Acidity and Salinization of Soil Following the Application of Ashes from Biomass Combustion Under Different Crop Plant Species Cultivation
Abstract
1. Introduction
2. Materials and Methods
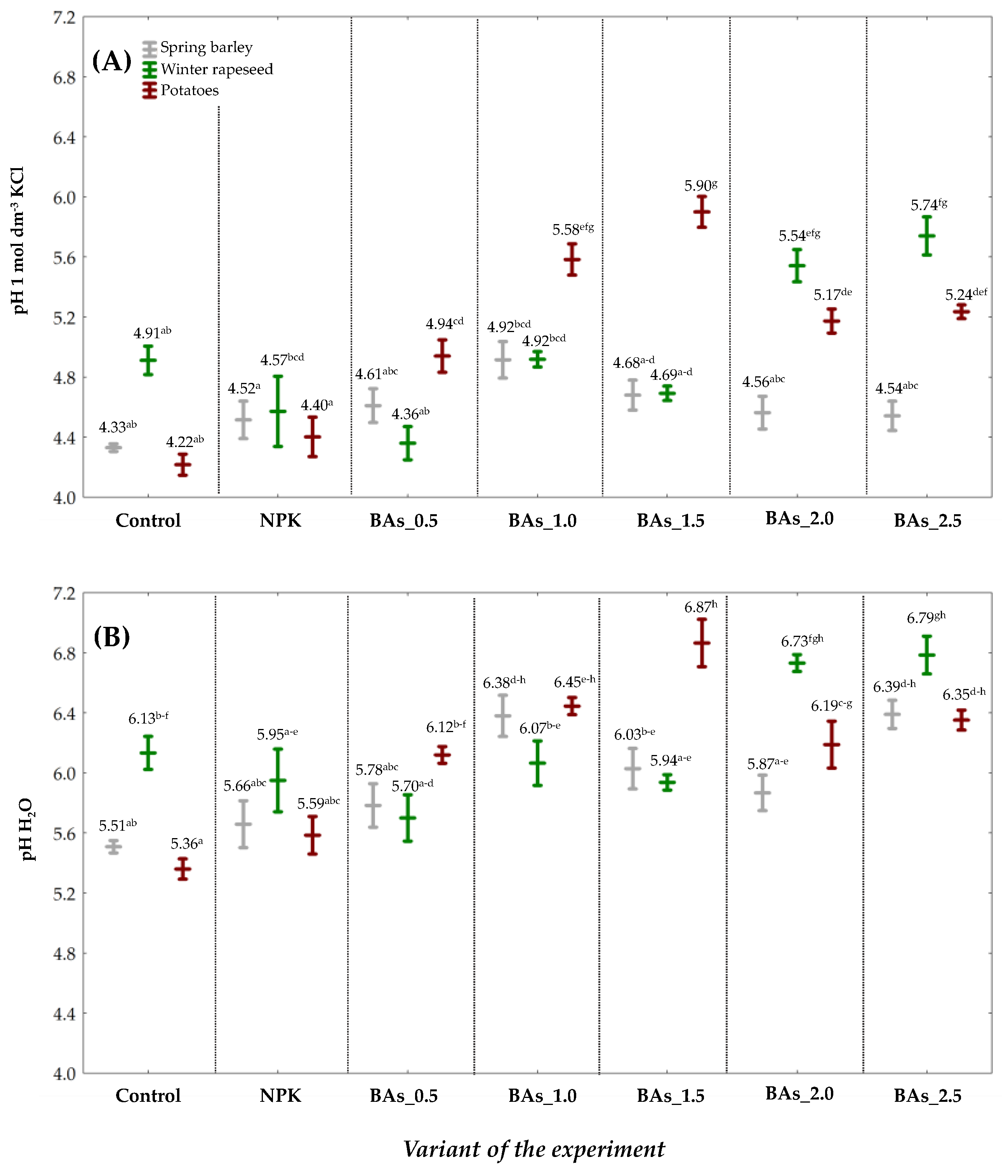
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehmusto, J.; Tesfaye, F.; Karlström, O.; Hupa, L. Ashes from Challenging Fuels in the Circular Economy. Waste Manag. 2024, 177, 211–231. [Google Scholar] [CrossRef] [PubMed]
- DIRECTIVE (EU) 2018/2001 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL—Of 11 December 2018—On the Promotion of the Use of Energy from Renewable Sources. Available online: https://eur-lex.europa.eu/eli/dir/2018/2001/oj (accessed on 28 September 2024).
