A Review of the Utilization of Recycled Powder from Concrete Waste as a Cement Partial Replacement in Cement-Based Materials: Fundamental Properties and Activation Methods
Abstract
1. Introduction
2. Research Methodology
3. Fundamental Properties of RP
3.1. RP Preparation Methods and Equipment
3.2. Particle Size Distribution of RP
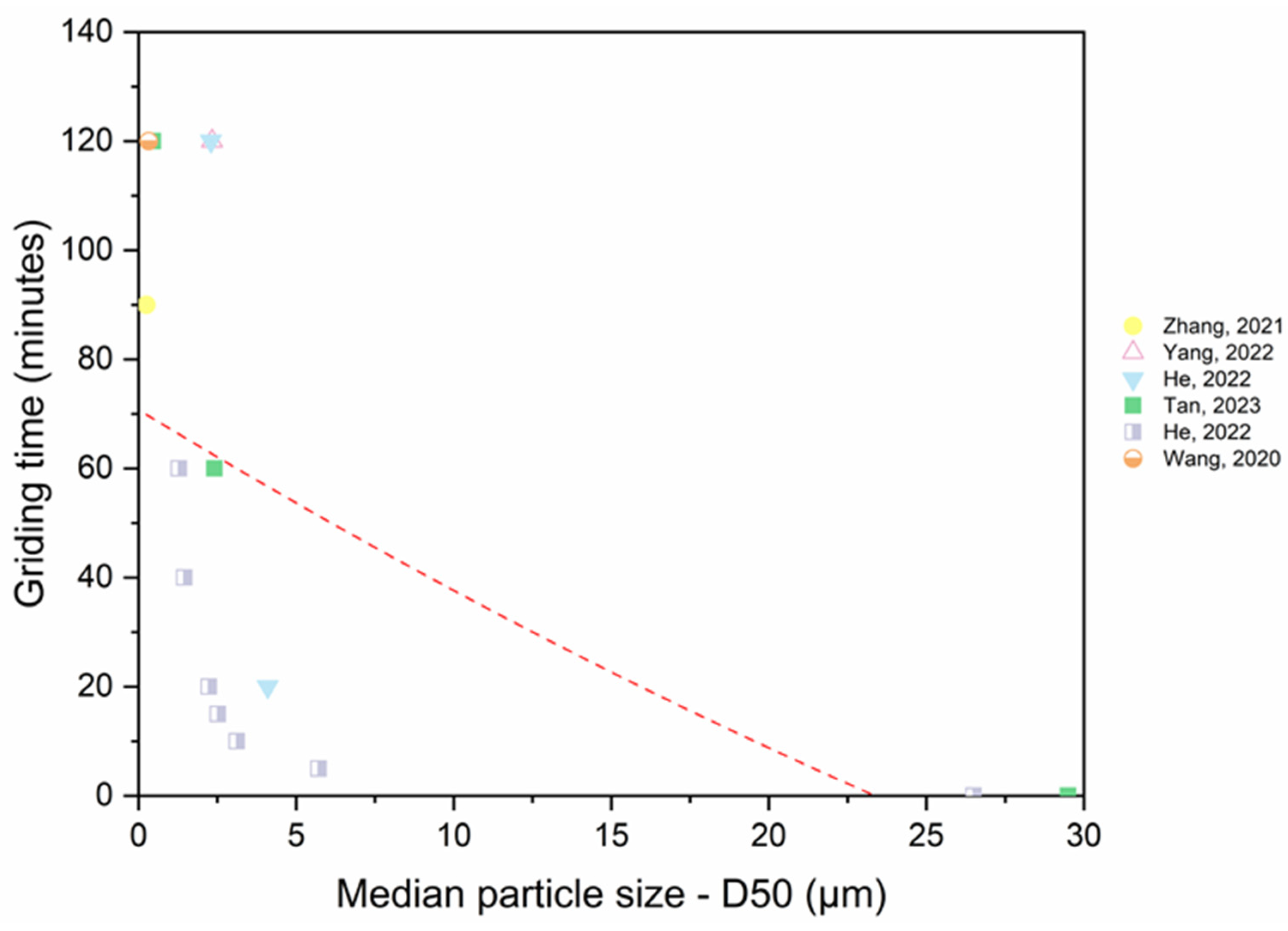
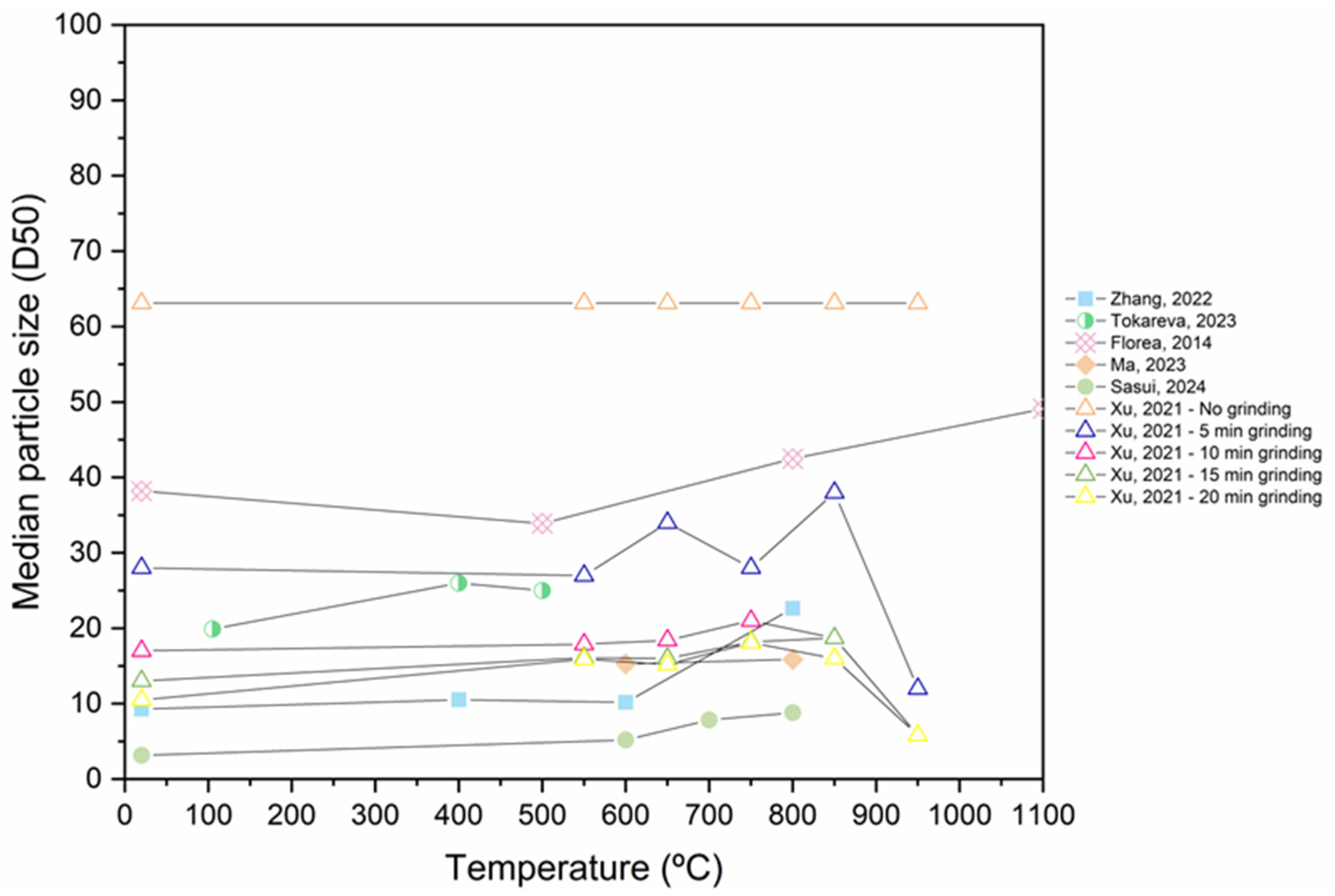
3.3. Specific Surface Area (SSA) and the Effect of Grinding Duration on RP’s Physical Properties
3.4. Physical and Chemical Properties of RP
3.4.1. Density
3.4.2. Appearance and Microstructure
3.4.3. Chemical Composition
3.5. Mineralogical Composition
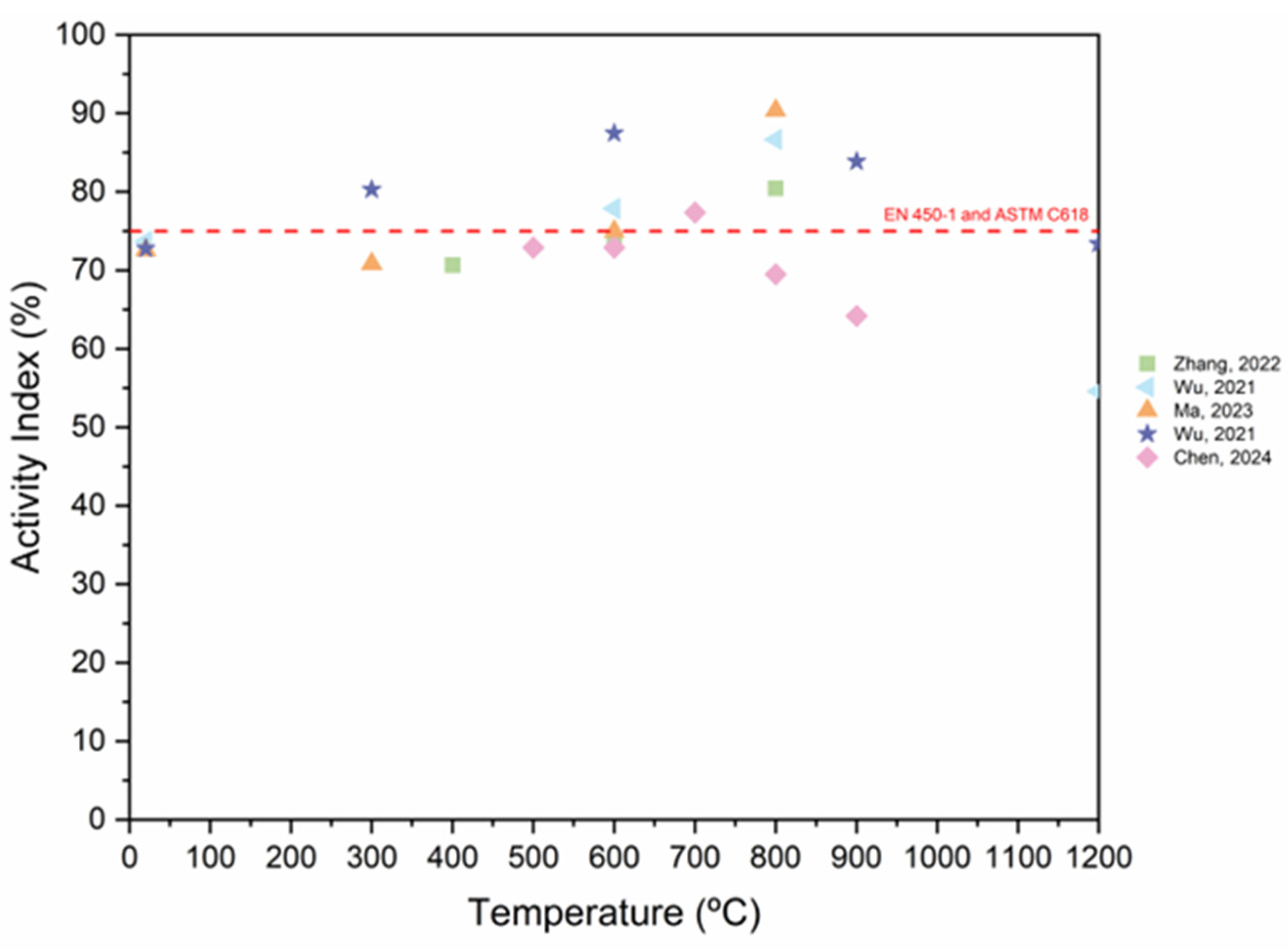
3.6. Activity Index
4. Activation Methods of RP
4.1. Thermal Activation
Activity Index of Thermal Activation on RP
4.2. CO2 Activation
4.2.1. Direct Carbonation Processes
4.2.2. Indirect Carbonation Process
4.3. Biomineralization Activation
4.4. Other Activation Methods
Activity Index of CO2, Biomineralization, and Other Activation Methods on RP
5. Micro-Properties of the Cementitious Materials with RP
5.1. Hydration Heat Analysis
5.2. Microstructural Analysis
5.3. Mineralogical Composition
5.4. Pore Structure
6. Fresh-State Properties in Cementitious Matrices with RP
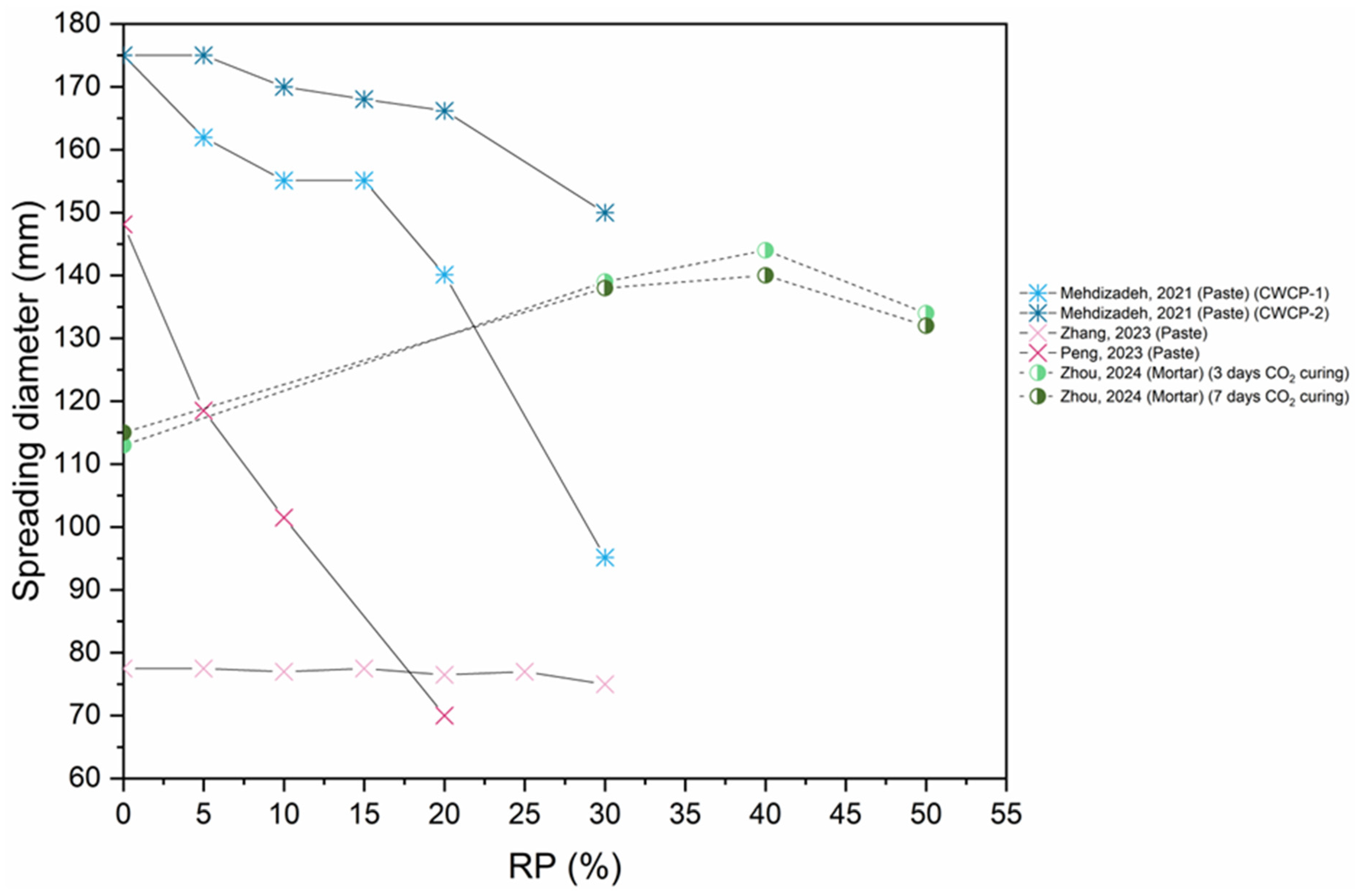
6.1. Workability
6.2. Setting Time
6.3. Air Content
6.4. Water Demand
6.5. Viscosity
6.6. Fresh Density
7. Conclusions
- When utilizing RP as an SCM, it is advisable to ensure it has a high reactivity and has a small particle size, comparable to or smaller than cement.
- RP reactivity has been shown to increase with higher SSA and fineness, grinding time, and grinding type. Enhancing the RP’s fineness can elevate the SSA of the RP, transform the stable SiO2 into amorphous SiO2, and therefore enhance the RP’s activity.
- Mechanical grinding can decrease the particle diameter and enhance the SSA of RP. However, the process of grinding to achieve fine RP requires a significant amount of energy, resulting in adverse environmental impacts and increased costs for RP. Furthermore, excessive grinding duration leads to an agglomeration of the material and reduces the strength of the specimen. Based on the analysis, 20 to 30 min is an appropriate duration for dry-grinding, whereas 60 to 80 min is ideal for wet grinding and the smallest RP particle sizes were produced by wet grinding.
- RP is a dry powder which has an off-white, grey color, and its look is comparable to that of cement, FA, SF, and LP. On the other hand, RP particles exhibit non-uniform shapes and uneven edges with sharp angles unlike cement, FA, SF, and LP particles.
- The primary chemical constituents of the RP are SiO2, CaO, Al2O3, and Fe2O3, whose contents vary according to waste origin. These chemical constituents closely resemble the composition of regular cement, FA, and GGBS which indicates an optimal distribution of oxides and an efficient source to be utilized as SCM in cement-based materials.
- SiO2 and Al2O3 are produced from sand and residues of coarse aggregate. CaO is formed through the hydration of cement particles, as well as from unhydrated cement particles and CaCO3.
- RP fulfills the necessary criteria to be classified as a pozzolanic material, with a composition of Fe2O3+Al2O3+SiO2 above 70%. On the other hand, the maximum LOI requirement, 6%, is not met for most of the cases.
- The mineralogical composition of RP is complex because of the various mineral phases found in cement-based materials, transitioning between crystalline and amorphous states.
- As the RP replacement ratio increases, the activity index of the RP decreases. Furthermore, extending the grinding period enhances the fineness and specific surface area of RP, hence enhancing the activity index of RP. In general, the AI of RP fulfills the standard requirement.
- Thermal activation effectively improves the hydration rate and maximizes the potential activity of RP. In general, an increase in temperature during the thermal treatment of RP results in a noticeable alteration in the size of the particles and on the minerology. To achieve a feasible thermal activation, it is recommended to maintain a temperature range of 600–900 °C. This temperature range will facilitate the generation of active components that can actively engage in the new hydration reaction. Regarding the effect of thermal treatment on the AI of RP, thermal activation increases the SAI up to 800 °C.
- The gas–solid and liquid–solid procedures of the direct carbonation method can effectively generate a suitable level of carbonation in RP by enhancing the microstructure of RP, resulting in reduced porosity because of the production of CaCO3 which also enhances the formation of stable CAHC, CAMC, and AFt, hence enhancing the microstructure of the cement matrix.
- Liquid–solid carbonation was found to be more effective in completing the carbonation process within a shorter timeframe (6 h), when the carbonation time for gas–solid carbonation was 24 h.
- Direct carbonation has several key benefits, including reduced costs and environmental consequences due to its straightforward nature and decreased use of chemicals compared to indirect carbonation.
- In general, direct carbonation increases the particle size and SSA of RP due to the particle agglomeration and chemical reaction of hydration products, and the carbonated RP exhibits a denser and smoother surface topology due to the carbonation of the hydration products which fills the micropores and voids with CaCO3 and silica gel.
- AI demonstrates a substantial enhancement while utilizing carbonated RP and RP treated with biomineralization in comparison to untreated RP.
- Biomineralization, chemical activation, TA activation, and the joint application of activation methods have been found to have positive results for different properties.
- RP addition decreases the alkalinity of the mixture, while the inclusion of an alkali exciter enhances the effectiveness of the RP. The use of an alkali activator can enhance the development of C–S–H, resulting in a more compact microstructure.
- The incorporation of mineral admixtures, and nano materialsis helpful to improve the performance of RP.
- The inclusion of the RP leads to a decrease in the peak time of the heat of hydration curves, suggesting that the addition of RP enhances the rate at which the paste hydrates due to its dilution effect. When the percentage of RP is below 30% and the fineness (D50) is below 20 μm, it enhances the early hydration of cement, leading to an increased rate of heat generation and a shorter induction duration. Similar to mechanical activation, thermal activation and CO2 activation have also a positive impact on enhancing the rate at which the RP hydrates.
- Typically, the microstructure of cementitious matrices including RP is less dense, more porous, and has more cracks compared to the reference mix. Increased looseness, more pores, and cracks can be observed when the RP ratio increases. However, when the RP content is below 30%, it does not have the ability to change the cement matrix. Instead, it forms a dense microstructure that is influenced by physical filling.
- The concentration of SiO2 and CaCO3 in the cementitious matrices increase, and CH and C–S–H decrease as the rate of RP replacement increases. The thermal activation and CO2 activation of RP have a limited impact on the mineral composition of the mixtures.
- As RP is involved and RP content increases, porosity increases. The presence of more RP content results in a proportional increase in the pore size and pore volume and an increase in the percentage of larger pores. High fineness of RP can enhance the hydration reaction of cement paste and increase the density of concrete by filling the pores.
- Thermal activation (up to 800 °C), CO2 activation, mineral admixtures, SCMs and nano material incorporation, and alkaline environment enhance the pore structure of the RP blended cementitious matrices.
- The addition of RP, the addition of nano materials, and thermal activation (up to 900 °C) result in a substantial decrease in workability, due to porous and uneven microstructure, the high specific surface area of fine particles, elevated initial hydration heat in the mixture because of the increased RP content, and the elevated water absorption of RP.
- Using an optimal combination of SCMs and CO2 activation enhances the workability of the mix.
