Nanostructured Magnetite Coated with BiOI Semiconductor: Readiness Level in Advanced Solar Photocatalytic Applications for the Remediation of Phenolic Compounds in Wastewater from the Wine and Pisco Industry
Abstract
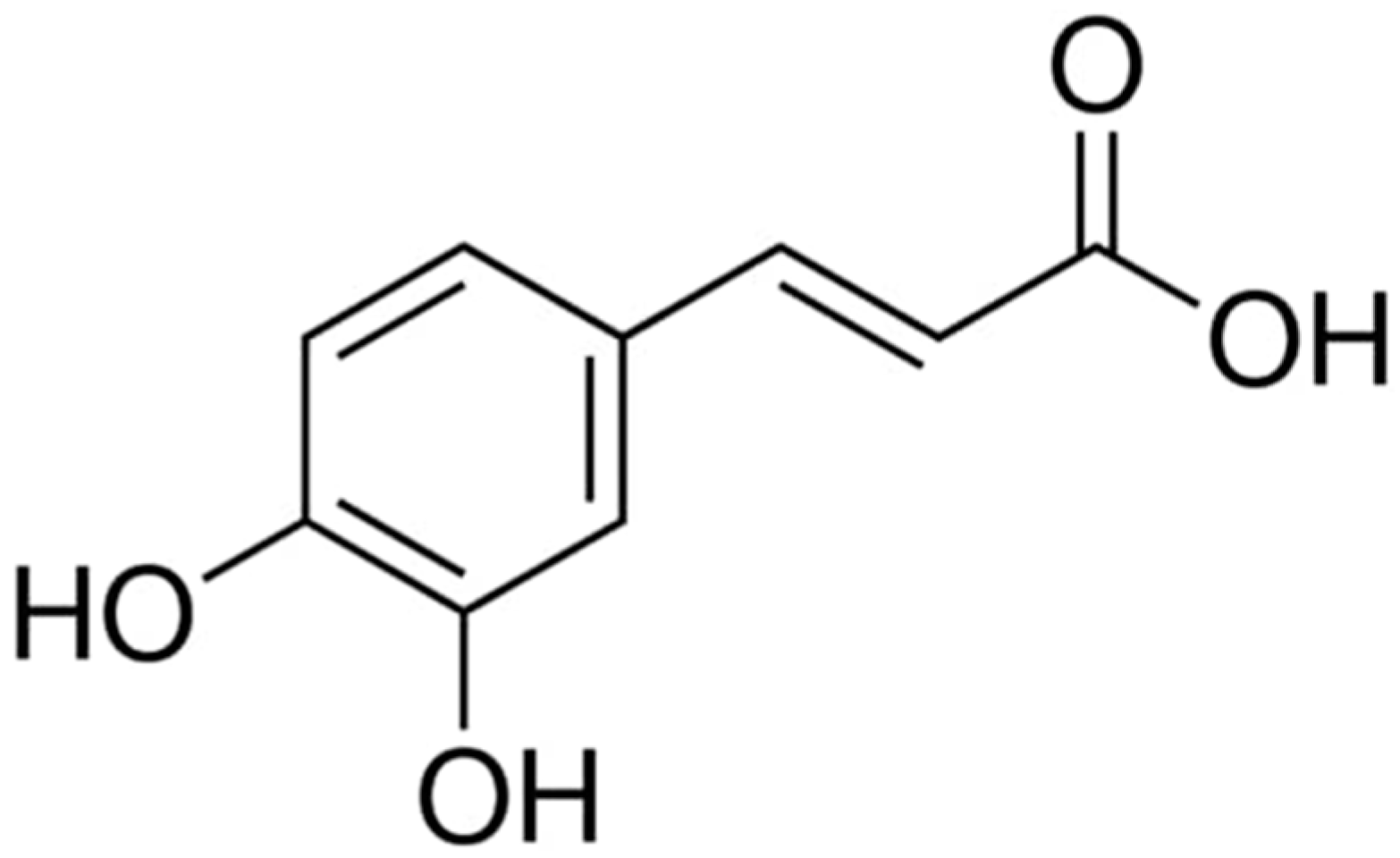
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Fe3O4 Nanoparticles
2.2.2. Synthesis of BiOI/Fe3O4 Nanoparticles
2.2.3. Characterization of Nanomaterials
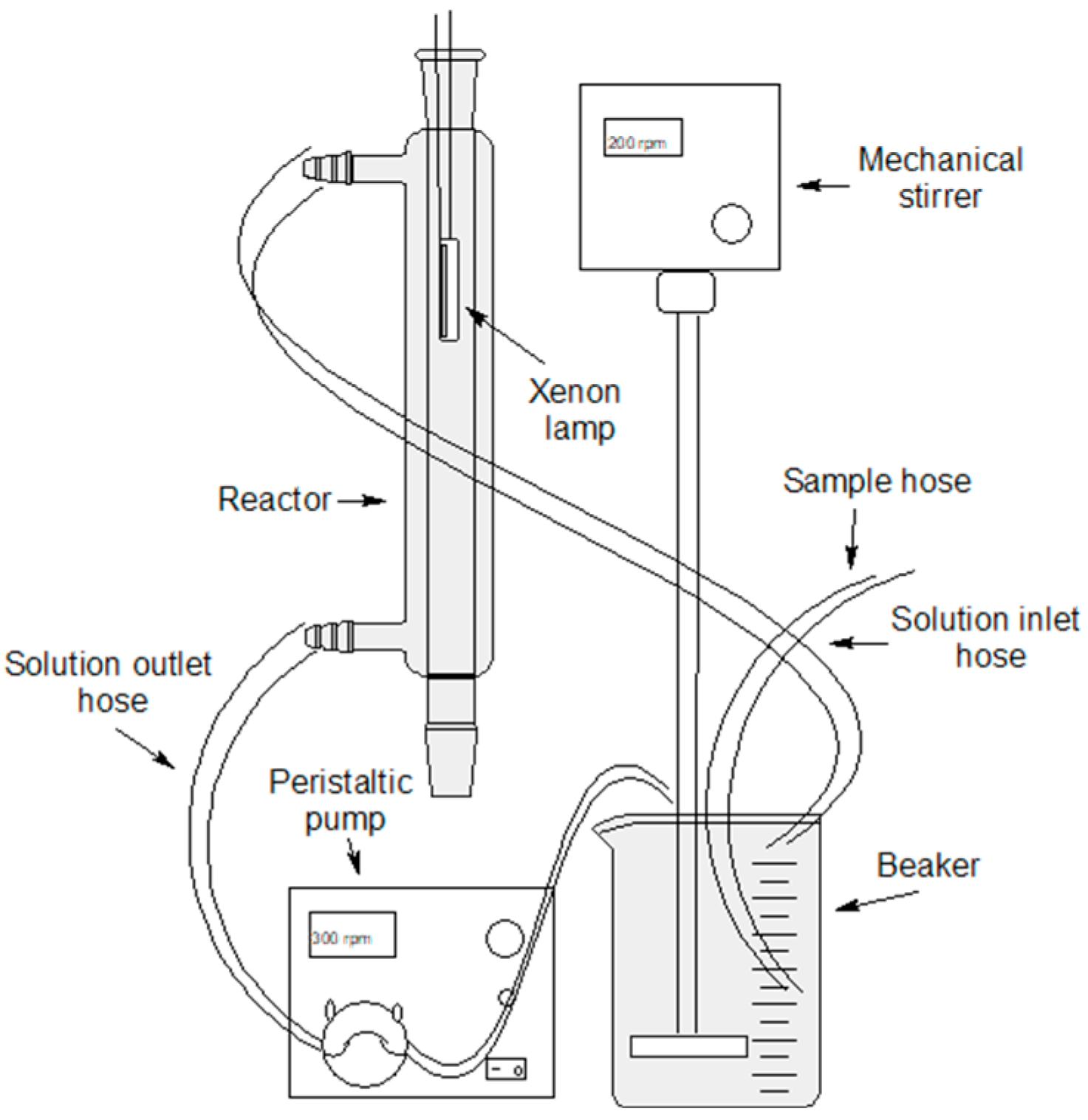
2.2.4. Measurement of Photocatalytic Activity
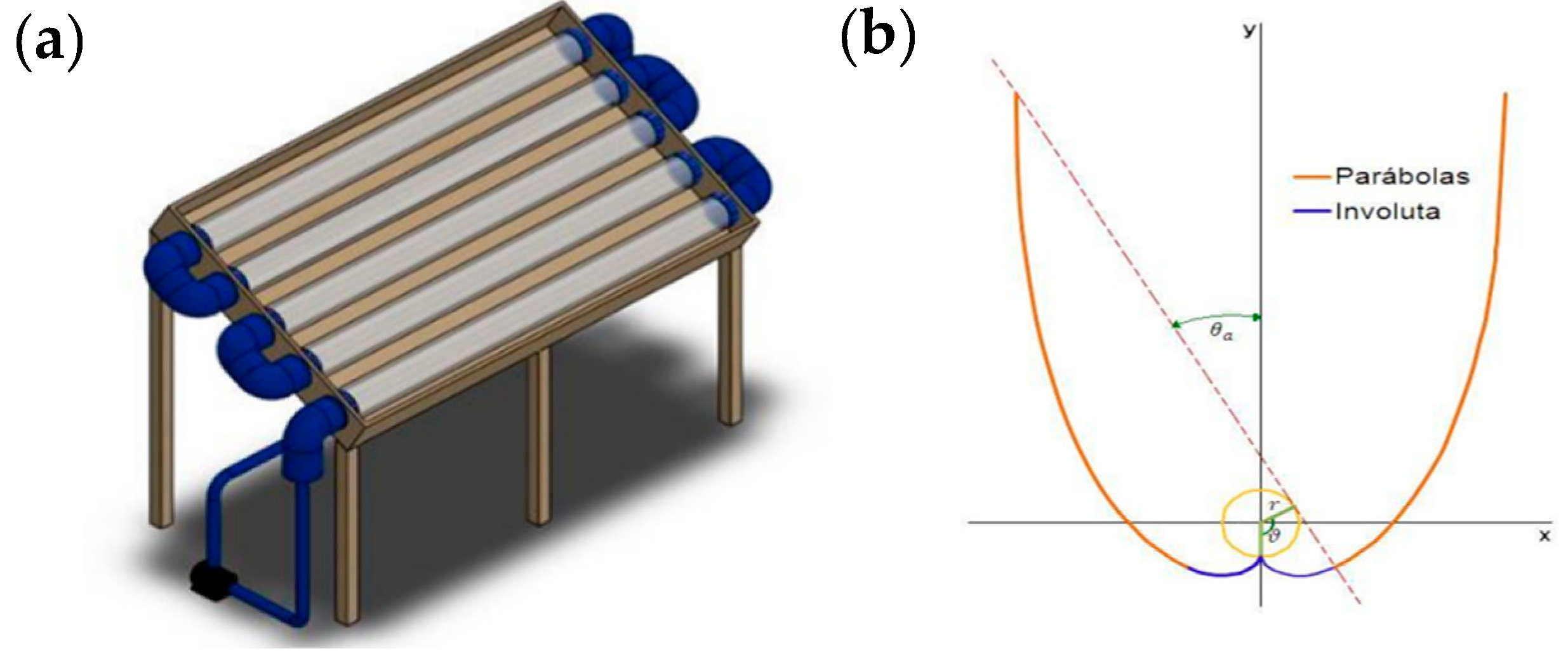
2.2.5. Solar Photocatalysis Preparation Level Using BF-5
3. Results and Discussion
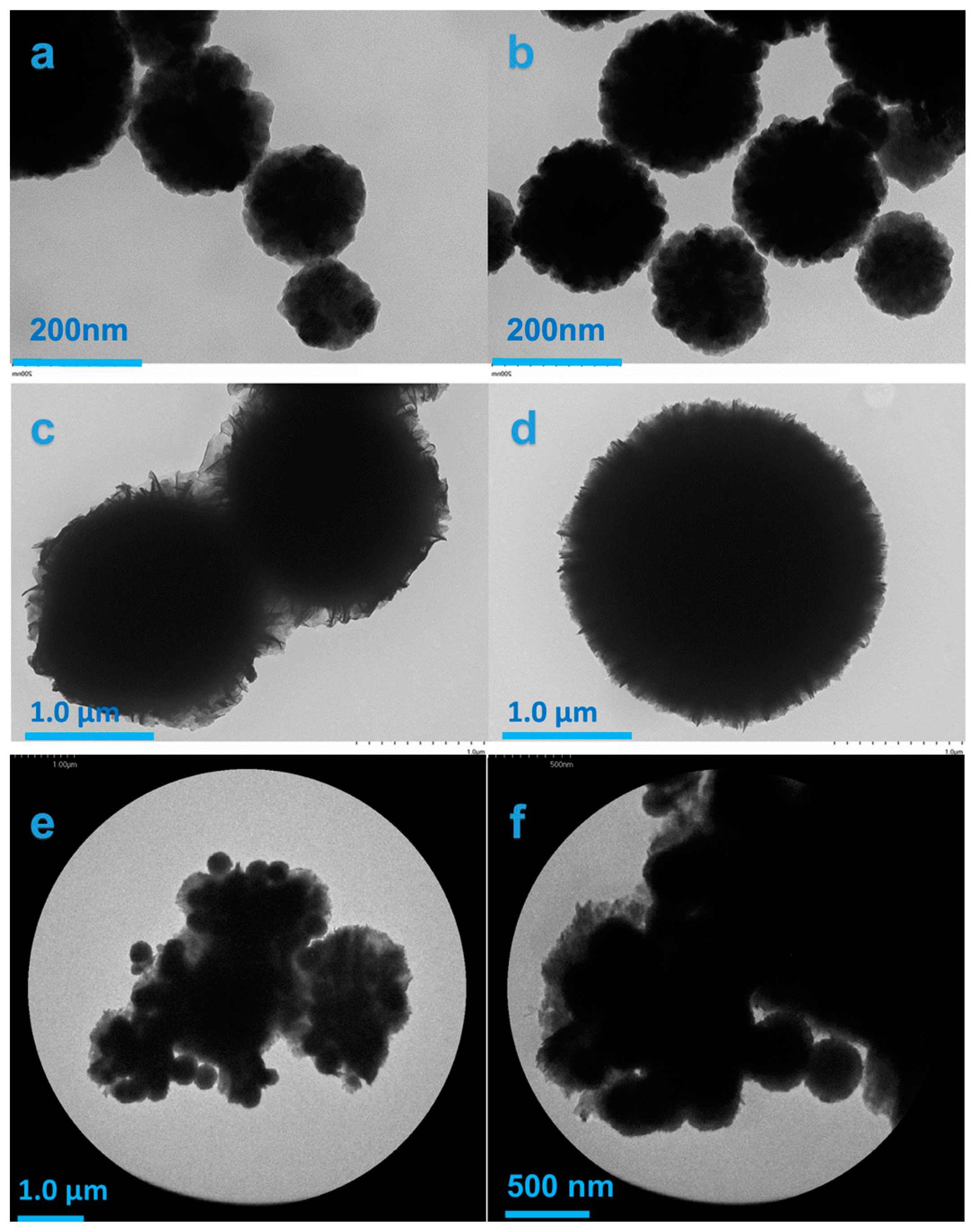
3.1. Physical and Optical Properties and Surface Chemistry of Fe3O4, BiOI, and BF-5
3.2. Magnetism Test
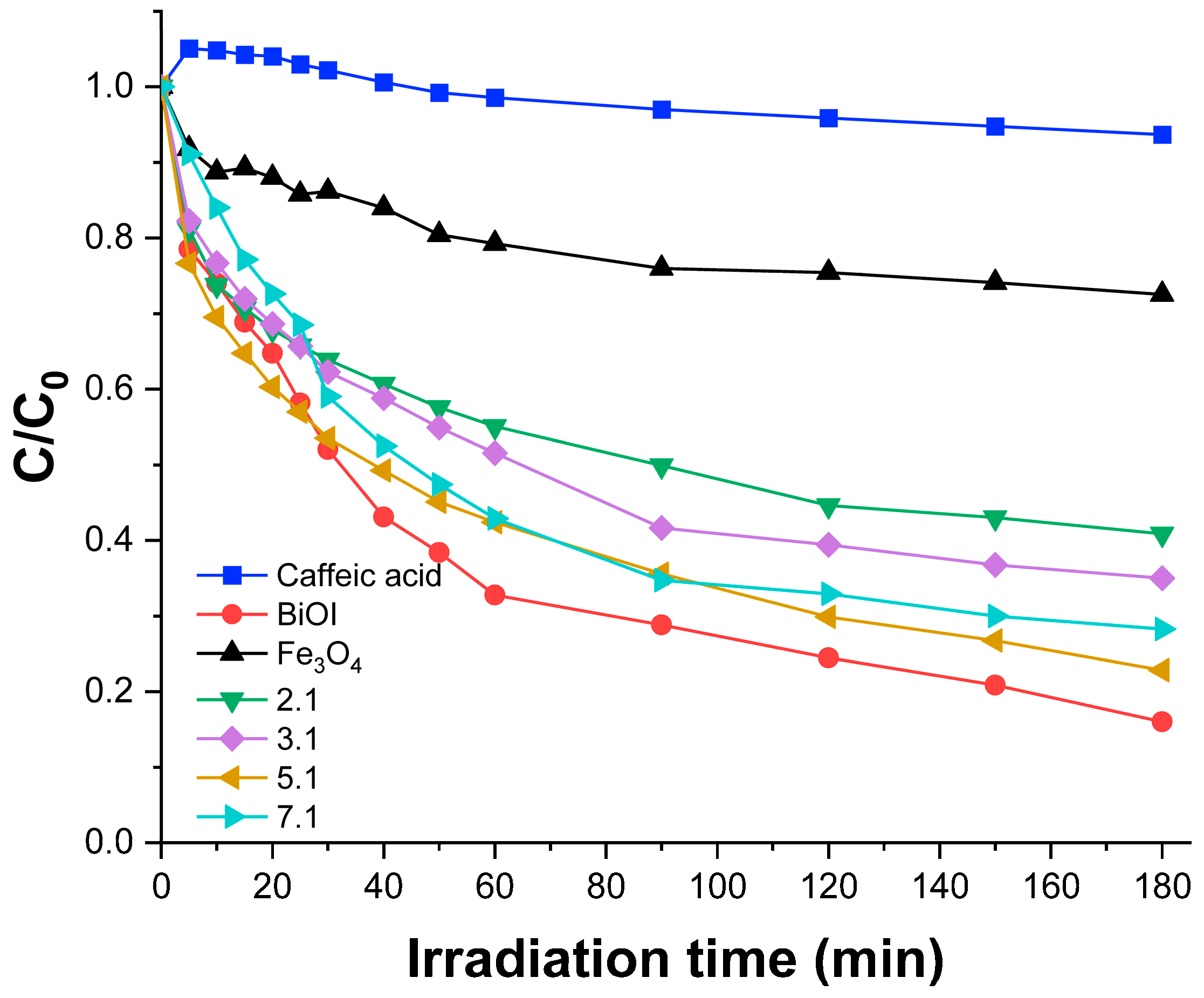
3.3. Photocatalytic Removal Efficiency