- Hubert, J.; Grigoletto, S.; Michel, F.; Zhao, Z.; Courard, L. Development and Properties of Recycled Biomass Fly Ashes Modified Mortars. Recycling 2024, 9, 46. [Google Scholar] [CrossRef]
- Communication from the Commission to the European Parliament, The Council, The European Economic and Social Committee and the Committee of the Regions Closing the Loop—An EU Action Plan for the Circular Economy. 2015. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:8a8ef5e8-99a0-11e5-b3b7-01aa75ed71a1.0012.02/DOC_1&format=PDF (accessed on 28 September 2024).
- Thomas, B.S.; Yang, J.; Mo, K.H.; Abdalla, J.A.; Hawileh, R.A.; Ariyachandra, E. Biomass Ashes from Agricultural Wastes as Supplementary Cementitious Materials or Aggregate Replacement in Cement/Geopolymer Concrete: A Comprehensive Review. J. Build. Eng. 2021, 40, 102332. [Google Scholar] [CrossRef]
- Schiemenz, K.; Eichler-Löbermann, B. Biomass Ashes and Their Phosphorus Fertilizing Effect on Different Crops. Nutr Cycl Agroecosyst 2010, 87, 471–482. [Google Scholar] [CrossRef]
- Latawiec, A.E.; Rodrigues, A.; Korys, K.A.; Medeiros, B. Methodical Aspects of Soil Ecosystem Services Valuation. Agric. Eng. 2022, 26, 39–49. [Google Scholar] [CrossRef]
- Patterson, S.J.; Acharya, S.N.; Thomas, J.E.; Bertschi, A.B.; Rothwell, R.L. Barley Biomass and Grain Yield and Canola Seed Yield Response to Land Application of Wood Ash. Agron. J. 2004, 96, 971–977. [Google Scholar] [CrossRef]
- Shi, R.; Li, J.; Jiang, J.; Mehmood, K.; Liu, Y.; Xu, R.; Qian, W. Characteristics of Biomass Ashes from Different Materials and Their Ameliorative Effects on Acid Soils. J. Environ. Sci. 2017, 55, 294–302. [Google Scholar] [CrossRef]
- Cruz, N.C.; Silva, F.C.; Tarelho, L.A.C.; Rodrigues, S.M. Critical Review of Key Variables Affecting Potential Recycling Applications of Ash Produced at Large-Scale Biomass Combustion Plants. Resour. Conserv. Recycl. 2019, 150, 104427. [Google Scholar] [CrossRef]
- Szostek, M.; Szpunar-Krok, E.; Jańczak-Pieniążek, M.; Ilek, A. Short-Term Effect of Fly Ash from Biomass Combustion on Spring Rape Plants Growth, Nutrient, and Trace Elements Accumulation, and Soil Properties. Int. J. Environ. Res. Public Health 2022, 20, 455. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Vicente, E.D.; Gomes, A.P.; Nunes, M.I.; Alves, C.; Tarelho, L.A.C. Effect of Industrial and Domestic Ash from Biomass Combustion, and Spent Coffee Grounds, on Soil Fertility and Plant Growth: Experiments at Field Conditions. Environ. Sci. Pollut. Res. 2017, 24, 15270–15277. [Google Scholar] [CrossRef]
- Demeyer, A.; Voundi Nkana, J.C.; Verloo, M.G. Characteristics of Wood Ash and Influence on Soil Properties and Nutrient Uptake: An Overview. Bioresour. Technol. 2001, 77, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Poluszyńska, J. Możliwości zastosowania popiołów ze spalania biomasy w gospodarowaniu osadami ściekowymi. Pract. Inst. Ceram. I Mater. Bud. 2013, 6, 49–59. [Google Scholar]
- Piekarczyk, M.; Kotwica, K.; Jaskulski, D. Effect of spring barley straw ash on the chemical properties of light soil. Fragm. Agron. 2011, 28, 91–99. Available online: https://pta.up.poznan.pl/pdf/2011/FA%2028(3)%202011%20Piekarczyk.pdf (accessed on 28 September 2024). (In Polish).