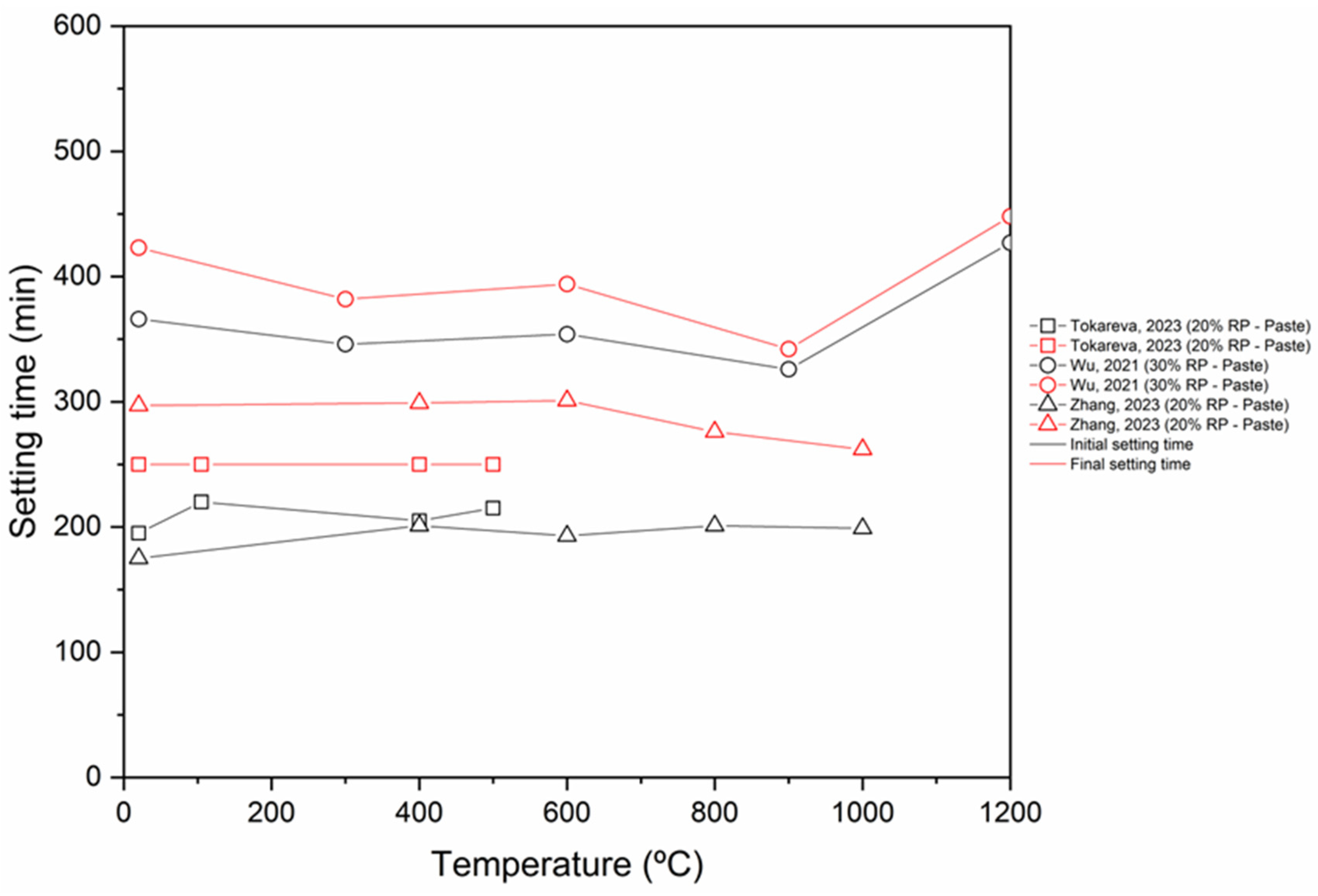
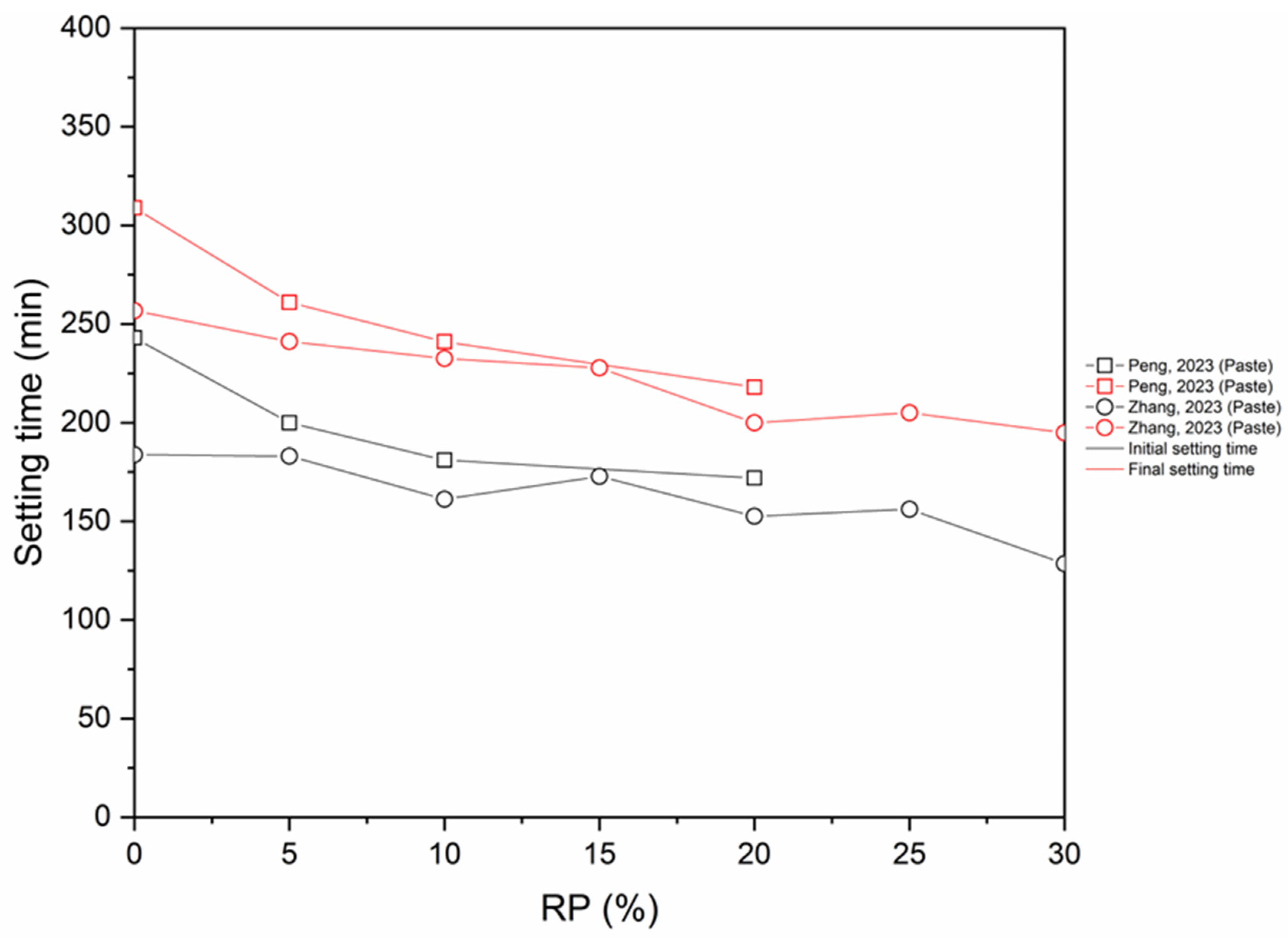
- The inclusion of RP has a dual effect on the setting time. On one side, the inclusion of RP reduces the quantity of hydration products in the mix, thereby prolonging the setting time. Conversely, RP particles function as nuclei for crystallization, thus facilitating the formation of hydration products and reducing the time it takes for the material to crystallize.
- Chemical activation and thermal activation (up to 900 °C) reduce the setting time (initial and final). On the other hand, CO2 activation causes an increase in the setting time.
- The incorporation of RP leads to an increase in air content due to the higher level of the porosity of the adhering mortar and to a more irregular form compared to the PC particles.
- As the incorporation of untreated RP or thermally activated RP increases, water demand increases due to the irregular shape, rough surface, micro pores, and the fineness of RP.
- The incorporation of SCMs and CO2 activation reduces the water demand.
- The inclusion of RP results in a significant increase in viscosity due to the rough and irregular morphology of the particles. The incorporation of SCMs also increases viscosity.
- As the substitution rate of RP increases, fresh density decreases marginally, due to the irregular nature, porosity, and low density of RP.
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, X.; Shang, Y.; Song, M. Industrial structure distortion and urban ecological efficiency from the perspective of green entrepreneurial ecosystems. Socio-Econ. Plan. Sci. 2020, 72, 100757. [Google Scholar] [CrossRef]
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Tripathi, D.K.; Chauhan, D.K.; Kumar, N.; Singh, G.S. Paradigms of climate change impacts on some major food sources of the world: A review on current knowledge and future prospects. Agric. Ecosyst. Environ. 2016, 216, 356–373. [Google Scholar] [CrossRef]
- IEA. Global Status Report for Buildings and Construction; IEA: Paris, France, 2019. [Google Scholar]
- Yang, Y.; Kang, Z.; Zhan, B.; Gao, P.; Yu, Q.; Wang, J.; Zhao, W.; Zhang, Y.; Bi, W.; Yang, C.; et al. Short review on the application of recycled powder in cement-based materials: Preparation, performance and activity excitation. Buildings 2022, 12, 1568. [Google Scholar] [CrossRef]
- Sun, C.; Chen, L.; Xiao, J.; Singh, A.; Zeng, J. Compound utilization of construction and industrial waste as cementitious recycled powder in mortar. Resour. Conserv. Recycl. 2021, 170, 105561. [Google Scholar] [CrossRef]
- Oliveira, D.R.B.; Leite, G.; Possan, E.; Marques Filho, J. Concrete powder waste as a substitution for Portland cement for environment-friendly cement production. Constr. Build. Mater. 2023, 397, 132382. [Google Scholar] [CrossRef]
- Wu, H.; Yang, D.; Ma, Z. Micro-structure, mechanical and transport properties of cementitious materials with high-volume waste concrete powder and thermal modification. Constr. Build. Mater. 2021, 313, 125477. [Google Scholar] [CrossRef]
- Rocha, J.H.A.; Toledo Filho, R.D. The utilization of recycled concrete powder as supplementary cementitious material in cement-based materials: A systematic literature review. J. Build. Eng. 2023, 76, 107319. [Google Scholar] [CrossRef]
- Xiao, J.; Hao, L.; Cao, W.; Ye, T. Influence of recycled powder derived from waste concrete on mechanical and thermal properties of foam concrete. J. Build. Eng. 2022, 61, 105203. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, S.H.; Kim, B. Properties of extrusion concrete panel using waste concrete powder. Appl. Sci. 2017, 7, 910. [Google Scholar] [CrossRef]
- Cantero, B.; Bravo, M.; de Brito, J.; Sáez del Bosque, I.F.; Medina, C. Thermal performance of concrete with recycled concrete powder as partial cement replacement and recycled CDW aggregate. Appl. Sci. 2020, 10, 4540. [Google Scholar] [CrossRef]
- Tanash, A.O.; Muthusamy, K.; Yahaya, F.M.; Ismail, M.A. Potential of recycled powder from clay Brick, sanitary Ware, and concrete waste as a cement substitute for Concrete: An overview. Constr. Build. Mater. 2023, 401, 132760. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Juenger, M.C.; Snellings, R.; Bernal, S.A. Supplementary cementitious materials: New sources, characterization, and performance insights. Cem. Concr. Res. 2019, 122, 257–273. [Google Scholar] [CrossRef]
- Rocha, J.H.A.; Toledo Filho, R.D.; Cayo-Chileno, N.G. Sustainable alternatives to CO2 reduction in the cement industry: A short review. Mater. Today Proc. 2022, 57, 436–439. [Google Scholar] [CrossRef]
- Campos, H.F.; Klein, N.S.; Marques Filho, J.; Bianchini, M. Low-cement high-strength concrete with partial replacement of Portland cement with stone powder and silica fume designed by particle packing optimization. J. Clean. Prod. 2020, 261, 121228. [Google Scholar] [CrossRef]
- John, V.M.; Damineli, B.L.; Quattrone, M.; Pileggi, R.G. Fillers in cementitious materials—Experience, recent advances and future potential. Cem. Concr. Res. 2018, 114, 65–78. [Google Scholar] [CrossRef]
- Carriço, A.; Bogas, J.A.; Guedes, M. Thermoactivated cementitious materials—A review. Constr. Build. Mater. 2020, 250, 118873. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Y.; Tam, V.W.; Tan, J.; Shen, A.; Zhang, C.; Zhang, J. Feasibility of recycled aggregates modified with a compound method involving sodium silicate and silane as permeable concrete aggregates. Constr. Build. Mater. 2022, 361, 129747. [Google Scholar] [CrossRef]
- Gartner, E.; Hirao, H. A review of alternative approaches to the reduction of CO2 emissions associated with the manufacture of the binder phase in concrete. Cem. Concr. Res. 2015, 78, 126–142. [Google Scholar] [CrossRef]
- Schneider, M. The cement industry on the way to a low-carbon future. Cem. Concr. Res. 2019, 124, 105792. [Google Scholar] [CrossRef]
- Zito, S.V.; Irassar, E.F.; Rahhal, V.F. Recycled Construction and Demolition Waste as Supplementary Cementing Materials in Eco-Friendly Concrete. Recycling 2023, 8, 54. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, M.; Duan, Z.; Liang, C.; Wu, H. Effects of active waste powder obtained from C&D waste on the microproperties and water permeability of concrete. J. Clean. Prod. 2020, 257, 120518. [Google Scholar]
- Noaman, A.S.; Alsaffar, A.E. A suggestion of a procedural method for the management of post-war waste. Civ. Eng. J. 2019, 5, 2143–2151. [Google Scholar] [CrossRef]
- European Commission. Construction and Demolition Waste (CDW). 2016. Available online: http://ec.europa.eu/environment/waste/construction_demolition.htm (accessed on 13 March 2024).
- Wang, J.; Wu, H.; Duan, H.; Zillante, G.; Zuo, J.; Yuan, H. Combining life cycle assessment and Building Information Modelling to account for carbon emission of building demolition waste: A case study. J. Clean. Prod. 2018, 172, 3154–3166. [Google Scholar] [CrossRef]
- Wu, H.; Zuo, J.; Yuan, H.; Zillante, G.; Wang, J. A review of performance assessment methods for construction and demolition waste management. Resour. Conserv. Recycl. 2019, 150, 104407. [Google Scholar] [CrossRef]
- European Commission. Construction and Demolition Waste (CDW). 2018. Available online: https://ec.europa.eu/newsroom/growth/items/455097/en (accessed on 17 March 2024).
- US EPA. Advancing Sustainable Materials Management: 2018 Fact Sheet; US EPA: Washington, DC, USA, 2020. [Google Scholar]
- Pickin, J.; Randell, P.; Trinh, J.; Grant, B. Australian National Waste Report. 2018, Australia. Available online: https://www.dcceew.gov.au/sites/default/files/documents/national-waste-report-2018.pdf (accessed on 20 October 2024).
- Du, J.; Zhang, T.; Chen, P.; Guo, Y.; Zhan, B.; Wei, J.; Yu, Q. Phase separation of recycled concrete powder during grinding and consequent influences on its hydration behaviors in cement paste. Cem. Concr. Compos. 2023, 142, 105203. [Google Scholar] [CrossRef]
- Tisi, Y.S.A.B.; Matos, F.A.; Carneiro, M.L.N.M. Development of waste-to-energy through integrated sustainable waste management: The case of ABREN WtERT Brazil towards changing status quo in Brazil. Waste Dispos. Sustain. Energy. 2023, 5, 295–308. [Google Scholar] [CrossRef]
- Oh, D.; Noguchi, T.; Kitagaki, R.; Choi, H. Proposal of demolished concrete recycling system based on performance evaluation of inorganic building materials manufactured from waste concrete powder. Renew. Sustain. Energy Rev. 2021, 135, 110147. [Google Scholar] [CrossRef]
- Li, Z.; Bian, Y.; Zhao, J.; Wang, Y.; Yuan, Z. Recycled concrete fine powder (RFP) as cement partial replacement: Influences on the physical properties, hydration characteristics, and microstructure of blended cement. J. Build. Eng. 2022, 62, 105326. [Google Scholar] [CrossRef]
- Jain, M.S. A mini review on generation, handling, and initiatives to tackle construction and demolition waste in India. Environ. Technol. Innov. 2021, 22, 101490. [Google Scholar] [CrossRef]
- Tam, V.W.; Soomro, M.; Evangelista, A.C.J. A review of recycled aggregate in concrete applications (2000–2017). Constr. Build. Mater. 2018, 172, 272–292. [Google Scholar] [CrossRef]
- Tang, Q.; Ma, Z.; Wu, H.; Wang, W. The utilization of eco-friendly recycled powder from concrete and brick waste in new concrete: A critical review. Cem. Concr. Compos. 2020, 114, 103807. [Google Scholar] [CrossRef]
- Pastrana-Ayala, J.; Silva-Urrego, Y.; Adrada-Molano, J.; Delvasto-Arjona, S. Physical mechanic properties of self-compacting concrete produced with concrete waste powder. Informador Técnico 2019, 83, 174–190. [Google Scholar]
- Liang, C.; Lu, N.; Ma, H.; Ma, Z.; Duan, Z. Carbonation behavior of recycled concrete with CO2-curing recycled aggregate under various environments. J. CO2 Util. 2020, 39, 101185. [Google Scholar] [CrossRef]
- Seror, N.; Portnov, B.A. Estimating the effectiveness of different environmental law enforcement policies on illegal C&D waste dumping in Israel. Waste Manag. 2020, 102, 241–248. [Google Scholar]
- Xiao, J.; Ma, Z.; Ding, T. Reclamation chain of waste concrete: A case study of Shanghai. Waste Manag. 2016, 48, 334–343. [Google Scholar] [CrossRef]
- Alsheyab, M.A.T. Recycling of construction and demolition waste and its impact on climate change and sustainable development. Int. J. Environ. Sci. Technol. 2022, 19, 2129–2138. [Google Scholar] [CrossRef]
- Ferreira, R.L.; Anjos, M.A.; Nóbrega, A.K.; Pereira, J.E.; Ledesma, E.F. The role of powder content of the recycled aggregates of CDW in the behaviour of rendering mortars. Constr. Build. Mater. 2019, 208, 601–612. [Google Scholar] [CrossRef]
- Li, S.; Gao, J.; Li, Q.; Zhao, X. Investigation of using recycled powder from the preparation of recycled aggregate as a supplementary cementitious material. Constr. Build. Mater. 2021, 267, 120976. [Google Scholar] [CrossRef]
- Wang, Y.; Burris, L.; Shearer, C.R.; Hooton, D.; Suraneni, P. Strength activity index and bulk resistivity index modifications that differentiate inert and reactive materials. Cem. Concr. Compos. 2021, 124, 104240. [Google Scholar] [CrossRef]
- Özalp, F.; Yılmaz, H.D.; Kara, M.; Kaya, Ö.; Şahin, A. Effects of recycled aggregates from construction and demolition wastes on mechanical and permeability properties of paving stone, kerb and concrete pipes. Constr. Build. Mater. 2016, 110, 17–23. [Google Scholar] [CrossRef]
- Liu, Q.; Tong, T.; Liu, S.; Yang, D.; Yu, Q. Investigation of using hybrid recycled powder from demolished concrete solids and clay bricks as a pozzolanic supplement for cement. Constr. Build. Mater. 2014, 73, 754–763. [Google Scholar] [CrossRef]
- Vieira, C.S.; Pereira, P.M. Use of recycled construction and demolition materials in geotechnical applications: A review. Resour. Conserv. Recycl. 2015, 103, 192–204. [Google Scholar] [CrossRef]
- Rahman, M.A.; Imteaz, M.; Arulrajah, A.; Disfani, M.M. Suitability of recycled construction and demolition aggregates as alternative pipe backfilling materials. J. Clean. Prod. 2014, 66, 75–84. [Google Scholar] [CrossRef]
- Pourkhorshidi, S.; Sangiorgi, C.; Torreggiani, D.; Tassinari, P. Using recycled aggregates from construction and demolition waste in unbound layers of pavements. Sustainability 2020, 12, 9386. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, S.; Huang, B.; Yang, Q.; Li, J. Comparison of mechanical, chemical, and thermal activation methods on the utilisation of recycled concrete powder from construction and demolition waste. J. Build. Eng. 2022, 61, 105295. [Google Scholar] [CrossRef]
- Marinković, S.; Radonjanin, V.; Malešev, M.; Ignjatović, I. Comparative environmental assessment of natural and recycled aggregate concrete. Waste Manag. 2010, 30, 2255–2264. [Google Scholar] [CrossRef]
- Xia, B.; Ding, T.; Xiao, J. Life cycle assessment of concrete structures with reuse and recycling strategies: A novel framework and case study. Waste Manag. 2020, 105, 268–278. [Google Scholar] [CrossRef]
- Li, L.G.; Lin, C.J.; Chen, G.M.; Kwan, A.K.H.; Jiang, T. Effects of packing on compressive behaviour of recycled aggregate concrete. Constr. Build. Mater. 2017, 157, 757–777. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, B.; Liu, S.; He, J.; Hernandez, A.G. Role of recycled concrete powder as sand replacement in the properties of cement mortar. J. Clean. Prod. 2022, 371, 133424. [Google Scholar] [CrossRef]
- Guo, H.; Shi, C.; Guan, X.; Zhu, J.; Ding, Y.; Ling, T.C.; Zhang, H.; Wang, Y. Durability of recycled aggregate concrete—A review. Cem. Concr. Compos. 2018, 89, 251–259. [Google Scholar] [CrossRef]
- Kancheva, Y.D.; Zaharieva, R.A. Recycling of concrete construction and demolition waste in alternative binders: Part 2–Environmental footprint. IOP Conf. Ser. Mater. Sci. Eng. 2020, 951, 012009. [Google Scholar] [CrossRef]
- Deng, X.; Li, J.; Lu, Z.; Zhang, J.; Luo, K.; Niu, Y.; Hu, J.; He, K. Rheological and early hydration of cementitious material containing recycled concrete powders collected from recycled aggregates. Constr. Build. Mater. 2023, 393, 132108. [Google Scholar] [CrossRef]
- Tam, V.W.; Gao, X.F.; Tam, C.M. Microstructural analysis of recycled aggregate concrete produced from two-stage mixing approach. Cem. Concr. Res. 2005, 35, 1195–1203. [Google Scholar] [CrossRef]
- Mehdizadeh, H.; Ling, T.C.; Cheng, X.; Mo, K.H. Effect of particle size and CO2 treatment of waste cement powder on properties of cement paste. Can. J. Civ. Eng. 2021, 48, 522–531. [Google Scholar]