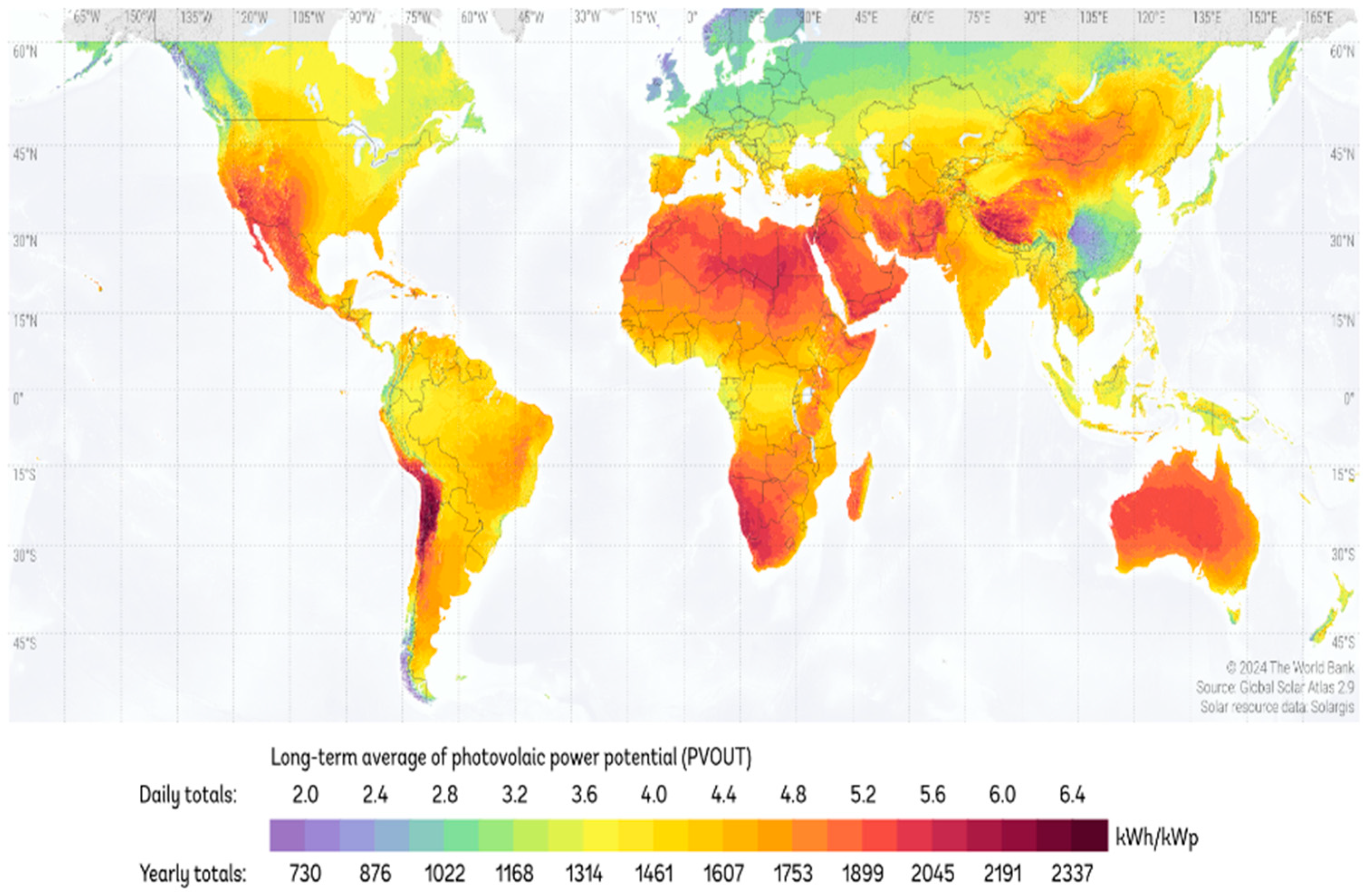
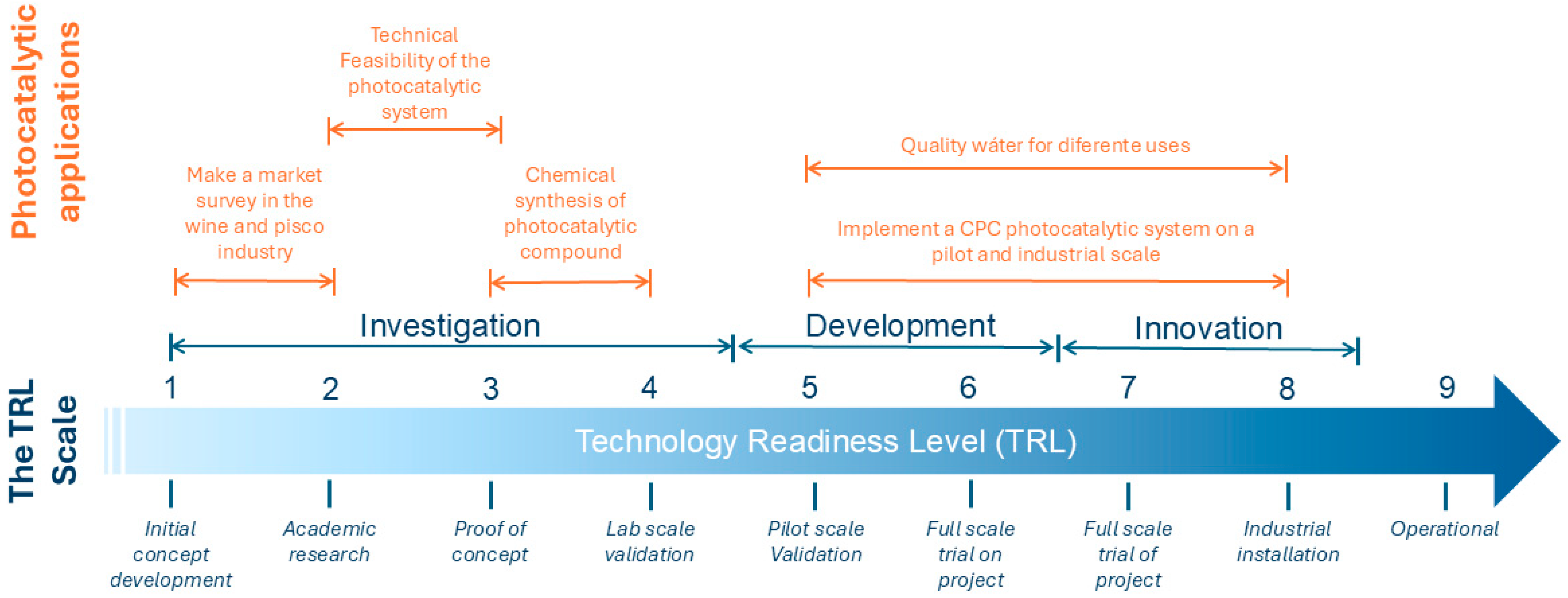
3.4. Future Projections Based on the Readiness Level of Solar Photocatalysis Using BF-5 Area of Study
3.5. Technical Feasibility
3.6. Cost Analysis of a Medium-Scale Photocatalytic System
3.7. Environmental Feasibility
3.8. Scalability of Heterogeneous Photocatalysis Using BF-5
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gallegos-Alcaíno, A.; Robles-Araya, N.; Avalos, C.; Alfonso-Alvarez, A.; Rodríguez, C.A.; Valdés, H.; Sánchez-Flores, N.A.; Durán-Alvarez, J.C.; Bizarro, M.; Romero-Salguero, F.J.; et al. Synthesis of BiOI/Mordenite Composites for Photocatalytic Treatment of Organic Pollutants Present in Agro-Industrial Wastewater. Nanomaterials 2022, 12, 1161. [Google Scholar] [CrossRef] [PubMed]
- Wines of Chile. Memoria Anual Vinos de Chile. 2018. Available online: https://www.winesofchile.org/wp-content/uploads/2019/05/MEMORIA-FINAL-EF.pdf (accessed on 4 August 2024).
- Adriana Valenzuela Palma. Antecedentes de Producción y Comercio del Pisco Chileno; Oficina de Estudios y Política Agraria (ODEPA)- Ministerio de Agricultura, Gobierno de Chile: Santiago de Chile, Chile, 2024; Available online: https://bibliotecadigital.odepa.gob.cl/bitstream/handle/20.500.12650/73529/Produccion-y-Comercio_del_PiscoChileno.pdf (accessed on 11 August 2024).
- Adriana Valenzuela Palma. Boletín del Vino: Producción, Precios y Comercio Exterior; Oficina de Estudios y Política Agraria (ODEPA)- Ministerio de Agricultura, Gobierno de Chile: Santiago de Chile, Chile, 2024; Available online: https://bibliotecadigital.odepa.gob.cl/bitstream/handle/20.500.12650/73301/BVino042024.pdf (accessed on 11 August 2024).
