The Development and Reliability of a Surface Electromyography-Based Index for Quantifying Knee Muscle Coactivation During the Lower Quarter Y-Balance Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Ethical Considerations
2.2. Studied Participants
2.3. Study Design
2.4. Surface Electromyography
2.5. Lower Quarter Y-Balance Test
2.6. The Calculation of the Surface Electromyography-Based Index for Quantifying Knee Muscle Coactivation During the Lower Quarter Y-Balance Test
2.7. Statistical Analysis
3. Results
3.1. Intra-Rater and Inter-Rater Reliability
3.2. Between-Limb Comparison
3.3. Between-Direction Comparison
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sonesson, S.; Lindblom, H.; Hägglund, M. Higher age and present injury at the start of the season are risk factors for in-season injury in amateur male and female football players-a prospective cohort study. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 4618–4630. [Google Scholar] [CrossRef] [PubMed]
- Torvaldsson, K.; Lindblom, H.; Sonesson, S.; Senorski, E.H.; Stigson, H.; Tamm, L.; Sandberg, J.; Hägglund, M. Swedish Olympic athletes report one injury insurance claim every second year: A 22-year insurance registry-based cohort study. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 4607–4617. [Google Scholar] [CrossRef] [PubMed]
- Astur, D.C.; Margato, G.F.; Zobiole, A.; Pires, D.; Funchal, L.F.Z.; Jimenez, A.E.; Freitas, E.V.; Cohen, M. The incidence of anterior cruciate ligament injury in youth and male soccer athletes: An evaluation of 17,108 players over two consecutive seasons with an age-based sub-analysis. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 2556–2562. [Google Scholar] [CrossRef] [PubMed]
- Paudel, Y.R.; Sommerfeldt, M.; Voaklander, D. Increasing incidence of anterior cruciate ligament reconstruction: A 17-year population-based study. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Al-Gburi, M.; Kristiansen, J.B.; Christensen, K.B.; Krogsgaard, M.R. Functional performance tests, clinical measurements, and patient-reported outcome measures do not correlate as outcomes 1 year after anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 5905–5912. [Google Scholar] [CrossRef]
- Velasquez Garcia, A.; Iida, N.; Kuroiwa, T.; Hsu, K.L.; de Marinis, R.; Abdo, G.; Ekdahl, M. Substantial influence of psychological factors on return to sports after anterior shoulder instability surgery: A systematic review and meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 5913–5923. [Google Scholar] [CrossRef]
- Królikowska, A.; Reichert, P.; Senorski, E.H.; Karlsson, J.; Becker, R.; Prill, R. Scores and sores: Exploring patient-reported outcomes for knee evaluation in orthopaedics, sports medicine and rehabilitation. Knee Surg. Sports Traumatol. Arthrosc. 2024. [Google Scholar] [CrossRef]