- Kim, Y.J.; Choi, Y.W. Utilization of waste concrete powder as a substitution material for cement. Constr. Build. Mater. 2012, 30, 500–504. [Google Scholar] [CrossRef]
- Silva, S.; Evangelista, L.; De Brito, J. Durability and shrinkage performance of concrete made with coarse multi-recycled concrete aggregates. Constr. Build. Mater. 2021, 272, 121645. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, J.; Qi, C.; Li, C. Rate sensitivity analysis of structural behaviors of recycled aggregate concrete frame. J. Build. Eng. 2022, 45, 103634. [Google Scholar] [CrossRef]
- Duan, Z.; Hou, S.; Xiao, J.; Li, B. Study on the essential properties of recycled powders from construction and demolition waste. J. Clean. Prod. 2020, 253, 119865. [Google Scholar] [CrossRef]
- Duan, Z.; Hou, S.; Xiao, J.; Singh, A. Rheological properties of mortar containing recycled powders from construction and demolition wastes. Constr. Build. Mater. 2020, 237, 117622. [Google Scholar] [CrossRef]
- Sun, D.; Huang, N.; Liu, K.; Tang, J.; Rong, N.; Wang, A.; Guan, Y.; Liang, P.; Deng, Y. Effect of recycled fine powder on autoclaved aerated concrete: Gas-foaming, physic-mechanical property and hydration products. J. Build. Eng. 2023, 67, 106013. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Ding, H.; Qiu, J.; Guo, Z. Recycling flue gas desulfurisation gypsum and phosphogypsum for cemented paste backfill and its acid resistance. Constr. Build. Mater. 2021, 275, 122170. [Google Scholar] [CrossRef]
- Yao, C.; Guo, Y.; Shen, A.; Cui, W.; He, Z. Recycling of fine-asphalt-pavement solid waste for low-shrinkage rapid hardening Portland cement concrete pavement. Constr. Build. Mater. 2021, 289, 123132. [Google Scholar] [CrossRef]
- Kim, J. Influence of quality of recycled aggregates on the mechanical properties of recycled aggregate concretes: An overview. Constr. Build. Mater. 2022, 328, 127071. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Bai, H.; Ma, L. Utilization of recycled concrete powder in cement composite: Strength, microstructure and hydration characteristics. J. Renew. Mater. 2021, 9, 2189–2208. [Google Scholar] [CrossRef]
- Ohemeng, E.A.; Ekolu, S.O.; Quainoo, H.; Naghizadeh, A. Economical and eco-friendly masonry mortar containing waste concrete powder as a supplementary cementitious material. Case Stud. Constr. Mater. 2022, 17, e01527. [Google Scholar] [CrossRef]
- Yao, T.; Tian, Q.; Zhang, M.; Qi, S.; Wang, C.; Ruan, M.; Xu, G.; Cai, J. Experimental research on the preparation and properties of foamed concrete using recycled waste concrete powder. Constr. Build. Mater. 2023, 407, 133370. [Google Scholar] [CrossRef]
- Villagrán-Zaccardi, Y.A.; Marsh, A.T.; Sosa, M.E.; Zega, C.J.; De Belie, N.; Bernal, S.A. Complete re-utilization of waste concretes–Valorisation pathways and research needs. Resour. Conserv. Recycl. 2022, 177, 105955. [Google Scholar] [CrossRef]
- Gebremariam, A.T.; Di Maio, F.; Vahidi, A.; Rem, P. Innovative technologies for recycling End-of-Life concrete waste in the built environment. Resour. Conserv. Recycl. 2020, 163, 104911. [Google Scholar] [CrossRef]
- Meng, Y.; Ling, T.C.; Mo, K.H. Recycling of wastes for value-added applications in concrete blocks: An overview. Resour. Conserv. Recycl. 2018, 138, 298–312. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Fan, M.X.; Zhou, Y.X.; Ji, D.D.; Li, J.H.; Yu, R. Development of a sustainable alkali activated ultra-high performance concrete (A-UHPC) incorporating recycled concrete fines. J. Build. Eng. 2023, 67, 105986. [Google Scholar] [CrossRef]
- Caneda-Martínez, L.; Monasterio, M.; Moreno-Juez, J.; Martínez-Ramírez, S.; García, R.; Frías, M. Behaviour and Properties of Eco-Cement Pastes Elaborated with Recycled Concrete Powder from Construction and Demolition Wastes. Materials 2021, 14, 1299. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, C.; Liu, H.; Hu, H.; He, C.; Song, L.; Huang, W. Pore structure and durability of green concrete containing recycled powder and recycled coarse aggregate. J. Build. Eng. 2022, 53, 104584. [Google Scholar] [CrossRef]
- Qian, X.; Xu, W.; Wang, Y.; Fang, H.; Jing, Z.; Chen, P. Revisiting the carbonation of recycled concrete fine: A pH-cycle carbonation method. J. Build. Eng. 2023, 77, 107438. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; Heikal, E.; El-Didamony, H.; Hashim, F.S.; Mohammed, A.H. Recycling of concrete waste to produce ready-mix alkali activated cement. Ceram. Int. 2018, 44, 7300–7304. [Google Scholar] [CrossRef]
- Kim, J.; Nciri, N.; Sicakova, A.; Kim, N. Characteristics of waste concrete powders from multi-recycled coarse aggregate concrete and their effects as cement replacements. Constr. Build. Mater. 2023, 398, 132525. [Google Scholar] [CrossRef]
- Deng, X.; Guo, H.; Tan, H.; He, X.; Zheng, Z.; Su, Y.; Yang, J. An accelerator prepared from waste concrete recycled powder and its effect on hydration of cement-based materials. Constr. Build. Mater. 2021, 296, 123767. [Google Scholar] [CrossRef]
- Sui, Y.; Liu, S.; Ou, C.; Liu, Q.; Meng, G. Experimental investigation for the influence of graphene oxide on properties of the cement-waste concrete powder composite. Constr. Build. Mater. 2021, 276, 122229. [Google Scholar] [CrossRef]
- Ji, Y.; Ji, W.; Li, W. Performance of Building Solid Waste Powder in Cement Cementitious Material: A Review. Materials 2022, 15, 5408. [Google Scholar] [CrossRef]
- Mucsi, G.; Halyag Papné, N.; Ulsen, C.; Figueiredo, P.O.; Kristály, F. Mechanical activation of construction and demolition waste in order to improve its pozzolanic reactivity. ACS Sustain. Chem. Eng. 2021, 9, 3416–3427. [Google Scholar] [CrossRef]
- Xiao, J.; Ma, Z.; Sui, T.; Akbarnezhad, A.; Duan, Z. Mechanical properties of concrete mixed with recycled powder produced from construction and demolition waste. J. Clean. Prod. 2018, 188, 720–731. [Google Scholar] [CrossRef]
- Pereira, P.; Evangelista, L.M.F.D.R.; De Brito, J.M.C.L. The effect of superplasticizers on the mechanical performance of concrete made with fine recycled concrete aggregates. Cem. Concr. Compos. 2012, 34, 1044–1052. [Google Scholar] [CrossRef]
- Zhao, Z.; Remond, S.; Damidot, D.; Xu, W. Influence of fine recycled concrete aggregates on the properties of mortars. Constr. Build. Mater. 2015, 81, 179–186. [Google Scholar] [CrossRef]
- Muñoz-Ruiperez, C.; Rodríguez, A.; Gutiérrez-González, S.; Calderón, V. Lightweight masonry mortars made with expanded clay and recycled aggregates. Constr. Build. Mater. 2016, 118, 139–145. [Google Scholar] [CrossRef]
- Wu, Y.; Mehdizadeh, H.; Mo, K.H.; Ling, T.C. High-temperature CO2 for accelerating the carbonation of recycled concrete fines. J. Build. Eng. 2022, 52, 104526. [Google Scholar] [CrossRef]
- Singh, A.; Miao, X.; Zhou, X.; Deng, Q.; Li, J.; Zou, S.; Duan, Z. Use of recycled fine aggregates and recycled powders in sustainable recycled concrete. J. Build. Eng. 2023, 77, 107370. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, J.; Tang, Y.; Duan, Z.; Poon, C.S. Long-term shrinkage and mechanical properties of fully recycled aggregate concrete: Testing and modelling. Cem. Concr. Compos. 2022, 130, 104527. [Google Scholar] [CrossRef]
- Nedeljkovic, M.; Visser, J.; Šavija, B.; Valcke, S.; Schlangen, E. Use of fine recycled concrete aggregates in concrete: A critical review. J. Build. Eng. 2021, 38, 102196. [Google Scholar] [CrossRef]
- Feng, Y.; Li, J.; Zhang, B.; Fu, H.; Chen, W.; Xue, Z.; Lu, Z.; Yang, J.; Xie, J. Concrete improvement incorporating recycled powder and aggregates treated via a combination of calcination and carbonation: The impact behaviors. J. Clean. Prod. 2023, 418, 138069. [Google Scholar] [CrossRef]
- Chinzorigt, G.; Lim, M.K.; Yu, M.; Lee, H.; Enkbold, O.; Choi, D. Strength, shrinkage and creep and durability aspects of concrete including CO2 treated recycled fine aggregate. Cem. Concr. Res. 2020, 136, 106062. [Google Scholar] [CrossRef]
- Zhang, B.; Feng, Y.; Xie, J.; Dai, J.; Chen, W.; Xue, Z.; Li, L.; Li, Y.; Li, J. Effects of pretreated recycled powder substitution on mechanical properties and microstructures of alkali-activated cement. Constr. Build. Mater. 2023, 406, 133360. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, C.; He, B.; Yi, S.; Tang, L. Recycling fine powder collected from construction and demolition wastes as partial alternatives to cement: A comprehensive analysis on effects, mechanism, cost and CO2 emission. J. Build. Eng. 2023, 71, 106507. [Google Scholar] [CrossRef]
- Zhang, D.; Hao, W.; Yang, Q. Experimental Study on the Application of Recycled Concrete Waste Powder in Alkali-Activated Foamed Concrete. Materials 2023, 16, 5728. [Google Scholar] [CrossRef]
- Wu, H.; Wang, C.; Yang, D.; Ma, Z. Utilizing nano-SiO2 for modifying the microstructure, strength and transport properties of sustainable cementitious materials with waste concrete powder. J. Build. Eng. 2023, 63, 105522. [Google Scholar] [CrossRef]
- Hu, R.; Wang, C.; Shen, J.; Ma, Z. Chloride transport and thermoactivated modification of sustainable cement-based materials with high-content waste concrete powder. J. Mater. Res. Technol. 2023, 23, 4012–4031. [Google Scholar] [CrossRef]
- Bu, C.; Tan, B.; Wu, Q.; Qiao, Y.; Sun, Y.; Yu, L.; Yang, Q. Activation Method and Reuse of Waste Concrete Powder—A Review. Sustainability 2023, 15, 5451. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Wang, H.; Fang, Y.; Shen, W.; Chen, P.; Xu, Y. Eco-friendly treatment of recycled concrete fines as supplementary cementitious materials. Constr. Build. Mater. 2022, 322, 126491. [Google Scholar] [CrossRef]
- Prziwara, P.; Breitung-Faes, S.; Kwade, A. Comparative study of the grinding aid effects for dry fine grinding of different materials. Miner. Eng. 2019, 144, 106030. [Google Scholar] [CrossRef]
- Qian, H.Y.; Kong, Q.G.; Zhang, B.L. The effects of grinding media shapes on the grinding kinetics of cement clinker in ball mill. Powder Technol. 2013, 235, 422–425. [Google Scholar] [CrossRef]
- Bu, X.; Chen, Y.; Ma, G.; Sun, Y.; Ni, C.; Xie, G. Wet and dry grinding of coal in a laboratory-scale ball mill: Particle-size distributions. Powder Technol. 2020, 359, 305–313. [Google Scholar] [CrossRef]
- Chelgani, S.C.; Parian, M.; Parapari, P.S.; Ghorbani, Y.; Rosenkranz, J. A comparative study on the effects of dry and wet grinding on mineral flotation separation—A review. J. Mater. Res. Technol. 2019, 8, 5004–5011. [Google Scholar] [CrossRef]
- Ogonowski, S.; Wolosiewicz-Glab, M.; Ogonowski, Z.; Foszcz, D.; Pawelczyk, M. Comparison of wet and dry grinding in electromagnetic mill. Minerals 2018, 8, 138. [Google Scholar] [CrossRef]
- Bu, X.; Chen, Y.; Ma, G.; Sun, Y.; Ni, C.; Xie, G. Differences in dry and wet grinding with a high solid concentration of coking coal using a laboratory conical ball mill: Breakage rate, morphological characterization, and induction time. Adv. Powder Technol. 2019, 30, 2703–2711. [Google Scholar] [CrossRef]
- Wang, Y.; He, X.; Su, Y.; Yang, J.; Strnadel, B.; Wang, X. Efficiency of wet-grinding on the mechano-chemical activation of granulated blast furnace slag (GBFS). Constr. Build. Mater. 2019, 199, 185–193. [Google Scholar] [CrossRef]
- ASTM C204; Standard Test Methods for Fineness of Hydraulic Cement by Air-Permeability Apparatus. Annual Book of ASTM. American Society of Testing and Materials: West Conshohocken, PA, USA, 2007.
- EN 196-6; Methods of Testing Cement—Part 6: Determination of Fineness. British Standards Institution: London, UK, 2010.
- Wang, T.; He, X.; Yang, J.; Zhao, H.; Su, Y. Nano-treatment of autoclaved aerated concrete waste and its usage in cleaner building materials. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2020, 35, 786–793. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, H.; He, X.; Zhao, R.; Yang, J.; Su, Y. Nano particles prepared from hardened cement paste by wet grinding and its utilization as an accelerator in Portland cement. J. Clean. Prod. 2021, 283, 124632. [Google Scholar] [CrossRef]
- He, Z.H.; Han, X.D.; Zhang, M.Y.; Yuan, Q.; Shi, J.Y.; Zhan, P.M. A novel development of green UHPC containing waste concrete powder derived from construction and demolition waste. Powder Tech. 2022, 398, 117075. [Google Scholar] [CrossRef]
- Wu, R.; Zhao, T.; Zhang, P.; Yang, D.; Liu, M.; Ma, Z. Tensile behavior of strain hardening cementitious composites (SHCC) containing reactive recycled powder from various C&D waste. J. Renew. Mater. 2021, 9, 743–765. [Google Scholar]
- Wu, H.; Liang, C.; Xiao, J.; Xu, J.; Ma, Z. Early-age behavior and mechanical properties of cement-based materials with various types and fineness of recycled powder. Struct. Concr. 2022, 23, 1253–1272. [Google Scholar] [CrossRef]
- Moreno-Juez, J.; Vegas, I.J.; Rojas, M.F.; de la Villa, R.V.; Guede-Vázquez, E. Laboratory-scale study and semi-industrial validation of viability of inorganic CDW fine fractions as SCMs in blended cements. Constr. Build. Mater. 2021, 271, 121823. [Google Scholar] [CrossRef]
- Li, S.; Li, Q.; Zhao, X.; Luo, J.; Gao, S.; Yue, G.; Su, D. Experimental study on the preparation of recycled admixtures by using construction and demolition waste. Materials 2019, 12, 1678. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ma, Q.; Su, Y.; Zheng, Z.; Tan, H.; Peng, K.; Zhao, R. Humid hardened concrete waste treated by multiple wet-grinding and its reuse in concrete. Constr. Build. Mater. 2022, 350, 128485. [Google Scholar] [CrossRef]
- Prošek, Z.; Tesárek, P.; Trejbal, J. Recycling and its use in concrete waste processing by high-speed milling. Acta Polytech. CTU Proc. 2019, 21, 28–32. [Google Scholar] [CrossRef]
- Oliveira, T.C.; Dezen, B.G.; Possan, E. Use of concrete fine fraction waste as a replacement of Portland cement. J. Clean. Prod. 2020, 273, 123126. [Google Scholar] [CrossRef]
- GB/T8074; Testing Method for Specific Surface of Cement-Blaine Method. National Standard of the People’s Republic of China: Beijing, China, 2008. (In Chinese)
- ASTM C204-18-1; Standard Test Methods for Fineness of Hydraulic Cement by Air-Permeability Apparatus. ASTM International: West Conshohocken, PA, USA, 2023.
- NBR 16372; Portland Cement and Other Powdered Materials—Determination of Fineness by the Air Permeability Method (Blaine Method). Associação Brasileira de Normas Técnicas–ABNT: Rio de Janeiro, Brazil, 2015.
