of which is to convert pyruvate molecules to acetyl-CoA, which is associated with a decrease in the formation of lactate. α-Lipoic acid has significant antioxidant and anti-inflammatory effects and the ability to inhibit the growth of various human cancer cell lines such as Jurkat leukemia, squamous carcinoma (FaDu), lung cancer (H460, A549), breast cancer (MCF-7, MDA-MB-231), colorectal cancer (HT29, Caco-2, HCT116), ovarian teratocarcinoma (CH1/PA-1), and glioblastoma (U-87). The research conducted shows that α-lipoic acid can be used in combination therapy with anticancer drugs (5-fluorouracil, paclitaxel), demonstrating a synergistic effect and reducing their toxicity. Taking into account the beneficial effects and low toxicity of α-lipoic acid, it can be considered a potential candidate in the treatment of cancer [22,23,24,25,26,27].
The research carried out was related to the synthesis of α-lipoic acid conjugates with a bioactive triterpene scaffold in order to obtain new anticancer agents. In silico methods were used to determine the physicochemical parameters and predict possible directions of biological activity of the synthesized compounds. Further experimental studies were carried out to determine the lipophilicity parameter and anticancer activity of the α-lipoic esters of triterpenoids.
2. Materials and Methods
2.1. Synthesis
2.1.1. Obtaining of Compounds 1–5
The materials and methods used in the synthesis of betulin derivatives are provided in the Supplementary Materials. Triterpenoids 1–5 were synthesized from betulin based on procedures previously described in the literature [28,29,30,31,32]. The chemical structures of compounds 1–5 were confirmed by comparing their melting points and NMR (1H, 13C) spectral data with published results.
2.1.2. General Procedure for the Synthesis of the α-Lipoic Esters of Triterpenoids 6–9
An appropriate triterpenoid like betulin, 3-acetylbetulin 2,3,28-diacetyl-30-hydroxybetulin 3, or 28-acetylbetulin 5 (0.5 mmol) was dissolved in dry dichloromethane (2.5 mL), and α-lipoic acid (0.122 g, 0.59 mmol) was added. The reaction mixture was cooled to −10 °C, and was then added dropwise to a solution of 0.5 mL dichloromethane containing N,N′-dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP) in amounts of 0.14 g (0.70 mmol) and 0.015 g (0.12 mmol), respectively. The reaction mixture was slowly brought to room temperature and then stirred for 24 h. The resulting precipitate was filtered off and washed with 6 mL of dichloromethane. The solvent was distilled from the filtrate using a rotary evaporator. The obtained crude product was purified by column chromatography (SiO2; dichloromethane–ethanol, 60:1, v/v).
28-(5-(1,2-Dithiolan-3-yl)pentanoyl))betulin 6 Yield 75%; mp 82–86 °C; Rf 0.27 (dichloromethane/ethanol, 60:1, v/v); 1H NMR (600 MHz, CDCl3) δ: 0.78 (s, 3H, CH3), 0.84 (s, 3H, CH3), 0.98 (s, 3H, CH3), 0.99 (s, 3H, CH3), 1.05 (s, 3H, CH3), 1.39–1.45 (m, 6H, CH2), 1.71 (s, 3H, CH3), 1.94 (m, 1H, CH2CHS), 0.69–2.02 (m, 25H, CH, CH2), 2.37 (t, 2H, J = 7.2 Hz, CH2S), 2.48 (m, 2H, CH2CHS, H-19), 3.13 (m, 1H, H-3), 3.19 (m, 2H, CH2C=O), 3.59 (m, 1H, CHS), 3.87 (m, 1H, H-28), 4.29 (m, 1H, H-28), 4.61 (m, 1H, H-29), 4.71 (m, 1H, H-29) (Figure S1, Supplementary Materials); 13C NMR (150 MHz, CDCl3) δ: 14.1, 14.8, 15.4, 16.0, 16.1, 18.3, 19.1, 20.8, 24.8, 25.2, 27.1, 27.4, 28.0, 28.8, 29.6, 29.8, 34.2, 34.3, 34.6, 37.2, 37.6, 38.5, 38.7, 38.9, 40.2, 40.9, 42.7, 46.4, 47.7, 48.8, 50.4, 55.3, 56.4, 62.6, 78.9, 109.9, 150.2, 174.0 (Figure S2, Supplementary Materials); IR (ν max cm−1, KBr): 3419, 2939, 1734, 1456, 1259, 881 (Figure S3, Supplementary Materials); HRMS (APCI) m/z (neg): 629.4052; C38H61O3S2 (Calculated 629.4062) (Figure S4, Supplementary Materials).
3-Acetyl-28-(5-(1,2-dithiolan-3-yl)pentanoyl))betulin 7 Yield 88%; mp 99–102 °C; Rf 0.61 (dichloromethane/ethanol, 60:1, v/v); 1H NMR (600 MHz, CDCl3) δ: 0.85 (s, 3H, CH3), 0.86 (s, 3H, CH3), 0.87 (s, 3H, CH3), 0.99 (s, 3H, CH3), 1.05 (s, 3H, CH3), 1.39–1.45 (m, 6H, CH2), 1.72 (s, 3H, CH3), 1.94 (m, 1H, CH2CHS), 0.79–1.95 (m, 24, CH, CH2), 2.06 (s, 3H, CH3C=O), 2.37 (t, 2H, J = 7.2 Hz, CH2S), 2.46 (m, 2H, CH2CHS, H-19), 3.14–3.23 (m, 2H, CH2C=O), 3.59 (m, 1H, CHS), 3.87 (d, 1H, J = 10.8 Hz, H-28), 4.29 (d, 1H, J = 10.8 Hz, H-28), 4.48 (m, 1H, H-3), 4.61 (m, 1H, H-29), 4.71 (m, 1H, H-29) (Figure S5, Supplementary Materials); 13C NMR (150 MHz, CDCl3) δ: 14.7, 16.0, 16.2, 16.5, 18.2, 19.1, 20.8, 21.3, 23.7, 23.7, 24.8, 25.2, 27.1, 27.9, 28.8, 29.6, 29.8, 34.1, 34.3, 34.6, 37.1, 37.6, 37.8, 38.4, 38.5, 40.2, 40.9, 42.7, 46.4, 47.7, 48.8, 50.3, 55.4, 56.4, 62.7, 80.9, 109.9, 150.2, 171.1, 174.0 (Figure S6, Supplementary Materials); IR (ν max cm−1, KBr): 2941, 1732, 1246 (Figure S7, Supplementary Materials); HRMS (APCI) m/z (neg): 671.4119; C40H63O4S2 (Calculated 671.4167) (Figure S8, Supplementary Materials).
3,28-Diacetyl-30-(5-(1,2-dithiolan-3-yl)pentanoyl))betulin 8 Yield 68%; mp 93–95 °C; Rf 0.43 (dichloromethane/ethanol, 60:1, v/v); 1H NMR (600 MHz, CDCl3) δ: 0.86–0.87 (m, 9H, 3×CH3), 0.99 (s, 3H, CH3), 1.09 (s, 3H, CH3), 1.42–1.49 (m, 6H, CH2), 0.80–1.90 (m, 25 H, CH,CH2), 1.94 (m, 1H, CH2CHS), 2.06 (s, 3H, CH3C=O), 2.09 (s, 3H, CH3C=O), 2.38–2.51 (m, 3H, CH2S, CH2CHS, H-19), 3.13–3.23 (m, 2H, CH2C=O), 3.60 (m, 1H, CHS), 3.84 (d, 1H, J = 10.8 Hz, H-28), 4.26 (d, 1H, J = 10.8 Hz, H-28), 4.48 (m, 1H, H-3), 4.58 (m, 2H, H-30), 4.96 (s, 1H, H-29), 4.98 (s, 1H, H-29) (Figure S9, Supplementary Materials); 13C NMR (150 MHz, CDCl3) δ: 14.8, 16.0, 16.2, 16.5, 18.2, 20.9, 21.1, 21.3, 23.7, 23.7, 24.7, 26.6, 27.0, 28.0, 28.8, 29.7, 31.1, 34.1, 34.4, 34.7, 37.1, 37.5, 37.8, 38.4, 38.5, 40.2, 40.9, 42.7, 43.8, 46.3, 49.5, 50.2, 55.4, 56.3, 62.5, 66.0, 80.9, 110.6, 148.9, 171.0, 171.6, 173.2 (Figure S10, Supplementary Materials); IR (ν max cm−1, KBr): 2943, 1734, 1458, 1244, 898 (Figure S11, Supplementary Materials); HRMS (APCI) m/z (neg): 729.4164; C42H65O6S2 (Calculated 729.4222) (Figure S12, Supplementary Materials).