- Poblete, R.; Bakit, J. Technical and economical assessment of the treatment of vinasse from Pisco production using the advanced oxidation process. Environ. Sci. Pollut. Res. 2023, 30, 70213–70228. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.F.R.; Lebron, Y.A.R.; Moreira, V.R.; Ribeiro, L.A.; Koch, K.; Amaral, M.C.S. High-retention membrane bioreactors for sugarcane vinasse treatment: Opportunities for environmental impact reduction and wastewater valorization. J. Environ. Manag. 2023, 329, 117001. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.P.; Gando-Ferreira, L.M.; Quina, M.J. Micellar enhanced ultrafiltration for the valorization of phenolic compounds and polysaccharides from winery wastewaters. J. Water Process Eng. 2020, 38, 101565. [Google Scholar] [CrossRef]
- Mohamad Said, K.A.; Ismail, A.F.; Abdul Karim, Z.; Abdullah, M.S.; Hafeez, A. A review of technologies for the phenolic compounds recovery and phenol removal from wastewater. Process Saf. Environ. Prot. 2021, 151, 257–289. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, Y.; Hou, M.; Xue, J.; Qin, R.; Zhou, M.; Zhang, Y. Properties of polyphenols and polyphenol-containing wastewaters and their treatment by Fenton/Fenton-like reactions. Sep. Purif. Technol. 2023, 317, 123905. [Google Scholar] [CrossRef]
- Pham, T.-H.; Lee, B.-K.; Kim, J. Improved adsorption properties of a nano zeolite adsorbent toward toxic nitrophenols. Process Saf. Environ. Prot. 2016, 104, 314–322. [Google Scholar] [CrossRef]
- Yáñez, E.; Santander, P.; Contreras, D.; Yáñez, J.; Cornejo, L.; Mansilla, H.D. Homogeneous and heterogeneous degradation of caffeic acid using photocatalysis driven by UVA and solar light. J. Environ. Sci. Health Part A 2016, 51, 78–85. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Ayekoe, C.Y.P.; Robert, D.; Lanciné, D.G. Combination of coagulation-flocculation and heterogeneous photocatalysis for improving the removal of humic substances in real treated water from Agbô River (Ivory-Coast). Catal. Today 2017, 281, 2–13. [Google Scholar] [CrossRef]
- Viet, N.M.; Trung, D.Q.; Giang, B.L.; Tri, N.L.M.; Thao, P.; Pham, T.H.; Kamand, F.Z.; Al Tahtamouni, T.M. Noble metal-doped graphitic carbon nitride photocatalyst for enhancement photocatalytic decomposition of antibiotic pollutant in wastewater under visible light. J. Water Process Eng. 2019, 32, 100954. [Google Scholar] [CrossRef]
- Long, Z.; Li, Q.; Wei, T.; Zhang, G.; Ren, Z. Historical development and prospects of photocatalysts for pollutant removal in water. J. Hazard. Mater. 2020, 395, 122599. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Guo, Y.; Guo, R.; Liu, X. A review of visible light-active photocatalysts for water disinfection: Features and prospects. Chem. Eng. J. 2019, 373, 624–641. [Google Scholar] [CrossRef]
- Chuaicham, C.; Pawar, R.R.; Karthikeyan, S.; Ohtani, B.; Sasaki, K. Fabrication and characterization of ternary sepiolite/g-C3N4/Pd composites for improvement of photocatalytic degradation of ciprofloxacin under visible light irradiation. J. Colloid. Interface Sci. 2020, 577, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Zhong, S.; Wang, S.; Lai, Y.; Yang, N.; Jiang, W. Promotion of phenol photodegradation based on novel self-assembled magnetic bismuth oxyiodide core-shell microspheres. RSC Adv. 2017, 7, 36653–36661. [Google Scholar] [CrossRef]
- Chang, M.-J.; Wang, H.; Li, H.-L.; Liu, J.; Du, H.-L. Facile preparation of novel Fe2O3/BiOI hybrid nanostructures for efficient visible light photocatalysis. J. Mater. Sci. 2018, 53, 3682–3691. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, S.; Zhao, L.; Zhao, S. Core/shell Fe3O4/BiOI nanoparticles with high photocatalytic activity and stability. J. Nanoparticle Res. 2016, 18, 318. [Google Scholar] [CrossRef]
- Gao, S.; Guo, C.; Lv, J.; Wang, Q.; Zhang, Y.; Hou, S.; Gao, J.; Xu, J. A novel 3D hollow magnetic Fe3O4/BiOI heterojunction with enhanced photocatalytic performance for bisphenol A degradation. Chem. Eng. J. 2017, 307, 1055–1065. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, H.; Zhang, Y.; Cheng, X.; Zhou, P.; Zhang, G.; Wang, J.; Tang, P.; Ke, T.; Li, W. Heterogeneous activation of persulfate for Rhodamine B degradation with 3D flower sphere-like BiOI/Fe3O4 microspheres under visible light irradiation. Sep. Purif. Technol. 2018, 192, 88–98. [Google Scholar] [CrossRef]
- Yongchun, T.; Min, F.; Jihong, W.; Qiaozhen, H.; Qingyun, W. Solvothermal synthesis and characterization of cobalt blue pigment. J. Solid. State Chem. 2023, 322, 123977. [Google Scholar] [CrossRef]
- Villabona-Leal, E.G.; Flores-Zuñiga, G.; Pérez-Valverde, M.I.; Negrete-Durán, S.E.; Ojeda-Galván, H.J.; Alanis, J.; Velázquez-Galván, Y.G.; Ocampo-Pérez, R.; Ovando-Medina, V.M.; Navarro-Contreras, H.R.; et al. Facile solvothermal synthesis of Exfoliated-Corrugated g-C3N4@BiOBr heterojunction for fast visible light Photocatalyst: A structural and optical study. Appl. Surf. Sci. 2024, 642, 158506. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Wang, J.; Jia, X.; Zhang, H.; Su, F.; Kong, C.; Yang, Z.; Wang, T.; Zhu, H. One-pot solvothermal synthesis of highly dispersed, morphology- and crystal structure-tunable copper-manganese oxide for catalytic combustion of toluene at low temperatures. Mater. Today Commun. 2023, 36, 106947. [Google Scholar] [CrossRef]
- Mehraj, O.; Pirzada, B.M.; Mir, N.A.; Khan, M.Z.; Sabir, S. A highly efficient visible-light-driven novel p-n junction Fe2O3/BiOI photocatalyst: Surface decoration of BiOI nanosheets with Fe2O3 nanoparticles. Appl. Surf. Sci. 2016, 387, 642–651. [Google Scholar] [CrossRef]
- Kamakshi, P.; Joshitha, C.; Chella, S.; Selvaraj, S. Synthesis, characterization of BiOI/rGO nanocomposite and its photocatalytic functionality analysis under visible light. Inorg. Chem. Commun. 2023, 150, 110545. [Google Scholar] [CrossRef]
- Mohamed, H.H.; Besisa, D.H.A.; Besisa, N.; Youssef, T.E. Green magnetite nanostructure for removing organic pollutants from water. Mater. Sci. Eng. B 2023, 296, 116634. [Google Scholar] [CrossRef]
- Epelle, E.I.; Okoye, P.U.; Roddy, S.; Gunes, B.; Okolie, J.A. Advances in the Applications of Nanomaterials for Wastewater Treatment. Environments 2022, 9, 141. [Google Scholar] [CrossRef]
- Reyes-García, J.L.; Arancibia-Bulnes, C.A.; Méndez-Arriaga, F.; Valadés-Pelayo, P.J.; Ramírez Cabrera, M.A. Optical and hydrodynamic performance of photocatalytic monoliths of different shapes in a solar photoreactor with compound parabolic collector. Catal. Today 2024, 429, 114498. [Google Scholar] [CrossRef]
- Younis, S.A.; Kim, K.H. Heterogeneous photocatalysis scalability for environmental remediation: Opportunities and challenges. Catalysts 2020, 10, 1109. [Google Scholar] [CrossRef]
- Ieropoulos, I.A.; Singh, A.; Zertuche Moreno, D.; Greenman, J. Bioelectrochemical systems and their readiness for commercialisation. Curr. Opin. Electrochem. 2024, 46, 101540. [Google Scholar] [CrossRef]
- Hekmatmehr, H.; Esmaeili, A.; Pourmahdi, M.; Atashrouz, S.; Abedi, A.; Ali Abuswer, M.; Nedeljkovic, D.; Latifi, M.; Farag, S.; Mohaddespour, A. Carbon capture technologies: A review on technology readiness level. Fuel 2024, 363, 130898. [Google Scholar] [CrossRef]
- Jiménez-Calvo, P. Synergy of visible-light responsive photocatalytic materials and device engineering for energy and environment: Minireview on hydrogen production and water decontamination. Mater. Today Catal. 2024, 4, 100040. [Google Scholar] [CrossRef]
- Devi, M.; Praharaj, S.; Rout, D. Industrial problems and solution towards visible light photocatalysis. In Nanostructured Materials for Visible Light Photocatalysis; Elsevier: Amsterdam, The Netherlands, 2022; pp. 535–567. [Google Scholar] [CrossRef]
- Li, J.; Yang, F.; Zhou, Q.; Ren, R.; Wu, L.; Lv, Y. A regularly combined magnetic 3D hierarchical Fe3O4/BiOBr heterostructure: Fabrication, visible-light photocatalytic activity and degradation mechanism. J. Colloid. Interface Sci. 2019, 546, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Banyal, R.; Raizada, P.; Ahamad, T.; Kaya, S.; Maslov, M.M.; Chaudhary, V.; Hussain, C.M.; Singh, P. Construction of novel BiOI/CuInS2/ZnO dual S-scheme charge transfer pathway for efficient antibiotic degradation. J. Phys. Chem. Solids 2024, 195, 112132. [Google Scholar] [CrossRef]
- Rueda-Marquez, J.J.; Levchuk, I.; Fernández Ibañez, P.; Sillanpää, M. A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J. Clean. Prod. 2020, 258, 120694. [Google Scholar] [CrossRef]
- Muscetta, M.; Ganguly, P.; Clarizia, L. Solar-powered photocatalysis in water purification: Applications and commercialization challenges. J. Environ. Chem. Eng. 2024, 12, 113073. [Google Scholar] [CrossRef]
- World Bank Group. Solargis. ESMAP: Global Solar Atlas. Available online: https://globalsolaratlas.info/map?s=-29.905867,-71.251772&m=site&c=-29.905867,-71.251772,11 (accessed on 26 August 2024).