- Ochal, P.; Smreczak, B. Soil acidification and current issues of liming. Studia i Raporty IUNG-PIB 2020, 63, 9–19. (In Polish) [Google Scholar] [CrossRef]
- Barwicki, J.; Borusiewicz, A.; Holden, L.; Kulcsar, L.; Skibko, Z.; Żuchowski, I.; Romaniuk, W. Leaching of Elements from Soil in Grassland Field Crops Treated with Raw and Acidified Slurry. Agric. Eng. 2022, 26, 145–156. [Google Scholar] [CrossRef]
- Mostafazadeh, B.; Fard; Heidarpour, M.; Aghakhani, A.; Feizi, M. Effect of Irrigation Water Salinity and Leaching on Soil Chemical Properties in an Arid Region. Int. J. Agric. Biol. 1560, 8530, 3–9. [Google Scholar]
- Trivedi, N.S.; Mandavgane, S.A.; Mehetre, S.; Kulkarni, B.D. Characterization and Valorization of Biomass Ashes. Environ. Sci. Pollut. Res. 2016, 23, 20243–20256. [Google Scholar] [CrossRef]
- Huotari, N.; Tillman-Sutela, E.; Kubin, E. Ground Vegetation Has a Major Role in Element Dynamics in an Ash-Fertilized Cut-Away Peatland. For. Ecol. Manag. 2011, 261, 2081–2088. [Google Scholar] [CrossRef]
- Kramar, V.G. PROBLEMS OF BIOMASS ASH UTILIZATION FROM BOILER HOUSES IN UKRAINE. Thermophys. Therm. Power Eng. 2021, 43, 71–77. [Google Scholar] [CrossRef]
- Grzebisz, W.; Zielewicz, W.; Przygocka-Cyna, K. Deficiencies of Secondary Nutrients in Crop Plants—A Real Challenge to Improve Nitrogen Management. Agronomy 2022, 13, 66. [Google Scholar] [CrossRef]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical Characteristics of Biomass Ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Yu, C.-L.; Deng, Q.; Jian, S.; Li, J.; Dzantor, E.K.; Hui, D. Effects of Fly Ash Application on Plant Biomass and Element Accumulations: A Meta-Analysis. Environ. Pollut. 2019, 250, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Harinarayanan, M.N.; Ramanatha, S.; Nagarajan, K.; Kalarani, M.K.; Sathyamoorthy, N.K.; Geethalakshmi, I.; Ramya, S. Soil and Water Conservation Engineering Techniques to Modify the Rhizosphere Climate of Cucumber (Cucumis sativus L.) for Improving Water Use Efficiency and Yield. Agric. Eng. 2022, 26, 201–213. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Qu, Z.; Fatehi, H.; Schmidt, F.M. Potassium Release from Biomass Particles during Combustion—Real-Time In Situ TDLAS Detection and Numerical Simulation. Appl. Sci. 2021, 11, 8887. [Google Scholar] [CrossRef]
- Szostek, M.; Szpunar-Krok, E.; Ilek, A. Chemical Speciation of Trace Elements in Soil Fertilized with Biomass Combustion Ash and Their Accumulation in Winter Oilseed Rape Plants. Agronomy 2023, 13, 942. [Google Scholar] [CrossRef]
- Szpunar-Krok, E.; Szostek, M.; Pawlak, R.; Gorzelany, J.; Migut, D. Effect of Fertilisation with Ash from Biomass Combustion on the Mechanical Properties of Potato Tubers (Solanum tuberosum L.) Grown in Two Types of Soil. Agronomy 2022, 12, 379. [Google Scholar] [CrossRef]