3-(5-(1,2-Dithiolan-3-yl)pentanoyl))-28-acetylbetulin 9 Yield 55%; mp 89–92 °C; Rf 0.65 (dichloromethane/ethanol, 60:1, v/v); 1H NMR (600 MHz, CDCl3) δ: 0.86–0.87 (m, 9H, 3×CH3), 0.99 (s, 3H, CH3), 1.05 (s, 3H, CH3), 1.40–1.51 (m, 6H, CH2), 1.70 (s, 3H, CH3), 0.80–1.90 (m, 24H, CH, CH2), 1.93 (m, 1H, CH2CHS), 2.09 (s, 3H, CH3C=O), 2.32 (t, 2H, J = 7.2 Hz, CH2S), 2.33–2.51 (m, 2H, CH2CHS, H-19), 3.13–3.23 (m, 2H, CH2C=O), 3.60 (m, 1H, CHS), 3,87 (d, 1H, J = 10.8 Hz, H-28), 4.27 (d, 1H, J = 10.8 Hz, H-28), 4.49 (m, 1H, H-3), 4.61 (m, 1H, H-29), 4.71 (m, 1H, H-29) (Figure S13, Supplementary Materials); 13C NMR (150 MHz, CDCl3) δ: 14.7, 16.0, 16.2, 16.6, 18.2, 19.1, 20.8, 21.1, 23.7, 24.9, 25.2, 27.1, 28.0, 28.8, 29.6, 29.7, 34.1, 34.5, 34.6, 34.7, 37.1, 37.6, 37.9, 38.4, 38.5, 40.2, 40.9, 42.7, 46.3, 47.7, 48.8, 50.3, 55.4, 56.4, 62.8, 80.8, 109.9, 150.2, 171.7, 173.3 (Figure S14, Supplementary Materials); IR (ν max cm−1, KBr): 2943, 1735, 1456, 1234, 883 (Figure S15, Supplementary Materials); HRMS (APCI) m/z (neg): 671.4287; C40H63O4S2 (Calculated 671.4168) (Figure S16, Supplementary Materials).
2.1.3. Preparation of Compound 10
The 28-tetrahydropyranyl ether of betulin 4 (0.91 mmol) was dissolved in dry dichloromethane (15 mL), and α-lipoic acid (0.24 g, 1.19 mmol) was added. The reaction mixture was cooled to −10 °C, and a solution of DCC (0.28 g, 1.40 mmol) and DMAP (0.03 g, 0.24 mmol) in 1 mL dichloromethane was added dropwise. The reaction mixture was slowly brought to room temperature and then stirred for 24 h. The resulting precipitate was filtered. The solvent was distilled from the filtrate using a rotary evaporator. The obtained intermediate (without isolation) was reacted with pyridinium p-toluenesulfonate (0.23 g, 0.95 mmol) in anhydrous ethanol (19 mL). The reaction mixture was stirred at room temperature for 7 days. Ethanol was distilled off using a rotary evaporator. The resulting residue was dissolved in dichloromethane (10 mL), and then washed with saturated sodium bicarbonate solution (5 mL) and water (10 mL). After drying the extract with anhydrous sodium sulfate, the solvent was distilled off. The crude product was purified by column chromatography (SiO2; dichloromethane–ethanol, 60:1, v/v).
3-(5-(1,2-Dithiolan-3-yl)pentanoyl))betulin 10 Yield 48%; mp 102–105 °C; Rf 0.32 (dichloromethane/ethanol, 60:1, v/v); 1H NMR (600 MHz, CDCl3) δ: 0.86–0.87 (m, 9H, 3×CH3), 1.00 (s, 3H, CH3), 1.04 (s, 3H, CH3), 1.42–1.52 (m, 6H, CH2), 1.71 (s, 3H, CH3), 1.93 (m, 1H, CH2CHS), 0.80–2.01 (m, 25H, CH,CH2), 2.35 (t, 2H, J = 7.2 Hz, CH2S), 2.41 (m, 1H, CH2CHS), 2.48 (m, 1H, H-19), 3.12–3.22 (m, 2H, CH2C=O), 3.35 (d, 1H, J = 10.8 Hz, H-28), 3.59 (m, 1H, CHS), 3.82 (d, 1H, J = 10.8 Hz, H-28), 4.50 (m, 1H, H-3), 4.60 (s, 1H, H-29), 4.70 (m, 1H, H-29) (Figure S17, Supplementary Materials); 13C NMR (150 MHz, CDCl3) δ: 14.7, 16.0, 16.2, 16.6, 18.2, 19.1, 20.9, 23.8, 24.9, 24.9, 25.2, 27.0, 28.0, 28.8, 29.2, 29.7, 34.0, 34.2, 34.6, 37.1, 37.3, 37.9, 38.4, 38.5, 40.2, 40.9, 42.7, 47.8 47.8, 48.8, 50.3, 55.4, 56.4, 60.6, 80.8, 109.7, 150.5, 173.3 (Figure S18, Supplementary Materials); IR (ν max cm−1, KBr): 3481, 2939, 1730, 1456, 1247, 881 (Figure S19, Supplementary Materials); HRMS (APCI) m/z (neg): 629.4153; C38H61O3S2 (Calculated 629.4062) (Figure S20, Supplementary Materials).
2.2. Experimental Lipophilicity
The lipophilicity of betulin derivatives was experimentally determined by reversed-phase thin-layer chromatography (RP-TLC). Aluminum plates covered with modified silica gel RP-18 with fluorescent indicator F254s (Merck, Darmstadt, Germany) were used. α-Lipoic acid, betulin, derivatives 2, 3, 5–10, and standard substances (presented in Table S1, Supplementary Materials) were dissolved in chloroform (concentration 1 mg/mL). In total, 2 µL of the obtained solutions were placed on chromatographic plates and then developed in chromatographic chambers saturated with eluent vapors. Mixtures of acetone (Merck, Darmstadt, Germany) and Tris buffer (aqueous solution of tris-hydroxymethyl)aminomethane (Merck, Darmstadt, Germany) with a concentration of 0.2 M, (pH 7.4) were used as mobile phases in volume proportions, taking into account the acetone content from 60 to 90% (every 5%). Chromatographic determinations were performed in triplicate for each compound. Chromatograms of betulin and its derivatives were sprayed with a solution of 10% sulfuric acid in ethanol, and then the plates were heated to 110 °C until the spots of the analyzed compounds were visible. Chromatograms with standard compounds were observed under UV light at a wavelength of λ = 254 nm. Spots on chromatograms with α-lipoic acid were visualized in iodine vapor. For all substances in each eluent system, the distances from the starting line to the center of the spot (a) and from the starting line to the eluent front line (b) were measured, and on this basis, the retardation coefficient was calculated (Rf = a/b). Then, the value of the RM coefficient was calculated based on Equation (1): RM = log [(1/Rf) − 1]. The dependence of the RM parameter on the percentage of acetone in the mobile phase is described by Equation (2): RM = RM0 + bC, where b is the slope of the regression graph. Extrapolating the acetone content in the eluent system to zero allows us to determine chromatographic lipophilicity parameter RM0. Seven standard compounds (acetanilide, prednisone, 4-bromoacetophenone, benzophenone, anthracene, dibenzyl, DDT- dichlorodiphenyltrichloroethane) were used to determine the calibration curve (dependence of logPlit. on RM0, Supplementary Materials). The literature logPlit values of these compounds are in the range of 1.21–6.38 [33]. RM0 values for the references and α-lipoic acid were determined analogously to those for the tested betulin derivatives (Table S1, Supplementary Materials).
2.3. In Silico Study
Carrying out analyses using in silico methods required the transformation of the chemical structures of the tested compounds into SMILES codes (simplified molecular linear input system) using the Chem Draw program (Perkin Elmer Informatics, Waltham, MA, USA). The theoretical values of the logP parameter (XLOGP2, MLOGP, ALOGPs, ACLogP, ALOGP, and XLOGP3) were calculated using the program VCCLAB [34]. The parameters necessary to assess the similarity of drugs and predict the biological activity of the tested betulin derivatives were calculated using SwissADME (Swiss Institute of Bioinformatics, Lausanne, Switzerland) [35] and Way2Drug (PASS Online software version 2.0) [36].
2.4. Biological Evaluation
2.4.1. Cell Lines and Cultured Mediums
All the cell lines were maintained at the Hirszfeld Institute of Immunology and Experimental Therapy, PAS, Wroclaw, Poland. Human cell lines (leukemia MV4-11, pancreas cancer MiaPaca-2 cells, melanoma Hs294T cells, colon cancer HCT116, and normal epithelial cells from mammary gland (MCF-10A)) were obtained from American Type Culture Collection (ATCC, Rockville, MD, USA). From the European Collection of Authenticated Cell Cultures (Culture Collections UK Health Security Agency, Porton Down, Salisbury, UK), human lung carcinoma A549, breast cancer MCF-7, and prostate adenocarcinoma PC-3 cell lines were obtained.