- Khairudin, K.; Abu Bakar, N.F.; Osman, M.S. Magnetically recyclable flake-like BiOI-Fe3O4 microswimmers for fast and efficient degradation of microplastics. J. Environ. Chem. Eng. 2022, 10, 108275. [Google Scholar] [CrossRef]
- Xu, H.-Y.; Wang, W.-S.; Li, B.; Zhang, L. Mechanism insights into the enhanced photocatatlytic peroxydisulfate activation by Fe3O4/BiOI heterojunction. Mater. Sci. Eng. B 2023, 294, 116509. [Google Scholar] [CrossRef]
- Yan, X.; Qian, J.; Pei, X.; Zhou, L.; Ma, R.; Zhang, M.; Du, Y.; Bai, L. Enhanced photodegradation of doxycycline (DOX) in the sustainable NiFe2O4/MWCNTs/BiOI system under UV light irradiation. Environ. Res. 2021, 199, 111264. [Google Scholar] [CrossRef]
- Premalatha, N.; Miranda, L.R. A magnetic separable 3D hierarchical BiOI/rGO/Fe3O4 catalyst for degradation of Rhodamine B under visible light: Kinetic studies and mechanism of degradation. Mater. Sci. Eng. B 2022, 276, 115576. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, J.; Wang, H.; Liu, L.; Li, L.; Zheng, S. Nanocomposites of polyethylene with Fe3O4 nanoparticles via surface-initiated ROMP: Thermomechanical, shape memory and photothermal properties. Compos. Part A Appl. Sci. Manuf. 2024, 177, 107923. [Google Scholar] [CrossRef]
- Manda, A.A.; Elsayed, K.A.; Gaya, U.I.; Haladu, S.A.; Ercan, İ.; Ercan, F.; Alheshibri, M.; Al Baroot, A.; Kayed, T.S.; Alshammery, S.; et al. Enhanced photocatalytic degradation of methylene blue by nanocomposites prepared by laser ablation of Bi on CNT-α-Fe2O3 nanoparticles. Opt. Laser Technol. 2022, 155, 108430. [Google Scholar] [CrossRef]
- Pan, M.; Zhang, H.; Gao, G.; Liu, L.; Chen, W. Facet-Dependent Catalytic Activity of Nanosheet-Assembled Bismuth Oxyiodide Microspheres in Degradation of Bisphenol A. Environ. Sci. Technol. 2015, 49, 6240–6248. [Google Scholar] [CrossRef]
- Siciliano, G.; Monteduro, A.G.; Turco, A.; Primiceri, E.; Rizzato, S.; Depalo, N.; Curri, M.L.; Maruccio, G. Polydopamine-Coated Magnetic Iron Oxide Nanoparticles: From Design to Applications. Nanomaterials 2022, 12, 1145. [Google Scholar] [CrossRef]
- Chang, M.-J.; Wang, H.; Liu, J.; Du, H.-L.; Li, H.-L. Facile Synthesis of Fe3O4@BiOI Core/Shell Nanostructures by Magnetic-Assisted Successive Ionic Layer Adsorption and Reaction for Catalytic Application. J. Nanosci. Nanotechnol. 2017, 17, 3759–3764. [Google Scholar] [CrossRef]
- Reyna-Cavazos, K.A.; la Cruz, A.M.; Longoria Rodríguez, F.E.; López-Cuellar, E. Synthesis of bismuth oxyiodide (BiOI) by means of microwaves in glycerol with high photocatalytic activity for the elimination of NOx and SO2. Res. Chem. Intermed. 2020, 46, 923–941. [Google Scholar] [CrossRef]
- Chander, S.; Yadav, S.; Sharma, H.R.; Gupta, A. Sequestration of Cd (II) utilizing biowaste-fabricated recyclable mesoporous magnetite (Fe3O4) nano-adsorbent: Process optimization, thermodynamic investigation, simulation modeling, and feasibility for electroplating effluent. J. Alloys Compd. 2024, 986, 174088. [Google Scholar] [CrossRef]
- Zhao, C.; Liang, Y.; Xu, S.; Fu, J. Photocatalytic activity of hollow mesoporous SiO2-BiOI/CQDs photocatalyst under visible light. J. Mol. Liq. 2024, 396, 124028. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Xie, X.; Li, C.; Si, Y.; Zhang, M.; Yan, Q. Synthesis flower-like BiVO4/BiOI core/shell heterostructure photocatalyst for tetracycline degradation under visible-light irradiation. J. Mater. Sci. Mater. Electron. 2019, 30, 9311–9321. [Google Scholar] [CrossRef]
- Wei, Z.; Zheng, N.; Dong, X.; Zhang, X.; Ma, H.; Zhang, X.; Xue, M. Green and controllable synthesis of one-dimensional Bi2O3/BiOI heterojunction for highly efficient visible-light-driven photocatalytic reduction of Cr(VI). Chemosphere 2020, 257, 127210. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.-H.; Zhang, X.-L.; Zhang, N.; Zhang, J.-Y.; Zhang, R.; Liu, Y.-F.; Fang, Y.-Z. A visible-light driven Bi2S3@ZIF-8 core–shell heterostructure and synergistic photocatalysis mechanism. Dalton Trans. 2018, 47, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Haryński, Ł.; Olejnik, A.; Grochowska, K.; Siuzdak, K. A facile method for Tauc exponent and corresponding electronic transitions determination in semiconductors directly from UV–Vis spectroscopy data. Opt. Mater. 2022, 127, 112205. [Google Scholar] [CrossRef]
- Delice, S.; Isik, M.; Gasanly, N.M. Temperature-dependent tuning of band gap of Fe3O4 nanoparticles for optoelectronic applications. Chem. Phys. Lett. 2024, 840, 141139. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, J.; Zhang, X.; Chen, F.; Tu, S.; Huang, H. Chemically bonded CdBiO2Br/BiOI heterojunction with strong interfacial electric field for enhanced photocatalysis. Appl. Surf. Sci. 2024, 672, 160869. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, R.; Ding, M.; Chen, G.; Liang, S.; Sun, S. Engineering of Bi2O3-BiOI 2D/2D S-scheme heterojunction for efficient photocatalytic organic hazards removal. Sep. Purif. Technol. 2024, 354, 129136. [Google Scholar] [CrossRef]
- Comak, G.; Bayram, G.; Görmez, Ö.; Çağlayan, U.; Gözmen, B. Synthesis of biomass-based BiOI@Hydrochar heterogeneous catalyst and investigation of its activity in sonocatalytic process. Desalination Water Treat. 2024, 320, 100625. [Google Scholar] [CrossRef]
- Arief, S.; Muldarisnur, M.; Usna, S.R. Enhancement in photoluminescence performance of carbon-based Fe3O4@ZnO–C nanocomposites. Vacuum 2023, 211, 111935. [Google Scholar] [CrossRef]
- Santos, G.T.A.D.; Estrada, A.C.; Amorim, C.O.; Amaral, J.S.; Deuermeier, J.; Duarte, A.C.; Santos, P.S.M. Hybrid nanocomposites of Fe3O4/SiO2-EDTA: Holistic comparison of one-step and two-step modification methods. Powder Technol. 2024, 444, 120046. [Google Scholar] [CrossRef]
- Yadav, S.; Chander, S.; Gupta, A. Microbially synthesised Zn@Fe3O4(M) and Zn@Fe3O4(ME) nanocomposites for textile effluent: Central composite design optimisation and kinetic modelling. Surf. Interfaces 2024, 46, 104058. [Google Scholar] [CrossRef]
- Xie, X.; Liu, Y.; Dong, X.; Lin, C.; Wen, X.; Yan, Q. Synthesis and characterization of Fe3O4/BiOI n-p heterojunction magnetic photocatalysts. Appl. Surf. Sci. 2018, 455, 742–747. [Google Scholar] [CrossRef]
- Fathollahi-Fard, A.M.; Ahmadi, A.; Al-e-Hashem, S.M.J.M. Sustainable closed-loop supply chain network for an integrated water supply and wastewater collection system under uncertainty. J. Environ. Manag. 2020, 275, 111277. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.; Gu, J.; Zhang, M.; Liu, Y. Towards carbon neutrality and water sustainability: An integrated anaerobic fixed-film MBR-reverse osmosis-chlorination process for municipal wastewater reclamation. Chemosphere 2022, 287, 132060. [Google Scholar] [CrossRef]
- Benra, F.; de Frutos, A.; Gaglio, M.; Álvarez-Garretón, C.; Felipe-Lucia, M.; Bonn, A. Mapping water ecosystem services: Evaluating InVEST model predictions in data scarce regions. Environ. Model. Softw. 2021, 138, 104982. [Google Scholar] [CrossRef]
- Jung, D.; Schönberger, F.; Moraga, F.; Ilgen, K.; Wieland, S. Floating photovoltaic in Chile: Potential for clean energy generation and water protection. Sustain. Energy Technol. Assess. 2024, 63, 103647. [Google Scholar] [CrossRef]
- Regional Water Center for Arid and Semi-Arid Zones in Latin America and the Caribbean (CAZALAC); Corporación Regional de Desarrollo Productivo (CRDP-Región de Coquimbo); Gobierno Regional de Coquimbo (GORE-Coquimbo). Estrategia Regional de Recursos Hídricos por Cuenca, 2014–2030. Available online: https://www.cazalac.org/wp-content/uploads/2023/06/Estrategia-Regional-de-Recursos-Hidricos.pdf (accessed on 1 September 2024).
- Rodríguez, C.; Carrasco, F.; Sánchez, R.; Rebolledo, N.; Schneider, N.; Serrano, J.; Leiva, E. Performance and treatment assessment of a pilot-scale decentralized greywater reuse system in rural schools of north-central Chile. Ecol. Eng. 2022, 174, 106460. [Google Scholar] [CrossRef]
- Orrego, C.; Muñoz, C.; Caballero, T.; Salinas, P.; Maldonado, P.; Molina, P.; Zavala, M.; Velasco, C.; Guerrero, J.; Guggiana, C.; et al. Boletín Climático CEAZA. August 2024. Available online: https://boletin.ceazamet.cl/images/boletin/boletin.ceazamet.2024.08.pdf (accessed on 6 September 2024).