- Thorolfsson, B.; Piussi, R.; Snaebjornsson, T.; Karlsson, J.; Samuelsson, K.; Beischer, S.; Thomeé, R.; Hamrin Senorski, E. Greater self-efficacy, psychological readiness and return to sport amongst paediatric patients compared with adolescents and young adults, 8 and 12 months after ACL reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 5629–5640. [Google Scholar] [CrossRef]
- Tischer, T.; Martens, G.; Cabri, J.; Thoreux, P.; Tscholl, P.; Edouard, P.; Leclerc, S.; Le Garrec, S.; Delvaux, F.; Croisier, J.L.; et al. The awareness of injury prevention programmes is insufficient among French- and German-speaking sports medicine communities in Europe. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 2563–2571. [Google Scholar] [CrossRef]
- Mir, B.; Vivekanantha, P.; Dhillon, S.; Cotnareanu, O.; Cohen, D.; Nagai, K.; de Sa, D. Fear of reinjury following primary anterior cruciate ligament reconstruction: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 2299–2314. [Google Scholar] [CrossRef]
- Kaarre, J.; Zsidai, B.; Winkler, P.W.; Narup, E.; Horvath, A.; Svantesson, E.; Senorski, E.H.; Musahl, V.; Samuelsson, K. Different patient and activity-related characteristics result in different injury profiles for patients with anterior cruciate ligament and posterior cruciate ligament injuries. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Obërtinca, R.; Meha, R.; Hoxha, I.; Shabani, B.; Meyer, T.; Aus der Fünten, K. Efficacy of a new injury prevention programme (FUNBALL) in young male football (soccer) players: A cluster-randomised controlled trial. Br. J. Sports Med. 2024, 58, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Magaña-Ramírez, M.; Gallardo-Gómez, D.; Álvarez-Barbosa, F.; Corral-Pernía, J.A. What exercise programme is the most appropriate to mitigate anterior cruciate ligament injury risk in football (soccer) players? A systematic review and network meta-analysis. J. Sci. Med. Sport 2024, 27, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Rommers, N.; Rössler, R.; Tassignon, B.; Verschueren, J.; De Ridder, R.; van Melick, N.; Longé, L.; Hendrikx, T.; Vaes, P.; Beckwée, D.; et al. Most amateur football teams do not implement essential components of neuromuscular training to prevent anterior cruciate ligament injuries and lateral ankle sprains. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 1169–1179. [Google Scholar] [CrossRef]
- Kim, S.; Glaviano, N.R.; Park, J. Exercise-induced fatigue affects knee proprioceptive acuity and quadriceps neuromuscular function more in patients with ACL reconstruction or meniscus surgery than in healthy individuals. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 5428–5437. [Google Scholar] [CrossRef]