MV4-11, Hs294T, and PC-3 cells were cultured in RPMI 1640 medium (Hirszfeld Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, Wrocław, Poland) with an addition of 1.0 mM sodium pyruvate (only MV4-11), 4.5 g/L glucose (only Hs294T) and 10% fetal bovine serum (FBS) (all from Merck, Darmstadt, Germany). A549 cells were cultured in RPMI 1640+Opti-MEM (1:1) (Hirszfeld Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, Wrocław, Poland and Gibco, Paisley, UK), supplemented with 5% FBS (Merck). The MCF-7 cells were cultured in Eagle medium (HIIET PAS, Poland) supplemented with 10% FBS, 8 µg/mL of insulin and 1% of MEM NON-Essential amino acid (all Merck). HCT116 cells were cultured in McCoy’s 5A medium (Gibco, Paisley, UK) supplemented with 10% FBS (Merck, Germany). MiaPaca-2 cells were cultured in Dulbecco medium (Gibco) supplemented with 10% FBS and 2.5% horse serum (HS) (all from Merck, Darmstadt, Germany). MCF-10A cells were cultured in Ham’s F12 medium (Gibco, UK) supplemented with 7.5% HS, 20 ng/mL of EGFh, 10 µg/mL of insulin, 0.5 µg/mL of Hydrocortisone, and 0.05 mg/mL of Cholera Toxin (from Vibrio cholerae) (all from Merck, Darmstadt, Germany). All culture media were supplemented with 2 mM L-glutamine (Merck, Germany), 100 units/mL penicillin, (Polfa Tarchomin S.A., Tarchomin, Poland) and 100 µg/mL streptomycin (Merck, Darmstadt, Germany). All cell lines were grown at 37 °C under a 5% CO2 humidified atmosphere.
2.4.2. MTT Assay
This technique was applied for the cytotoxicity screening against leukemia cells growing in suspension culture. An assay was performed after 72 h of exposure to varying concentrations of the tested agents. For the last 4 h of incubation, 20 µL of MTT solution was added to each well (MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; stock solution: 5 mg/mL, Merck, Darstadt, Germany). The mitochondria of viable cells reduce the pale yellow MTT to a navy-blue formazan: the more viable cells present in the well, the more MTT will be reduced to formazan. At the end of the incubation time, 80 µL of the lysing mixture was added to each well (lysing mixture: 225 mL dimethylformamide (Avantor, Gliwice, Poland), 67.5 g sodium dodecyl sulfate (Merck), and 275 mL of distilled water). After 24 h, when formazan crystals had been dissolved, the optical densities of the samples were read on a Synergy H4 photometer (BioTek Instruments, Winooski, VT, USA) at a 570 nm wavelength. The background optical density was measured in the wells filled with culture medium, without the cells [37].
2.4.3. SRB Assay
This technique was applied for the cytotoxicity screening against cells growing in adherent culture. The cytotoxicity assay was performed after 72 h of exposure of the cultured cells to varying concentrations of the tested agents using an authomatic Washer dyspenser EL406, BioTek (Agilent Technologies, Santa Clara, CA, USA). In the first step, the washer dyspenser collected 40 µL of cultured medium from each well, and then the cells attached to the plastic were fixed by gently layering 20 µL per well of cold 25% TCA (trichloroacetic acid) on the top of the culture medium in each well. The plates were incubated for 40 min and then washed five times with tap water. The cellular material fixed with TCA was stained with 20 µL per well of 0.1% sulforhodamine B (SRB, Merck) dissolved in 1% acetic acid (Avantor, Poland) for 30 min. Unbound dye was removed by rinsing (4×) with 1% acetic acid. The protein-bound dye was extracted by 30 min. using 70 µL per well of 10 mM unbuffered Tris base (Merck, Germany) for determination of optical density (at 540 nm) on Synergy H4 photometer (BioTek Instruments, Winooski, VT, USA). The background optical density was measured in the wells filled with culture medium, without the cells [37].
2.4.4. Determination of Antiproliferative Activity
The solutions of tested compounds were prepared by dissolving the substances in dimethyl sulfoxide (DMSO) to the desired concentration (10 mg/mL). Then, the tested compounds were diluted in culture medium to reach the final concentrations. Before adding the tested compounds (24 h prior), the cells were plated in 384-well plates (Greiner Bio One, Kremsmünster, Austria) or in 96-well plates (MV4-11 cell; Sarstedt, Nümbrecht, Germany) at a density of 1 × 103, 2 × 103 (MCF-10A) or 5 × 103 (MV4-11) cells per well. The assay was performed after 72 h of exposure to tested agents at a concentration in the range of 100–0.03 µg/mL. The in vitro cytotoxic effect of all agents was examined using the MTT (MV4-11) or SRB assay, described previously [37]. The results were calculated as an IC50 (inhibitory concentration of 50%) for the concentration of the tested agent, which is cytotoxic for 50% of the cancer cells. IC values were calculated separately for each experiment using the Prolab-3 system, which is based on Cheburator 0.4 software [37]. Each compound in each concentration was tested in triplicate in a single experiment, which was repeated 3–5 times. Doxorubicin was used as a reference compound. The IC50 values in µg/mL were calculated in µM, and the mean values ± SD are presented.
2.5. Mechanistic Studies for Compound 9
2.5.1. Apoptosis Determination by Annexin V Staining
The MV4-11 cells were seeded at a density of 2.25 × 105 (for 24 h) or 0.75 × 105 (for 72 h) cells/well on 12-well plates (Greiner Bio-One) in culture medium to the final volume of 3 mL. The cells were exposed for 24 or 72 h to compound 9 at the following concentrations: 55 µM and 75 µM (about 1.5 × IC50 and 2 × IC50 value). Campthotecin at a concentration of 0.05 µM was used as a positive control. After incubation, the cells were collected and counted, and 1 × 105 of cells were washed twice with PBS and then suspended in 100 µL of binding buffer (Hepes buffer: 10 mM HEPES/NaOH, pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, (IIET, Wrocław, Poland)) with 4 µL of APC-Annexin V (Becton Dickinson, Pharmingen, Franklin Lakes, NJ, USA). After 15 min of incubation in the dark at room temperature, prior to the analysis, propidium iodide (PI) solution was added (final concentration of 4 µg/mL). Data acquisition was performed by flow cytometry using a BD LSRFortessa cytometer (BD Bioscience, San Jose, CA, USA). Compounds at each concentration and combination were tested at least three times independently. The results were analyzed using Flowing software 2.5.1 (Turku, Finland). The data were displayed as a two-color dot plot with an APC-Annexin V (AnV) vs. PI. Double-negative cells were live cells, PI+/AnV+ cells were late apoptotic, PI−/AnV+ cells were early apoptotic, and PI+/AnV− cells were necrotic.
2.5.2. Cell Cycle Analysis
The MV4-11 cells were seeded at a density of 3 × 105 (for 24 h) or 1 × 105 (for 72 h) cells/well on 6-well plates (Greiner Bio-One) in culture medium to the final volume of 4 mL. The cells were exposed to compound 9 at a concentration of 55 µM (about 1.5 × IC50) by 24 h or 72 h. After incubation, the cells were collected, and 1 × 106 of cells were washed twice in cold PBS and fixed for 24 h in 70% ethanol at −20 °C. Then, the cells were washed twice in PBS and were incubated with RNAse (8 μg/mL, Thermo Scientific, Waltham, MA, USA) at 37 °C for 1 h. The cells were stained for 30 min. with propidium iodide (50 μg/mL, Merck, Germany) at 4 °C, and the cellular DNA content was analyzed by flow cytometry using a BD LSRFortessa cytometer (BD Bioscience, USA). The obtained results were analyzed using Flowing software 2.5.1 (Turku, Finland).
2.5.3. Statistical Analysis
To indicate significant differences, Kruskal–Wallis tests were performed to compare two groups using GraphPad Prism 7. A statistically significant difference was considered at p ≤ 0.05.