- Maeseele, C.; Roux, P. An LCA framework to assess environmental efficiency of water reuse: Application to contrasted locations for wastewater reuse in agriculture. J. Clean. Prod. 2021, 316, 128151. [Google Scholar] [CrossRef]
- Tahmasebi, A.; Ghaffari, S.-B.; Sarrafzadeh, M.-H. Techno-economic comparison of two hydroxyl and sulfate radicals based advanced oxidation process for enhancing biodegradability of pulp and paper mill wastewater. Chem. Eng. Sci. 2024, 293, 120095. [Google Scholar] [CrossRef]
- Wei, G.; Wei, T.; Li, Z.; Wei, C.; Kong, Q.; Guan, X.; Qiu, G.; Hu, Y.; Wei, C.; Zhu, S.; et al. BOD/COD ratio as a probing index in the O/H/O process for coking wastewater treatment. Chem. Eng. J. 2023, 466, 143257. [Google Scholar] [CrossRef]
- Bendeif, S.; Kadi, K.; Arhab, R.; Ziegler-Devin, I.; Brosse, N.; Addad, D. HPAEC-PAD, biochemical characterization, and evaluation of the antioxidants activities of polysaccharides extracted from Olive Mill Wastewater of two endemic varieties of Khenchela region, Algeria. Bioact. Carbohydr. Diet. Fibre 2023, 30, 100363. [Google Scholar] [CrossRef]
- Rice, E.W.; Bridgewater, L.; American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Maffessoni, D.; Grazziotin, I.C.; Klauck, C.R.; Benvenuti, T.; da Silva, S.W.; Meneguzzi, A. Heterogeneous photocatalysis of moxifloxacin at a pilot solar compound parabolic collector: Elimination of the genotoxicity. J. Environ. Manag. 2021, 297, 113296. [Google Scholar] [CrossRef]
- Bhargava, N.; Bahadur, N.; Kansal, A. Techno-economic assessment of integrated photochemical AOPs for sustainable treatment of textile and dyeing wastewater. J. Water Process Eng. 2023, 56, 104302. [Google Scholar] [CrossRef]
- Moreira, V.R.; Carpanez, T.G.; Santos, F.S.; dos Santos, L.S.; Fernandes, D.d.S.; França-Neta, L.S.; Lange, L.C.; Amaral, M.C.S. Circular economy in biorefineries: Scale-up of anaerobic/aerobic membrane bioreactors for vinasse recycling. J. Clean. Prod. 2022, 377, 134448. [Google Scholar] [CrossRef]
- Silva, A.F.R.; Magalhães, N.C.; Cunha, P.V.M.; Amaral, M.C.S.; Koch, K. Influence of COD/SO42− ratio on vinasse treatment performance by two-stage anaerobic membrane bioreactor. J. Environ. Manag. 2020, 259, 110034. [Google Scholar] [CrossRef]
- Carpanez, T.G.; Moreira, V.R.; Magalhães, N.C.; Assis, I.R.; Lange, L.C.; Amaral, M.C.S. Integrated membrane-based processes to obtain organo-mineral fertilizer, water, and energy from sugarcane vinasse. Sep. Purif. Technol. 2022, 302, 122180. [Google Scholar] [CrossRef]
- An, Z.; Zhu, J.; Zhang, M.; Zhou, Y.; Su, X.; Lin, H.; Sun, F. Anaerobic membrane bioreactor for the treatment of high-strength waste/wastewater: A critical review and update. Chem. Eng. J. 2023, 470, 144322. [Google Scholar] [CrossRef]
- Ngulube, K.F.; Abdelhaleem, A.; Osman, A.I.; Peng, L.; Nasr, M. Advancing sustainable water treatment strategies: Harnessing magnetite-based photocatalysts and techno-economic analysis for enhanced wastewater management in the context of SDGs. Environ. Sci. Pollut. Res. 2024, 1–37. [Google Scholar] [CrossRef]
- Osman, A.I.; Elgarahy, A.M.; Eltaweil, A.S.; Abd El-Monaem, E.M.; El-Aqapa, H.G.; Park, Y.; Hwang, Y.; Ayati, A.; Farghali, M.; Ihara, I.; et al. Biofuel production, hydrogen production and water remediation by photocatalysis, biocatalysis and electrocatalysis. Environ. Chem. Lett. 2023, 21, 1315–1379. [Google Scholar] [CrossRef]
- Abdel Maksoud, M.I.A.; Elgarahy, A.M.; Farrell, C.; Al-Muhtaseb, A.H.; Rooney, D.W.; Osman, A.I. Insight on water remediation application using magnetic nanomaterials and biosorbents. Coord. Chem. Rev. 2020, 403, 213096. [Google Scholar] [CrossRef]
- Tawfik, A.; Alalm, M.G.; Awad, H.M.; Islam, M.; Qyyum, M.A.; Al-Muhtaseb, A.H.; Osman, A.I.; Lee, M. Solar photo-oxidation of recalcitrant industrial wastewater: A review. Environ. Chem. Lett. 2022, 20, 1839–1862. [Google Scholar] [CrossRef]
- Rivera, N.M.; Ruiz-Tagle, J.C.; Spiller, E. The health benefits of solar power generation: Evidence from Chile. J. Environ. Econ. Manag. 2024, 126, 102999. [Google Scholar] [CrossRef]
- Moreno-SanSegundo, J.; Martín-Sómer, M.; Marugán, J. Dynamic concentration factor: A novel parameter for the rigorous evaluation of solar compound parabolic collectors. Chem. Eng. J. 2022, 437, 135360. [Google Scholar] [CrossRef]
- Sreedhar, A.; Ta, Q.T.H.; Noh, J.-S. Advancements in the photocatalytic activity of various bismuth-based semiconductor/Ti3C2 MXene interfaces for sustainable environmental management: A review. J. Ind. Eng. Chem. 2022, 115, 26–47. [Google Scholar] [CrossRef]
- Leontyeva, X.A.; Puzikova, D.S.; Dergacheva, M.B.; Khussurova, G.M.; Panchenko, P.V. Synthesis and properties of semiconductor bismuth sulfide iodide for photoelectrochemical applications. J. Saudi Chem. Soc. 2023, 27, 101694. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, Q.; Liu, Y.; Shi, Y.; Qin, Z. Preparation of Fe3O4/TiO2 magnetic photocatalyst for photocatalytic degradation of phenol. J. Mater. Sci. Mater. Electron. 2018, 29, 8258–8266. [Google Scholar] [CrossRef]
- Li, Z.-J.; Huang, Z.-W.; Guo, W.-L.; Wang, L.; Zheng, L.-R.; Chai, Z.-F.; Shi, W.-Q. Enhanced Photocatalytic Removal of Uranium (VI) from Aqueous Solution by Magnetic TiO2/Fe3O4 and Its Graphene Composite. Environ. Sci. Technol. 2017, 51, 5666–5674. [Google Scholar] [CrossRef]
- Jogaiah, S.; Paidi, M.K.; Venugopal, K.; Geetha, N.; Mujtaba, M.; Udikeri, S.S.; Govarthanan, M. Phytotoxicological effects of engineered nanoparticles: An emerging nanotoxicology. Sci. Total Environ. 2021, 801, 149809. [Google Scholar] [CrossRef]
- Liu, M.; Ye, Y.; Ye, J.; Gao, T.; Wang, D.; Chen, G.; Song, Z. Recent Advances of Magnetite (Fe3O4)-Based Magnetic Materials in Catalytic Applications. Magnetochemistry 2023, 9, 110. [Google Scholar] [CrossRef]
- Bondarenko, L.; Kahru, A.; Terekhova, V.; Dzhardimalieva, G.; Uchanov, P.; Kydralieva, K. Effects of Humic Acids on the Ecotoxicity of FeFe3O4 Nanoparticles and Fe-Ions: Impact of Oxidation and Aging. Nanomaterials 2020, 10, 2011. [Google Scholar] [CrossRef]
- Zulkiflee, A.; Khan, M.M.; Harunsani, M.H. Bismuth oxyhalides: Recent progress and its applications in photocatalysis, hydrogen production, antibacterial studies, and sensors. Mater. Sci. Semicond. Process 2023, 163, 107547. [Google Scholar] [CrossRef]
- Suzuki, H.; Matano, Y. Introduction. In Organobismuth Chemistry; Elsevier: Amsterdam, The Netherlands, 2001; pp. 1–20. [Google Scholar] [CrossRef]
- EPA. United States Enviromental Protection Agency. Available online: https://www.epa.gov/ (accessed on 4 August 2024).
- EPA. RCRA 94-580 Resource Conservation and Recovery Act. 1976. Available online: https://www.epa.gov/rcra (accessed on 26 August 2024).
- 2008/98/EC. Directive of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives. 2008. Available online: http://data.europa.eu/eli/dir/2008/98/oj (accessed on 26 August 2024).
- LWP 2014/955/EUEU. Commission Decision of 18 December 2014 Amending Decision 2000/532/EC on the List of Waste Pursuant to Directive 2008/98/EC of the European Parliament and of the Council Text with EEA Relevance. 2014. Available online: http://data.europa.eu/eli/dec/2014/955/oj (accessed on 26 August 2024).
- (EC) No 1272/2008. Regulation of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006. 2008. Available online: http://data.europa.eu/eli/reg/2008/1272/oj (accessed on 26 August 2024).
- DS. 148, Art. 11. Aprueba Reglamento Sanitario Sobre Manejo de Residuos Peligroso. 2004. Available online: https://www.bcn.cl/leychile/navegar?idNorma=226458 (accessed on 26 August 2024).
- RE. 777. Aprueba Listado Oficial de Clasificación de Sustancias, Según Artículo 6° del DS NN° 57, de 2019, del Ministerio de Salud. 2021. Available online: https://www.bcn.cl/leychile/navegar?idNorma=1164063 (accessed on 26 August 2024).