- Hunger, M.; Brouwers, H.J.H. Flow analysis of water–powder mixtures: Application to specific surface area and shape factor. Cem. Concr. Compos. 2009, 31, 39–59. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Shen, W.; Cao, K.; Sun, L.; Wang, P.; Cui, L. Compressive strength, hydration and pore structure of alkali-activated slag mortars integrating with recycled concrete powder as binders. KSCE J. Civ. Eng. 2022, 26, 795–805. [Google Scholar] [CrossRef]
- Lei, B.; Xiong, Q.; Zhao, H.; Dong, W.; Tam, V.W.; Sun, Z.; Li, W. Performance of asphalt mortar with recycled concrete powder under different filler-to-asphalt weight ratios. Case Stud. Constr. Mater. 2023, 18, 01834. [Google Scholar] [CrossRef]
- Li, J.; Zhan, B.; Gao, P.; Hu, L.; Qiao, M.; Sha, H.; Yu, Q. Effects of recycled concrete powders on the rheology, setting and early age strength of cement paste. Constr. Build. Mater. 2023, 401, 132899. [Google Scholar] [CrossRef]
- Peng, L.; Jiang, Y.; Ban, J.; Shen, Y.; Ma, Z.; Zhao, Y.; Shen, P.; Poon, C.S. Mechanism underlying early hydration kinetics of carbonated recycled concrete fines-ordinary portland cement (CRCF-OPC) paste. Cem. Concr. Compos. 2023, 144, 105275. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Yu, C.; Li, Z.; Xie, W. Study on the performance of ternary blended cement with calcined clay and recycled concrete powder. Low-carbon Mater. Green Constr. 2024, 2, 4. [Google Scholar] [CrossRef]
- Sasui, S.; Kim, G.; van Riessen, A.; Alam, S.F.; Nam, J.; Ishak, S.; Eu, H. Influence of elevated temperature on waste concrete powder and its application in alkali activated materials. J. Clean. Prod. 2024, 434, 140423. [Google Scholar] [CrossRef]
- Liao, G.; Yao, W. Upcycling of waste concrete powder into a functionalized host for nano-TiO2 photocatalyst: Binding mechanism and enhanced photocatalytic efficiency. J. Clean. Prod. 2022, 366, 132918. [Google Scholar] [CrossRef]
- Arvaniti, E.C.; Juenger, M.C.G.; Bernal, S.A.; Duchesne, J.; Courard, L.; Leroy, S.; Provis, J.L.; Klemm, A.; Belie, N. Physical characterization methods for supplementary cementitious materials. Mater. Struct. 2015, 48, 3675–3686. [Google Scholar] [CrossRef]
- Mohan, V.B.; Jayaraman, K.; Bhattacharyya, D. Brunauer–Emmett–Teller (BET) specific surface area analysis of different graphene materials: A comparison to their structural regularity and electrical properties. Solid State Commun. 2020, 320, 114004. [Google Scholar] [CrossRef]
- Huo, W.; Zhu, Z.; Sun, H.; Gao, Q.; Zhang, J.; Wan, Y.; Zhang, C. Reaction kinetics, mechanical properties, and microstructure of nano-modified recycled concrete fine powder/slag based geopolymers. J. Clean. Prod. 2022, 372, 133715. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Tong, X.; Gong, Y. Investigation of Mineral Admixtures on Mechanical Properties of Alkali-Activated Recycled Concrete Powders Cement. Buildings 2022, 12, 1234. [Google Scholar] [CrossRef]
- Topič, J.; Prošek, Z. Properties and microstructure of cement paste including recycled concrete powder. Acta Polytech. 2017, 57, 49–57. [Google Scholar] [CrossRef]
- Nežerka, V.; Havlásek, P.; Trejbal, J. Mitigating inclusion-induced shrinkage cracking in cementitious composites by incorporating recycled concrete fines. Constr. Build. Mater. 2020, 248, 118673. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Lyu, K.; Li, T.; Zhao, P.; Liu, R.; Zuo, J.; Fu, F.; Shah, S.P. Enhanced early hydration and mechanical properties of cement-based materials with recycled concrete powder modified by nano-silica. J. Build. Eng. 2022, 50, 104175. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, C.; Liu, H.; Zhang, W.; Hu, T. Deterioration mechanism understanding of recycled powder concrete under coupled sulfate attack and freeze–thaw cycles. Constr. Build. Mater. 2023, 388, 131718. [Google Scholar] [CrossRef]
- Oksri-Nelfia, L.; Mahieux, P.Y.; Amiri, O.; Turcry, P.; Lux, J. Reuse of recycled crushed concrete fines as mineral addition in cementitious materials. Mater. Struct. 2016, 49, 3239–3251. [Google Scholar] [CrossRef]
- Huo, W.; Zhu, Z.; Chen, W.; Zhang, J.; Kang, Z.; Pu, S.; Wan, Y. Effect of synthesis parameters on the development of unconfined compressive strength of recycled waste concrete powder-based geopolymers. Constr. Build. Mater. 2021, 292, 123264. [Google Scholar] [CrossRef]
- Rocha, J.H.A.; Tinoco, M.P.; Toledo Filho, R.D. The effect of recycled concrete powder (RCP) from precast concrete plant on fresh and mechanical properties of cementitious pastes. Mater. Construcción 2023, 73, 325. [Google Scholar] [CrossRef]
- Prošek, Z.; Nežerka, V.; Hlůžek, R.; Trejbal, J.; Tesárek, P.; Karra’a, G. Role of lime, fly ash, and slag in cement pastes containing recycled concrete fines. Constr. Build. Mater. 2019, 201, 702–714. [Google Scholar] [CrossRef]
- Sun, C.; Chen, L.; Xiao, J.; Zuo, J.; Wu, H. Effects of eco powders from solid waste on freeze-thaw resistance of mortar. Constr. Build. Mater. 2022, 333, 127405. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Zhu, Z.; Ma, L. Evaluation of waste concrete recycled powder (WCRP) on the preparation of low-exothermic cement. J. Build. Eng. 2022, 53, 104511. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, L. Effect of recycled concrete fine powder after calcination on the properties of autoclaved aerated concrete. Case Stud. Constr. Mater. 2024, 20, 02961. [Google Scholar] [CrossRef]
- Pešta, J.; Ženíšek, M.; Kočí, V.; Pavlů, T. Environmental perspectives of recycled concrete powder as cement replacement. AIP Conf. Proc. 2021, 2322, 1. [Google Scholar]
- Dacić, A.; Fenyvesi, O. Packing density investigation of blended cement pastes incorporating waste perlite and recycled concrete powder. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Zhu, H.; Shen, Z.; Li, Z.; Zhao, Z.; Liang, S.; Yang, S.; Ruan, Z.; Ding, Y. Properties study of artificial aggregate with high-content recycled concrete powder. J. Build. Eng. 2023, 78, 107697. [Google Scholar] [CrossRef]
- Prošek, Z.; Trejbal, J.; Nežerka, V.; Goliáš, V.; Faltus, M.; Tesárek, P. Recovery of residual anhydrous clinker in finely ground recycled concrete. Resour. Conserv. Recycl. 2020, 155, 104640. [Google Scholar] [CrossRef]
- Likes, L.; Markandeya, A.; Haider, M.M.; Bollinger, D.; McCloy, J.S.; Nassiri, S. Recycled concrete and brick powders as supplements to Portland cement for more sustainable concrete. J. Clean. Prod. 2022, 364, 132651. [Google Scholar] [CrossRef]
- Ma, Z.; Duan, Z.; Ba, G. Effects of an applied load on the chloride penetration of concrete with recycled aggregates and recycled powder. Adv. Civ. Eng. 2019, 1, 1340803. [Google Scholar] [CrossRef]
- Kim, Y.J. Quality properties of self-consolidating concrete mixed with waste concrete powder. Constr. Build. Mater. 2017, 135, 177–185. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Kang, X.; Fan, Y. Study on recycled concrete powders as mineral admixture in recycled concrete. IOP Conf. Ser. Earth Environ. Sci. 2019, 330, 022113. [Google Scholar] [CrossRef]
- Shen, Z.; Zhu, H.; Zhao, Z.; Pang, S.; Li, Z.; Yang, S.; Cao, P.; Lin, S. High-performance artificial aggregate prepared with recycled concrete powder and its impact on concrete properties. Constr. Build. Mater. 2023, 404, 133151. [Google Scholar] [CrossRef]
- Hlůžek, R.; Trejbal, J.; Nežerka, V.; Demo, P.; Prošek, Z.; Tesárek, P. Improvement of bonding between synthetic fibers and a cementitious matrix using recycled concrete powder and plasma treatment: From a single fiber to FRC. Eur. J. Environ. Civ. Eng. 2022, 26, 3880–3897. [Google Scholar] [CrossRef]
- Li, Z.; Yu, C.; Qiao, M.; Xie, W.; Yu, J. Effect of recycled powder on the yield stress of cement paste with varied superplasticizers. Low-carbon Mater. Green Constr. 2023, 1, 24. [Google Scholar]
- Zhao, Y.; Gao, J.; Liu, C.; Chen, X.; Xu, Z. The particle-size effect of waste clay brick powder on its pozzolanic activity and properties of blended cement. J. Clean. Prod. 2020, 242, 118521. [Google Scholar] [CrossRef]
- Kaya, Y.; Aytekin, B.; Kaya, T.; Mardani, A. Investigation of pozzolanic activity of recycled concrete powder: Effect of cement fineness, grain size distribution and water/cement ratio. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Wu, H.; Liang, C.; Wang, C.; Ma, Z. Properties of green mortar blended with waste concrete-brick powder at various components, replacement ratios and particle sizes. Constr. Build. Mater. 2022, 342, 128050. [Google Scholar] [CrossRef]
- Li, Q.; Xu, Q.; Ai, W. Study on Present Situation of Active Stimulation of Recycled Fine Powder. IOP Conf. Ser. Earth Environ. Sci. 2020, 512, 012056. [Google Scholar] [CrossRef]
- Wu, H.; Yang, D.; Xu, J.; Liang, C.; Ma, Z. Water transport and resistance improvement for the cementitious composites with eco-friendly powder from various concrete wastes. Constr. Build. Mater. 2021, 290, 123247. [Google Scholar] [CrossRef]
- Cantero, B.; Bravo, M.; De Brito, J.; del Bosque, I.S.; Medina, C. Mechanical behaviour of structural concrete with ground recycled concrete cement and mixed recycled aggregate. J. Clean. Prod. 2020, 275, 122913. [Google Scholar] [CrossRef]
- Xiao, J.; Tang, Y.; Chen, H.; Zhang, H.; Xia, B. Effects of recycled aggregate combinations and recycled powder contents on fracture behavior of fully recycled aggregate concrete. J. Clean. Prod. 2022, 366, 132895. [Google Scholar] [CrossRef]
- Kursula, K.; Perumal, P.; Ohenoja, K.; Illikainen, M. Production of artificial aggregates by granulation and carbonation of recycled concrete fines. J. Mater. Cycles Waste Manag. 2022, 24, 2141–2150. [Google Scholar] [CrossRef]
- Dun, Z.; Wang, M.; Ren, L.; Dun, Z. Tests Research on Grouting Materials of Waste-Concrete-Powder Cement for Goaf Ground Improvement. Adv. Mater. Sci. Eng. 2021, 1, 9598418. [Google Scholar] [CrossRef]
- Dong, W.; Zhan, Q.; Zhang, X.; Su, Y.; Zhou, J. Study on accelerating activation of recycled hardened concrete powder based on microbial induced calcium precipitation. Constr. Build. Mater. 2024, 411, 134710. [Google Scholar] [CrossRef]
- Tan, H.; Yang, Z.; Deng, X.; Guo, H.; Zhang, J.; Zheng, Z.; Li, M.; Chen, P.; He, X.; Yang, J.; et al. Surface reinforcement of recycled aggregates by multi-diameter recycled powder blended cement paste. J. Build. Eng. 2023, 64, 105609. [Google Scholar] [CrossRef]
- Real, S.; Carriço, A.; Bogas, J.A.; Guedes, M. Influence of the Treatment Temperature on the Microstructure and Hydration Behavior of Thermoactivated Recycled Cement. Materials 2020, 13, 3937. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cui, X.; Lu, N.; Hou, S.; He, Z.; Liang, C. Effect of recycled powders on the mechanical properties and durability of fully recycled fiber-reinforced mortar. J. Build. Eng. 2022, 45, 103574. [Google Scholar] [CrossRef]
- Liu, Q.; Li, B.; Xiao, J.; Singh, A. Utilization potential of aerated concrete block powder and clay brick powder from C&D waste. Constr. Build. Mater. 2020, 238, 117721. [Google Scholar]
- Xu, J.; Kang, A.; Wu, Z.; Gong, Y.; Xiao, P. The effect of mechanical-thermal synergistic activation on the mechanical properties and microstructure of recycled powder geopolymer. J. Clean. Prod. 2021, 327, 129477. [Google Scholar] [CrossRef]
- Sun, C.; Chen, L.; Xiao, J.; Liu, Q.; Zuo, J. Low-Carbon and Fundamental Properties of Eco-Efficient Mortar with Recycled Powders. Materials 2021, 14, 7503. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, Y.; Wu, R.; Yang, D.; Ma, Z. The utilization of active recycled powder from various construction wastes in preparing ductile fiber-reinforced cementitious composites: A case study. Case Stud. Constr. Mater. 2021, 15, 00650. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Wu, J. Effect of recycled mixed powder on the mechanical properties and microstructure of concrete. J. Renew. Mater. 2022, 10, 1397. [Google Scholar] [CrossRef]
- Yanga, D.; Liu, M.; Zhang, Z.; Yao, P.; Ma, Z. Properties and modification of sustainable foam concrete including eco-friendly recycled powder from concrete waste. Case Stud. Constr. Mater. 2022, 16, 00826. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, B.; Tang, L.; Zeng, W. Analysis of two processing techniques applied on powders from recycling of clay bricks and concrete, in terms of efficiency, energy consumption, and cost. Constr. Build. Mater. 2023, 385, 131517. [Google Scholar] [CrossRef]
- Zhou, Z.; Xiao, J.; Ye, T.; Wang, J.; Choi, D. Influence of recycled concrete powder and CO2 curing on the properties of thermal insulation mortars. Constr. Build. Mater. 2024, 414, 134907. [Google Scholar] [CrossRef]
- He, X.; Zheng, Z.; Yang, J.; Su, Y.; Wang, T.; Strnadel, B. Feasibility of incorporating autoclaved aerated concrete waste for cement replacement in sustainable building materials. J. Clean. Prod. 2020, 250, 119455. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X. Recycling of waste autoclaved aerated concrete powder in Portland cement by accelerated carbonation. Waste Manag. 2019, 89, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Singh, A.; Xiao, J.; Hou, S. Combined use of recycled powder and recycled coarse aggregate derived from construction and demolition waste in self-compacting concrete. Constr. Build. Mater. 2020, 254, 119323. [Google Scholar] [CrossRef]
- Kaliyavaradhan, S.K.; Ling, T.C.; Mo, K.H. Valorization of waste powders from cement-concrete life cycle: A pathway to circular future. J. Clean. Prod. 2020, 268, 122358. [Google Scholar] [CrossRef]
- GB/T208; Test Method for Determinging Cement Density. National Standard of the People’s Republic of China: Beijing, China, 2014. (In Chinese)
- ASTM C188-17; Standard Test Method for Density of Hydraulic Cement. ASTM International: West Conshohocken, PA, USA, 2023.
- NBR 16605; Portland Cement and Other Powdered Material—Determination of the Specific Gravity. ABNT: Rio de Janeiro, Brazil, 2017.
- C188-15; Standard Test Method for Density of Hidraulic Cement. ASTM International: West Conshohocken, PA, USA, 2015.
- IS 4031-11; Methods of Physical Tests for Hydraulic Cement, Part 11: Determination of Density. Bureau of Indian Standards: New Delhi, India, 2019.
- EN 1097-7:2022; Tests for Mechanical and Physical Properties of Aggregates—Part 7: Determination of the Particle Density of Filler—Pyknometer Method. European Standard: Brussels, Belgium, 2022.
- Hou, Y.; Yu, Z.; Zhang, J.; Yang, H.; Song, W. Gray Model Study of Strength and Pore Structure of Recycled Concrete Powder (RCP) Concrete Based on Low-Field NMR Technology. Materials 2023, 16, 6058. [Google Scholar] [CrossRef]
- Wu, H.; Wang, C.; Ma, Z. Drying shrinkage, mechanical and transport properties of sustainable mortar with both recycled aggregate and powder from concrete waste. J. Build. Eng. 2022, 49, 104048. [Google Scholar] [CrossRef]
- Horsakulthai, V. Effect of recycled concrete powder on strength, electrical resistivity, and water absorption of self-compacting mortars. Case Stud. Constr. Mater. 2021, 15, 00725. [Google Scholar] [CrossRef]
- Li, X.; Lv, X.; Zhou, X.; Meng, W.; Bao, Y. Upcycling of waste concrete in eco-friendly strain-hardening cementitious composites: Mixture design, structural performance, and life-cycle assessment. J. Clean. Prod. 2022, 330, 129911. [Google Scholar] [CrossRef]
- Wang, C.Q.; Cheng, L.X.; Ying, Y.; Yang, F.H. Utilization of all components of waste concrete: Recycled aggregate strengthening, recycled fine powder activity, composite recycled concrete and life cycle assessment. J. Build. Eng. 2024, 82, 108255. [Google Scholar] [CrossRef]
- Wang, C.; Yu, Q.; Zhan, B.; Gao, P.; Guo, B.; Chu, Y.; Chen, Y.; Bian, P. Activity quantification and assessment of recycled concrete powder based on the contributions of the dilution effect, physical effect and chemical effect. J. Clean. Prod. 2024, 442, 140918. [Google Scholar] [CrossRef]
- Yuan, T.; Yao, W. Preparation and Properties of g-C3N4-TiO2 Cement-Based Materials Supported by Recycled Concrete Powder. Catalysts 2023, 13, 312. [Google Scholar] [CrossRef]
- Bian, Y.; Qiu, X.; Zhao, J.; Li, Z.; Ouyang, J. Influence of Recycled Concrete Fine Powder on Durability of Cement Mortar. Fluid Dyn. Mater. Process. 2024, 20, 45–58. [Google Scholar] [CrossRef]
- Liu, M.; Hu, R.; Zhang, Y.; Wang, C.; Ma, Z. Effect of ground concrete waste as green binder on the micro-macro properties of eco-friendly metakaolin-based geopolymer mortar. J. Build. Eng. 2023, 68, 106191. [Google Scholar] [CrossRef]
- Ma, X.; Li, H.; Wang, D.; Li, C.; Wei, Y. Simulation and Experimental Substantiation of the Thermal Properties of Non-Autoclaved Aerated Concrete with Recycled Concrete Powder. Materials 2022, 15, 8341. [Google Scholar] [CrossRef]
- Wan, X.; Li, H.; Che, X.; Xu, P.; Li, C.; Yu, Q. A Study on the Application of Recycled Concrete Powder in an Alkali-Activated Cementitious System. Processes 2023, 11, 203. [Google Scholar] [CrossRef]
- Lin, Y.; He, T.; Da, Y.; Yang, R.; Zheng, D. Effects of recycled micro-powders mixing methods on the properties of recycled concrete. J. Build. Eng. 2023, 80, 107994. [Google Scholar] [CrossRef]
- Jagadesh, P.; Oyebisi, S.; Muthu, A.H.; Sarulatha, A.; Supikshaa, K.; Sor, N.A.; Mydin, M.A.O. Recycled concrete powder on cement mortar: Physico-mechanical effects and lifecycle assessments. J. Build. Eng. 2024, 86, 108507. [Google Scholar] [CrossRef]
- Maaze, M.R.; Shrivastava, S. Development of framework in the selection and reuse of concrete waste and brick waste powder as pozzolanic material in cement concrete application using analytical hierarchy process technique. Constr. Build. Mater. 2023, 393, 132056. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, Y.; Zhang, Z.; Ma, Z. Development of Sustainable Cement-Based Materials with Ultra-High Content of Waste Concrete Powder: Properties and Improvement. Sustainability 2023, 15, 14812. [Google Scholar] [CrossRef]
- Boudali, S.; Kerdal, D.E.; Ayed, K.; Abdulsalam, B.; Soliman, A.M. Performance of self-compacting concrete incorporating recycled concrete fines and aggregate exposed to sulphate attack. Constr. Build. Mater. 2016, 124, 705–713. [Google Scholar] [CrossRef]
- Ye, T.; Xiao, J.; Duan, Z.; Li, S. Geopolymers made of recycled brick and concrete powder—A critical review. Constr. Build. Mater. 2022, 330, 127232. [Google Scholar] [CrossRef]
- Ohemeng, E.A.; Naghizadeh, A. Alternative cleaner production of masonry mortar from fly ash and waste concrete powder. Constr. Build. Mater. 2023, 374, 130859. [Google Scholar] [CrossRef]
- Lan, C.; Lu, J.; Chen, J.; Wu, H.; Liu, D. Study on the application of recycled fine powder in ready-mixed concrete. MATEC Web Conf. 2019, 278, 01010. [Google Scholar]
- Cantero, B.; Bravo, M.; De Brito, J.; Del Bosque, I.S.; Medina, C. Water transport and shrinkage in concrete made with ground recycled concrete-additioned cement and mixed recycled aggregate. Cem. Concr. Compos. 2021, 118, 103957. [Google Scholar] [CrossRef]
- Tokareva, A.; Kaassamani, S.; Waldmann, D. Fine demolition wastes as Supplementary cementitious materials for CO2 reduced cement production. Constr. Build. Mater. 2023, 392, 131991. [Google Scholar] [CrossRef]
- Wang, X.; Yu, R.; Shui, Z.; Song, Q.; Liu, Z.; Liu, Z.; Wu, S. Optimized treatment of recycled construction and demolition waste in developing sustainable ultra-high performance concrete. J. Clean. Prod. 2019, 221, 805–816. [Google Scholar] [CrossRef]
- Darweesh, H.H.M. Recycling of Concrete Demolition Waste Powder as a Sustainable Material in Portland Cement Pastes Modified with Nano-silica. JSMPM 2022, 2, 78–89. [Google Scholar] [CrossRef]
- Vashistha, P.; Oinam, Y.; Pyo, S. Valorization of waste concrete powder (WCP) through silica fume incorporation to enhance the reactivity and hydration characteristics. Dev. Built Environ. 2023, 16, 100272. [Google Scholar] [CrossRef]
- Sui, Y.; Ou, C.; Liu, S.; Zhang, J.; Tian, Q. Study on Properties of Waste Concrete Powder by Thermal Treatment and Application in Mortar. Appl. Sci. 2020, 10, 998. [Google Scholar] [CrossRef]
- Ren, P.; Li, B.; Yu, J.G.; Ling, T.C. Utilization of recycled concrete fines and powders to produce alkali-activated slag concrete blocks. J. Clean. Prod. 2020, 267, 122115. [Google Scholar] [CrossRef]
- Liu, X.; Liu, R.; Xie, X.; Zuo, J.; Lyu, K.; Shah, S.P. Chloride corrosion resistance of cement mortar with recycled concrete powder modified by nano-silica. Constr. Build. Mater. 2023, 364, 129907. [Google Scholar] [CrossRef]
- Song, S.; Li, X.; Wang, Z.; Wang, W. Orthogonal experimental and theoretical study on mechanical properties of fiber-reinforced recycled powder concrete. Case Stud. Constr. Mater. 2022, 17, 01546. [Google Scholar] [CrossRef]
- Kaliyavaradhan, S.K.; Li, L.; Ling, T.C. Response surface methodology for the optimization of CO2 uptake using waste concrete powder. Constr. Build. Mater. 2022, 340, 127758. [Google Scholar] [CrossRef]
- Jiang, Y.; Ling, T.C.; Shi, M. Strength enhancement of artificial aggregate prepared with waste concrete powder and its impact on concrete properties. J. Clean. Prod. 2020, 257, 120515. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, L.; Lu, J.X.; Shen, P.; Ling, T.C.; Poon, C.S. Mechanism of carbonating recycled concrete fines in aqueous environment: The particle size effect. Cem. Concr. Compos. 2022, 133, 104655. [Google Scholar] [CrossRef]
- He, Z.H.; Xu, H.; Huynh, T.P.; Shi, J.Y.; Ma, Z.M.; Hu, Y.J.; Chen, F.J. Multi-scale characteristics of magnesium potassium phosphate cement enhanced by waste concrete powder. Ceram. Int. 2023, 49, 15480–15491. [Google Scholar] [CrossRef]
- Aninda, S.S.; Islam, M.S. Effectiveness of waste concrete powder in fabricating compressed stabilized earth blocks: Strength, durability and thermal assessment. J. Build. Eng. 2023, 80, 107989. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.; Wu, H.; Yang, D.; Ma, Z. Reusing recycled powder as eco-friendly binder for sustainable GGBS-based geopolymer considering the effects of recycled powder type and replacement rate. J. Clean. Prod. 2022, 364, 132656. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, H.; Wu, K.; Wang, F.; Zhao, X.; Liao, Q. Understanding the effect of particle size of waste concrete powder on phosphorus removal efficiency. Constr. Build. Mater. 2020, 236, 117526. [Google Scholar] [CrossRef]
- Afrin, H.; Huda, N.; Abbasi, R. An overview of eco-friendly alternatives as the replacement of cement in concrete. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1200, 012003. [Google Scholar] [CrossRef]
- Real, S.; Bogas, J.A.; Carriço, A.; Hu, S. Mechanical Characterisation and Shrinkage of Thermoactivated Recycled Cement Concrete. Appl. Sci. 2021, 11, 2454. [Google Scholar] [CrossRef]
- Shen, P.; Sun, Y.; Liu, S.; Jiang, Y.; Zheng, H.; Xuan, D.; Lu, J.; Poon, C.S. Synthesis of amorphous nano-silica from recycled concrete fines by two-step wet carbonation. Cem. Concr. Res. 2021, 147, 106526. [Google Scholar] [CrossRef]
- Liu, M.; Wu, H.; Yao, P.; Wang, C.; Ma, Z. Microstructure and macro properties of sustainable alkali-activated fly ash mortar with various construction waste fines as binder replacement up to 100%. Cem. Concr. Compos. 2022, 134, 104733. [Google Scholar] [CrossRef]
- ASTM C618; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. American Society for Testing and Materials: West Conshohocken, PA, USA, 2003.
- Chen, L.; Wei, M.; Lei, N.; Li, H. Effect of chemical–thermal activation on the properties of recycled fine powder cementitious materials. Case Stud. Constr. Mater. 2024, 20, 02956. [Google Scholar] [CrossRef]
- Li, Y.; Kang, X. Effect of Physical Excitation on the Properties of Recycled Fine Powder. IOP Conf. Ser. Earth Environ. Sci. 2019, 219, 012025. [Google Scholar] [CrossRef]
- Gencel, O.; Nodehi, M.; Hekimoğlu, G.; Ustaoğlu, A.; Sarı, A.; Kaplan, G.; Bayraktar, O.Y.; Sutcu, M.; Ozbakkaloglu, T. Foam Concrete Produced with Recycled Concrete Powder and Phase Change Materials. Sustainability 2022, 14, 7458. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, P.; Kapoor, K. Utilization of recycled fine powder as an activator in fly ash based geopolymer mortar. Constr. Build. Mater. 2022, 323, 126581. [Google Scholar] [CrossRef]
- Yao, P.; Yang, D.; Wang, C.; Ma, Z. Upcycling of construction waste powder for sustainable ultra-high performance engineered cementitious composites: Effects of waste powder source and content. Constr. Build. Mater. 2022, 349, 128789. [Google Scholar] [CrossRef]
- Donatello, S.; Tyrer, M.; Cheeseman, C.R. Comparison of test methods to assess pozzolanic activity. Cem. Concr. Compos. 2010, 32, 121–127. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Zhang, Z. Pozzolanic activity evaluation methods of solid waste: A review. J. Clean. Prod. 2023, 402, 136783. [Google Scholar] [CrossRef]
- Mostafa, N.Y.; Brown, P.W. Heat of hydration of high reactive pozzolans in blended cements: Isothermal conduction calorimetry. Thermochim. Acta 2005, 435, 162–167. [Google Scholar] [CrossRef]
- Tseng, Y.S.; Huang, C.L.; Hsu, K.C. The pozzolanic activity of a calcined waste FCC catalyst and its effect on the compressive strength of cementitious materials. Cem. Concr. Res. 2005, 35, 782–787. [Google Scholar] [CrossRef]
- GB/T 1596–2017; Fly Ash Used for Cement and Concrete. Chinese Standard: Beijing, China, 2017.
- EN 450–1:2012; Fly Ash for Concrete Definition, Specifications and Conformity Criteria. European Standard: Brussels, Belgium, 2012.
- NBR 12653; Pozzolanic Materials—Requirements. ABNT (Associação Brasileira de Normas Técnicas): Rio de Janerio, Brazil, 2015.
- GB/T2847–2005; Pozzolanic Materials Used for Cement Production. Chinese Standard: Beijing, China, 2005.
- BS EN 196-5:2011; Methods of Testing Cement Pozzolanicity Test for Pozzolanic Cement. European Standard: Brussels, Belgium, 2011.