3. Results and Discussion
3.1. Chemistry
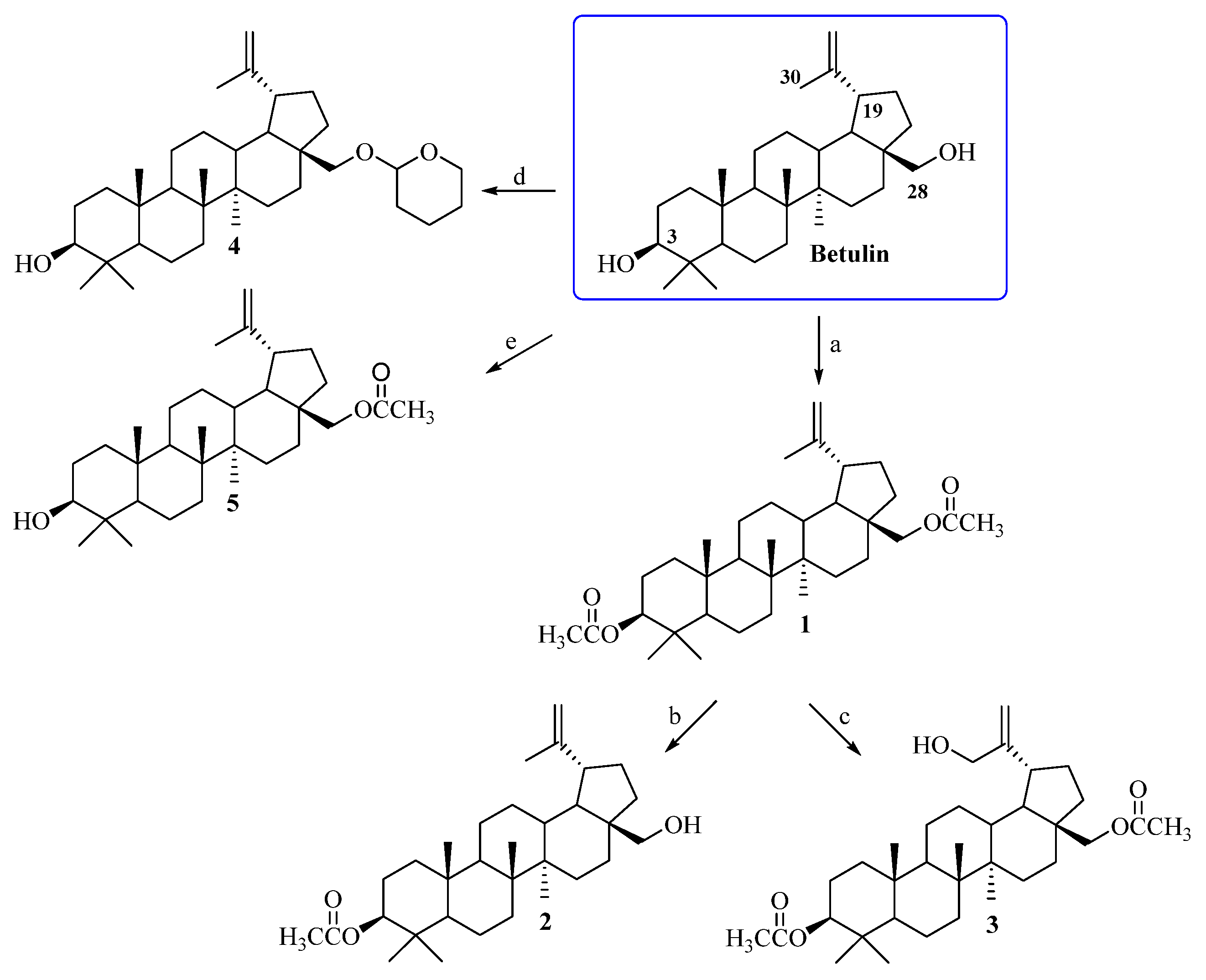
Betulin, a pentacyclic lupane-type triterpene, occurs in many plant species. The largest amounts, of up to 20–30%, of this natural bioactive substance are isolated from the outer bark of birches [38]. Obtaining the target compounds required a preliminary modification of the triterpenoid skeleton of betulin at the C3, C28, and C30 positions (Scheme 1). In the first step, the C3 and C28 hydroxyl groups of betulin were subjected to an acylation reaction with acetic anhydride ((CH3CO)2O) in pyridine, leading to 3,28-diacetylbetulin 1 [28]. Then, compound 1 was transformed into 3-acetylbetulin 2 as a result of the alkaline hydrolysis reaction in a 0.2 M solution of sodium hydroxide in CH3OH/THF/H2O (1:2:1, v/v) [29]. 3,28-Diacetylbetulin 1 was also reacted with 3-chloroperbenzoic acid (m-CPBA) in chloroform at the 60 °C to yield 3,28-diacetyl-30-hydroxybetulin 3 [30]. Betulin was also a substrate for the synthesis of 28-tetrahydropyranyl ether 4 and 28-acetylbetulin 5 according procedures described by Pohjala and Tietze [31,32] (Scheme 1). Changing the conditions of the betulin acylation reaction allowed us to obtain 28-acetylbetulin 5. Acylation with acetic anhydride in pyridine medium enables the reaction of both the primary (C28) and secondary (C3) hydroxyl groups (obtaining compound 1). On the other hand, the use of chloroform as a solvent in the acylation reaction and the presence of imidazole as a catalyst allowed us to obtain monoacetylated derivative 5. The chemical structures of substrates 1–5 were confirmed on the basis of NMR spectra (1H, 13C) and compared with the relevant data described in the literature [28,30,31,32,39].
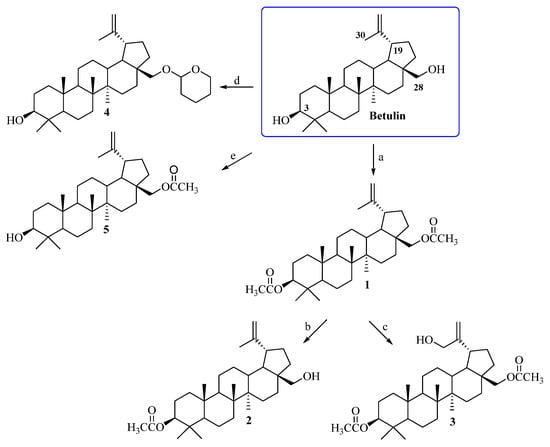
Scheme 1.
Synthesis of substrates 1–5. Conditions: a. (CH3CO)2O, pyridine, room temp., 18 h; b. 0.2 M solution of sodium hydroxide, CH3OH: THF: H2O (1:2:1, v/v), room temp., 30 min.; c. 3-chloroperbenzoic acid (m-CPBA), CHCl3, reflux, 8 h; d. dihydropyran (DHP), pyridinium p-toluenesulfonate (PPTS), CH2Cl2, room temp., 3 days; e. (CH3CO)2O, imidazole, chloroform, 50 °C, 1 h.
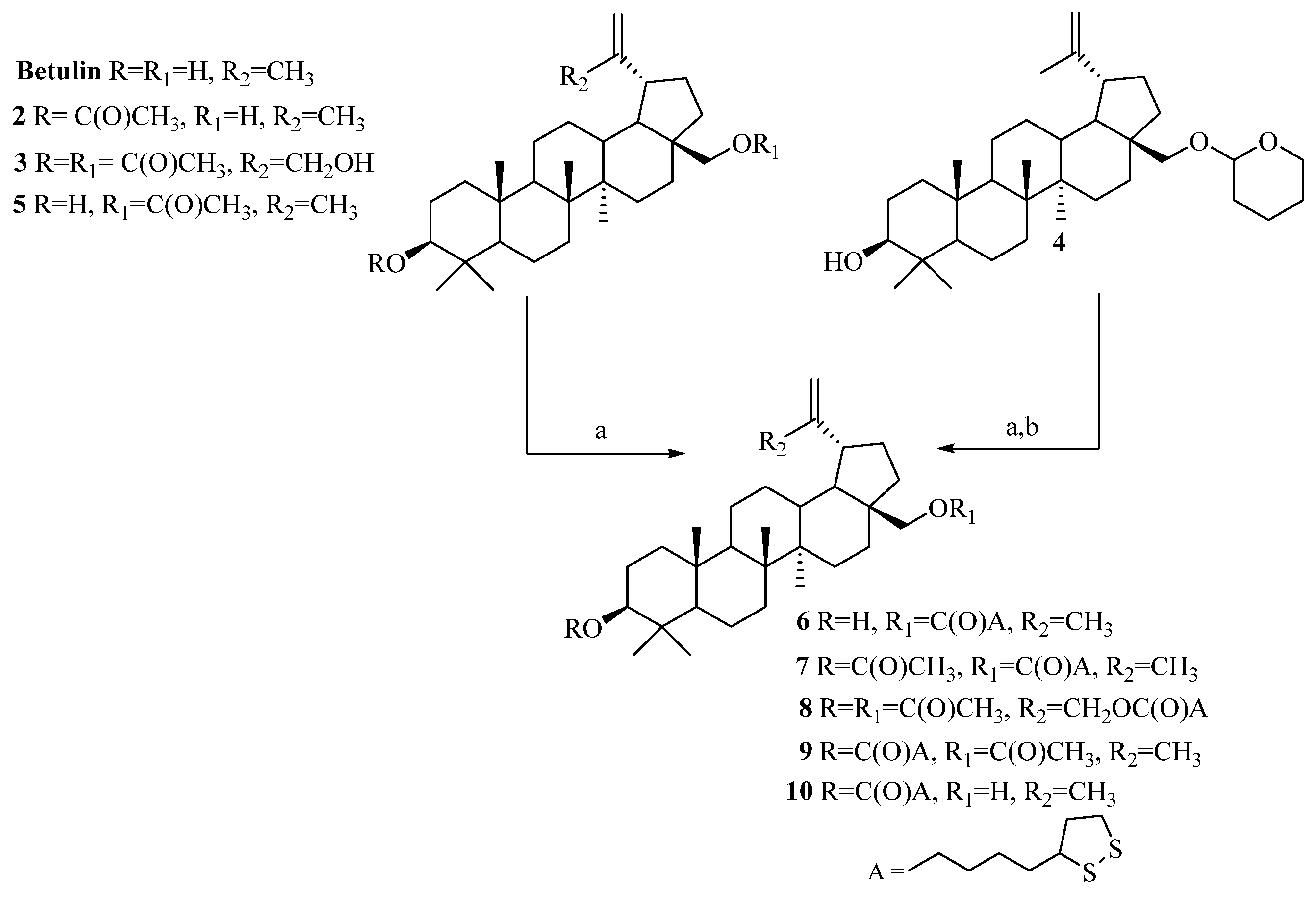
The α-lipoic esters of triterpenoids 6–10 were obtained using the Steglich method. In this procedure, betulin and triterpenoids 2–5 were treated with α-lipoic acid in the presence of DCC and DMAP in dry dichloromethane.
