Inactivation Efficiency of Bacillus atrophaeus Spores on Seeds of Barley, Wheat, Lupine and Rapeseed by Direct Cold Atmospheric Pressure Plasma
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Material
2.2. Artificial Inoculation of Seeds with Bacillus atrophaeus Spores and Recovery of Viable Spores from Crop Seeds
2.3. Plasma Sources and Treatment of Seeds
2.4. Seed Germination Tests
2.5. Water Contact Angle (WCA) Analysis
2.6. Statistical Analysis
3. Results
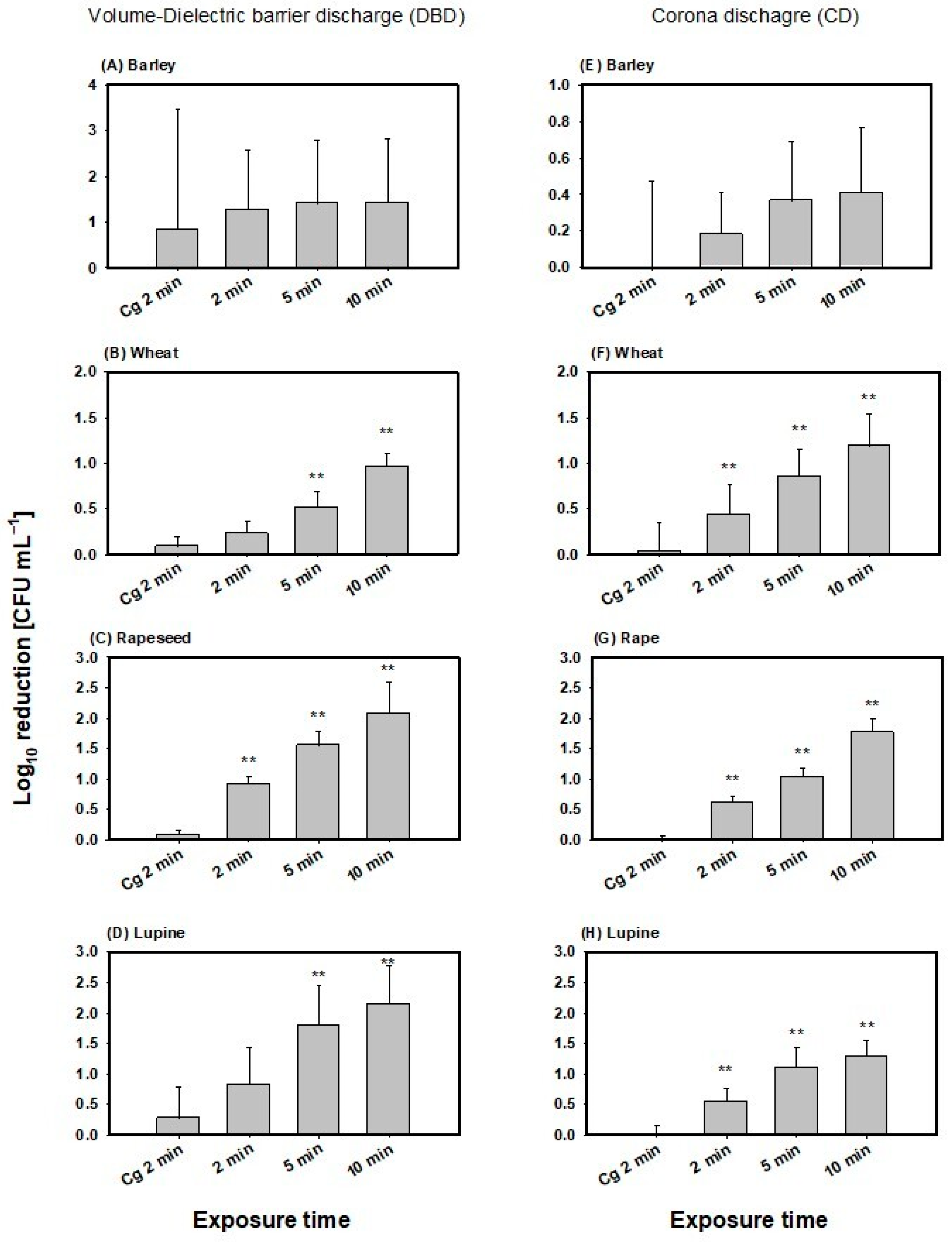
3.1. Inactivation Efficiency of B. atrophaeus by Direct CAPP
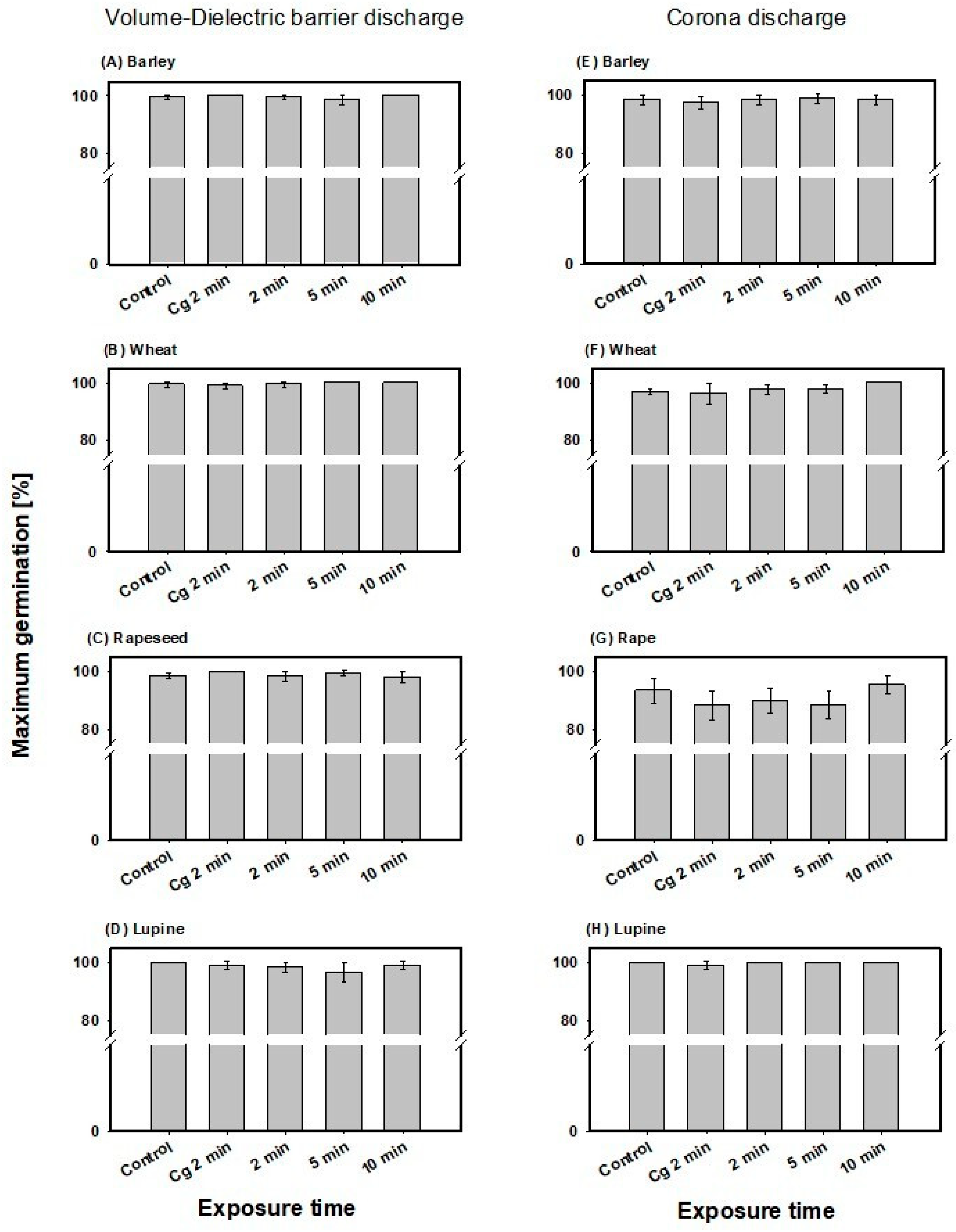
3.2. Impact of Direct CAPP Treatment on Seed Viability
3.3. Impact of Direct CAPP Treatment on Seed Surface Hydrophobicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richard, B.; Qi, A.; Fitt, B.D. Control of crop diseases through Integrated Crop Management to deliver climate-smart farming systems for low-and high-input crop production. Plant Pathol. 2022, 71, 187–206. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.-C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Oerke, E.-C.; Dehne, H.-W. Safeguarding production—Losses in major crops and the role of crop protection. Crop Prot. 2004, 23, 275–285. [Google Scholar] [CrossRef]