- Zając, B.; Olszewski, M.; Mika, A. Influence of protocol variables on outcomes of the star excursion balance test group (SEBT, mSEBT, YBT-LQ) in healthy individuals: A systematic review. Front. Physiol. 2024, 15, 1415887. [Google Scholar] [CrossRef]
- Melzer, I.; Benjuya, N.; Kaplanski, J.; Alexander, N. Association between ankle muscle strength and limit of stability in older adults. Age Ageing 2009, 38, 119–123. [Google Scholar] [CrossRef]
- Gribble, P.A.; Hertel, J.; Plisky, P. Using the Star Excursion Balance Test to assess dynamic postural-control deficits and outcomes in lower extremity injury: A literature and systematic review. J. Athl. Train 2012, 47, 339–357. [Google Scholar] [CrossRef]
- Plisky, P.; Schwartkopf-Phifer, K.; Huebner, B.; Garner, M.B.; Bullock, G. Systematic Review and Meta-Analysis of the Y-Balance Test Lower Quarter: Reliability, Discriminant Validity, and Predictive Validity. Int. J. Sports Phys. Ther. 2021, 16, 1190–1209. [Google Scholar] [CrossRef]
- Chaabene, H.; Negra, Y.; Sammoud, S.; Moran, J.; Ramirez-Campillo, R.; Granacher, U.; Prieske, O. The Effects of Combined Balance and Complex Training Versus Complex Training Only on Measures of Physical Fitness in Young Female Handball Players. Int. J. Sports Physiol. Perform. 2021, 16, 1439–1446. [Google Scholar] [CrossRef]
- Powden, C.J.; Dodds, T.K.; Gabriel, E.H. The reliability of the star excursion balance test and lower quarter y-balance test in healthy adults: A systematic review. Int. J. Sports Phys. Ther. 2019, 14, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Molina-Rueda, F.; Fernández-Vázquez, D.; Navarro-López, V.; López-González, R.; Carratalá-Tejada, M. Muscle Coactivation Index during Walking in People with Multiple Sclerosis with Mild Disability, a Cross-Sectional Study. Diagnostics 2023, 13, 2169. [Google Scholar] [CrossRef] [PubMed]
- Bandini, V.; Carpinella, I.; Marzegan, A.; Jonsdottir, J.; Frigo, C.A.; Avanzino, L.; Pelosin, E.; Ferrarin, M.; Lencioni, T. Surface-Electromyography-Based Co-Contraction Index for Monitoring Upper Limb Improvements in Post-Stroke Rehabilitation: A Pilot Randomized Controlled Trial Secondary Analysis. Sensors 2023, 23, 7320. [Google Scholar] [CrossRef]
- Rodrigues, C.; Correia, M.; Abrantes, J.; Benedetti, M.; Nadal, J. Muscle coactivation analysis for neuromuscular control assessment of lower limb stretch-shortening cycle. Gait Posture 2023, 106, S175–S176. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Kim, J. Electromyography-signal-based muscle fatigue assessment for knee rehabilitation monitoring systems. Biomed. Eng. Lett. 2018, 8, 345–353. [Google Scholar] [CrossRef]