- Montwedi, M.; Munyaradzi, M.; Pinoy, L.; Dutta, A.; Ikumi, D.S.; Motoasca, E.; Van der Bruggen, B. Resource recovery from and management of wastewater in rural South Africa: Possibilities and practices. J. Water Process Eng. 2021, 40, 101978. [Google Scholar] [CrossRef]
- Mei, J.; Gao, X.; Zou, J.; Pang, F. Review Research on Photocatalytic Wastewater Treatment Reactors: Design, Optimization, and Evaluation Criteria. Catalysts 2023, 13, 974. [Google Scholar] [CrossRef]

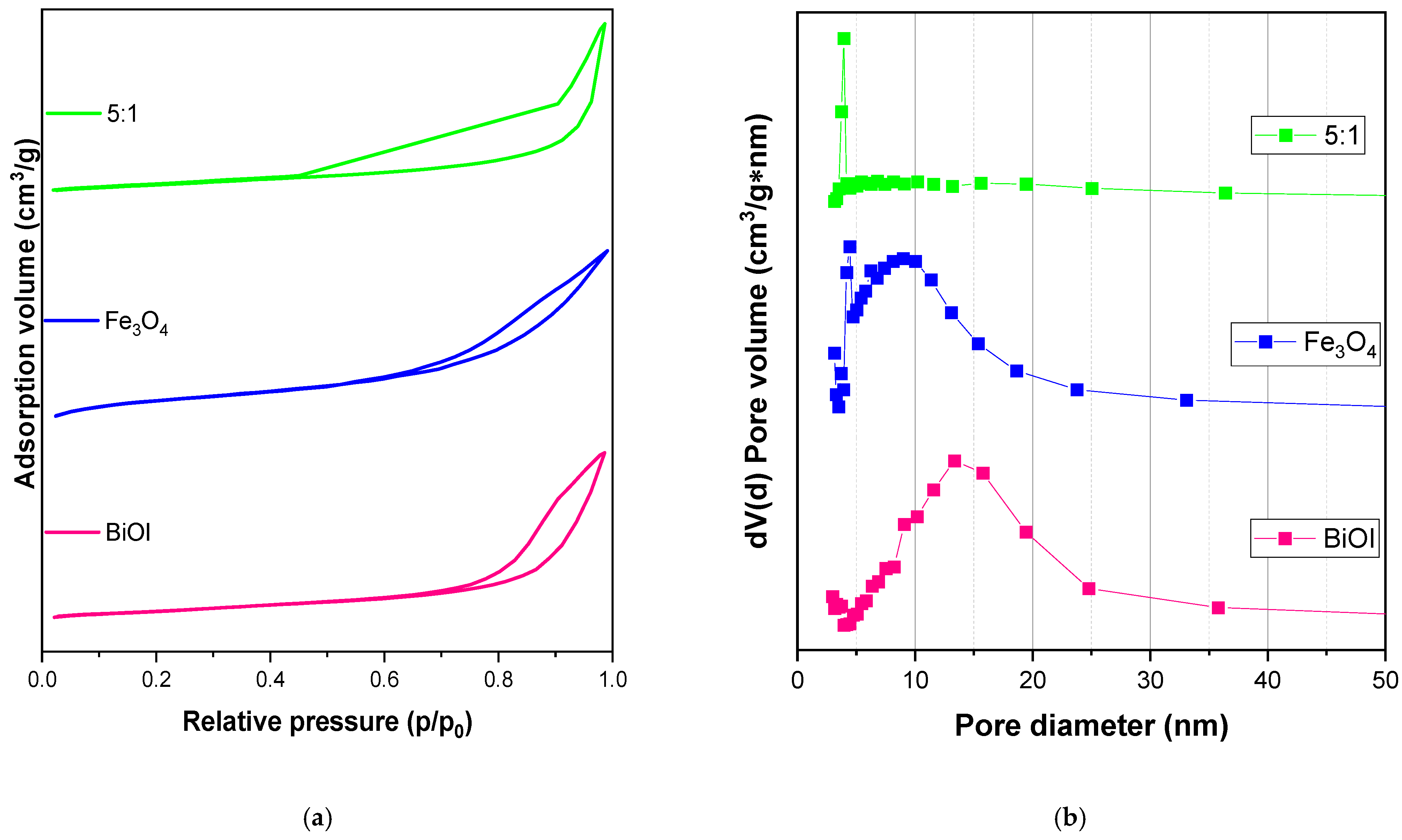
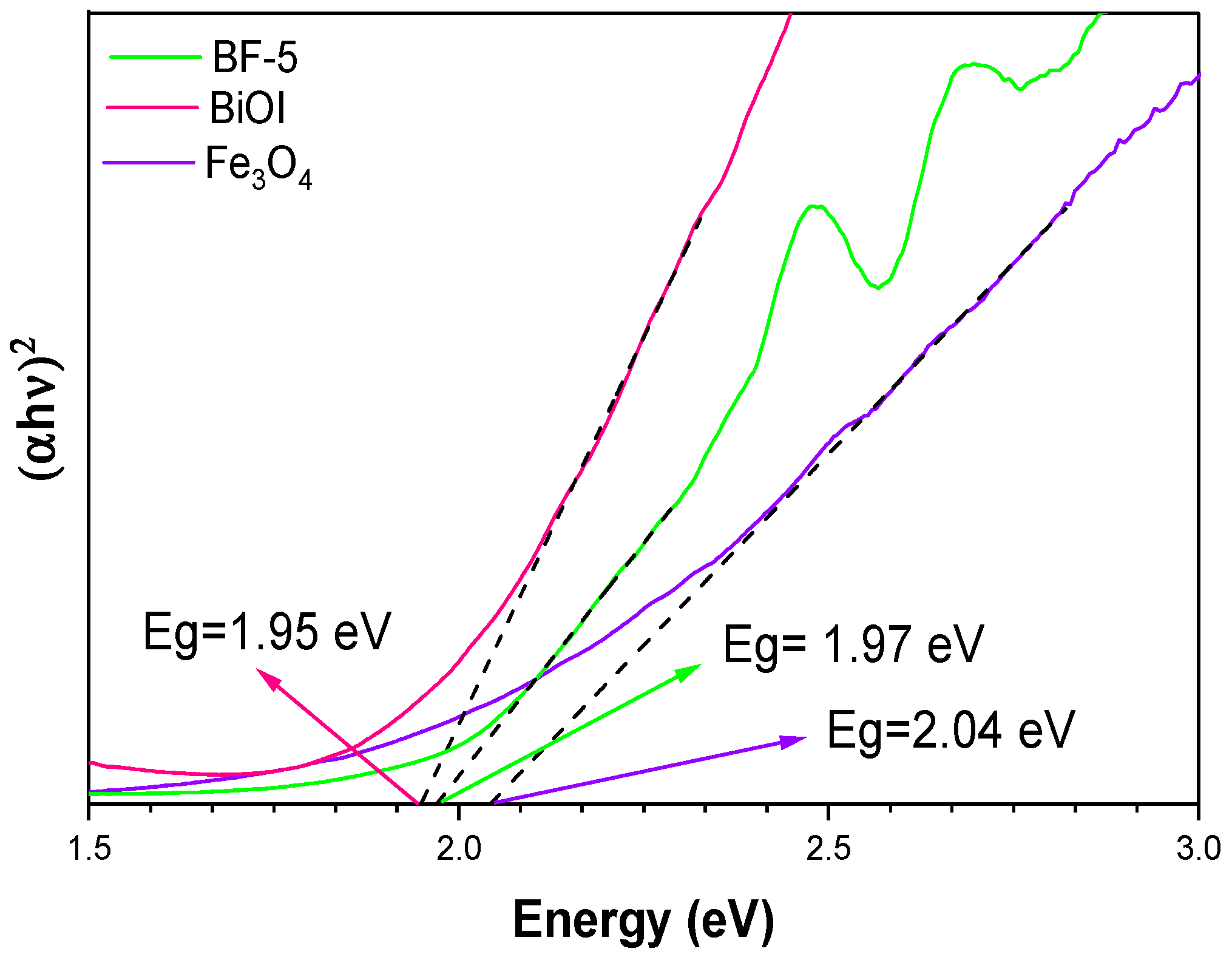
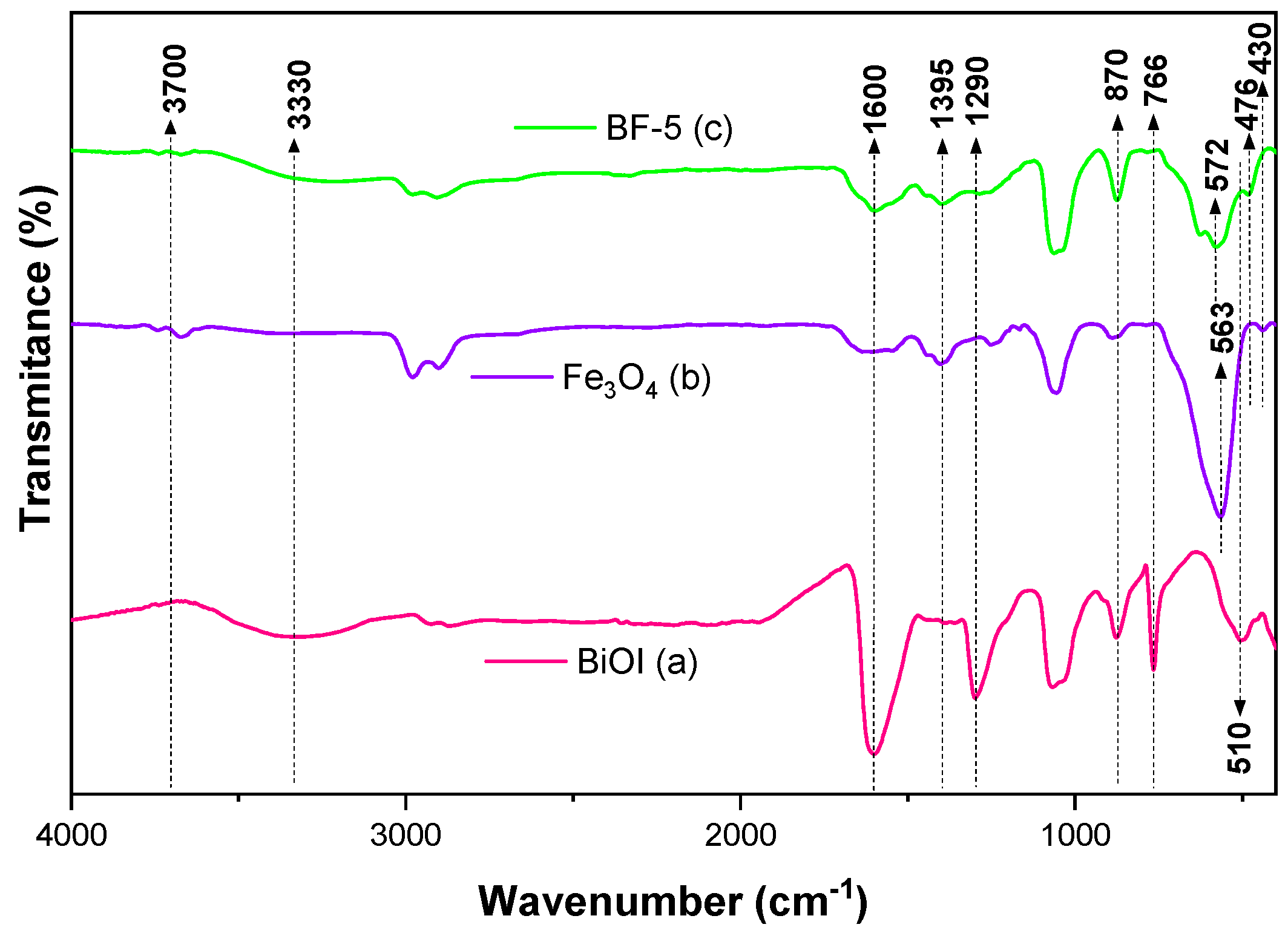

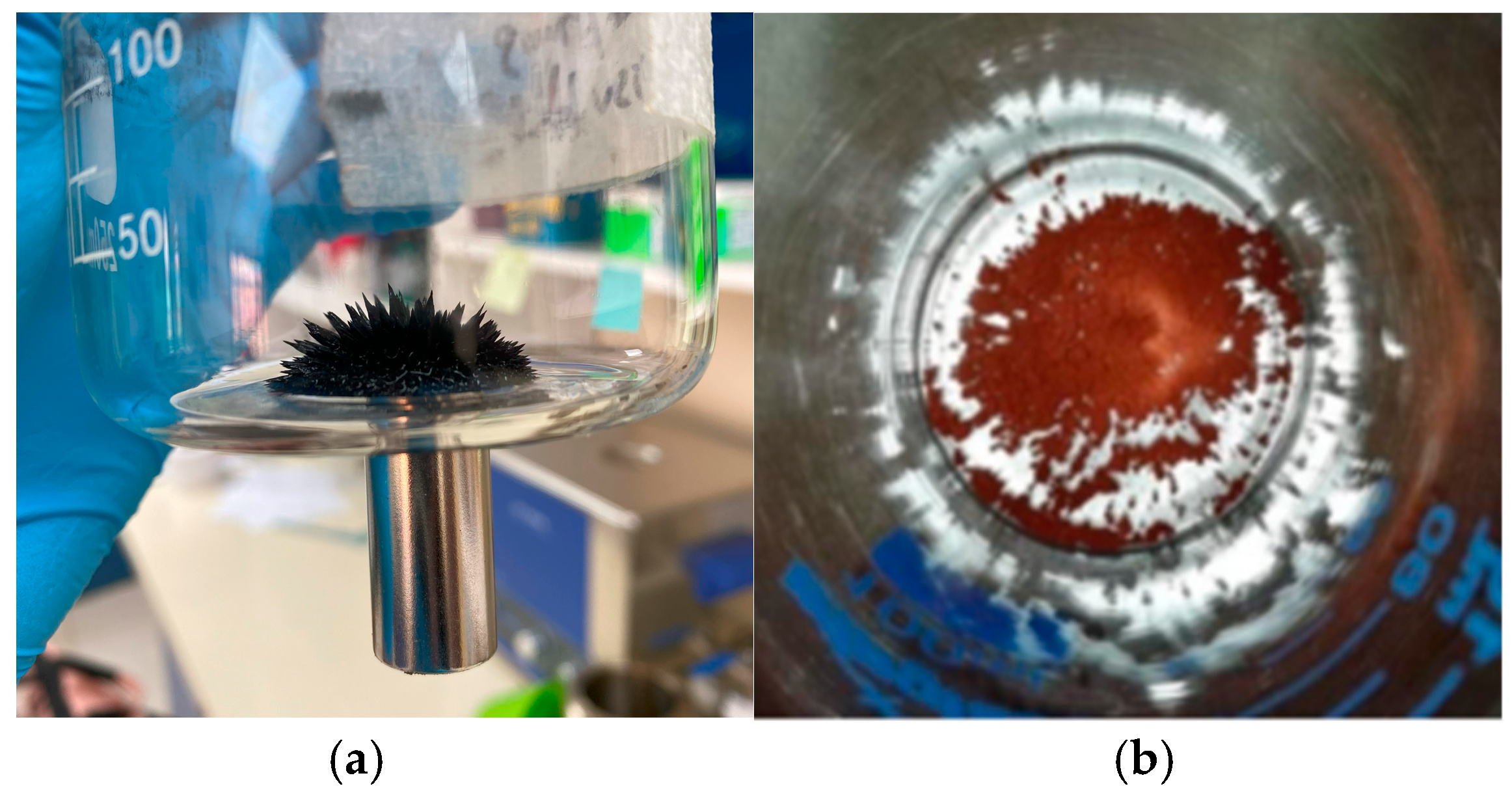
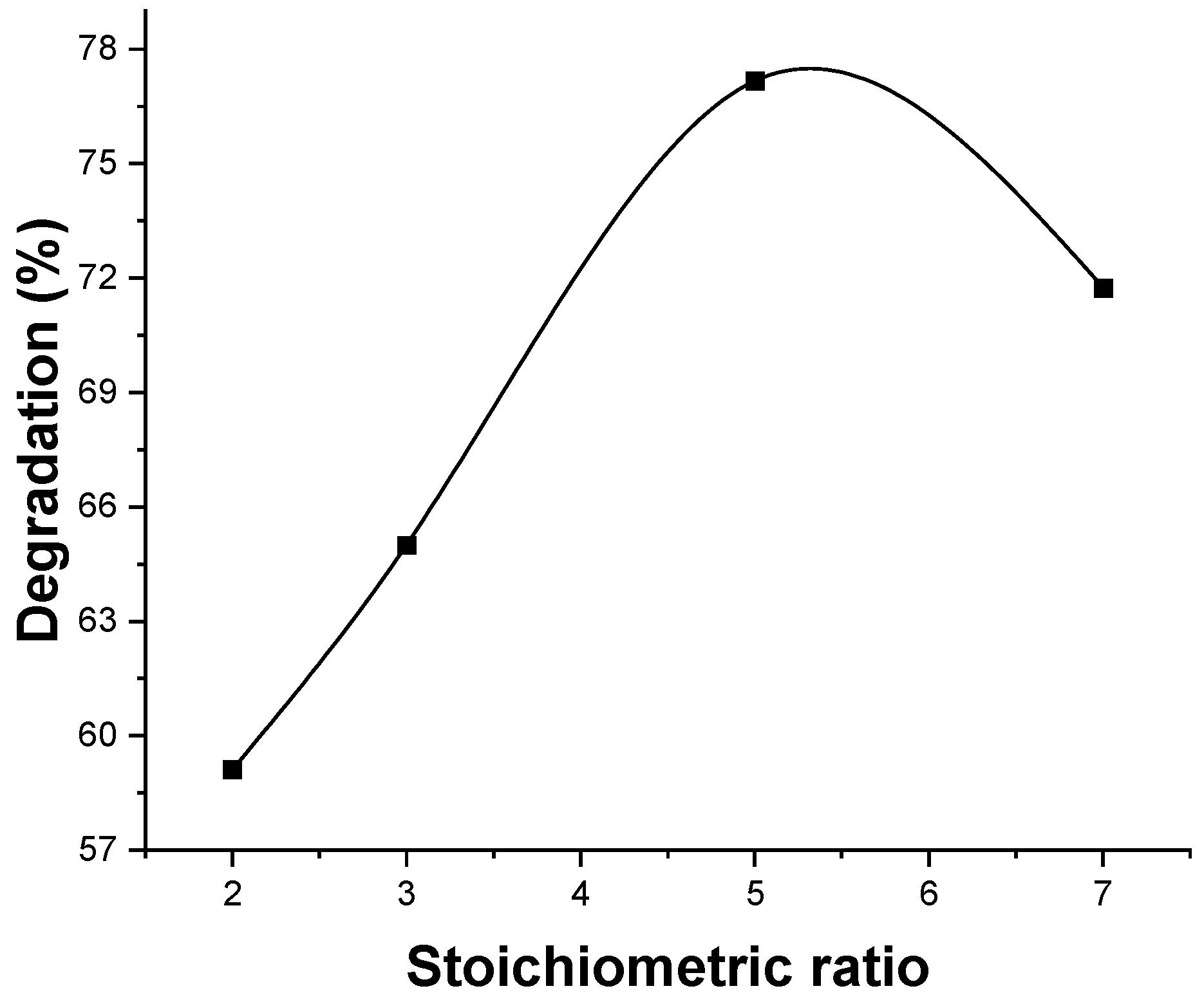

| Materials | BET (m2 g−1) | Pore Diameter (nm) | Pore Volume (cm3 g−1) | Eg (eV) |
|---|---|---|---|---|
| Pure BiOI | 61 | 13.34 | 0.250 | 1.95 |
| Fe3O4 | 16 | 4.42 | 0.032 | 2.04 |
| BiOI/Fe3O4 | 20 | 3.95 | 0.056 | 1.97 |
| Parameter | Unit | Value |
|---|---|---|
| Chemical oxygen demand (COD) | mg/L | 11978 |
| Biochemical oxygen demand (BOD5) | mg/L | 2400 |
| BOD5/COD | - | 0.20 |
| pH | - | 3.73 |
| Turbidity | NTU | 309 |
| Electrical conductivity (EC) | mS/cm | 2.97 |
| Total suspended solids (TSS) | mg/L | 733.30 |
| Description | Cost * (USD/h) Solvothermal | Cost * (USD/h) Hydrothermal |
|---|---|---|
| A photocatalytic system with a solar CPC reactor, includes a hydraulic pump (USD 6807 a, service life 15 years) | 0.218 | 0.218 |
| High gradient magnetic separator, operates 1 h/day (USD 68.98, service life 15 years) | 0.002 | 0.002 |
| Wastewater feeding system, considering hydraulic passage pump and PVC pipes. (USD 96.26, service life—5 years) | 0.009 | 0.009 |
| Energy cost (step pumps and CPC system) | 0.004 | 0.004 |
| Labor b, considers system operation and maintenance. | 2.900 | 2.900 |
| Raw material c | 13.044 | 1.100 |