- Brauer, V.S.; Rezende, C.P.; Pessoni, A.M.; De Paula, R.G.; Rangappa, K.S.; Nayaka, S.C.; Gupta, V.K.; Almeida, F. Antifungal Agents in Agriculture: Friends and Foes of Public Health. Biomolecules 2019, 9, 521. [Google Scholar] [CrossRef]
- Jørgensen, L.N.; Heick, T.M. Azole Use in Agriculture, Horticulture, and Wood Preservation–Is It Indispensable? Front. Cell. Infect. Microbiol. 2021, 11, 730297. [Google Scholar] [CrossRef]
- Lopez-Antia, A.; Ortiz-Santaliestra, M.E.; Mougeot, F.; Camarero, P.R.; Mateo, R. Birds Feeding on Tebuconazole Treated Seeds Have Reduced Breeding Output. Environ. Pollut. 2021, 271, 116292. [Google Scholar] [CrossRef]
- Bryson, R.; Brix, H. Challenges and prospects for fungicidal control of wheat diseases. In Integrated Disease Management of Wheat and Barley; Burleigh Dodds Science Publishing: Cambridge, UK, 2018; pp. 239–254. [Google Scholar]
- Chakraborty, S. Migrate or evolve: Options for plant pathogens under climate change. Glob. Change Biol. 2013, 19, 1985–2000. [Google Scholar] [CrossRef]
- Garrett, K.A.; Thomas-Sharma, S.; Forbes, G.A.; Nopsa, J.H. Climate change and plant pathogen invasions. In Invasive Species and Global Climate Change; CABI: Wallingford, UK, 2014; pp. 22–44. [Google Scholar]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Corrales, D.C.; Soltani, E. Biological seed treatments promote crop establishment and yield: A global meta-analysis. Agron. Sustain. Dev. 2022, 42, 45. [Google Scholar] [CrossRef]