- Pycia, K.; Szupnar-Krok, E.; Szostek, M.; Pawlak, R.; Juszczak, L. Effect of Soil Type and Application of Ecological Fertilizer Composed of Ash from Biomass Combustion on Selected Physicochemical, Thermal, and Rheological Properties of Potato Starch. Molecules 2022, 27, 4318. [Google Scholar] [CrossRef]
- Pycia, K.; Szpunar-Krok, E.; Szostek, M.; Pawlak, R.; Juszczak, L. Selected Physicochemical, Thermal, and Rheological Properties of Barley Starch Depending on the Type of Soil and Fertilization with Ash from Biomass Combustion. Foods 2023, 13, 49. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Modrzewska, B. Acidity and sorption properties of zinc-contaminated soil following the application of neutralising substances added. J. Ecol. Eng. 2016, 17, 63–68. [Google Scholar] [CrossRef]
- Sommers, L.E.; Nelson, D.W. Determination of Total Phosphorus in Soils: A Rapid Perchloric Acid Digestion Procedure. Soil Sci. Soc. Am. J. 1972, 36, 902–904. [Google Scholar] [CrossRef]
- Perucci, P.; Monaci, E.; Onofri, A.; Vischetti, C.; Casucci, C. Changes in Physico-Chemical and Biochemical Parameters of Soil Following Addition of Wood Ash: A Field Experiment. Eur. J. Agron. 2008, 28, 155–161. [Google Scholar] [CrossRef]
- Meller, E.; Bilenda, E. Effects of biomass ash on the physicochemical properties of light soil. Polityka Energetyczna—Energy Policy J. 2012, 15, 287–292. [Google Scholar]
- Saletnik, B.; Zagula, G.; Bajcar, M.; Czernicka, M.; Puchalski, C. Biochar and Biomass Ash as a Soil Ameliorant: The Effect on Selected Soil Properties and Yield of Giant Miscanthus (Miscanthus x Giganteus). Energies 2018, 11, 2535. [Google Scholar] [CrossRef]
- Schönegger, D.; Gómez-Brandón, M.; Mazzier, T.; Insam, H.; Hermanns, R.; Leijenhorst, E.; Bardelli, T.; Fernández-Delgado Juárez, M. Phosphorus Fertilising Potential of Fly Ash and Effects on Soil Microbiota and Crop. Resour. Conserv. Recycl. 2018, 134, 262–270. [Google Scholar] [CrossRef]
- Jacobson, S.; Högbom, L.; Ring, E.; Nohrstedt, H.-Ö. Effects of Wood Ash Dose and Formulation on Soil Chemistry at Two Coniferous Forest Sites. Water Air Soil Pollut. 2004, 158, 113–125. [Google Scholar] [CrossRef]
- Pasquali, M.; Zanoletti, A.; Benassi, L.; Federici, S.; Depero, L.E.; Bontempi, E. Stabilized Biomass Ash as a Sustainable Substitute for Commercial P-fertilizers. Land Degrad. Dev. 2018, 29, 2199–2207. [Google Scholar] [CrossRef]
- Hansen, M.; Kepfer-Rojas, S.; Bjerager, P.E.R.; Holm, P.E.; Skov, S.; Ingerslev, M. Effects of Ash Application on Nutrient and Heavy Metal Fluxes in the Soil and Soil Solution in a Norway Spruce Plantation in Denmark. For. Ecol. Manag. 2018, 424, 494–504. [Google Scholar] [CrossRef]
- Małuszyńska, I.; Małuszyński, M.J. Study of soil salinity on growth and advancement of selected plants species. Inżynieria Ekol. 2009, 21, 32–39. Available online: http://www.archive.ecoeet.com/pdf/21/5.pdf (accessed on 28 September 2024). (In Polish).