After purification by column chromatography, target compounds 6–10 were obtained in a 48–88% yield. The chemical structures of α-lipoic acid conjugates with a triterpenoids 6–10 were confirmed based on 1H NMR, 13C NMR, IR, and HRMS spectra (Supplementary Materials, Figures S1–S20). The synthesis (reagents and conditions) of compounds 6–10 is presented in Scheme 2.
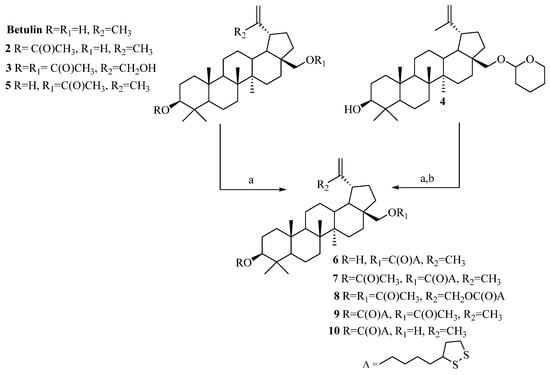
Scheme 2.
Synthesis of α-lipoic esters of triterpenoids 6–10. Conditions: a. α-lipoic acid, N,N′-dicyclohexylcarbodiimide (DCC), 4-aminopyridine (DMAP), CH2Cl2, from −10 °C to room temp., 24 h; b. pyridinium p-toluenesulfonate (PPTS), ethanol, room temp., 7 days.
3.2. Lipophilicity—Experimental and Theoretical Studies
Lipophilicity is one of the most important physicochemical parameters related to the pharmacodynamic, pharmacokinetic, and toxic properties of medicinal substances. It is of key importance in the initial stage of developing new drugs and plays a role in binding ligands to proteins and determining the ADMET (absorption, distribution, metabolism, excretion, toxicity) characteristics that are the basis for determining the therapeutic dose. These properties affect the passive permeability of the membrane, which determines the drug absorption process after oral administration, as well as the possibility of access to intracellular areas and penetration into tissues. Determining theoretical and experimental lipophilicity values allows for the design of compounds characterized by appropriate potency obtained using a low dose of the drug [40].
Lipophilicity is most often presented as the decimal logarithm of the partition coefficient P (log P), which expresses the affinity of the active compound molecules for the organic and aqueous phases. Reversed-phase thin-layer chromatography (RP-TLC) is a commonly used chromatographic method for determining the lipophilicity of drug-like compounds. This method is characterized by the ability to analyze a large number of compounds in a relatively short time [41].
Chromatographic lipophilicity parameters RM0 for α-lipoic acid, betulin, 3-acetylbetulin 2, 3,28-diacetyl-30-hydroxybetulin 3, 28-acetylbetulin 5, and the α-lipoic esters of triterpenoids 6–10 were determined using the RP-TLC method. The value of the RM0 parameter of the tested compounds was calculated from the retardation factor (Rf) according to Equations (1) and (2) described in Section 2.2. RM0 and b in Equation (2) represent the intercept and slope of the simple linear regression between acetone concentration (C) and RM, respectively. The RM0 and log Plit values of the reference substances were used to establish a calibration curve (Supplementary Materials, Table S1), based on which the experimental log PTLC values of α-lipoic acid, betulin, and derivatives 2–3, 5–10 were calculated. The experimentally determined values of lipophilicity (RM0, log PTLC) are presented in Table 1.

Table 1.
Experimentally determined values of lipophilicity (RM0, log PTLC) for α-lipoic acid, betulin, and derivatives 2–3, 5–10.
The values of log PTLC of the α-lipoic esters of triterpenoids 6–10 are in the range of 7.66–9.34. The most lipophilic compounds are diesters 7 and 9 with an acetyl and (1,2-dithiolan-3-yl)pentanoyl groups at the C3 and/or C28 position. The introduction of the (1,2-dithiolan-3-yl)pentanoyl moiety at the C30 position into the structure of 3,28-diacetyl-30-hydroxybetulin 3 (log PTLC = 7.25) leads to an increase in the lipophilicity parameter of derivative 8 (log PTLC = 8.12). The lowest lipophilic properties from the group of α-lipoate esters were found for compound 6 (log PTLC = 7.66), with a hydroxyl group at the C3 carbon atom. From six computer programs, in the case of the analog of compound 6 with a hydroxyl group at position C28 (derivative 10), an increase in the log PTLC value is visible (log PTLC = 8.29).
The theoretical lipophilicity values (log P) of the tested compounds were obtained (XLOGP2, MLOGP, ALOGPs, ACLogP, ALOGP, and XLOGP3) based on various computational algorithms. The values of log P ranged from 7.00 to 11.45 (Table 2). This indicates agreement with the results obtained from the RP-TLC method. For triterpenoids 6–10, the lowest lipophilicity log P values were obtained using ALOGPs, while the highest log P values were obtained using XLOGP2 and XLOGP3.

Table 2.
Theoretical lipophilicity parameters (log P) for α-lipoic acid, betulin, and derivatives 2–3, 5–10 calculated using online VCCLAB database [34].
The last stage of the lipophilicity analysis concerned a comparison of the theoretical log p values of the tested compounds. The highest correlation coefficient was obtained by comparing the theoretical values of XLOGP2 and XLOGP3 (R = 0.999, Table S2, Supplementary Materials). Furthermore, experimental log PTLC values were correlated with theoretically calculated log p values using linear regression analysis. The obtained correlation coefficients are presented in Table S2 (Supplementary Materials). The best correlation coefficient was obtained by comparing the log PTLC with the values calculated from XLOGP2 (R = 0.861).
3.3. Computational Studies
3.3.1. Drug-Likeness Properties
Currently, the discovery of new medicinal substances is associated with the use of in silico methods estimating the similarity of a chemical compound to a drug. Drug-likeness assessments are useful at the initial stage of testing active substances by determining their pharmacokinetic properties and safety of use. Lipinski’s rule of five (RO5), introduced in the 20th century, is used to assess compounds absorbed after oral administration. RO5 is based on simple molecular properties, assuming that compounds exhibit poor absorption or permeation when the MW is over 500; the Log P is over 5 or MLOGP is over 4.15; and there are over 10 hydrogen bond acceptors (HAs) and over 5 hydrogen bond donors (HDs). In addition to RO5, other methods of testing the reliability of drugs, proposed by Ghose and Veber were introduced [42,43].
The drug-likeliness properties of triterpenoids 6–10 based on their chemical structure and physicochemical properties were evaluated using Lipinski’s rule of five and Ghose’s and Veber’s rules (Table 3).

Table 3.
Results of drug-likeness analysis of tested compounds based on Lipinski’s, Ghose’s, and Veber’s rules.
Molecular descriptors (MW—molecular weight; HAs—hydrogen bond acceptors; HDs—hydrogen bond donors; RBs—rotational bonds; TPSA—topological polar surface; and MR—molar refractivity) for derivatives 2–3 and 5–10 were obtained using the SwissADME web server [35] (Table S3, Supplementary Materials).
The tested triterpenoids 6–10 violate two Lipinski criteria regarding the permissible value of molar mass (MW > 500) and lipophilicity (MLOGP > 4.15). These compounds violate all criteria formulated by Ghose. The molecular weight of the compounds ranges from 631.03 to 731.10 g/mol (MW > 480), and the lipophilicity parameter value ranges from 10.26 to 10.83 (WLOGP > 5.6). The calculated molar refractivity for compounds 6–10 ranges from 187.95 to 208.59 (MR > 130), and the number of atoms is higher than 70 [35]. The rule developed by Veber takes into account two parameters such as the number of rotatable bonds (RB < 10) and the topological polar surface of the molecule (TPSA < 140 Å2) [35]. Veber’s rule is met by compounds 6 and 10 (RB = 9; TPSA = 97.10 Å2). The remaining derivatives 7–9 do not meet this rule, exceeding the number of rotational bonds (RB is 11–14).
3.3.2. Prediction of Biological Activity
An interesting issue regarding the characterization of the synthesized compounds was the in silico analysis in terms of predicting their potential pharmacological activity. For this purpose, the “PASS online” program was used, which, based on the similarity of the chemical structure of the tested substance with a database of over 250,000 chemical compounds with tested biological activity, is able to predict, with a certain probability, the spectrum of activity that the tested substance may exhibit. The PASS program predicts the potential biological activity of a molecule based on its chemical structure. The results are presented as the probability that the molecule is active (Pa) or inactive (Pi), ranging from 0.000 (no probability at all) to 1.000 (certainty of activity) [36].
Table 4 includes the directions of activity of compounds 6–10 for which the Pa calculation results are above 0.7.

Table 4.
Predicted directions of biological activity of tested compounds calculated using PASS program version 2.0.
The predicted directions of biological activity of the synthesized α-liponate esters 6–10 include hepatoprotective, anti-inflammatory, and anticancer effects (probability Pa = 0.861–0.893). Additionally, analysis using the PASS program indicates potential activity against melanoma, colorectal cancer, colon cancer, lung cancer, and cervical cancer (Table 4).
The presented results of the in silico study can be treated as a starting point for the initial selection of cancer cells sensitive to the tested compounds. However, this is not always reflected in activity tests in the in vitro model.