- Rosa, S.; Pesaresi, P.; Mizzotti, C.; Bulone, V.; Mezzetti, B.; Baraldi, E.; Masiero, S. Game-changing alternatives to conventional fungicides: Small RNAs and short peptides. Trends Biotechnol. 2022, 40, 320–337. [Google Scholar] [CrossRef] [PubMed]
- Bewley, J.D.; Black, M.; Halmer, P. The Encyclopedia of Seeds: Science, Technology and Uses; CABI: Wallingford, UK, 2006. [Google Scholar]
- Jahn, M.; Koch, E.; Blum, H.; Nega, E.; Wilbois, K.-P. Leitfaden Saatgutgesundheit im Ökologischen Landbau-Gemüsekulturen; Forschungsinstitut für biologischen Landbau eV, FiBL Deutschland eV: Frankfurt am Main, Germany, 2007. [Google Scholar]
- Yamagishi, N.; Fujinaga, M.; Ishiyama, Y.; Ogiso, H.; Sato, T.; Tosa, Y. Life cycle and control of Colletotrichum nymphaeae, the causal agent of celery stunt anthracnose. J. Gen. Plant Pathol. 2015, 81, 279–286. [Google Scholar] [CrossRef]
- Schaal, R. Beizung auf Elektronisch. agrarzeitung. 11 August 2023. Available online: https://www.agrarzeitung.de/pflanzenbautipps/getreide/physikalisch-biologisches-verfahren-beizung-auf-elektronisch-108324 (accessed on 28 August 2024).