- Telesiński, A. The effect of salinity on some biochemical indices of soil fertility. Water-Environ.-Rural A Reas 2012, 12, 209–217. [Google Scholar]
- Zhang, R.; Wienhold, B.J. The Effect of Soil Moisture on Mineral Nitrogen, Soil Electrical Conductivity, and pH. Nutr. Cycl. Agroecosystems 2002, 63, 251–254. [Google Scholar] [CrossRef]
- Allison, L.E.; Bernstein, L.; Bower, C.A.; Brown, J.W.; Fireman, M.; Hatcher, J.T.; Hayward, H.E.; Pearson, G.A.; Reeve, R.C.; Richards, A. United States Salinity Laboratory Staff. Available online: https://www.ars.usda.gov/ARSUserFiles/20360500/hb60_pdf/hb60complete.pdf (accessed on 28 September 2024).
- Matsi, T.; Keramidas, V.Z. Fly Ash Application on Two Acid Soils and Its Effect on Soil Salinity, pH, B, P and on Ryegrass Growth and Composition. Environ. Pollut. 1999, 104, 107–112. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Tarelho, L.; Gomes, A.P. Incorporation of Biomass Fly Ash and Biological Sludge in the Soil: Effects along the Soil Profile and in the Leachate Water. J. Soils Sediments 2018, 18, 2023–2031. [Google Scholar] [CrossRef]
- Ondrasek, G.; Zovko, M.; Kranjčec, F.; Savić, R.; Romić, D.; Rengel, Z. Wood Biomass Fly Ash Ameliorates Acidic, Low-Nutrient Hydromorphic Soil & Reduces Metal Accumulation in Maize. J. Clean. Prod. 2021, 283, 124650. [Google Scholar] [CrossRef]
- Tiecher, T.; Gatiboni, L.; dos Santos, D.R.; Bissani, C.A.; Martins, A.P.; Gianello, C.; Dick, D.P.; Bortoluzzi, E.C.; Escosteguy, P.A.V.; da Silva, L.S.; et al. Base Saturation Is an Inadequate Term for Soil Science. Rev. Bras. Ciênc. Solo 2023, 46, e0220125. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Chemical Composition of Biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Baxter, D. Trace Element Concentrations and Associations in Some Biomass Ashes. Fuel 2014, 129, 292–313. [Google Scholar] [CrossRef]
- Khanna, P.K.; Raison, R.J.; Falkiner, R.A. Chemical Properties of Ash Derived from Eucalyptus Litter and Its Effects on Forest Soils. For. Ecol. Manag. 1994, 66, 107–125. [Google Scholar] [CrossRef]
- Singh, V.K.; Dwivedi, B.S.; Rathore, S.S.; Mishra, R.P.; Satyanarayana, T.; Majumdar, K. Timing Potassium Applications to Synchronize with Plant Demand. In Improving Potassium Recommendations for Agricultural Crops; Murrell, T.S., Mikkelsen, R.L., Sulewski, G., Norton, R., Thompson, M.L., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 363–384. ISBN 978-3-030-59196-0. [Google Scholar]
- Czyżyk, F.; Rajmund, A. Leaching of Biogenic Elements (NPK) from Fertilized Light Soil. Proc. ECOpole 2014, 8, 369–375. [Google Scholar] [CrossRef]
- Singh, Y.-S.; Singh, B.; Timsina, J. Crop Residue Management for Nutrient Cycling and Improving Soil Productivity in Rice-Based Cropping Systems in the Tropics. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2005; Volume 85, pp. 269–407. ISBN 978-0-12-000783-7. [Google Scholar]
- Kramar, V. POSSIBLE UTILIZATION DIRECTIONS OF ASH FROM BIOMASS COMBUSTION. BIOMASS ASH AS FERTILIZER IN AGRICULTURE. 2020. Available online: https://uabio.org/wp-content/uploads/2020/12/AZ_Kramar_Zastosuvannya-zoly-biomasy-yak-dobryva_fin_en2.pdf (accessed on 28 September 2024).