- Czamara, A.; Królikowska, A.; Szuba, Ł.; Widuchowski, W.; Kentel, M. Single- vs. double-bundle anterior cruciate ligament reconstruction: A new aspect of knee assessment during activities involving dynamic knee rotation. J. Strength. Cond. Res. 2015, 29, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Królikowska, A.; Mika, A.; Plaskota, B.; Daszkiewicz, M.; Kentel, M.; Kołcz, A.; Kentel, M.; Prill, R.; Diakowska, D.; Reichert, P.; et al. Reliability and Validity of the Athletic Shoulder (ASH) Test Performed Using Portable Isometric-Based Strength Training Device. Biology 2022, 11, 577. [Google Scholar] [CrossRef] [PubMed]
- Królikowska, A.; Maj, A.; Dejnek, M.; Prill, R.; Skotowska-Machaj, A.; Kołcz, A. Wrist motion assessment using Microsoft Azure Kinect DK: A reliability study in healthy individuals. Adv. Clin. Exp. Med. 2023, 32, 203–209. [Google Scholar] [CrossRef]
- Oleksy, Ł.; Królikowska, A.; Mika, A.; Reichert, P.; Kentel, M.; Kentel, M.; Poświata, A.; Roksela, A.; Kozak, D.; Bienias, K.; et al. A Reliability of Active and Passive Knee Joint Position Sense Assessment Using the Luna EMG Rehabilitation Robot. Int. J. Environ. Res. Public Health 2022, 19, 15885. [Google Scholar] [CrossRef]
- Królikowska, A.; Czamara, A.; Kentel, M. Does Gracilis Tendon Harvest During ACL Reconstruction with a Hamstring Autograft Affect Torque of Muscles Responsible for Shin Rotation? Med. Sci. Monit. 2015, 21, 2084–2093. [Google Scholar] [CrossRef]
- Czamara, A.; Markowska, I.; Krolikowska, A.; Szopa, A.; Domagalska Szopa, M. Kinematics of Rotation in Joints of the Lower Limbs and Pelvis during Gait: Early Results-SB ACLR Approach versus DB ACLR Approach. BioMed Res. Int. 2015, 2015, 707168. [Google Scholar] [CrossRef] [PubMed]
- Prill, R.; Królikowska, A.; Becker, R.; Karlsson, J. Why there is a need to improve evaluation standards for clinical studies in orthopaedic and sports medicine. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Królikowska, A.; Reichert, P.; Karlsson, J.; Mouton, C.; Becker, R.; Prill, R. Improving the reliability of measurements in orthopaedics and sports medicine. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 5277–5285. [Google Scholar] [CrossRef]
- Daly, L.; Bourke, G.J. Interpretation and Uses of Medical Statistics; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Bruton, A.; Conway, J.H.; Holgate, S.T. Reliability: What is it, and how is it measured? Physiotherapy 2000, 86, 94–99. [Google Scholar] [CrossRef]
- Lee, K.M.; Lee, J.; Chung, C.Y.; Ahn, S.; Sung, K.H.; Kim, T.W.; Lee, H.J.; Park, M.S. Pitfalls and important issues in testing reliability using intraclass correlation coefficients in orthopaedic research. Clin. Orthop. Surg. 2012, 4, 149–155. [Google Scholar] [CrossRef]
- Peacock, J.; Peacock, P. Oxford Handbook of Medical Statistics; OUP: Oxford, UK, 2011. [Google Scholar]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef]
- Schlüter, I.-M.; Prill, R.; Królikowska, A.; Cruysen, C.; Becker, R. A Pilot Study on the Reliability of Ultrasound-Based Assessment of Patella Diameter and Sulcus Angle. Diagnostics 2022, 12, 3164. [Google Scholar] [CrossRef]