- Wagner, R.; Weihe, T.; Winter, H.; Weit, C.; Ehlbeck, J.; Schnabel, U. Reducing Storage Losses of Organic Apples by Plasma Processed Air (PPA). Appl. Sci. 2023, 13, 12654. [Google Scholar] [CrossRef]
- Ziuzina, D.; Misra, N.; Cullen, P.; Keener, K.M.; Mosnier, J.; Vilaró, I.; Gaston, E.; Bourke, P. Demonstrating the potential of industrial scale in-package atmospheric cold plasma for decontamination of cherry tomatoes. Plasma Med. 2016, 6, 397–412. [Google Scholar] [CrossRef]
- Cullen, P.J.; Lalor, J.; Scally, L.; Boehm, D.; Milosavljević, V.; Bourke, P.; Keener, K. Translation of plasma technology from the lab to the food industry. Plasma Process. Polym. 2018, 15, 1700085. [Google Scholar] [CrossRef]
- Deng, X.; Shi, J.; Kong, M.G. Physical mechanisms of inactivation of Bacillus subtilis spores using cold atmospheric plasmas. IEEE Trans. Plasma Sci. 2006, 34, 1310–1316. [Google Scholar] [CrossRef]
- Laroussi, M. Low temperature plasma-based sterilization: Overview and state-of-the-art. Plasma Process. Polym. 2005, 2, 391–400. [Google Scholar] [CrossRef]
- Moisan, M.; Barbeau, J.; Crevier, M.-C.; Pelletier, J.; Philip, N.; Saoudi, B. Plasma sterilization. Methods and mechanisms. Pure Appl. Chem. 2002, 74, 349–358. [Google Scholar] [CrossRef]
- Fridman, G.; Brooks, A.D.; Balasubramanian, M.; Fridman, A.; Gutsol, A.; Vasilets, V.N.; Ayan, H.; Friedman, G. Comparison of direct and indirect effects of non-thermal atmospheric-pressure plasma on bacteria. Plasma Process. Polym. 2007, 4, 370–375. [Google Scholar] [CrossRef]
- Adhikari, B.; Pangomm, K.; Veerana, M.; Mitra, S.; Park, G. Plant Disease Control by Non-Thermal Atmospheric-Pressure Plasma. Front. Plant Sci. 2020, 11, 77. [Google Scholar] [CrossRef]
- Brust, H.; Wannicke, N.; Park, G. Agriculture and Food Processing Applications. In Plasma Biosciences and Medicine; Springer: Singapore, 2023; pp. 111–227. [Google Scholar]
- Jo, Y.-K.; Cho, J.; Tsai, T.-C.; Staack, D.; Kang, M.-H.; Roh, J.-H.; Shin, D.-B.; Cromwell, W.; Gross, D. A Non-thermal Plasma Seed Treatment Method for Management of a Seedborne Fungal Pathogen on Rice Seed. Crop Sci. 2014, 54, 796–803. [Google Scholar] [CrossRef]
- Zahoranová, A.; Henselová, M.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Medvecká, V.; Černák, M. Effect of Cold Atmospheric Pressure Plasma on the Wheat Seedlings Vigor and on the Inactivation of Microorganisms on the Seeds Surface. Plasma Chem. Plasma Process. 2016, 36, 397–414. [Google Scholar] [CrossRef]
- Zahoranová, A.; Hoppanová, L.; Šimončicová, J.; Tučeková, Z.; Medvecká, V.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Černák, M. Effect of Cold Atmospheric Pressure Plasma on Maize Seeds: Enhancement of Seedlings Growth and Surface Microorganisms Inactivation. Plasma Chem. Plasma Process. 2018, 38, 969–988. [Google Scholar] [CrossRef]
- Kim, J.-W.; Puligundla, P.; Mok, C. Effect of corona discharge plasma jet on surface-borne microorganisms and sprouting of broccoli seeds. J. Sci. Food Agric. 2017, 97, 128–134. [Google Scholar] [CrossRef]
- Puligundla, P.; Kim, J.-W.; Mok, C. Effect of corona discharge plasma jet treatment on decontamination and sprouting of rapeseed (Brassica napus L.) seeds. Food Control 2017, 71, 376–382. [Google Scholar] [CrossRef]
- Nishime, T.M.C.; Wannicke, N.; Horn, S.; Weltmann, K.D.; Brust, H. A Coaxial Dielectric Barrier Discharge Reactor for Treatment of Winter Wheat Seeds. Appl. Sci. 2020, 10, 7133. [Google Scholar] [CrossRef]
- Nishime, T.M.; Werner, J.; Wannicke, N.; Mui, T.S.; Kostov, K.G.; Weltmann, K.-D.; Brust, H. Characterization and optimization of a conical corona reactor for seed treatment of rapeseed. Appl. Sci. 2022, 12, 3292. [Google Scholar] [CrossRef]
- ISO 11138-4; Sterilization of Health Care Products—Biological Indicators—Part 4: Biological Indicators for Dry Heat Sterilization Processes. International Organization for Standardization: Geneva, Switzerland, 2017.