3.3.3. Antiproliferative Activity
Designing hybrid molecules based on the combination of two biologically active compounds of natural origin seems to be justified due to the possibility of their simultaneous delivery to target organs. Moreover, hybrid molecules provide better dosage compliance and reduction in drug–drug interactions. An example of a hybrid molecule is the combination of α-lipoic acid and tacrine. The obtained hybrid compound has a synergistic effect on three target pathways (cholinergic hypothesis, the amyloid pathway, and oxidative stress), which may allow it to be used in the treatment of Alzheimer’s disease [44]. Naturally occurring α-lipoic acid and its ester derivatives are also the subject of extensive research regarding anticancer activity [25,45,46]. Sheng et al. described the synthesis and studies of the anticancer activity of conjugates of pentacyclic triterpenes of the oleanane type with α-lipoic acid. The synthesized compounds were tested against a human liver cancer cell line (BEL-7402), chronic myeloid leukemia cell line (K562), and normal human liver cell line (L-O2). Lipoate derivatives were characterized by moderate cytotoxic activity against chronic myeloid leukemia cells (K562) with IC50 values ranging from 64.3 µM to >200 µM. Unfortunately, none of the tested compounds turned out to be active against human liver cancer cells (BEL-7402). It is worth noting, however, that all tested α-lipoate esters showed no toxic effect on normal liver cell lines (L-O2) [47].
The new triterpenoids 6–10, betulin, and α-lipoic acid were tested for antiproliferative activity in vitro against seven human cancer cell lines like biphenotypic B myelomonocytic leukemia (MV4-11), lung carcinoma (A549), breast cancer (MCF-7), prostate adenocarcinoma (PC-3), colon cancer (HCT116), pancreas cancer (MiaPaca-2), and melanoma (Hs294T). The results of inhibiting the proliferation of cancer cells and normal breast epithelial MCF-10A cells with the tested compounds are presented in Table 5.

Table 5.
Antiproliferative activity of α-lipoic acid, betulin, and its derivatives 6–10.
Biphenotypic B myelomonocytic leukemia cells are the most sensitive to the action of α-lipoate derivatives 8–10. The IC50 values of compounds 8–10 against MV4-11 cells are in the range of 37.9–76.5 µM. The compound with activity against prostate adenocarcinoma (PC-3) and colon cancer (HCT116) cell lines is derivative 10 with (1,2-dithiolan-3-yl)pentanoyl moiety at the C3 position. The presence of a (1,2-dithiolan-3-yl)pentanoyl group at the C28 position (compounds 6 and 7) leads to a decrease in activity against MV4-11 cells. All synthesized compounds do not have cytotoxic effects on human normal epithelial cells from mammary gland (MCF-10A).
SI selectivity indices are determined by dividing the IC50 value for the normal line (MCF-10A) using the IC50 value for the specific tumor line being tested. A favorable SI (>1.0) indicates that the substance has greater effectiveness against cancer cells than toxicity against normal cells.
This study did not obtain IC50 values for normal cells (MCF-10A) and cancer cells for compounds 6, 7 and α-lipoic acid; therefore, we could not calculate the selectivity index (SI). For the betulin SI, the values were 3 (MV4-11), 7 (A549), 2.3 (MCF-7), 1.9 (PC-3), 2.6 (HCT116), and 1.68 (Hs294T). No activity or selectivity towards MiaPaca-2 cells was observed.
The antiproliferative effect may vary depending on the cancer lines tested. Previously conducted studies described by Kvasnica et al. indicated that in the case of leukemia cell lines (CEM, K562), the activity of betulin was lower than that of its 28-acetyl derivative. The introduction of an additional ester substituent at the C3 position in 28-acetylbetuline gave the compound with the highest activity. This relationship was not confirmed in our study [39].
3.3.4. Mechanistic Studies
Programmed cell death (apoptosis) is a process in which all unnecessary cells in the body are eliminated. The apoptosis pathway can be activated by intracellular signals (mitochondrial pathway) and extracellular signals (death receptor pathways). Apoptosis occurs with the participation of caspases, which have the ability to cleave various proteins [46]. Currently, many drugs are being designed to trigger apoptosis in cancer cells that normally do not undergo it. Among the molecules of this nature are betulin derivatives active in various types of cancer [48,49,50,51].
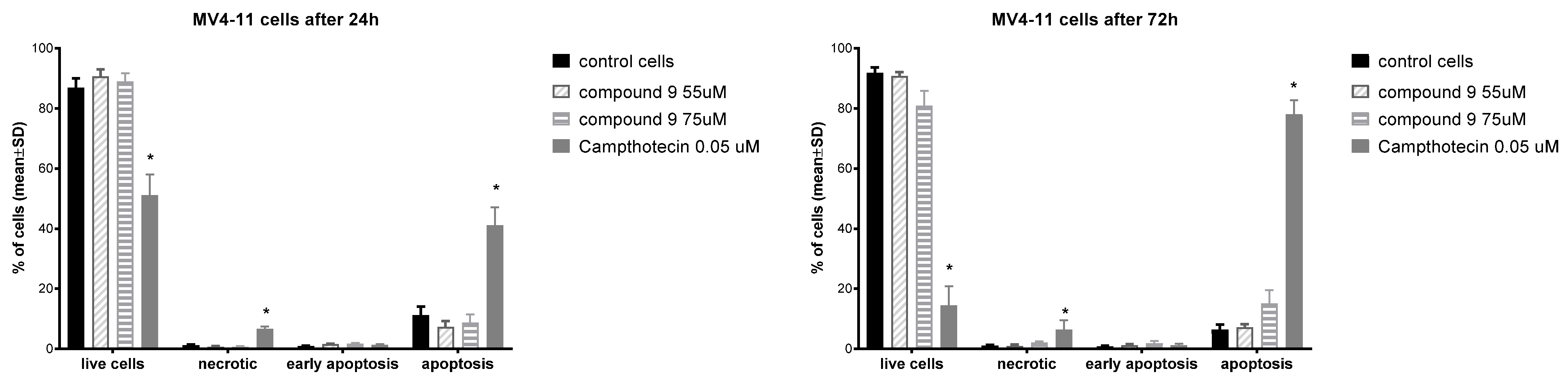
To evaluate the mechanism of antiproliferative action, compound 9 was selected, showing the highest activity against MV-4-11 cells. An apoptosis induction test was performed using the Annexin V (AnV)/Propidium iodide (PI) staining method after 24 and 72 h of incubation with derivative 9 at the following concentrations: 55 µM and 75 µM (about 1.5 × IC50 and 2 × IC50 value). The obtained results are presented in Figure 2.

Figure 2.
Induction of apoptosis with derivative 9 after 24 and 72 h. * statistically significant versus control cells, p < 0.05.
The incubation of MV-4-11 cells with derivative 9 for 24 h did not induct cell apoptosis. An incubation time extended to 72 h slightly increased the level of apoptotic cells using compound 9 at a concentration of 75 µM. Betulin had much higher pro-apoptotic properties than compound 9. After 48 h at a concentration of 40 mM, betulin induced the death of 39.1% leukemia U937 cells [52]. On other types of cancers cells (e.g., melanoma, breast, lung, gastric, bladder, colon, prostate), betulin also induced apoptosis [53].
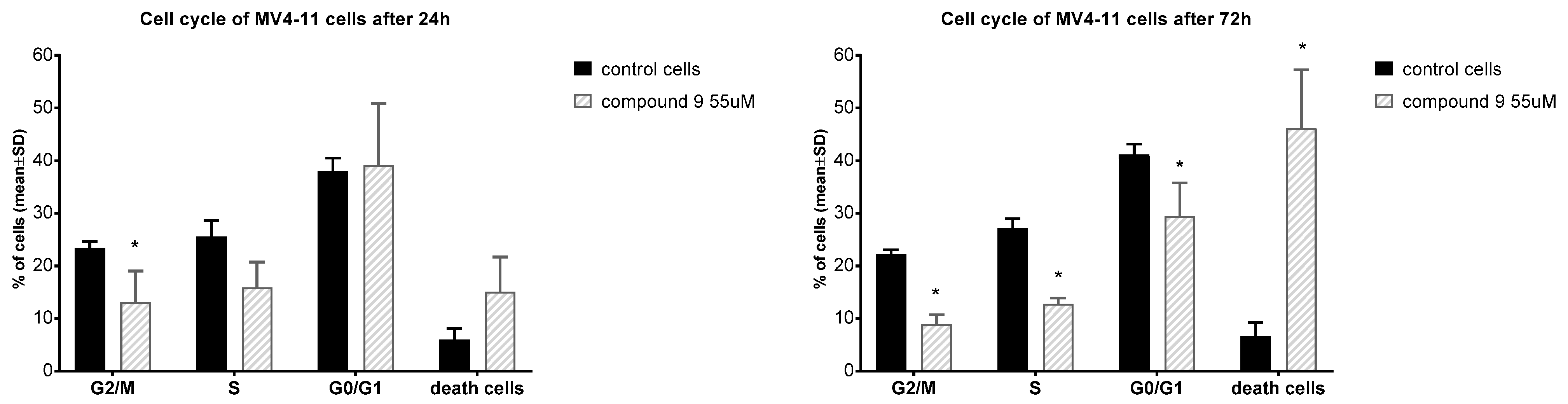
In order to better understand the effect of compound 9, an analysis of its effect on the cell cycle of MV4-11 cells was performed. The cell exposure time was 24 and 72 h (Figure 3).