- Ma, Z.; Zhang, Z.; Hu, R.; Liu, X.; Shen, J.; Wang, C. Chloride resistance and improvement of fully recycled cementitious materials with both recycled aggregate and recycled powder. J. Sustain. Cem.-Based Mater. 2024, 13, 49–67. [Google Scholar] [CrossRef]
- Yu, X.X.; Li, R.Y.; Dong, X.; Li, G.; Zhang, S. Effect of mechanical force grinding on the properties of recycled powder. J. Synth. Cryst. 2017, 46, 688–692. [Google Scholar]
- Florea, M.V.A.; Ning, Z.; Brouwers, H.J.H. Activation of liberated concrete fines and their application in mortars. Constr. Build. Mater. 2014, 50, 1–12. [Google Scholar] [CrossRef]
- Lu, B.; Shi, C.; Zhang, J.; Wang, J. Effects of carbonated hardened cement paste powder on hydration and microstructure of Portland cement. Constr. Build. Mater. 2018, 186, 699–708. [Google Scholar] [CrossRef]
- Vashistha, P.; Park, S.; Pyo, S. A review on sustainable fabrication of futuristic cementitious binders based on application of waste concrete powder, steel slags, and coal bottom ash. Int. J. Concr. Struct. Mater. 2022, 16, 51. [Google Scholar] [CrossRef]
- Wu, H.; Xu, J.; Yang, D.; Ma, Z. Utilizing thermal activation treatment to improve the properties of waste cementitious powder and its newmade cementitious materials. J. Clean. Prod. 2021, 322, 129074. [Google Scholar] [CrossRef]
- Liu, D.; Quan, X.; Zhu, H.; Huang, Q.; Zhou, L. Evaluation of modified waste concrete powder used as a novel phosphorus remover. J. Clean. Prod. 2020, 257, 120646. [Google Scholar] [CrossRef]
- Ma, Z.; Xue, R.; Li, J.S.; Zhao, Y.; Xue, Q.; Chen, Z.; Wang, Q.; Poon, C.S. Use of thermally modified waste concrete powder for removal of Pb (II) from wastewater: Effects and mechanism. Environ. Pollut. 2021, 277, 116776. [Google Scholar] [CrossRef]
- Vashistha, P.; Oinam, Y.; Kim, H.K.; Pyo, S. Effect of thermo-mechanical activation of waste concrete powder (WCP) on the characteristics of cement mixtures. Constr. Build. Mater. 2023, 362, 129713. [Google Scholar] [CrossRef]
- Zheng, Y.; Xi, X.; Liu, H.; Du, C.; Lu, H. A review: Enhanced performance of recycled cement and CO2 emission reduction effects through thermal activation and nanosilica incorporation. Constr. Build. Mater. 2024, 422, 135763. [Google Scholar] [CrossRef]
- Ma, Z.; Yao, P.; Yang, D.; Shen, J. Effects of fire-damaged concrete waste on the properties of its preparing recycled aggregate, recycled powder and newmade concrete. J. Mater. Res. Technol. 2021, 15, 1030–1045. [Google Scholar] [CrossRef]
- Ma, Z.; Hu, R.; Yao, P.; Wang, C. Utilizing heat-mechanical synergistic treatment for separating concrete waste into high-quality recycled aggregate, active recycled powder and new concrete. J. Build. Eng. 2023, 68, 106161. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, M.; Wang, Y.; Zhang, M. Roles of carbonated recycled fines and aggregates in hydration, microstructure and mechanical properties of concrete: A critical review. Cem. Concr. Compos. 2023, 138, 104994. [Google Scholar] [CrossRef]
- Georget, F.; Prévost, J.H.; Huet, B. Reactive transport modelling of cement paste leaching in brines. Cem. Concr. Res. 2018, 111, 183–196. [Google Scholar] [CrossRef]
- Zajac, M.; Skibsted, J.; Bullerjahn, F.; Skocek, J. Semi-dry carbonation of recycled concrete paste. J. CO2 Util. 2022, 63, 102111. [Google Scholar] [CrossRef]
- Ho, H.J.; Iizuka, A.; Shibata, E. Chemical recycling and use of various types of concrete waste: A review. J. Clean. Prod. 2021, 284, 124785. [Google Scholar] [CrossRef]
- Shen, P.; Zhang, Y.; Jiang, Y.; Zhan, B.; Lu, J.; Zhang, S.; Xuan, D.; Poon, C.S. Phase assemblance evolution during wet carbonation of recycled concrete fines. Cem. Concr. Res. 2022, 154, 106733. [Google Scholar] [CrossRef]
- Poon, C.S.; Shen, P.; Jiang, Y.; Ma, Z.; Xuan, D. Total recycling of concrete waste using accelerated carbonation: A review. Cem. Concr. Res. 2023, 173, 107284. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Xu, B.; Yuan, J. Influences of Carbonated Recycled Concrete Fines on Cement Hydration. Buildings 2023, 13, 926. [Google Scholar] [CrossRef]
- Ho, H.J.; Iizuka, A.; Shibata, E.; Tomita, H.; Takano, K.; Endo, T. CO2 utilization via direct aqueous carbonation of synthesized concrete fines under atmospheric pressure. ACS Omega 2020, 5, 15877–15890. [Google Scholar] [CrossRef]
- Saade, M.R.M.; Yahia, A.; Amor, B. Is crushed concrete carbonation significant enough to be considered as a carbon mitigation strategy? Environ. Res. Lett. 2022, 17, 104049. [Google Scholar] [CrossRef]
- Tam, V.W.; Butera, A.; Le, K.N.; Da Silva, L.C.; Evangelista, A.C. A prediction model for compressive strength of CO2 concrete using regression analysis and artificial neural networks. Constr. Build. Mater. 2022, 324, 126689. [Google Scholar] [CrossRef]
- Veetil, S.P.; Hitch, M. Recent developments and challenges of aqueous mineral carbonation: A review. Int. J. Environ. Sci. Technol. 2020, 17, 4359–4380. [Google Scholar] [CrossRef]
- Jiang, Y.; Peng, L.; Ma, Z.; Lu, J.X.; Shen, P.; Poon, C.S. Enhancing the treatment efficiency of recycled concrete fines with aqueous carbonation. Cem. Concr. Res. 2023, 174, 107338. [Google Scholar] [CrossRef]
- Qian, X.; Wang, J.; Fang, Y.; Wang, L. Carbon dioxide as an admixture for better performance of OPC-based concrete. J. CO2 Util. 2018, 25, 31–38. [Google Scholar] [CrossRef]
- Ouyang, X.; Wang, L.; Xu, S.; Ma, Y.; Ye, G. Surface characterization of carbonated recycled concrete fines and its effect on the rheology, hydration and strength development of cement paste. Cem. Concr. Compos. 2020, 114, 103809. [Google Scholar] [CrossRef]
- Zhan, B.J.; Poon, C.S.; Shi, C.J. Materials characteristics affecting CO2 curing of concrete blocks containing recycled aggregates. Cem. Concr. Compos. 2016, 67, 50–59. [Google Scholar] [CrossRef]
- Shah, V.; Scrivener, K.; Bhattacharjee, B.; Bishnoi, S. Changes in microstructure characteristics of cement paste on carbonation. Cem. Concr. Res. 2018, 109, 184–197. [Google Scholar] [CrossRef]
- Kong, Y.K.; Song, Y.; Kurumisawa, K.; Wang, T.; Yan, D.; Zeng, Q.; Zhou, X.; Ruan, S. Use of hydrated cement pastes (HCP) as a CO2 sponge. J. CO2 Util. 2022, 55, 101804. [Google Scholar] [CrossRef]
- Iizuka, A.; Sasaki, T.; Honma, M.; Yoshida, H.; Hayakawa, Y.; Yanagisawa, Y.; Yamasaki, A. Pilot-scale operation of a concrete sludge recycling plant and simultaneous production of calcium carbonate. Chem. Eng. Commun. 2017, 204, 79–85. [Google Scholar] [CrossRef]
- Shuto, D.; Igarashi, K.; Nagasawa, H.; Iizuka, A.; Inoue, M.; Noguchi, M.; Yamasaki, A. CO2 fixation process with waste cement powder via regeneration of alkali and acid by electrodialysis: Effect of operation conditions. Ind. Eng. Chem. Res. 2015, 54, 6569–6577. [Google Scholar] [CrossRef]
- Mun, M.; Cho, H.; Kwon, J.; Kim, K.; Kim, R. Investigation of the CO2 sequestration by indirect aqueous carbonation of waste cement. Am. J. Clim. Chang. 2017, 6, 132. [Google Scholar] [CrossRef]
- Vanderzee, S.; Zeman, F. Recovery and carbonation of 100% of calcium in waste concrete fines: Experimental results. J. Clean. Prod. 2018, 174, 718–727. [Google Scholar] [CrossRef]
- Lee, M.G.; Kang, D.; Jo, H.; Park, J. Carbon dioxide utilization with carbonation using industrial waste-desulfurization gypsum and waste concrete. J. Mater. Cycles Waste Manag. 2016, 18, 407–412. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, Y.; Feng, H.; Wang, Q.; Wang, Y.; Xu, T. Simultaneous CO2 capture and amino acid production using bipolar membrane electrodialysis (BMED). J. Membr. Sci. 2017, 542, 264–271. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Y.; Shen, J.; Van der Bruggen, B. Application of Bipolar Membrane Electrodialysis in Environmental Protection and Resource Recovery: A Review. Membranes 2022, 12, 829. [Google Scholar] [CrossRef]
- Yadav, S.; Mehra, A. A review on ex situ mineral carbonation. Environ. Sci. Pollut. Res. 2021, 28, 12202–12231. [Google Scholar] [CrossRef]
- Joshi, S.; Goyal, S.; Reddy, M.S. Influence of biogenic treatment in improving the durability properties of waste amended concrete: A review. Constr. Build. Mater. 2020, 263, 120170. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Tong, T.; Wang, C. Study of the effect of temperature on microbially induced carbonate precipitation. Acta Geotech. 2019, 14, 627–638. [Google Scholar] [CrossRef]
- Shan, B.; Hao, R.; Xu, H.; Li, J.; Li, Y.; Xu, X.; Zhang, J. A review on mechanism of biomineralization using microbial-induced precipitation for immobilizing lead ions. Environ. Sci. Pollut. Res. 2021, 28, 30486–30498. [Google Scholar] [CrossRef] [PubMed]
- Nežerka, V.; Holeček, P.; Somr, M.; Tichá, P.; Domonkos, M.; Stiborová, H. On the possibility of using bacteria for recycling finest fractions of concrete waste: A critical review. Rev. Environ. Sci. Biotechnol. 2023, 22, 427–450. [Google Scholar] [CrossRef]
- Proudfoot, D.; Brooks, L.; Gammons, C.H.; Barth, E.; Bless, D.; Nagisetty, R.M.; Lauchnor, E.G. Investigating the potential for microbially induced carbonate precipitation to treat mine waste. J. Hazard. Mater. 2022, 424, 127490. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, L.; Zeng, W.; sun Poon, C.; Lu, Z. Improvement in properties of concrete with modified RCA by microbial induced carbonate precipitation. Cem. Concr. Compos. 2021, 124, 104251. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Xu, Z.; Zheng, S.; Xue, M. Adsorption of tannic acid from aqueous solution by aminopropyl functionalized SBA-15. Desalin. Water Treat. 2015, 56, 475–484. [Google Scholar] [CrossRef]
- Kanda, Y.; Harada, S. Synthesis of zeolites from calcareous waste concrete powder. Results Mater. 2022, 13, 100250. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; Rashad, A.M.; Heikal, M. Sustainable utilization of pretreated concrete waste in the production of one-part alkali-activated cement. J. Clean. Prod. 2019, 232, 318–328. [Google Scholar] [CrossRef]
- ASTM C311/C311M-18; Test Methods for Sampling and Testing Fly Ash or Natural Pozzolans for Use in Portland-Cement Concrete. ASTM International: West Conshohocken, PA, USA, 2022. [CrossRef]
- JG/T573-2020; Recycled Micro-Powder for Concrete and Mortar. Ministry of Construction of the People’s Republic of China: Beijing, China, 2020.
- Liang, G.; Liu, T.; Li, H.; Wu, K. Shrinkage mitigation, strength enhancement and microstructure improvement of alkali-activated slag/fly ash binders by ultrafine waste concrete powder. Compos. B Eng. 2022, 231, 109570. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Xu, Y.; Cao, K.; Ge, Y.; Wang, X.; Li, Q. Accelerating the Reaction Kinetics of Na2CO3-Activated Slag Mortars by Calcined Recycled Concrete Fines. Materials 2022, 15, 5375. [Google Scholar] [CrossRef]
- Wu, H.; Yang, D.; Wang, C.; Ma, Z. Effects of construction waste powder on micro–macro properties of green high-strength cement paste with low water-to-binder ratio. Constr. Build. Mater. 2023, 385, 131493. [Google Scholar] [CrossRef]
- Belkadi, A.A.; Kessal, O.; Berkouche, A.; Noui, A.; Daguiani, S.E.; Dridi, M.; Benaniba, S.; Tayebi, T. Experimental investigation into the potential of recycled concrete and waste glass powders for improving the sustainability and performance of cement mortars properties. Sustain. Energy Technol. 2024, 64, 103710. [Google Scholar]
- Huashan, Y.A.N.G.; Yujun, C.H.E. Influences of waste concrete powder on the strength development and hydration products of mortar containing fly ash. Mater. Sci. 2022, 28, 106–112. [Google Scholar]
- Ma, X.; Wang, Z. Effect of ground waste concrete powder on cement properties. Adv. Mater. Sci. Eng. 2013, 1, 918294. [Google Scholar] [CrossRef]
- Nedunuri, A.S.S.S.; yar Mohammed, A.; Muhammad, S. Carbonation potential of concrete debris fines and its valorisation through mineral carbonation. Constr. Build. Mater. 2021, 310, 125162. [Google Scholar] [CrossRef]
- Diliberto, C.; Lecomte, A.; Aissaoui, C.; Mechling, J.M.; Izoret, L. The incorporation of fine recycled concrete aggregates as a main constituent of cement. Mater. Struct. 2021, 54, 1–14. [Google Scholar] [CrossRef]
- Bogas, J.A.; Carriço, A.; Pereira, M.F.C. Mechanical characterization of thermal activated low-carbon recycled cement mortars. J. Clean. Prod. 2019, 218, 377–389. [Google Scholar] [CrossRef]
- Wu, Z. High Performance Concrete; China Railway Publishing House: Beijing, China, 1999. [Google Scholar]
- Qian, H.; Hua, S.; Yue, H.; Feng, G.; Qian, L.; Jiang, W. Utilization of recycled construction powder in 3D concrete printable materials through particle packing optimization. J. Build. Eng. 2022, 61, 105236. [Google Scholar] [CrossRef]
- Bian, Y.; Li, Z.; Zhao, J.; Wang, Y. Synergistic enhancement effect of recycled fine powder (RFP) cement paste and carbonation on recycled aggregates performances and its mechanism. J. Clean. Prod. 2022, 344, 130848. [Google Scholar] [CrossRef]
- Deng, S.; Ren, P.; Jiang, Y.; Shao, X.; Ling, T.C. Use of CO2-active BOFS binder in the production of artificial aggregates with waste concrete powder. Resour. Conserv. Recycl. 2022, 182, 106332. [Google Scholar] [CrossRef]
- Wang, K.; Ren, L.; Yang, L. Excellent Carbonation Behavior of Rankinite Prepared by Calcining the C-S-H: Potential Recycling of Waste Concrete Powders for Prefabricated Building Products. Materials 2018, 11, 1474. [Google Scholar] [CrossRef] [PubMed]
- GB/T 50080-2002; Standard for Test Method of Performance on Ordinary Fresh Concrete, China Quality and Standards Pressing & Media. Ministry of Construction of the People’s Republic of China: Beijing, China, 2002. (In Chinese)
- GB/T 50081-2002; Standard for Test Method of Mechanical Properties on Ordinary Concrete. National Standard of the People’s Republic of China: Beijing, China, 2002; p. 60.
- GB/T 2419-2005; Test Method for Fluidity of Cement Mortar. National Standard of the People’s Republic of China: Beijing, China, 2005. (In Chinese)
- GB/T 1346-2011; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cement. Chinese National Standard: Beijing, China, 2011. (In Chinese)
- GB/T8077; Methods for Testing Uniformity of Concrete Admixture. China National Standards: Beijing, China, 2012. (In Chinese)
- BS EN 1015-6; Methods of Test for Mortar for Masonry: Determination of Bulk Density of Fresh Mortar. British Standards Institution: London, UK, 1999.
- EN 1015-3:2000; Methods of Test for Mortars for Masonry. Part 3: Determination of Consistency of Fresh Mortar (by Flow Table). European Standard: Brussels, Belgium, 2000.
- GB/T 17671-2021; Test Method of Cement Mortar Strength (IOS Method). National Standard of the People’s Republic of China: Beijing, China, 2021. Available online: https://www.chinesestandard.net/PDF/English.aspx/GBT17671-2021 (accessed on 1 June 2024).
- ASTM C1437-15; Standard Test Method for Flow of Hydraulic Cement Mortar. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM C230/C230M-21; Standard Specification for Flow Table for Use in Tests of Hydraulic Cement. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM C143/C143M; Standard Test Method for Slump of Hydraulic-Cement Concrete. ASTM International: West Conshohocken, PA, USA, 2020.
- Liu, Q.; Xiao, J.; Singh, A. Quantification of plastic shrinkage and cracking in mortars containing different recycled powders using digital image correlation technique. Constr. Build. Mater. 2021, 293, 123509. [Google Scholar] [CrossRef]
- GB 175-2007; Common Portland Cement. National Standard of the People’s Republic of China: Beijing, China, 2007.
- ASTM C187-16; Standard Test Method for Amount of Water Required for Normal Consistency of Hydraulic Cement Paste. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM C191; Standard Test Methods for Time of Setting of Hydraulic Cement by Vicat Needle. ASTM International: West Conshohocken, PA, USA, 2013.
- BS EN 196; Part 3. Methods of Testing Cement. Determination of Setting Time and Soundness. British Standards Institution: London, UK, 2005.
- JC/T 727-2005; Apparatus for Determining Normal Consistency and Setting Time of Cement Paste. National Standardization Technical Committee Cement: Beijing, China, 2005.
- Larsen, O.; Samchenko, S.; Naruts, V. Blended binder based on Portland cement and recycled concrete powder. Mag. Civ. Eng. 2022, 113, 11306. [Google Scholar]
- Yao, T.; Tian, Q.; Zhang, M.; Qi, S.; Wang, C.; Ruan, M. Laboratory investigation of foamed concrete prepared by recycled waste concrete powder and ground granulated blast furnace slag. J. Clean. Prod. 2023, 426, 139095. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.; Li, K.; Lin, S.; Li, M.; Hao, T.; Ling, Z.; Xiang, D.; Wang, T. A systematic review of factors affecting properties of thermal-activated recycled cement. Resour. Conserv. Recycl. 2022, 185, 106432. [Google Scholar] [CrossRef]
- ASTM C1749-17a; Standard Guide for Measurement of the Rheological Properties of Hydraulic Cementious Paste Using a Rotational Rheometer. American Society for Testing and Materials: West Conshohocken, PA, USA, 2017.
- ASTM C1874-20; Standard Test Method for Measuring Rheological Properties of Cementitious Materials Using Coaxial Rotational Rheometer. ASTM International: West Conshohocken, PA, USA, 2020.