- Wannicke, N.; Wagner, R.; Stachowiak, J.; Nishime, T.M.C.; Ehlbeck, J.; Weltmann, K.-D.; Brust, H. Efficiency of plasma-processed air for biological decontamination of crop seeds on the premise of unimpaired seed germination. Plasma Process. Polym. 2021, 18, 2000207. [Google Scholar] [CrossRef]
- Braşoveanu, M.; Nemţanu, M.; Surdu-Bob, C.; Karaca, G.; Erper, I. Effect of glow discharge plasma on germination and fungal load of some cereal seeds. Rom. Rep. Phys. 2015, 67, 617–624. [Google Scholar]
- Leadley, C.E.; Williams, A. Pulsed electric field processing, power ultrasound and other emerging technologies. Food Process. Handb. 2006, 201, 5–20. [Google Scholar]
- Los, A.; Ziuzina, D.; Akkermans, S.; Boehm, D.; Cullen, P.J.; Van Impe, J.; Bourke, P. Improving microbiological safety and quality characteristics of wheat and barley by high voltage atmospheric cold plasma closed processing. Food Res. Int. 2018, 106, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Hoppanová, L.; Medvecká, V.; Dylíková, J.; Hudecová, D.; Kaliňáková, B.; Kryštofová, S.; Zahoranová, A. Low-temperature plasma applications in chemical fungicide treatment reduction. Acta Chim. Slovaca 2020, 13, 26–33. [Google Scholar] [CrossRef]
- Hertwig, C.; Reineke, K.; Ehlbeck, J.; Knorr, D.; Schluter, O. Decontamination of whole black pepper using different cold atmospheric pressure plasma applications. Food Control 2015, 55, 221–229. [Google Scholar] [CrossRef]
- Reineke, K.; Langer, K.; Hertwig, C.; Ehlbeck, J.; Schlüter, O. The impact of different process gas compositions on the inactivation effect of an atmospheric pressure plasma jet on Bacillus spores. Innov. Food Sci. Emerg. Technol. 2015, 30, 112–118. [Google Scholar] [CrossRef]
- Lim, J.-P.; Uhm, H.S.; Li, S.-Z. Influence of oxygen in atmospheric-pressure argon plasma jet on sterilization of Bacillus atrophaeous spores. Phys. Plasmas 2007, 14, 093504. [Google Scholar] [CrossRef]
- Liao, X.; Liu, D.; Xiang, Q.; Ahn, J.; Chen, S.; Ye, X.; Ding, T. Inactivation mechanisms of non-thermal plasma on microbes: A review. Food Control 2017, 75, 83–91. [Google Scholar] [CrossRef]
- Moisan, M.; Barbeau, J.; Moreau, S.; Pelletier, J.; Tabrizian, M.; Yahia, L. Low-temperature sterilization using gas plasmas: A review of the experiments and an analysis of the inactivation mechanisms. Int. J. Pharm. 2001, 226, 1–21. [Google Scholar] [CrossRef]
- Laroussi, M.; Leipold, F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrom. 2004, 233, 81–86. [Google Scholar] [CrossRef]
- Ehlbeck, J.; Brandenburg, R.; von Woedtke, T.; Krohmann, U.; Stieber, M.; Weltmann, K.-D. PLASMOSE-antimicrobial effects of modular atmospheric plasma sources. GMS Krankenhaushygiene Interdiszip. 2008, 3, 1–12. [Google Scholar]
- Adhikari, B.; Adhikari, M.; Park, G. The Effects of Plasma on Plant Growth, Development, and Sustainability. Appl. Sci. 2020, 10, 6045. [Google Scholar] [CrossRef]