| Parameter | Unit | |
|---|---|---|
| Sand (2.0–0.05 mm) | % | 17.0 ± 2.0 |
| Silt (0.05–0.002 mm) | 75.0 ± 4.0 | |
| Clay (<0.002 mm) | 8.0 ± 2.0 | |
| Granulometric group | - | Silt (Si) |
| pH KCl | - | 4.98 ± 0.16 |
| pH H2O | - | 5.95 ± 0.05 |
| EC | µS cm−1 | 68.0 ± 15.5 |
| HAC | cmol(+) kg−1 | 1.23 ± 0.16 |
| TEC | 5.18 ± 0.40 | |
| CEC | 6.41 ± 0.47 | |
| BS | % | 80.78 ± 2.04 |
| Ca | mg kg−1 | 1295 ± 75.6 |
| Mg | 826 ± 46.1 | |
| K | 1778 ± 79.5 | |
| Na | 212 ± 18.9 |
| Variable | BAs_D | CPS | BAs_ D × CPS | |
|---|---|---|---|---|
| pH KCl | F | 25.63 | 36.46 | 14.83 |
| p | 0.000 | 0.000 | 0.000 | |
| pH H2O | F | 21.56 | 7.52 | 7.81 |
| p | 0.000 | 0.001 | 0.000 | |
| HAC | F | 6.19 | 2.86 | 3.89 |
| p | 0.000 | 0.061 | 0.001 | |
| EC | F | 0.52 | 7.82 | 0.54 |
| p | 0.814 | 0.001 | 0.907 | |
| Ca | F | 3.01 | 18.57 | 5.46 |
| p | 0.006 | 0.000 | 0.000 | |
| Mg | F | 3.33 | 0.62 | 2.52 |
| p | 0.003 | 0.539 | 0.004 | |
| K | F | 4.61 | 0.90 | 2.33 |
| p | 0.000 | 0.409 | 0.007 | |
| Na | F | 7.86 | 57.04 | 2.85 |
| p | 0.000 | 0.000 | 0.001 |
| Plants | Variant of the Experiment | Ca | Mg | K | Na |
|---|---|---|---|---|---|
| (mg kg−1) | |||||
| Spring barley | Control | 1278 a–c ± 23.8 | 800 a–c ± 18.6 | 1777 a–c ± 23.1 | 192 b–g ± 8.5 |
| NPK | 1274 ab ± 28.5 | 758 a–c ± 24.1 | 1739 a–c ± 17.4 | 216 g ± 14.3 | |
| BAs_0.5 | 1284 a–d ± 16.4 | 754 a–c ± 18.8 | 1739 a–c ± 17.1 | 206 c–g ± 3.9 | |
| BAs_1.0 | 1332 a–e ± 19.2 | 875 bc ± 65.7 | 1925 c ± 59.3  | 208 d–g ± 2.7 | |
| BAs_1.5 | 1280 a–c ± 10.2 | 760 a–c ± 13.9 | 1685 ab ± 45.0 | 215 e–g ± 5.2 | |
| BAs_2.0 | 1257 a ± 17.4  | 733 ab ± 23.5 | 1659 ab ± 12.2 | 216 fg ± 3.1  | |
| BAs_2.5 | 1268 ab ± 12.3 | 744 a–c ± 30.7 | 1714 a–c ± 63.8 | 215 fg ± 6.1  | |
| Winter rapeseed | Control | 1401 de ± 18.7 | 802 a–c ± 15.7 | 1794 a–c ± 46.0 | 186 a–f ±5.7 |
| NPK | 1307 a–d ± 37.2 | 845 a–c ± 25.7 | 1616 a ± 40.4  | 186 a–g ± 4.0 | |
| BAs_0.5 | 1290 a–d ± 19.6 | 720 a ± 18.2  | 1745 a–c ± 39.3 | 191 b–g ± 2.9 | |
| BAs_1.0 | 1292 a–d ± 14.9 | 770 a–c ± 24.3 | 1720 a–c ± 43.1 | 185 a–e ± 2.2 | |