| Total (USD/hour) | 16.178 | 4.234 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallegos-Alcaíno, A.; Barría, G.P.; Moreno, Y.; Fernández, I.; Poblete, R.; Maureira-Cortés, H.; Figueroa Alvarado, A.C.; Hernández, C.B.; Flores, J. Nanostructured Magnetite Coated with BiOI Semiconductor: Readiness Level in Advanced Solar Photocatalytic Applications for the Remediation of Phenolic Compounds in Wastewater from the Wine and Pisco Industry. Appl. Sci. 2024, 14, 9898. https://doi.org/10.3390/app14219898
Gallegos-Alcaíno A, Barría GP, Moreno Y, Fernández I, Poblete R, Maureira-Cortés H, Figueroa Alvarado AC, Hernández CB, Flores J. Nanostructured Magnetite Coated with BiOI Semiconductor: Readiness Level in Advanced Solar Photocatalytic Applications for the Remediation of Phenolic Compounds in Wastewater from the Wine and Pisco Industry. Applied Sciences. 2024; 14(21):9898. https://doi.org/10.3390/app14219898
Chicago/Turabian StyleGallegos-Alcaíno, Alejandra, Gabriela Paz Barría, Yanko Moreno, Iván Fernández, Rodrigo Poblete, Héctor Maureira-Cortés, Antonia Cristal Figueroa Alvarado, Constanza Belén Hernández, and José Flores. 2024. "Nanostructured Magnetite Coated with BiOI Semiconductor: Readiness Level in Advanced Solar Photocatalytic Applications for the Remediation of Phenolic Compounds in Wastewater from the Wine and Pisco Industry" Applied Sciences 14, no. 21: 9898. https://doi.org/10.3390/app14219898
APA StyleGallegos-Alcaíno, A., Barría, G. P., Moreno, Y., Fernández, I., Poblete, R., Maureira-Cortés, H., Figueroa Alvarado, A. C., Hernández, C. B., & Flores, J. (2024). Nanostructured Magnetite Coated with BiOI Semiconductor: Readiness Level in Advanced Solar Photocatalytic Applications for the Remediation of Phenolic Compounds in Wastewater from the Wine and Pisco Industry. Applied Sciences, 14(21), 9898. https://doi.org/10.3390/app14219898