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M. Plasma Agriculture from Laboratory to Farm: A Review. Processes 2020, 8, 1002. [Google Scholar] [CrossRef]
- Starič, P.; Vogel-Mikuš, K.; Mozetič, M.; Junkar, I. Effects of Nonthermal Plasma on Morphology, Genetics and Physiology of Seeds: A Review. Plants 2020, 9, 1736. [Google Scholar] [CrossRef] [PubMed]
- Brust, H.; Nishime, T.M.C.; Wannicke, N.; Mui, T.S.M.; Horn, S.; Quade, A.; Weltmann, K.D. A medium-scale volume dielectric barrier discharge system for short-term treatment of cereal seeds indicates improved germination performance with long-term effects. J. Appl. Phys. 2021, 129, 044904. [Google Scholar] [CrossRef]
- Gómez-Ramírez, A.; López-Santos, C.; Cantos, M.; García, J.L.; Molina, R.; Cotrino, J.; Espinós, J.; González-Elipe, A.R. Surface chemistry and germination improvement of Quinoa seeds subjected to plasma activation. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Molina, R.; Lalueza, A.; López-Santos, C.; Ghobeira, R.; Cools, P.; Morent, R.; de Geyter, N.; González-Elipe, A.R. Physicochemical surface analysis and germination at different irrigation conditions of DBD plasma-treated wheat seeds. Plasma Process. Polym. 2020, 18, e2000086. [Google Scholar] [CrossRef]
- Štěpánová, V.; Slavíček, P.; Kelar, J.; Prášil, J.; Smékal, M.; Stupavská, M.; Jurmanová, J.; Černák, M. Atmospheric pressure plasma treatment of agricultural seeds of cucumber (Cucumis sativus L.) and pepper (Capsicum annuum L.) with effect on reduction of diseases and germination improvement. Plasma Process. Polym. 2018, 15, 1700076. [Google Scholar] [CrossRef]
- Recek, N.; Vesel, A.; Zaplotnik, R.; Paul, D.; Primc, G.; Gselman, P.; Mozetič, M. Hydrophilization of corn seeds by non-equilibrium gaseous plasma. Chem. Biol. Technol. Agric. 2021, 8, 32. [Google Scholar] [CrossRef]
- Švubová, R.; Kyzek, S.; Medvecká, V.; Slováková, Ľ.; Gálová, E.; Zahoranová, A. Novel insight at the Effect of Cold Atmospheric Pressure Plasma on the Activity of Enzymes Essential for the Germination of Pea (Pisum sativum L. cv. Prophet) Seeds. Plasma Chem. Plasma Process. 2020, 40, 1221–1240. [Google Scholar] [CrossRef]
- Švubová, R.; Slováková, Ľ.; Holubová, Ľ.; Rovňanová, D.; Gálová, E.; Tomeková, J. Evaluation of the Impact of Cold Atmospheric Pressure Plasma on Soybean Seed Germination. Plants 2021, 10, 177. [Google Scholar] [CrossRef]
- Los, A.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Bourke, P. Investigation of mechanisms involved in germination enhancement of wheat (Triticum aestivum) by cold plasma: Effects on seed surface chemistry and characteristics. Plasma Process. Polym. 2019, 16, 1800148. [Google Scholar] [CrossRef]