- Wise, K.L.; Kelly, B.J.; Knudsen, M.L.; Macalena, J.A. Reliability Studies and Surveys. In Basic Methods Handbook for Clinical Orthopaedic Research: A Practical Guide and Case Based Research Approach; Musahl, V., Karlsson, J., Hirschmann, M.T., Ayeni, O.R., Marx, R.G., Koh, J.L., Nakamura, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 343–358. [Google Scholar]
- Nene, A.; Byrne, C.; Hermens, H. Is rectus femoris really a part of quadriceps? Assessment of rectus femoris function during gait in able-bodied adults. Gait Posture 2004, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Plisky, P.J.; Rauh, M.J.; Kaminski, T.W.; Underwood, F.B. Star Excursion Balance Test as a predictor of lower extremity injury in high school basketball players. J. Orthop. Sports Phys. Ther. 2006, 36, 911–919. [Google Scholar] [CrossRef]
- Bujang, M.A.; Baharum, N. A simplified guide to determination of sample size requirements for estimating the value of intraclass correlation coefficient: A review. Arch. Orofac. Sci. 2017, 12, 1–11. [Google Scholar]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Madjarova, S.J.; Pareek, A.; Eckhardt, C.M.; Khorana, A.; Kunze, K.N.; Ollivier, M.; Karlsson, J.; Williams, R.J., 3rd; Nwachukwu, B.U. Fragility Part I: A guide to understanding statistical power. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 3924–3928. [Google Scholar] [CrossRef]
- Cicchetti, D.V.; Sparrow, S.A. Developing criteria for establishing interrater reliability of specific items: Applications to assessment of adaptive behavior. Am. J. Ment. Defic 1981, 86, 127–137. [Google Scholar] [PubMed]
- Madjarova, S.J.; Williams, R.J., 3rd; Nwachukwu, B.U.; Martin, R.K.; Karlsson, J.; Ollivier, M.; Pareek, A. Picking apart p values: Common problems and points of confusion. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 3245–3248. [Google Scholar] [CrossRef]
- Bailey, C.A.; Corona, F.; Pilloni, G.; Porta, M.; Fastame, M.C.; Hitchcott, P.K.; Penna, M.P.; Pau, M.; Côté, J.N. Sex-dependent and sex-independent muscle activation patterns in adult gait as a function of age. Exp. Gerontol. 2018, 110, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Santuz, A.; Janshen, L.; Brüll, L.; Munoz-Martel, V.; Taborri, J.; Rossi, S.; Arampatzis, A. Sex-specific tuning of modular muscle activation patterns for locomotion in young and older adults. PLoS ONE 2022, 17, e0269417. [Google Scholar] [CrossRef]
- Jeon, W.; Ramadan, A.; Whitall, J.; Alissa, N.; Westlake, K. Age-related differences in lower limb muscle activation patterns and balance control strategies while walking over a compliant surface. Sci. Rep. 2023, 13, 16555. [Google Scholar] [CrossRef]