| BAs_1.5 | 1291 a–d ± 13.7 | 774 a–c ± 13.2 | 1738 a–c ± 37.8 | 177 a–c ± 4.2 | |
| BAs_2.0 | 1394 c–e ± 22.8 | 803 a–c ± 16.1 | 1760 a–c ± 35.1 | 177 a–c ± 3.0 | |
| BAs_2.5 | 1402 de ± 32.5 | 777 a–c ± 10.2 | 1868 bc ± 25.3 | 178 a–d ± 4.6 | |
| Control | 1263 ab ± 6.9 | 708 a ± 19.6 | 1670 ab ± 34.5 | 158 a ±4.2 | |
| NPK | 1292 a–d ± 33.0 | 740 ab ± 37.2 | 1687 ab ± 61.1  | 172 ab ± 4.0 | |
| BAs_0.5 | 1357 a–e ± 8.7  | 798 a–c ± 20.5 | 1669 ab ± 38.3  | 182 a ± 2.8  | |
| BAs_1.0 | 1394 c–e ± 15.5 | 895 c ± 82.3  | 1859 bc ± 101.9 | 176 a–c ± 4.9 | |
| Potatoes | BAs_1.5 | 1445 e ± 36.5  | 831 a–c ± 22.7 | 1744 a–c ± 51.2  | 178 a–d ± 4.8 |
| BAs_2.0 | 1371 a–e ± 9.6  | 780 a–c ± 11.9 | 1620 a ± 42.9  | 181 a–d ± 5.9 | |
| BAs_2.5 | 1381 b–e ± 5.6  | 802 a–c ± 15.7 | 1786 a–c ± 26.6  | 191 b–g ± 2.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szostek, M.; Szpunar-Krok, E.; Matłok, N.; Ilek, A.; Słowik, K.; Kuboń, M. Acidity and Salinization of Soil Following the Application of Ashes from Biomass Combustion Under Different Crop Plant Species Cultivation. Appl. Sci. 2024, 14, 9812. https://doi.org/10.3390/app14219812
Szostek M, Szpunar-Krok E, Matłok N, Ilek A, Słowik K, Kuboń M. Acidity and Salinization of Soil Following the Application of Ashes from Biomass Combustion Under Different Crop Plant Species Cultivation. Applied Sciences. 2024; 14(21):9812. https://doi.org/10.3390/app14219812
Chicago/Turabian StyleSzostek, Małgorzata, Ewa Szpunar-Krok, Natalia Matłok, Anna Ilek, Klaudia Słowik, and Maciej Kuboń. 2024. "Acidity and Salinization of Soil Following the Application of Ashes from Biomass Combustion Under Different Crop Plant Species Cultivation" Applied Sciences 14, no. 21: 9812. https://doi.org/10.3390/app14219812
APA StyleSzostek, M., Szpunar-Krok, E., Matłok, N., Ilek, A., Słowik, K., & Kuboń, M. (2024). Acidity and Salinization of Soil Following the Application of Ashes from Biomass Combustion Under Different Crop Plant Species Cultivation. Applied Sciences, 14(21), 9812. https://doi.org/10.3390/app14219812