- Ji, S.H.; Ki, S.H.; Kang, M.H.; Choi, J.S.; Park, Y.; Oh, J.; Kim, S.B.; Yoo, S.J.; Choi, E.H.; Park, G. Characterization of physical and biochemical changes in plasma treated spinach seed during germination. J. Phys. D Appl. Phys. 2018, 51, 145205. [Google Scholar] [CrossRef]
- Pawlat, J.; Starek, A.; Sujak, A.; Kwiatkowski, M.; Terebun, P.; Budzeń, M. Effects of atmospheric pressure plasma generated in GlidArc reactor on Lavatera thuringiaca L. seeds’ germination. Plasma Process. Polym. 2018, 15, 1700064. [Google Scholar] [CrossRef]
- Bewley, J.; Bradford, K.J.; Hilhorst, H.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013; Volume 10, p. 978-1. [Google Scholar]

| Plant Species | Treatment | Volume-DBD | CD |
|---|---|---|---|
| WCA (°) | |||
| Barely | Control | 115.5 ± 6.0 | 115.7 ± 5.6 |
| Control gas | 111.3 ± 9.6 | 115.3 ± 5.4 | |
| CAPP 2 min | 89.1 ± 9.1 | 80.2 ± 34.1 | |
| CAPP 5 min | 63.4 ± 9.5 | 89.6 ± 31.3 | |
| CAPP 10 min | 56.2 ± 12.6 | 68.1 ± 39.7 | |
| Wheat | Control | 110.1 ± 8.4 | 105.7 ± 5.6 |
| Control gas | 116.7 ± 5.4 | 108.1 ± 8.3 | |
| CAPP 2 min | 66.4 ± 11.4 | 89.3 ± 12.6 | |
| CAPP 5 min | 66.4 ± 11.3 | 91.6 ± 14.4 | |
| CAPP 10 min | 68.8 ± 18 | 54.0 ± 16.9 | |
| Rapeseed | Control | 108.9 ± 2.9 | 109.2 ± 4.2 |
| Control gas | 109.5 ± 5.3 | 107.8 ± 5.6 | |
| CAPP 2 min | 62.2 ± 8.4 | 63.0 ± 13.7 | |
| CAPP 5 min | 57.4 ± 10.8 | 49.9 ± 11.5 | |
| CAPP 10 min | 52.7 ± 12.5 | 45.7 ± 7.5 | |
| Lupine | Control | 125.6 ± 7.77 | 122.6 ± 6.5 |
| Control gas | 126.2 ± 7.21 | 121.5 ± 6.4 | |
| CAPP 2 min | 57.4 ± 7.2 | 77.3 ± 38.7 | |
| CAPP 5 min | 60.4 ± 6.6 | 69.6 ± 50.4 | |
| CAPP 10 min | 56.3 ± 5.3 | 75.6 ± 45.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wannicke, N.; Martins Dias, J.; Nishime, T.M.C.; Brust, H. Inactivation Efficiency of Bacillus atrophaeus Spores on Seeds of Barley, Wheat, Lupine and Rapeseed by Direct Cold Atmospheric Pressure Plasma. Appl. Sci. 2024, 14, 9793. https://doi.org/10.3390/app14219793
Wannicke N, Martins Dias J, Nishime TMC, Brust H. Inactivation Efficiency of Bacillus atrophaeus Spores on Seeds of Barley, Wheat, Lupine and Rapeseed by Direct Cold Atmospheric Pressure Plasma. Applied Sciences. 2024; 14(21):9793. https://doi.org/10.3390/app14219793
Chicago/Turabian StyleWannicke, Nicola, Jasmin Martins Dias, Thalita M. C. Nishime, and Henrike Brust. 2024. "Inactivation Efficiency of Bacillus atrophaeus Spores on Seeds of Barley, Wheat, Lupine and Rapeseed by Direct Cold Atmospheric Pressure Plasma" Applied Sciences 14, no. 21: 9793. https://doi.org/10.3390/app14219793
APA StyleWannicke, N., Martins Dias, J., Nishime, T. M. C., & Brust, H. (2024). Inactivation Efficiency of Bacillus atrophaeus Spores on Seeds of Barley, Wheat, Lupine and Rapeseed by Direct Cold Atmospheric Pressure Plasma. Applied Sciences, 14(21), 9793. https://doi.org/10.3390/app14219793