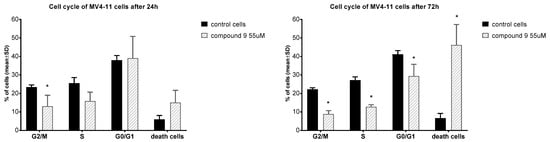
Figure 3.
Influence of compound 9 on cell cycle after 24 and 72 h of incubation. * statistically significant versus control cells, p < 0.05.
In the case of MV-4-11 cells, after 24 h of incubation, compound 9 decreased the number of cells in the S and G2/M phases in comparison to control cells and increased the number of dead cells observed. The extension of the incubation time to 72 h with compound 9 resulted in a strong increase in the percentage of dead cells with a statistically significant decrease in the cell number in the G2/M, S and G0/G1 phases. Park et al., in studies on U937 leukemia, showed that betulin caused cell cycle arrest in the G2/M phase and strongly increased the percentage of cells in the subG1 phase after 48 h at concentrations of 20–40 mM [52]. On ovarian OVACAR-3 cancer cells, betulin, after 24 h at concentrations of 15, 60, and 120 mM, also arrested cells in the G2/M phase [54]. Compound 9 appears to have a different effect on the cell cycle than betulin.
4. Conclusions
Phytochemicals constitute a large group of compounds characterized by a diverse chemical structure and the resulting multidirectionality of biological action. Therefore, the use of compounds of natural origin in the design of new medicinal substances is one of the leading directions of research in medicinal chemistry. The use of mild Steglich reaction conditions enabled the combination of the triterpene system with lipoic acid, leading to the formation of conjugates 6–10 with good yields (48–88%). The initial prediction of biological activity indicating anticancer activity was verified by in vitro tests on various cancer cell lines. The most beneficial effect was observed in relation to MV4-11 leukemia cells. The combination of the betulin system with an ester bond at the C28 position with the cyclic dithiolane system via a long alkyl linker yielded inactive derivatives (compounds 6 and 7). The presence of this substituent at the C3 position (compounds 9 and 10) increased the activity. The second important factor influencing the activity was the presence of a small ester (acetyl) group at the C28 position. Compound 9 was characterized by activity comparable to betulin and much higher than compound 10 with a free hydroxyl group at the C28 carbon atom. All lipoate derivatives did not show cytotoxic effects on the MCF-10A normal cells.
To sum up, the modification of the betulin molecule by introducing a fragment of α-lipoic acid at the C28 and C3 positions did not result in the expected increase in antiproliferative activity. Future studies should aim to introduce other anticancer pharmacophores into the betulin molecule to ensure greater activity of the new conjugates.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app14219970/s1: Figure S1. 1H NMR, compound 6; Figure S2. 13C NMR, compound 6; Figure S3. IR, compound 6; Figure S4. HRMS, compound 6; Figure S5. 1H NMR, compound 7; Figure S6. 13C NMR, compound 7; Figure S7. IR, compound 7; Figure S8. HRMS, compound 7; Figure S9. 1H NMR, compound 8; Figure S10. 13C NMR, compound 8; Figure S11. IR, compound 8; Figure S12. HRMS, compound 8; Figure S13. 1H NMR, compound 9; Figure S14. 13C NMR, compound 9; Figure S15. IR, compound 9; Figure S16. HRMS, compound 9; Figure S17. 1H NMR, compound 10; Figure S18. 13C NMR, compound 10; Figure S19. IR, compound 10; Figure S20. HRMS, compound 10; Table S1. Lipophilicity parameters of standard compounds determined experimentally (RM0; mobile phase acetone–buffer Tris; pH 7.4) and based on literature values (Log Plit); Table S2. The correlation matrix for theoretically and experimentally obtained lipophilicity parameters of the tested betulin derivatives; Table S3. Molecular descriptors for betulin and its derivatives 2–3 and 5–10.
Author Contributions
E.C. and E.B.: conceptualization, methodology, validation, writing—original draft preparation, project administration, and funding acquisition; M.Ś.: methodology, validation, writing—review and editing, and project administration; J.W.: supervision and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical University of Silesia grant number (BNW-1-100/K/4/F).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chaachouay, N.; Zidane, L. Plant-derived natural products: A source for drug discovery and development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Davis, C.C.; Choisy, P. Medicinal plants meet modern biodiversity science. Curr. Biol. 2024, 34, R158–R173. [Google Scholar] [CrossRef] [PubMed]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’Arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S.; et al. Beneficial role of phytochemicals on oxidative stress and age-related diseases. Biomed. Res. Int. 2019, 2019, 8748253. [Google Scholar] [CrossRef] [PubMed]
- AlSheikh, H.M.A.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-Sheikh, H.; Tasleem Jan, A.; Haq, Q.M.R. Plant-based phytochemicals as possible alternative to antibiotics in combating bacterial drug resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef] [PubMed]
- Koche, D.; Shirsat, R.; Kawale, M. An overview of major classes of phytochemicals: Their types and role in disease prevention. Hislopia J. 2016, 9, 1–11. [Google Scholar]
- Ahmed, M.H.; Karkush, S.I.; Ali, S.A.; Mohammed, A.A. Phytochemicals: A new arsenal in drug discovery. Int. J. Med. Sci. Dent. Health 2024, 10, 29–44. [Google Scholar] [CrossRef]
- Patra, S.; Nayak, R.; Patro, S.; Pradhan, B.; Sahu, B.; Behera, C.; Bhutia, S.K.; Jena, M. Chemical diversity of dietary phytochemicals and their mode of chemoprevention. Biotechnol. Rep. 2021, 30, e00633. [Google Scholar] [CrossRef]
- Yadav, N.; Parveen, S.; Banerjee, M. Potential of nano-phytochemicals in cervical cancer therapy. Clin. Chim. Acta 2020, 505, 60–72. [Google Scholar] [CrossRef]
- Swain, S.S.; Panda, S.K.; Luyten, W. Phytochemicals against SARS-CoV as potential drug leads. Biomed. J. 2021, 44, 74–85. [Google Scholar] [CrossRef]
- Suganya, T.; Packiavathy, I.A.S.V.; Aseervatham, G.S.B.; Carmona, A.; Rashmi, V.; Mariappan, S.; Devi, N.R.; Ananth, D.A. Tackling multiple-drug-resistant bacteria with conventional and complex phytochemicals. Front. Cell. Infect. Microbiol. 2022, 12, 883839. [Google Scholar] [CrossRef]
- Alam, S.; Sarker, M.M.R.; Sultana, T.N.; Chowdhury, M.N.R.; Rashid, M.A.; Chaity, N.I.; Zhao, C.; Xiao, J.; Hafez, E.E.; Khan, S.A.; et al. Antidiabetic phytochemicals from medicinal plants: Prospective candidates for new drug discovery and development. Front. Endocrinol. 2022, 13, 800714. [Google Scholar] [CrossRef] [PubMed]
- Nisar, A.; Jagtap, S.; Vyavahare, S.; Deshpande, M.; Harsulkar, A.; Ranjekar, P.; Prakash, O. Phytochemicals in the treatment of inflammation associated diseases: The journey from preclinical trials to clinical practice. Front. Pharmacol. 2023, 14, 1177050. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, A.; Hussain, W.; Rasool, N. Probing the pharmacological binding properties, and reactivity of selective phytochemicals as potential HIV-1 protease inhibitors. Univ. Sci. 2019, 24, 441–464. [Google Scholar] [CrossRef]
- Soural, M.; Hodon, J.; Dickinson, N.J.; Sidova, V.; Gurska, S.; Dzubak, P.; Hajduch, M.; Sarek, J.; Urban, M. Preparation of Conjugates of Cytotoxic Lupane Triterpenes with Biotin. Bioconjug. Chem. 2015, 26, 2563–2570. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef]
- Gür, F.M.; Bilgiç, S. Silymarin, an antioxidant flavonoid, protects the liver from the toxicity of the anticancer drug paclitaxel. Tissue Cell 2023, 83, 102158. [Google Scholar] [CrossRef]
- Demets, O.V.; Takibayeva, A.T.; Kassenov, R.Z.; Aliyeva, M.R. Methods of betulin extraction from birch bark. Molecules 2022, 27, 3621. [Google Scholar] [CrossRef]
- Adepoju, F.O.; Duru, K.C.; Li, E.; Kovaleva, E.G.; Tsurkan, M.V. Pharmacological potential of betulin as a multitarget compound. Biomolecules 2023, 13, 1105. [Google Scholar] [CrossRef]