- Srivastava, S.; Patten, C.; Kautz, S.A. Altered muscle activation patterns (AMAP): An analytical tool to compare muscle activity patterns of hemiparetic gait with a normative profile. J. NeuroEng. Rehabil. 2019, 16, 21. [Google Scholar] [CrossRef]
- Almeida, M.B.d.; Moreira, M.; Miranda-Oliveira, P.; Moreira, J.; Família, C.; Vaz, J.R.; Moleirinho-Alves, P.; Oliveira, R. Evolving Dynamics of Neck Muscle Activation Patterns in Dental Students: A Longitudinal Study. Sensors 2024, 24, 5689. [Google Scholar] [CrossRef]
- Newcomer, K.L.; Jacobson, T.D.; Gabriel, D.A.; Larson, D.R.; Brey, R.H.; An, K.-N. Muscle activation patterns in subjects with and without low back pain. Arch. Phys. Med. Rehabil. 2002, 83, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Alcan, V.; Zinnuroğlu, M. Current developments in surface electromyography. Turk. J. Med. Sci. 2023, 53, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
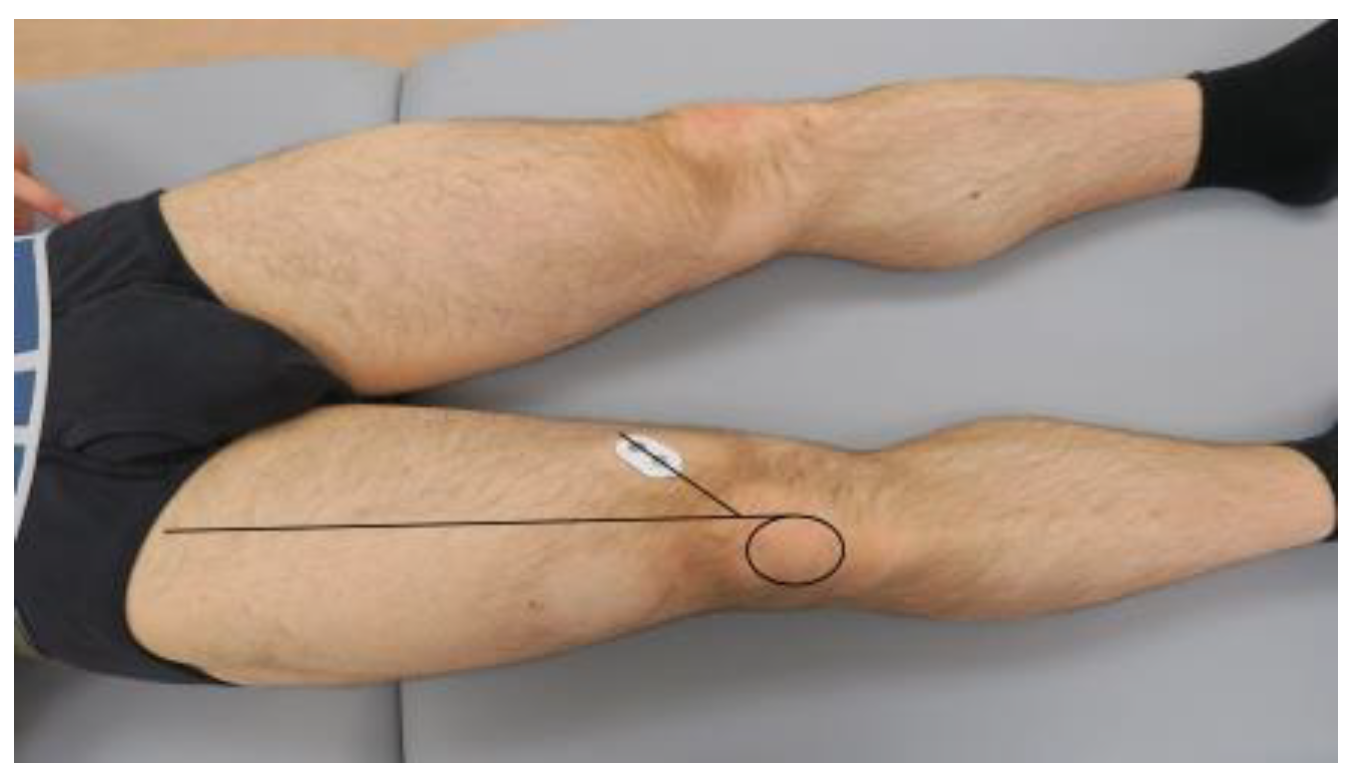

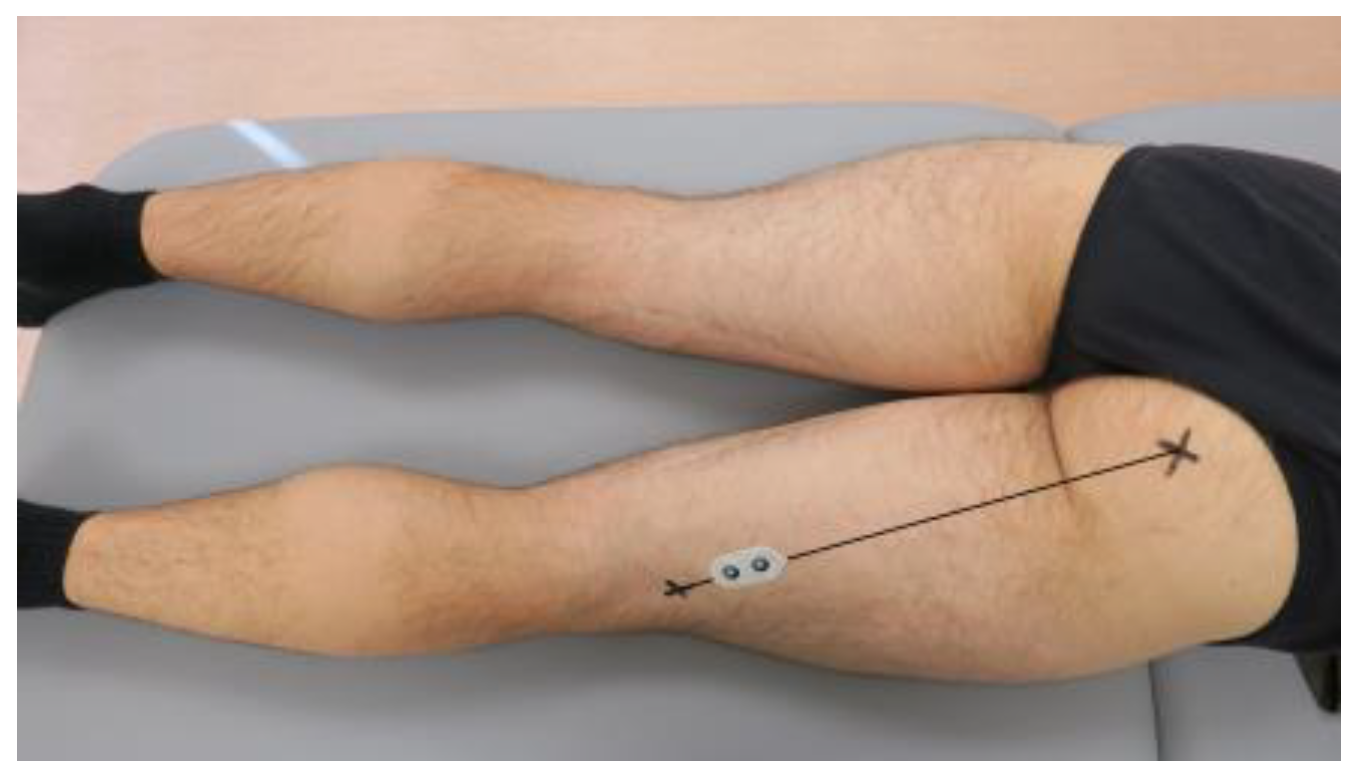

| Surface Electromyography-Based CoAI1 (ST/VM) | ||||
|---|---|---|---|---|
| Intra-Rater Reliability | Inter-Rater Reliability | |||
| YBT-LQ Direction | Right Lower Limb | Left Lower Limb | Right Lower Limb | Left Lower Limb |
| Anterior | 0.742 (0.561, 0.855) | 0.613 (0.379, 0.774) | 0.315 (0.002, 0.570) | 0.660 (0.445, 0.804) |
| Posteromedial | 0.636 (0.406, 0.790) | 0.585 (0.339, 0.756) | 0.620 (0.385, 0.780) | 0.667 (0.451, 0.809) |
| Posterolateral | 0.720 (0.528, 0.842) | 0.495 (0.227, 0.695) | 0.756 (0.583, 0.863) | 0.790 (0.633, 0.884) |
| Surface Electromyography-Based CoAI2 (ST/VL) | ||||
|---|---|---|---|---|
| Intra-Rater Reliability | Inter-Rater Reliability | |||
| YBT-LQ Direction | Right Lower Limb | Left Lower Limb | Right Lower Limb | Left Lower Limb |
| Anterior | 0.765 (0.598, 0.869) | 0.707 (0.512, 0.833) | 0.503 (0.227, 0.703) | 0.675 (0.465, 0.813) |
| Posteromedial | 0.761 (0.591, 0.867) | 0.743 (0.565, 0.856) | 0.712 (0.520, 0.836) | 0.743 (0.563, 0.855) |
| Posterolateral | 0.690 (0.487, 0.823) | 0.567 (0.317, 0.744) | 0.779 (0.620, 0.877) | 0.732 (0.544, 0.849) |
| Surface Electromyography-Based CoA3 (BF/VM) | ||||
|---|---|---|---|---|
| Intra-Rater Reliability | Inter-Rater Reliability | |||
| YBT-LQ Direction | Right Lower Limb | Left Lower Limb | Right Lower Limb | Left Lower Limb |
| Anterior | 0.554 (0.296, 0.737) | 0.551 (0.293, 0.734) | 0.488 (0.208, 0.693) | 0.671 (0.455, 0.811) |
| Posteromedial | 0.703 (0.504, 0.831) | 0.829 (0.700, 0.905) | 0.629 (0.399, 0.784) | 0.662 (0.447, 0.805) |
| Posterolateral | 0.279 (−0.026, 0.538) | 0.707 (0.511, 0.834) | 0.568 (0.312, 0.746) | 0.793 (0.642, 0.885) |
| Surface Electromyography-Based CoAI4 (BF/VL) | ||||
|---|---|---|---|---|
| Intra-Rater Reliability | Inter-Rater Reliability | |||
| YBT-LQ Direction | Right Lower Limb | Left Lower Limb | Right Lower Limb | Left Lower Limb |