- Ibrahim, M.; Alimi, W.; Assaggaf, R.; Salami, B.A.; Oladapo, E.A. An overview of factors influencing the properties of concrete incorporating construction and demolition wastes. Constr. Build. Mater. 2023, 367, 130307. [Google Scholar] [CrossRef]
- Han, F.; Pu, S.; Zhou, Y.; Zhang, H.; Zhang, Z. Effect of ultrafine mineral admixtures on the rheological properties of fresh cement paste: A review. J. Build. Eng. 2022, 51, 104313. [Google Scholar] [CrossRef]
- Perrot, A.; Rangeard, D. Effects of mix design parameters on consolidation behaviour of fresh cement-based materials. Mater. Struct. 2017, 50, 1–9. [Google Scholar] [CrossRef]
- Roussel, N.; Bessaies-Bey, H.; Kawashima, S.; Marchon, D.; Vasilic, K.; Wolfs, R. Recent advances on yield stress and elasticity of fresh cement-based materials. Cem. Concr. Res. 2019, 124, 105798. [Google Scholar] [CrossRef]
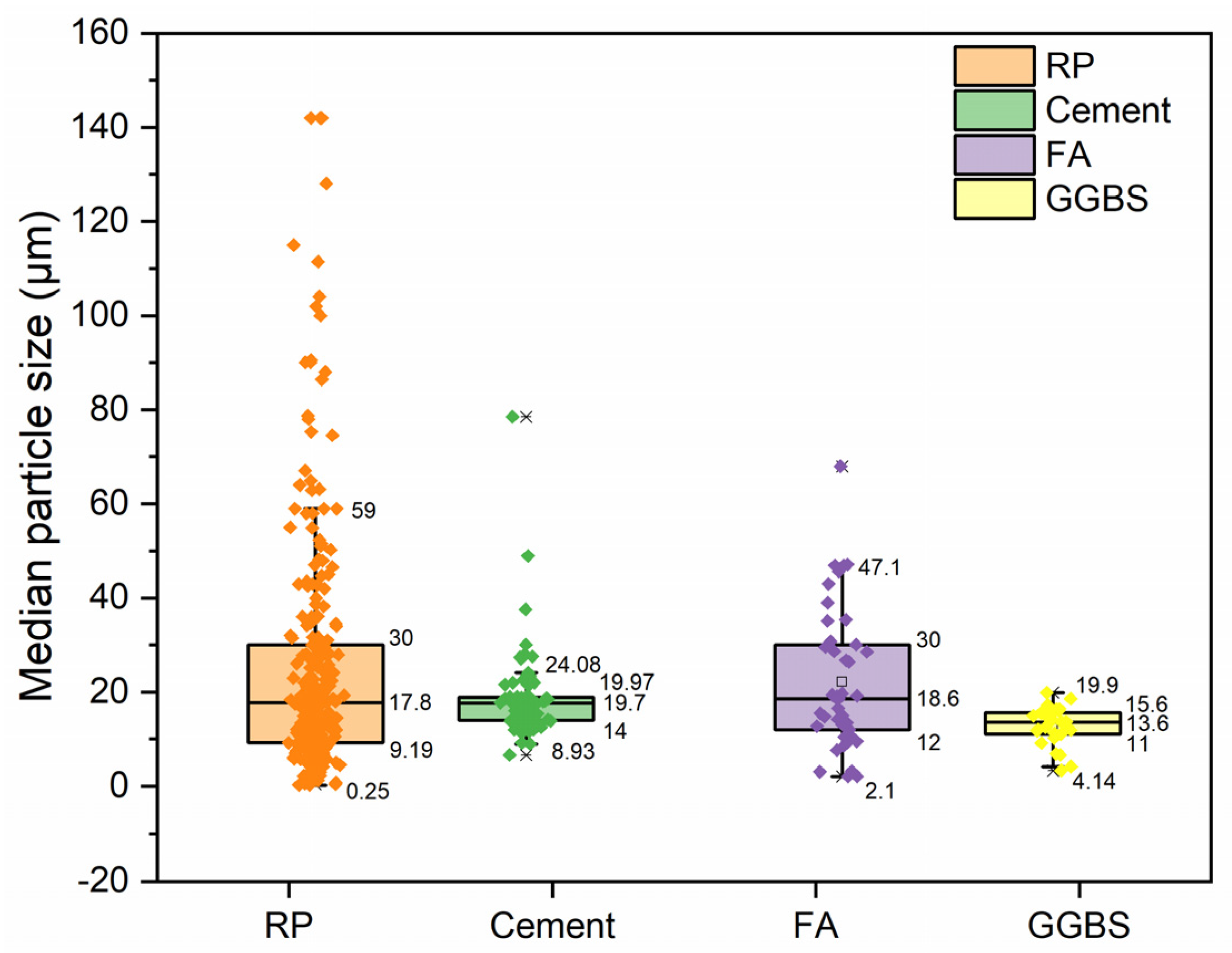


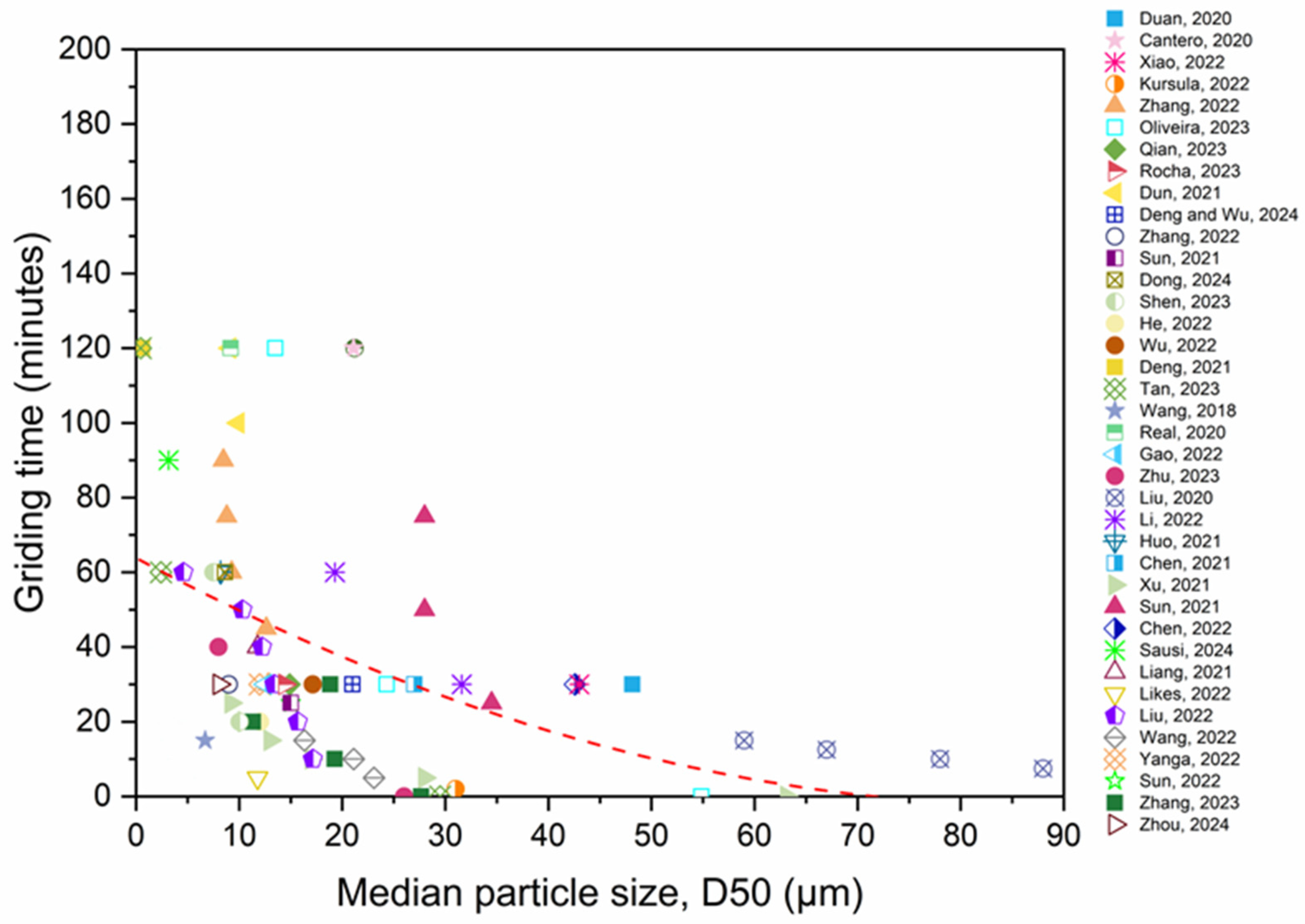

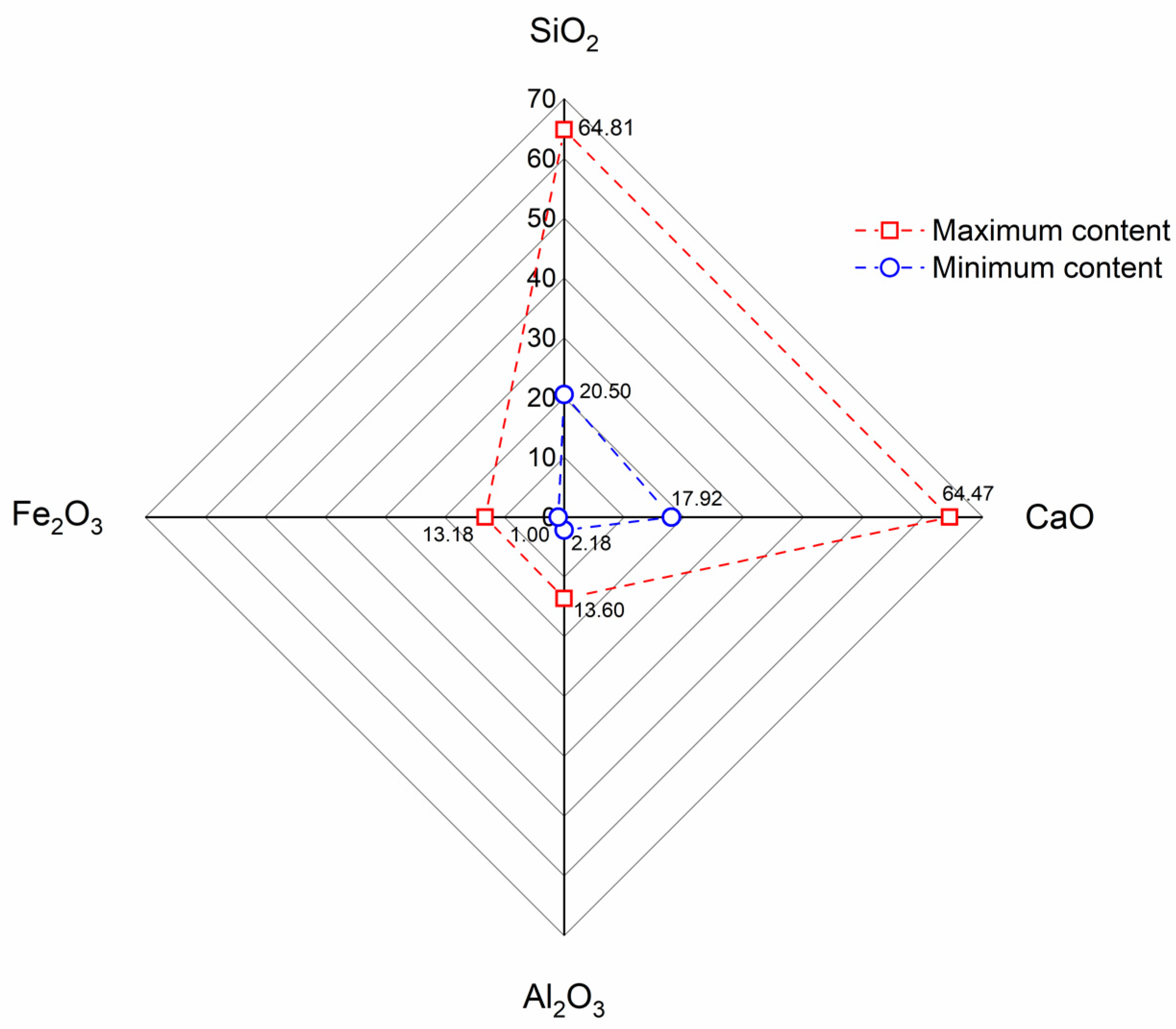
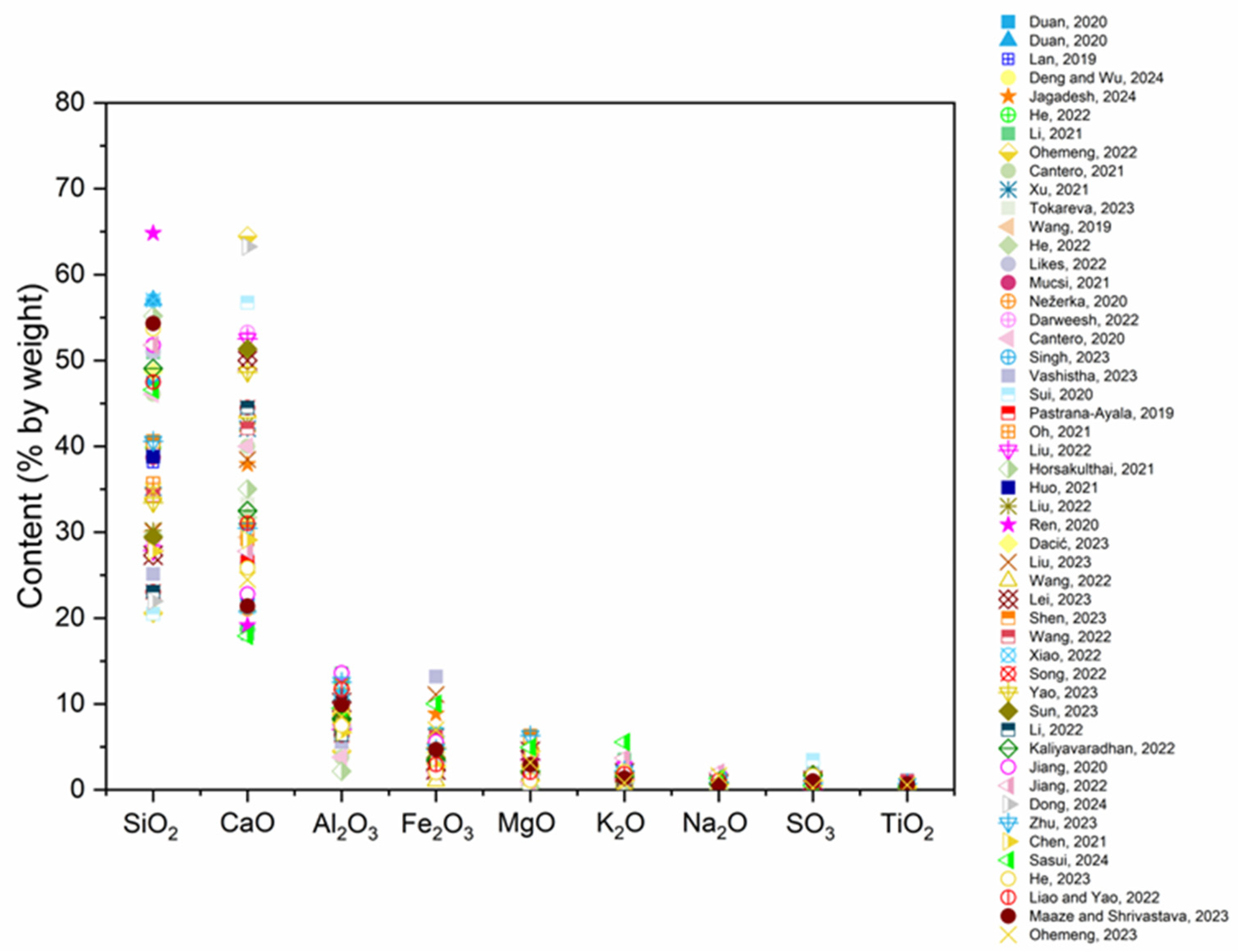

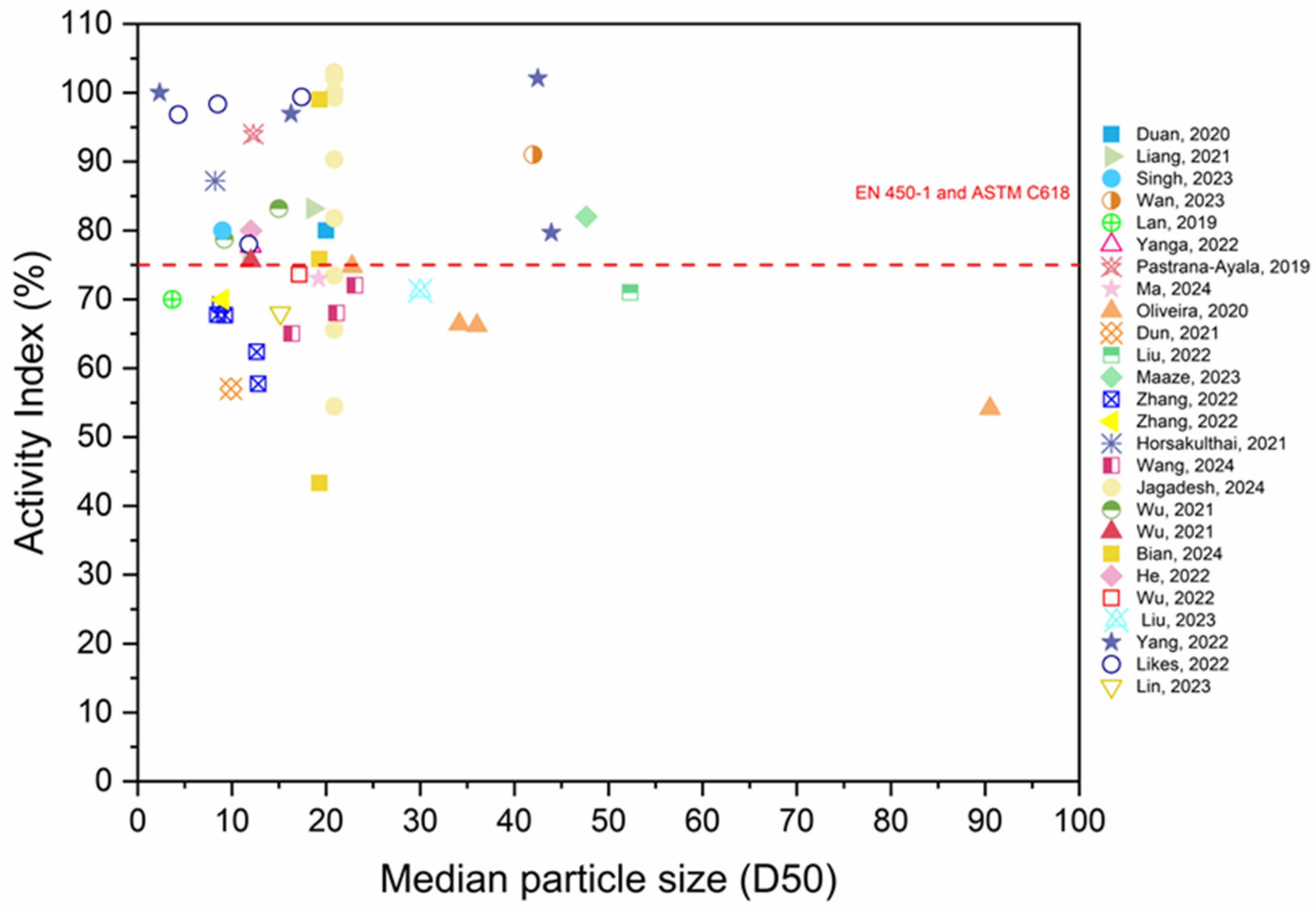
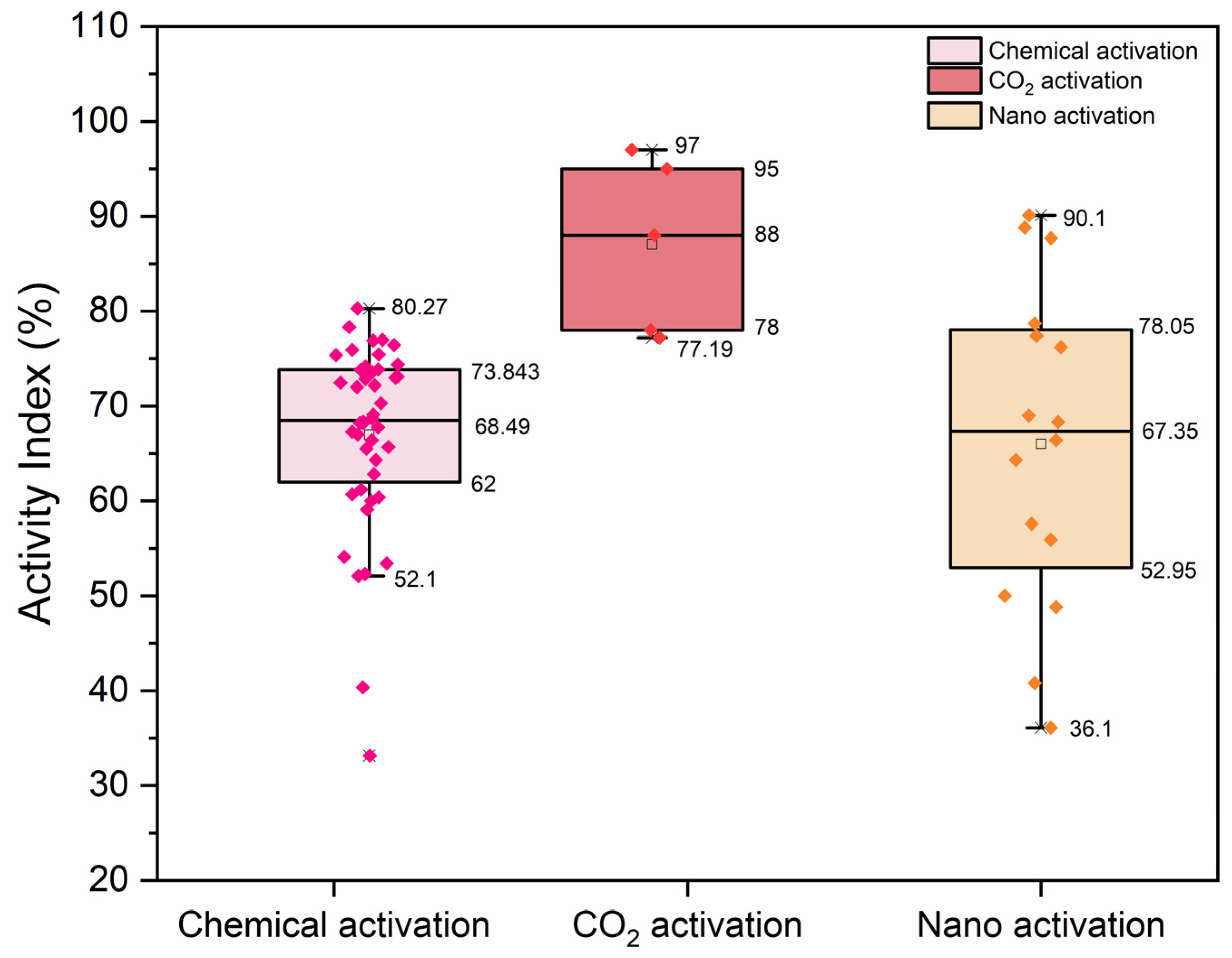