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.R.; Machaj, F.; Ghamsari, M.; Rosik, J.; et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotech. Adv. 2020, 38, 107409. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef]
- Lv, S.; He, S.; Ling, X.; Wang, Y.; Huang, C.; Long, J.; Wang, J.; Qin, Y.; Wei, H. Review of lipoic acid: From a clinical therapeutic agent to various emerging biomaterials. Int. J. Pharm. 2022, 627, 122201. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Berkay Yılmaz, Y.; Antika, G.; Boyunegmez Tumer, T.; Fawzi Mahomoodally, M.; Lobine, D.; Akram, M.; Riaz, M.; Capanoglu, E.; Sharopov, F.; et al. Insights on the use of α-lipoic acid for therapeutic purposes. Biomolecules 2019, 9, 356. [Google Scholar] [CrossRef] [PubMed]
- Tibullo, D.; Li Volti, G.; Giallongo, C.; Grasso, S.; Tomassoni, D.; Anfuso, C.D.; Lupo, G.; Amenta, F.; Avola, R.; Bramanti, V. Biochemical and clinical relevance of alpha lipoic acid: Antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential. Inflamm. Res. 2017, 66, 947–959. [Google Scholar] [CrossRef]
- Dörsam, B.; Fahrer, J. The disulfide compound α-lipoic acid and its derivatives: A novel class of anticancer agents targeting mitochondria. Cancer Lett. 2016, 371, 12–19. [Google Scholar] [CrossRef]
- Izadi, A.; Sadeghi, A.; Jalili-Nik, M.; Mirzavi, F.; Afshari, A.R.; Soukhtanloo, M. Combination of alpha-lipoic acid and auraptene induces apoptosis and prevents proliferation of the human U-87 glioblastoma cells. Rev. Bras. Farmacogn. 2023, 33, 1177–1186. [Google Scholar] [CrossRef]
- Liu, X.; Barth, M.C.; Cseh, K.; Kowol, C.R.; Jakupec, M.A.; Keppler, B.K.; Gibson, D.; Weigand, W. Oxoplatin-based Pt(IV) lipoate complexes and their biological activity. Chem. Biodivers. 2022, 19, e202200695. [Google Scholar] [CrossRef]
- Deng, Y.; Snyder, J.K. Preparation of a 24-nor-1,4-dien-3-one triterpene derivative from betulin: A new route to 24-nortriterpene analogues. J. Org. Chem. 2002, 67, 2864–2873. [Google Scholar] [CrossRef]
- Chrobak, E.; Marciniec, K.; Dąbrowska, A.; Pęcak, P.; Bębenek, E.; Kadela-Tomanek, M.; Bak, A.; Jastrzębska, M.; Boryczka, S. New phosphorus analogs of bevirimat: Synthesis, evaluation of anti-HIV-1 activity and molecular docking study. Int. J. Mol. Sci. 2019, 20, 5209. [Google Scholar] [CrossRef]
- Huang, F.Y.; Chung, B.Y.; Bentley, M.D.; Alford, A.R. Colorado potato beetle antifeedants by simple modification of the birchbark triterpene betulin. J. Agric. Food. Chem. 1995, 43, 2513–2516. [Google Scholar] [CrossRef]
- Pohjala, L.; Alakurtti, S.; Ahola, T.; Yli-Kauhaluoma, J.; Tammela, P. Betulin-derived compounds as inhibitors of alphavirus replication. J. Nat. Prod. 2009, 72, 1917–1926. [Google Scholar] [CrossRef]
- Tietze, L.F.; Heinzen, H.; Moyna, P.; Rischer, M.; Neunaber, H. Synthesis of [13C]- and [2H] betulin for biological transformations. Lieb. Ann. Chem. 1991, 1991, 1245–1249. [Google Scholar] [CrossRef]
- Pęcak, P.; Świtalska, M.; Chrobak, E.; Boryczka, G.; Bębenek, E. Betulin acid ester derivatives inhibit cancer cell growth by inducing apoptosis through caspase cascade activation: A comprehensive in vitro and in silico study. Int. J. Mol. Sci. 2023, 24, 196. [Google Scholar] [CrossRef] [PubMed]
- Tetko, I.V.; Tanchuk, V.Y. Application of associative neural networks for prediction of lipophilicity in ALOGPS 2.1 program. J. Chem. Inf. Comput. Sci. 2002, 42, 1136–1145. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Lagunin, A.; Stepanchikova, A.; Filimonov, D.; Poroikov, V. PASS: Prediction of activity spectra for biologically active substances. Bioinformatics 2000, 16, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, M.; Świtalska, M.; Wietrzyk, J.; Maciejewska, G.; Gliszczyńska, A. Synthesis, characterization, and in vitro cancer cell growth inhibition evaluation of novel phosphatidylcholines with anisic and veratric acids. Molecules 2018, 23, 2022. [Google Scholar] [CrossRef]
- Šiman, P.; Filipová, A.; Tichá, A.; Niang, M.; Bezrouk, A.; Havelek, R. Effective method of purification of betulin from birch bark: The importance of its purity for scientific and medicinal use. PLoS ONE 2016, 11, e0154933. [Google Scholar] [CrossRef]
- Kvasnica, M.; Sarek, J.; Klinotova, E.; Dzubak, P.; Hajduch, M. Synthesis of phthalates of betulinic acid and betulin with cytotoxic activity. Bioorg. Med. Chem. 2005, 13, 3447–3454. [Google Scholar] [CrossRef]
- Johnson, T.W.; Gallego, R.A.; Edwards, M.P. Lipophilic Efficiency as an Important Metric in Drug Design. J. Med. Chem. 2018, 61, 6401–6420. [Google Scholar] [CrossRef]
- Bakht, M.A.; Alajmi, M.F.; Alam, P.; Alam, A.; Alam, P.; Aljarba, T.M. Theoretical and experimental study on lipophilicity and wound healing activity of ginger compounds. Asian Pac. J. Trop Biomed. 2014, 4, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Jia, C.Y.; Li, J.Y.; Hao, G.F.; Yang, G.F. A drug-likeness toolbox facilitates ADMET study in drug discovery. Drug Discov. Today. 2020, 25, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Singh, P.K.; Verma, H.; Singh, H.; Silakari, O. Success stories of natural product-based hybrid molecules for multi-factorial diseases. Eur. J. Med. Chem. 2018, 151, 62–97. [Google Scholar] [CrossRef] [PubMed]
- Olejarz, W.; Wrzosek, M.; Jóźwiak, M.; Grosicka-Maciąg, E.; Roszkowski, P.; Filipek, A.; Cychol, A.; Nowicka, G.; Struga, M. Synthesis and anticancer effects of α-lipoic ester of alloxanthoxyletin. Med. Chem. Res. 2019, 28, 788–796. [Google Scholar] [CrossRef]
- Śmiłowicz, D.; Slootweg, J.C.; Metzler-Nolte, N. Bioconjugation of cyclometalated gold(III) lipoic acid fragments to linear and cyclic breast cancer targeting peptides. Mol. Pharm. 2019, 16, 4572–4581. [Google Scholar] [CrossRef]
- Sheng, L.X.; Huang, J.Y.; Liu, C.M.; Zang, J.Z.; Cheng, K.G. Synthesis of oleanolic acid/ursolic acid/glycyrrhetinic acid-hydrogen sulfide donor hybrids and their antitumor activity. Med. Chem. Res. 2019, 28, 1212–1222. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Yang, S.J.; Liu, M.C.; Xiang, H.M.; Zhao, Q.; Xue, W.; Yang, S. Synthesis and in vitro antitumor evaluation of betulin acid ester derivatives as novel apoptosis inducers. Eur. J. Med. Chem. 2015, 102, 249–255. [Google Scholar] [CrossRef]
- Wang, J.; Wu, J.; Han, Y.; Zhang, J.; Lin, Y.; Wang, H.; Wang, J.; Liu, J.; Bu, M. Design and synthesis of novel betulin derivatives containing thio-/semicarbazone moieties as apoptotic inducers through mitochindria-related pathways. Molecules 2021, 26, 6356. [Google Scholar] [CrossRef]
- Mihoub, M.; Pichette, A.; Sylla, B.; Gauthier, C.; Legault, J. Bidesmosidic betulin saponin bearing L-rhamnopyranoside moieties induces apoptosis and inhibition of lung cancer cells growth in vitro and in vivo. PLoS ONE 2018, 13, e0193386. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Jeong, J.W.; Han, M.H.; Lee, H.; Kim, G.Y.; Jin, S.; Park, J.H.; Kwon, H.J.; Kim, B.W.; Choi, Y.H. The anti-cancer effect of betulinic acid in u937 human leukemia cells is mediated through ROS-dependent cell cycle arrest and apoptosis. Anim. Cells Syst. 2021, 25, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Sak, K.; Gupta, D.S.; Kaur, G.; Aggarwal, D.; Chaturvedi Parashar, N.; Choudhary, R.; Yerer, M.B.; Kaur, J.; Kumar, M.; et al. Anti-inflammatory and anticancer properties of birch bark-derived betulin: Recent developments. Plants 2021, 10, 2663. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Fei, Z.; Huang, C. Betulin terpenoid targets OVCAR-3 human ovarian carcinoma cells by inducing mitochondrial mediated apoptosis, G2/M phase cell cycle arrest, inhibition of cell migration and invasion and modulating mTOR/PI3K/AKT signalling pathway. Cell. Mol. Biol. 2021, 67, 14–19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).