| Anterior | 0.370 (0.067, 0.610) | 0.544 (0.280, 0.730) | 0.515 (0.243, 0.712) | 0.559 (0.300, 0.741) |
| Posteromedial | 0.617 (0.384, 0.776) | 0.818 (0.682, 0.900) | 0.612 (0.378, 0.773) | 0.616 (0.381, 0.776) |
| Posterolateral | 0.303 (−0.004, 0.559) | 0.733 (0.547, 0.849) | 0.560 (0.301, 0.741) | 0.713 (0.519, 0.837) |
| Surface Electromyography-Based CoAIs | ||||
|---|---|---|---|---|
| CoAI | YBT-LQ Direction | Right Limb | Left Limb | Between-Limb p-Value |
| CoAI1 (ST/VM) | Anterior | 54.55 ± 29.17 | 56.30 ± 46.45 | 0.826 |
| Posteromedial | 43.44 ± 28.08 | 48.69 ± 37.25 | 0.318 | |
| Posterolateral | 43.86 ± 27.55 | 41.81 ± 24.54 | 0.624 | |
| CoAI2 (ST/VL) | Anterior | 60.32 ± 31.79 | 63.56 ± 41.81 | 0.681 |
| Posteromedial | 40.79 ± 20.35 | 46.64 ± 27.06 | 0.130 | |
| Posterolateral | 41.24 ± 25.49 | 42.39 ± 29.86 | 0.839 | |
| CoAI3 (BF/VM) | Anterior | 40.82 ± 27.52 | 41.47 ± 27.15 | 0.906 |
| Posteromedial | 48.95 ± 33.74 | 54.27 ± 42.49 | 0.383 | |
| Posterolateral | 53.27 ± 27.92 | 50.53 ± 35.50 | 0.565 | |
| CoAI4 (BF/VL) | Anterior | 44.48 ± 27.59 | 47.78 ± 31.23 | 0.523 |
| Posteromedial | 45.88 ± 25.75 | 51.87 ± 32.67 | 0.280 | |
| Posterolateral | 51.43 ± 27.65 | 49.30 ± 32.22 | 0.708 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daszkiewicz, M.; Prill, R.; Reichert, P.; Becker, R.; Oleksy, Ł.; Kuźniecow, M.; Lech, M.; Kułakowski, M.; Kentel, M.; Kentel, M.; et al. The Development and Reliability of a Surface Electromyography-Based Index for Quantifying Knee Muscle Coactivation During the Lower Quarter Y-Balance Test. Appl. Sci. 2024, 14, 9788. https://doi.org/10.3390/app14219788
Daszkiewicz M, Prill R, Reichert P, Becker R, Oleksy Ł, Kuźniecow M, Lech M, Kułakowski M, Kentel M, Kentel M, et al. The Development and Reliability of a Surface Electromyography-Based Index for Quantifying Knee Muscle Coactivation During the Lower Quarter Y-Balance Test. Applied Sciences. 2024; 14(21):9788. https://doi.org/10.3390/app14219788
Chicago/Turabian StyleDaszkiewicz, Maciej, Robert Prill, Paweł Reichert, Roland Becker, Łukasz Oleksy, Mateusz Kuźniecow, Marcin Lech, Michał Kułakowski, Monika Kentel, Maciej Kentel, and et al. 2024. "The Development and Reliability of a Surface Electromyography-Based Index for Quantifying Knee Muscle Coactivation During the Lower Quarter Y-Balance Test" Applied Sciences 14, no. 21: 9788. https://doi.org/10.3390/app14219788
APA StyleDaszkiewicz, M., Prill, R., Reichert, P., Becker, R., Oleksy, Ł., Kuźniecow, M., Lech, M., Kułakowski, M., Kentel, M., Kentel, M., Kowal, M., Dejnek, M., & Królikowska, A. (2024). The Development and Reliability of a Surface Electromyography-Based Index for Quantifying Knee Muscle Coactivation During the Lower Quarter Y-Balance Test. Applied Sciences, 14(21), 9788. https://doi.org/10.3390/app14219788