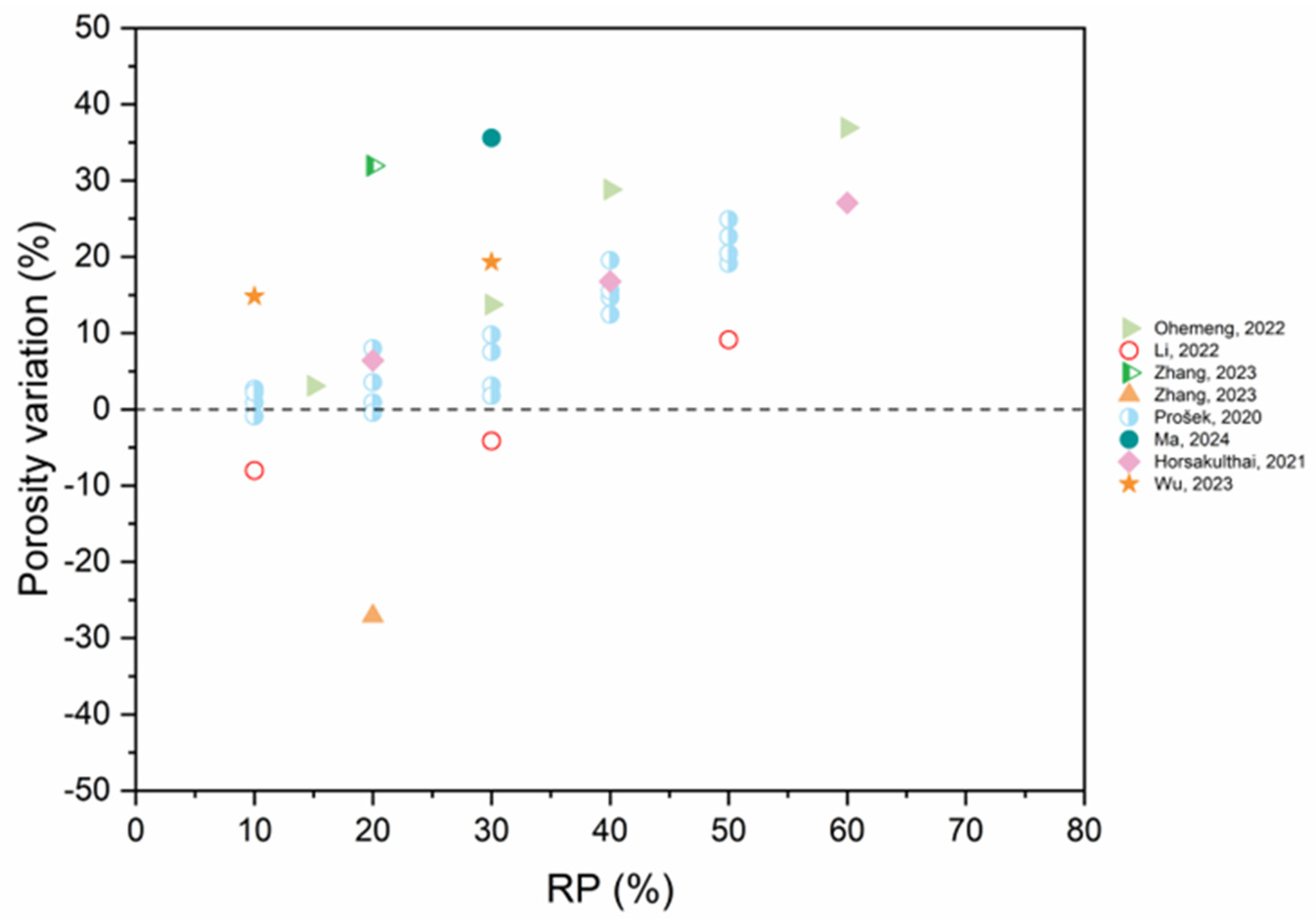
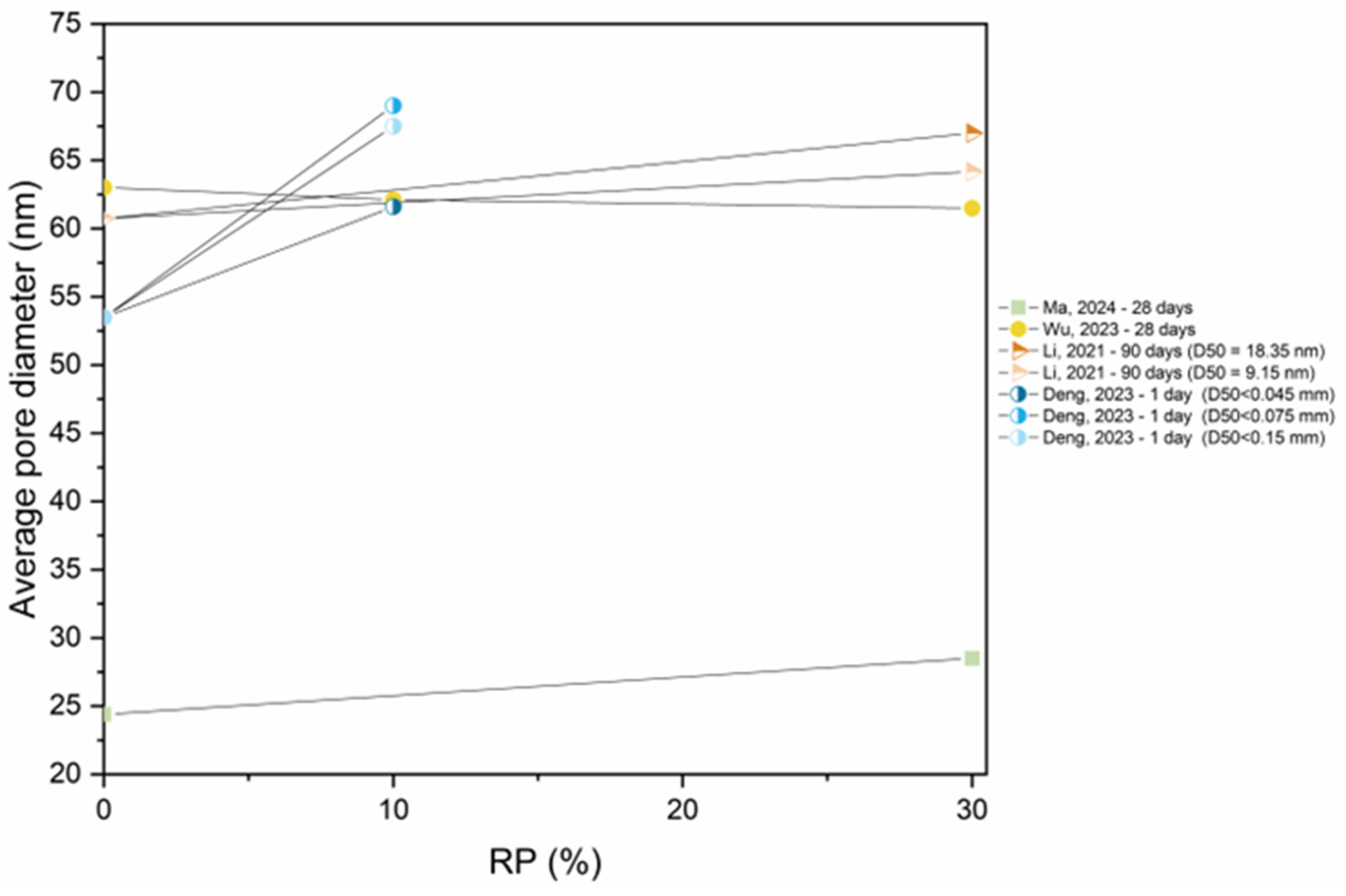
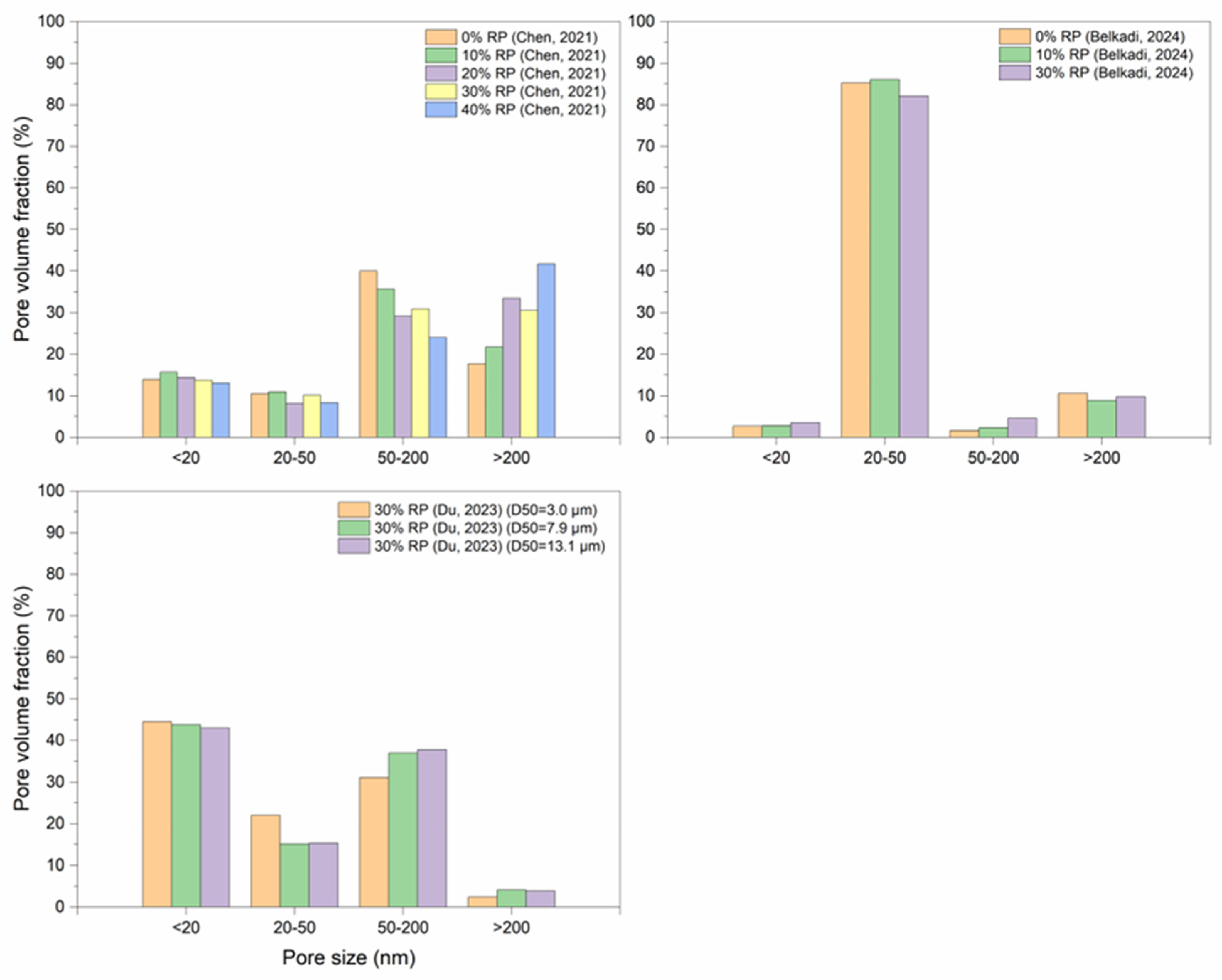

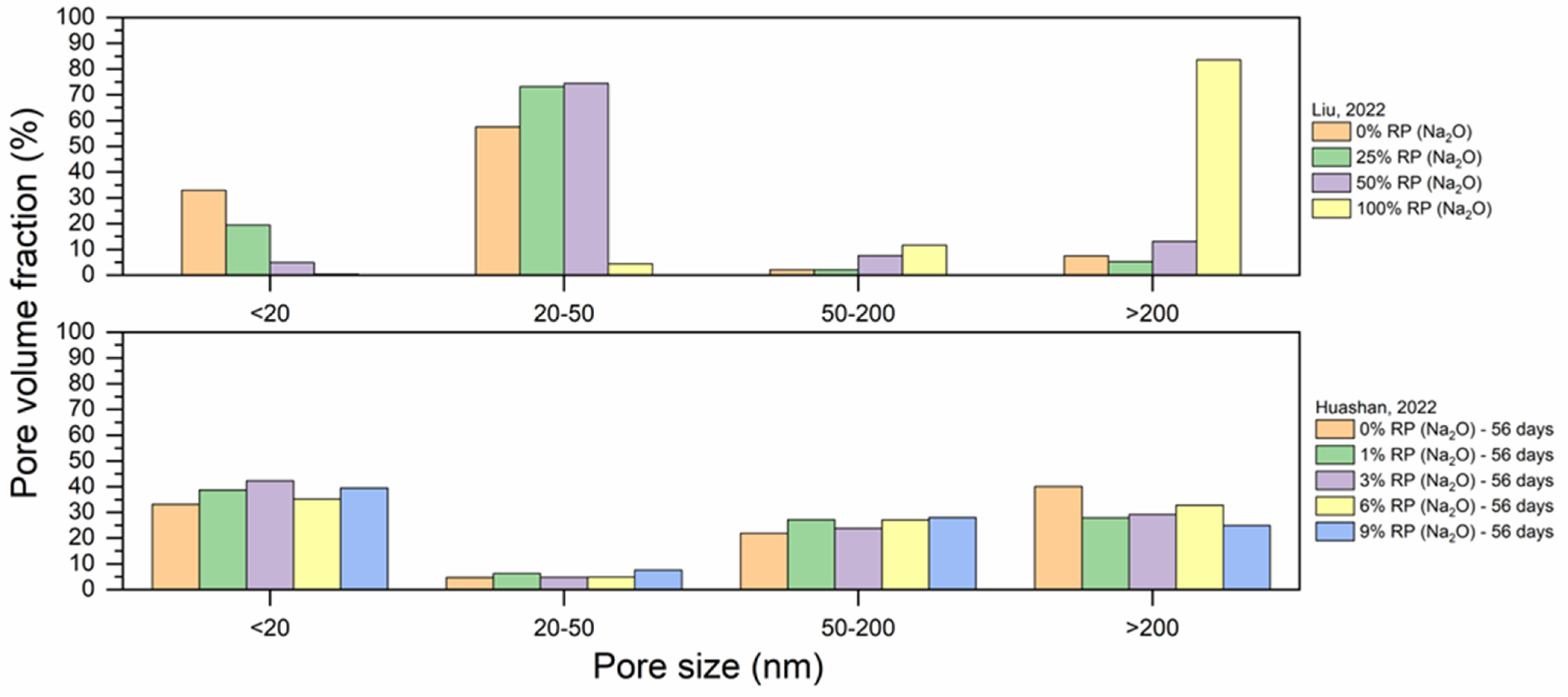
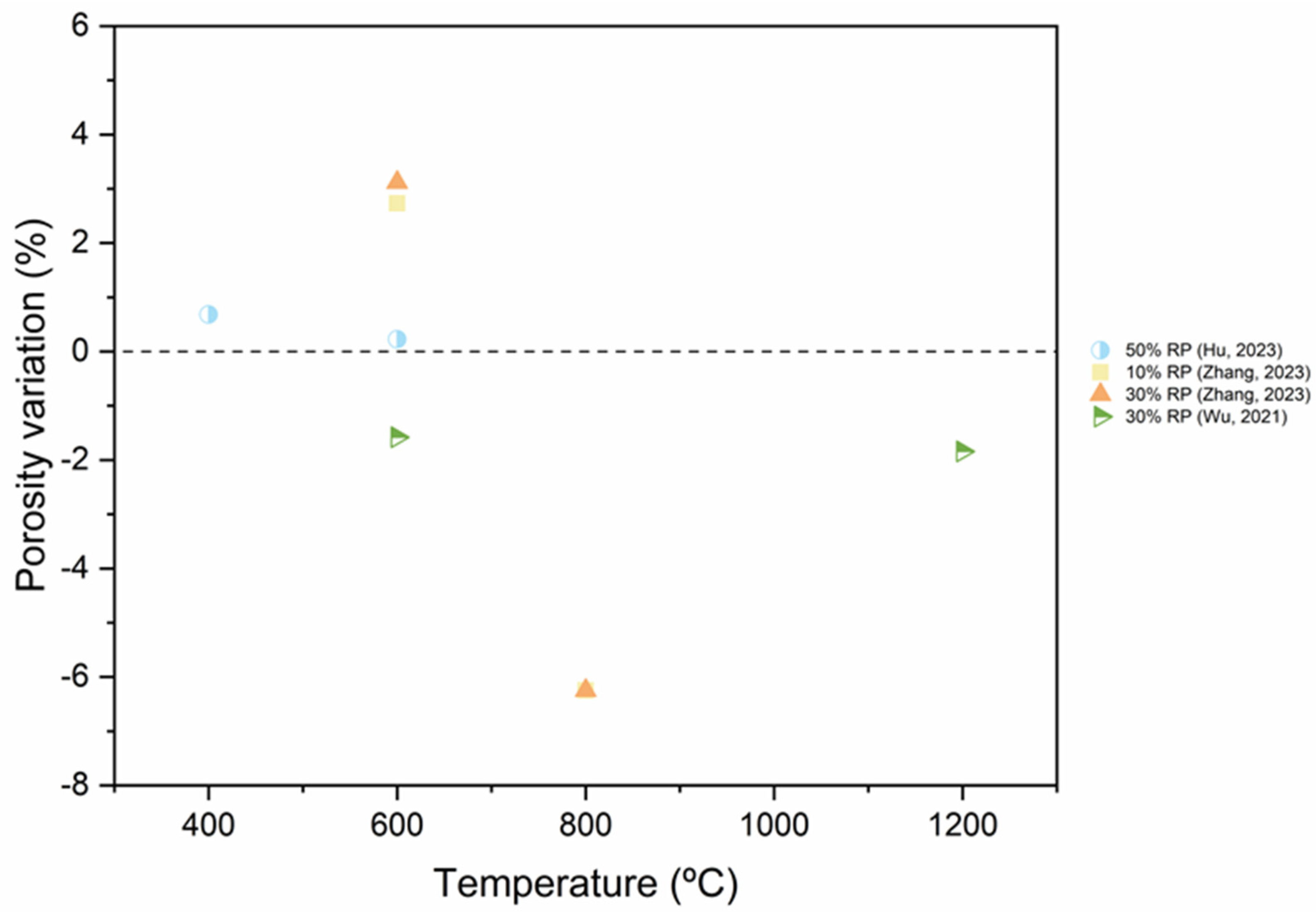
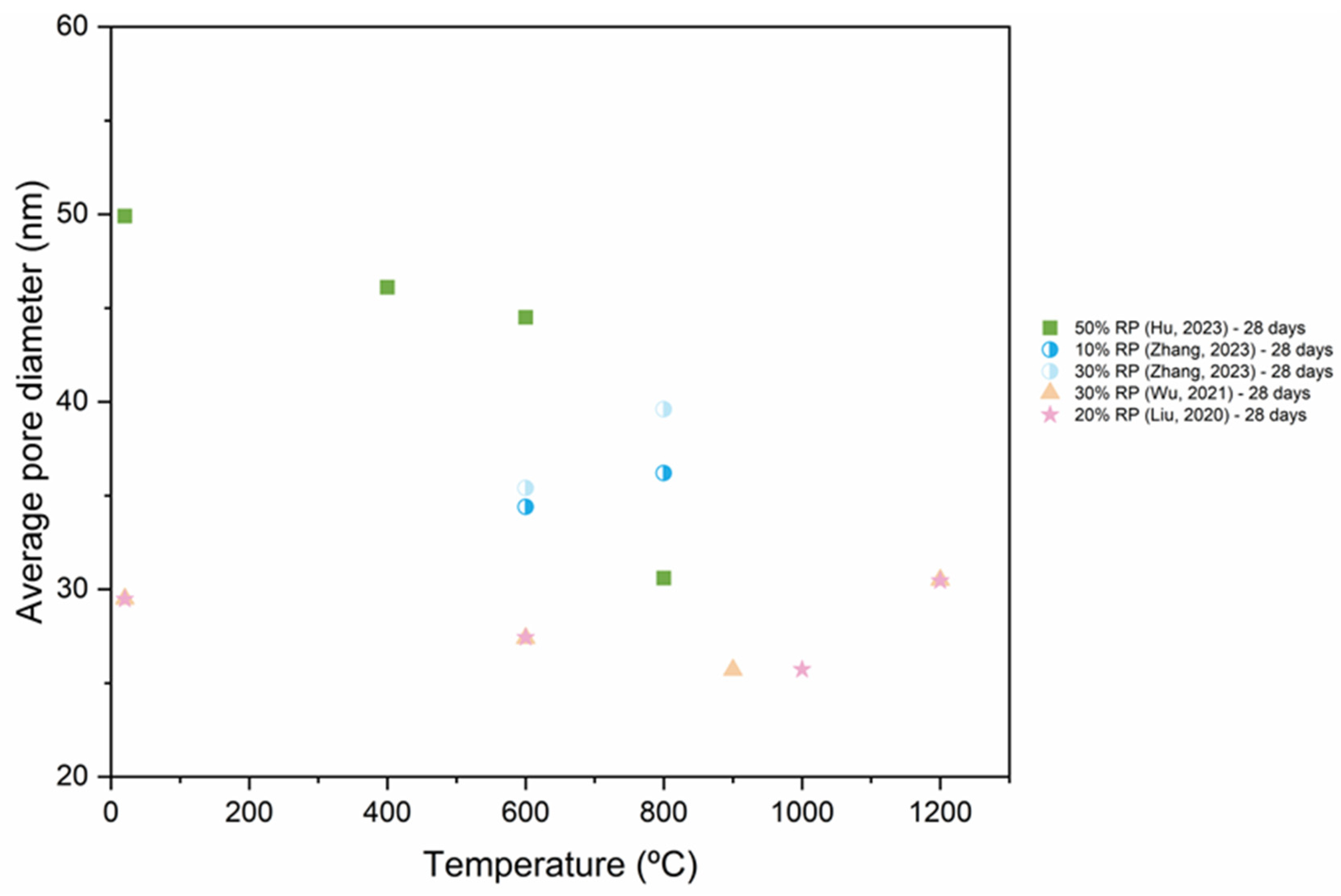

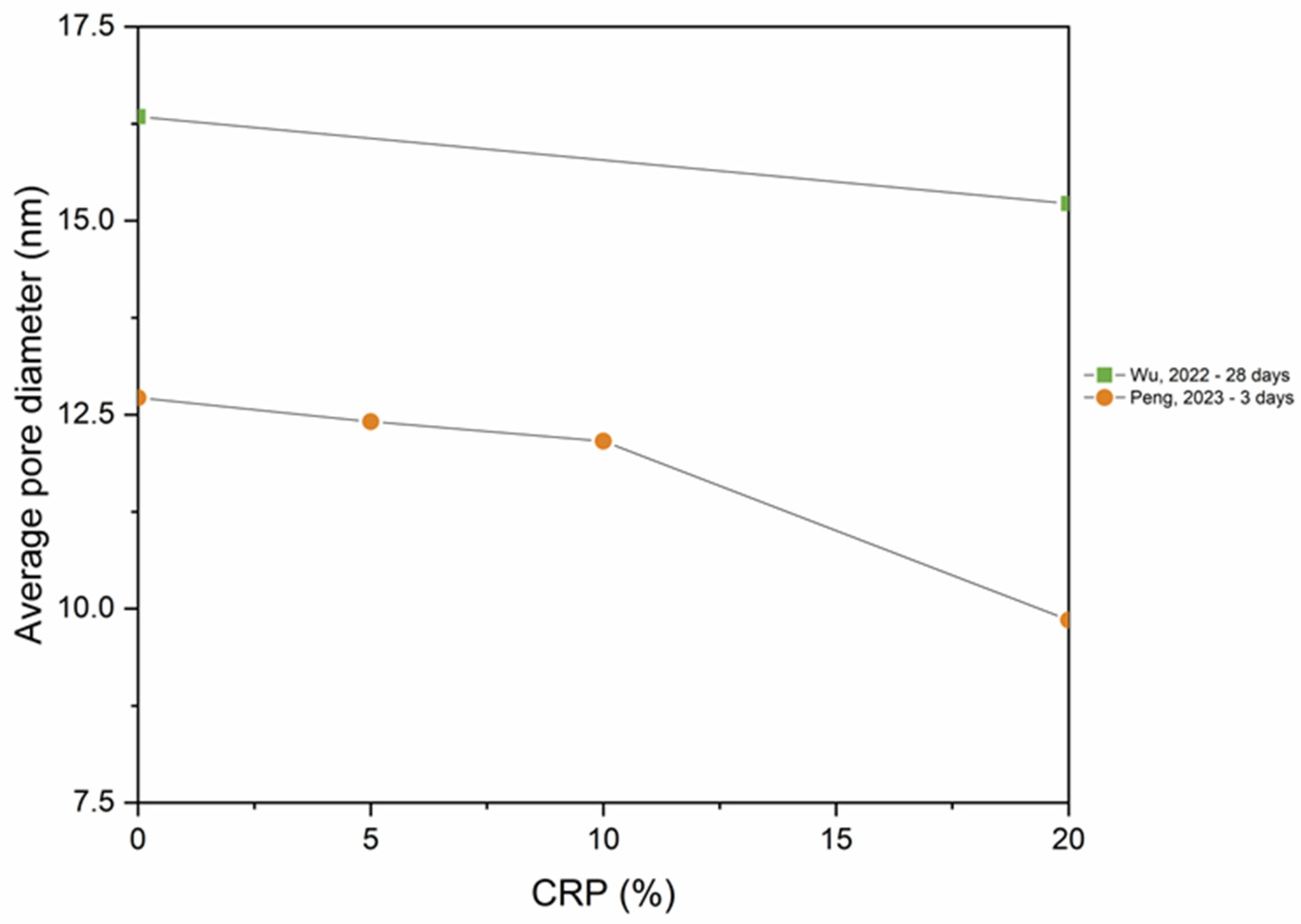
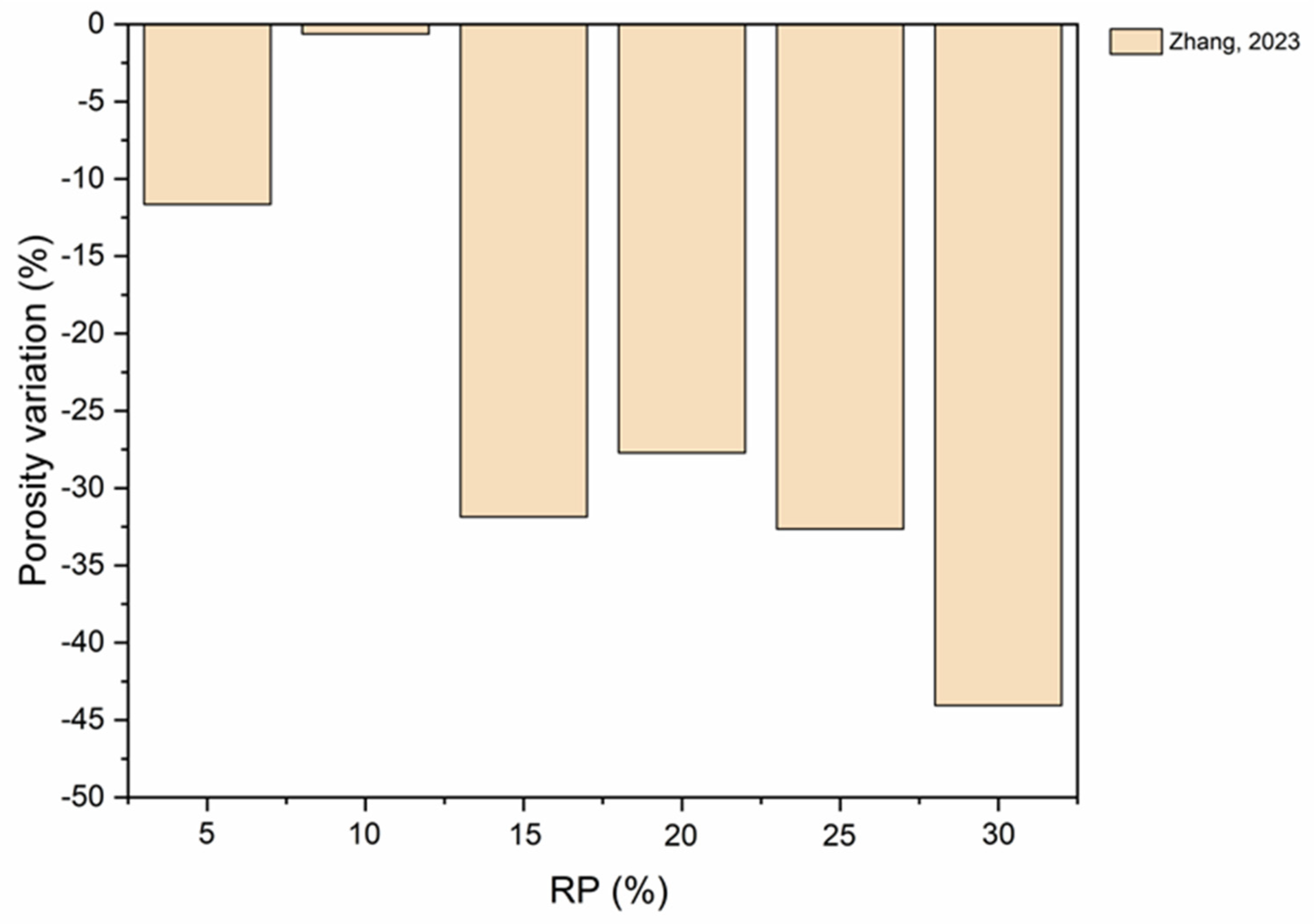
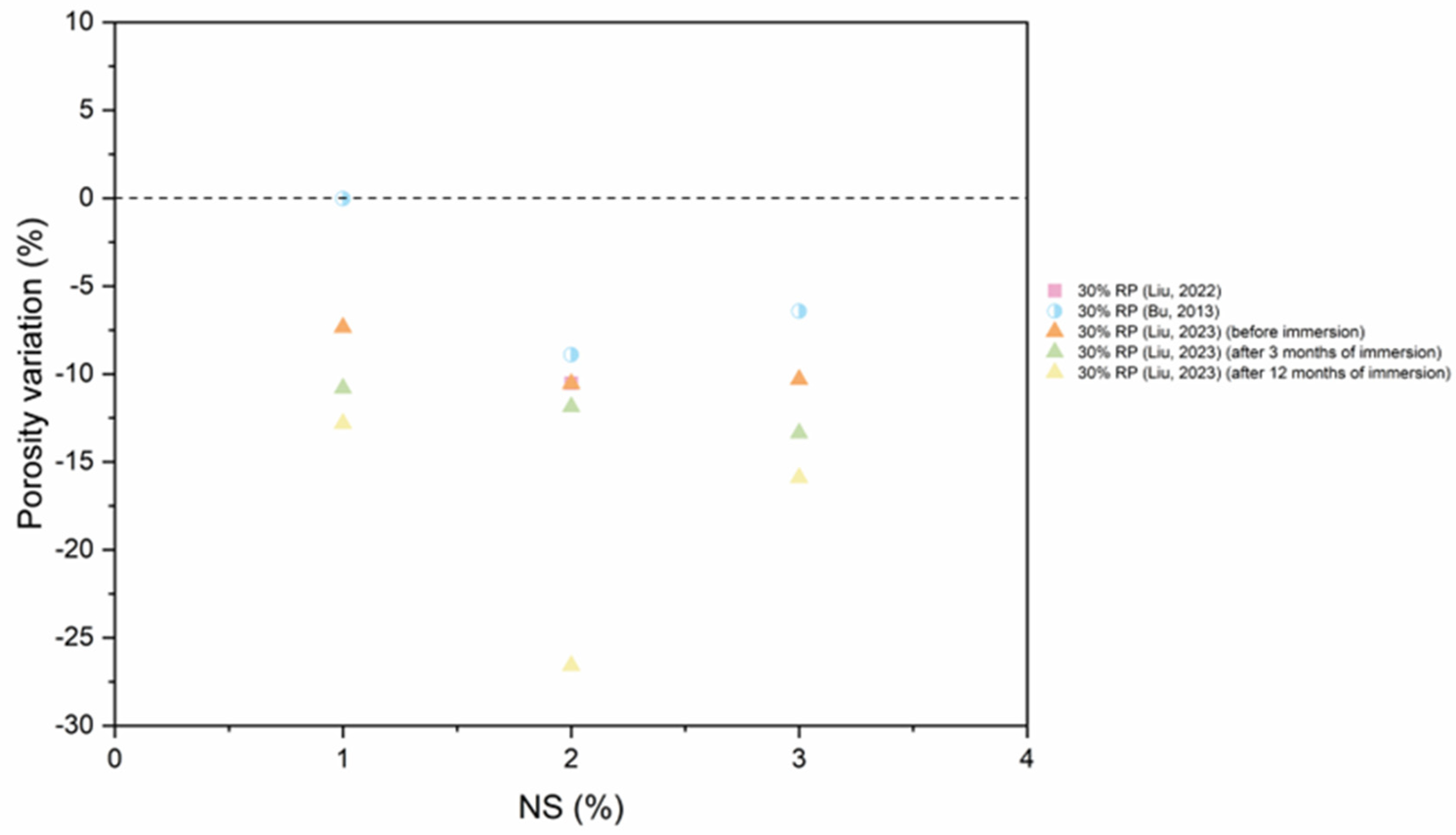
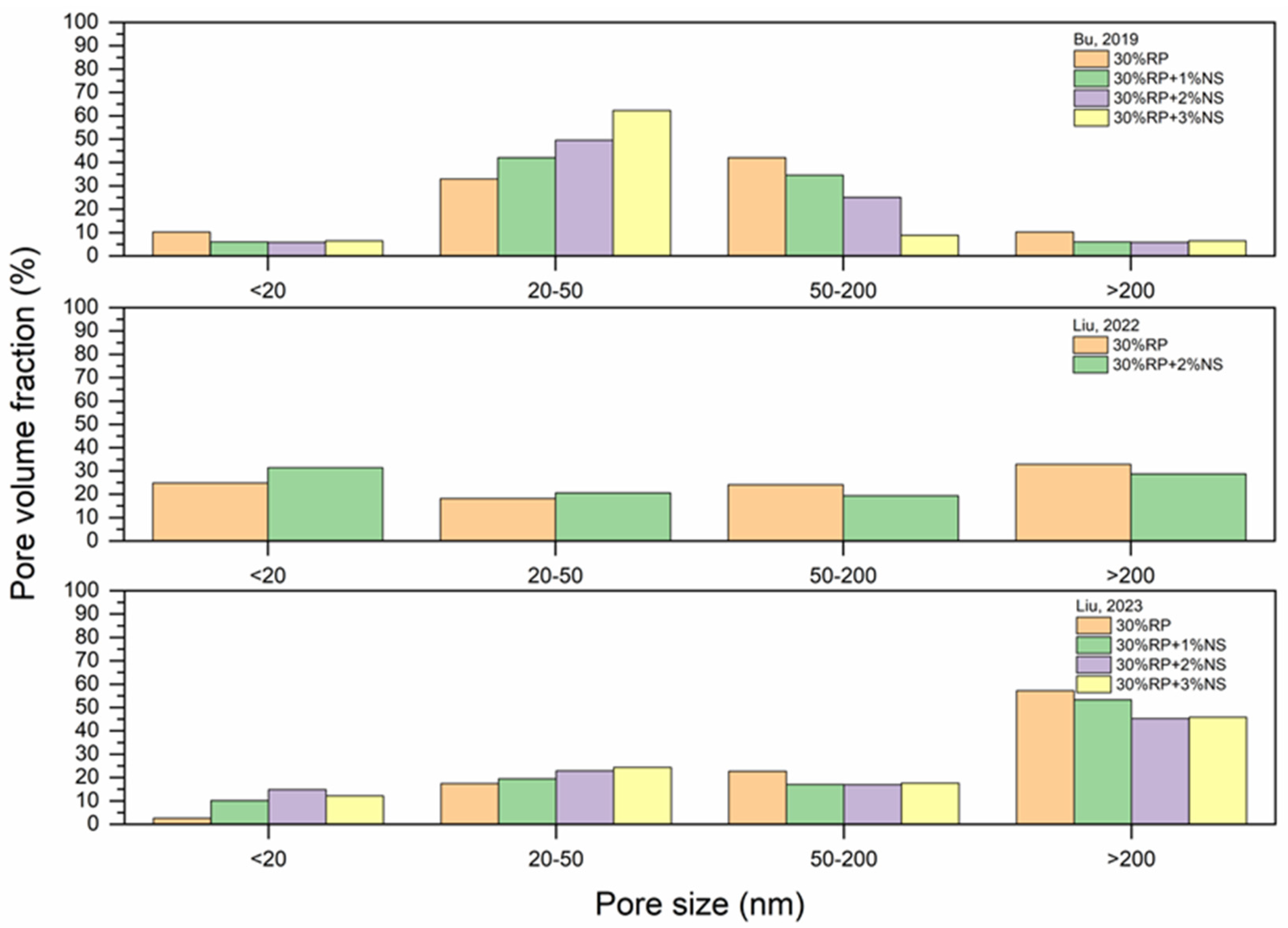
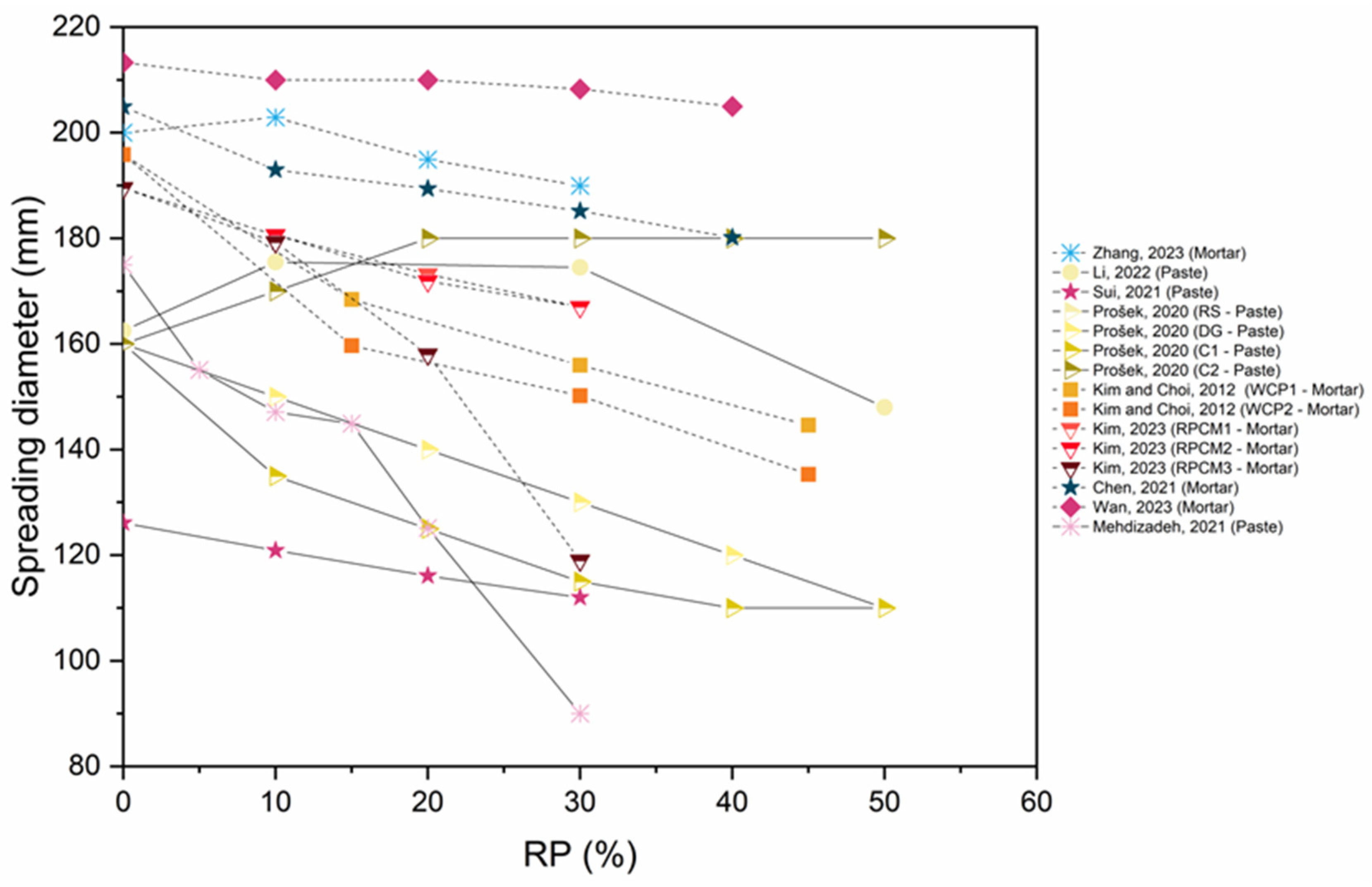
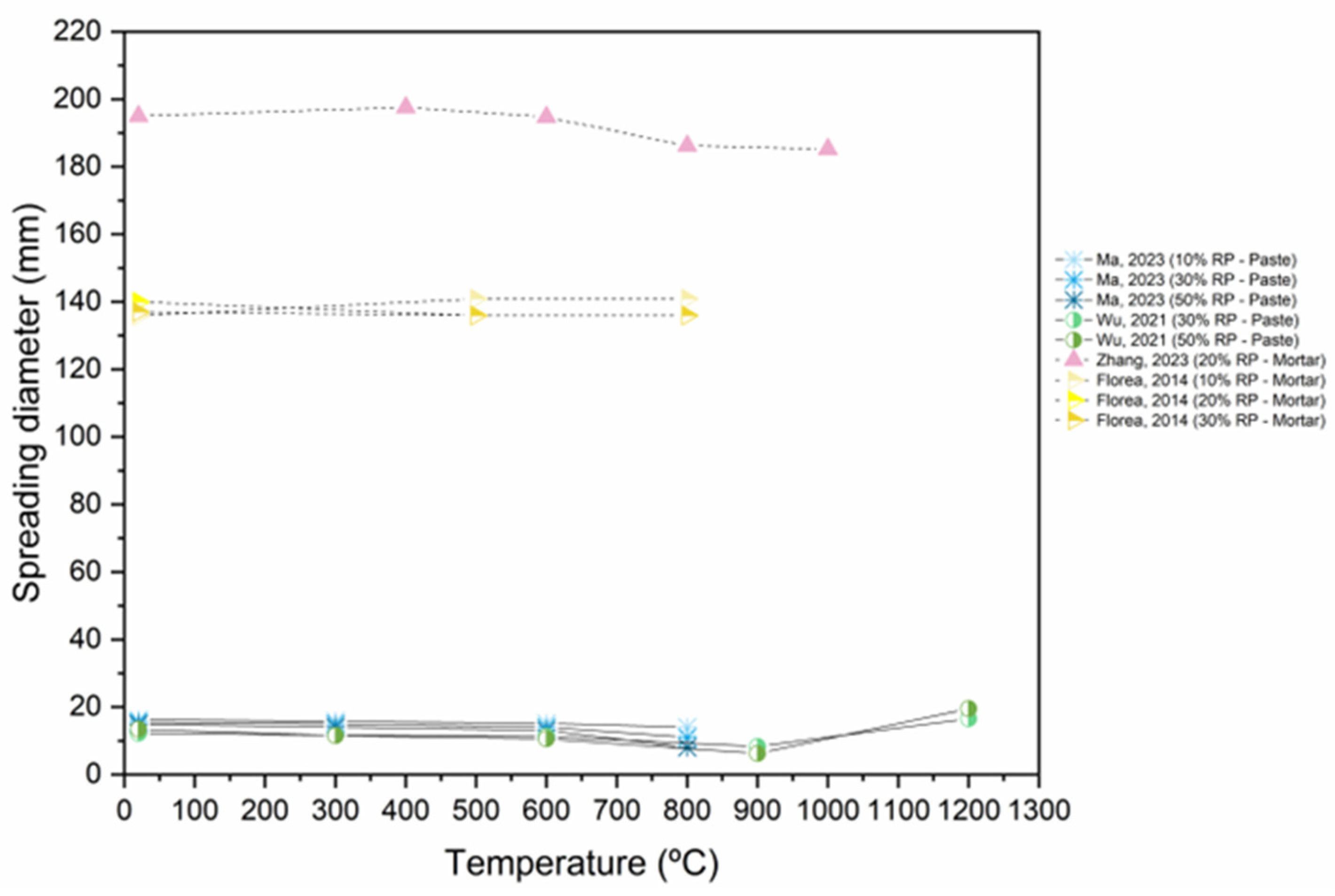
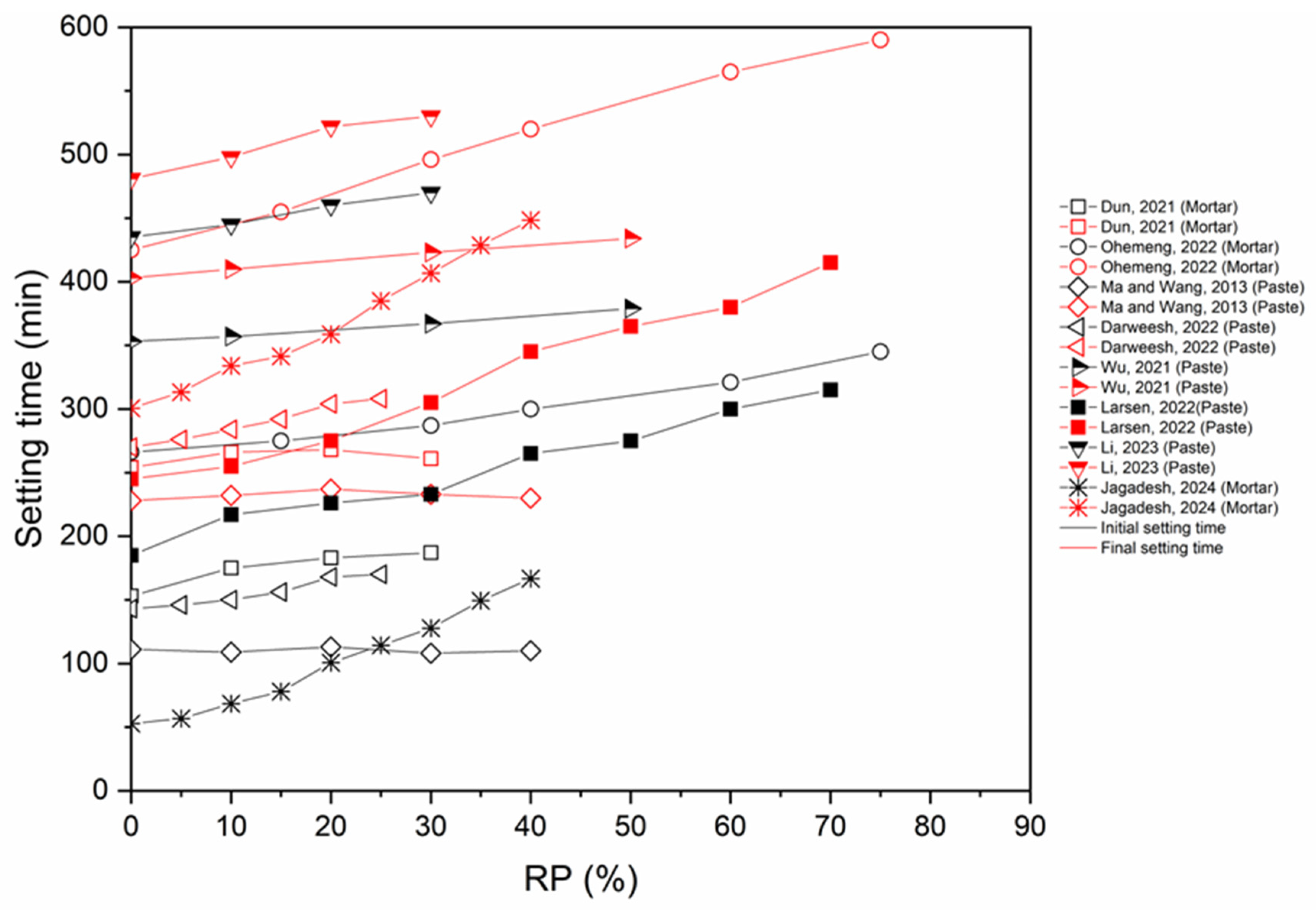
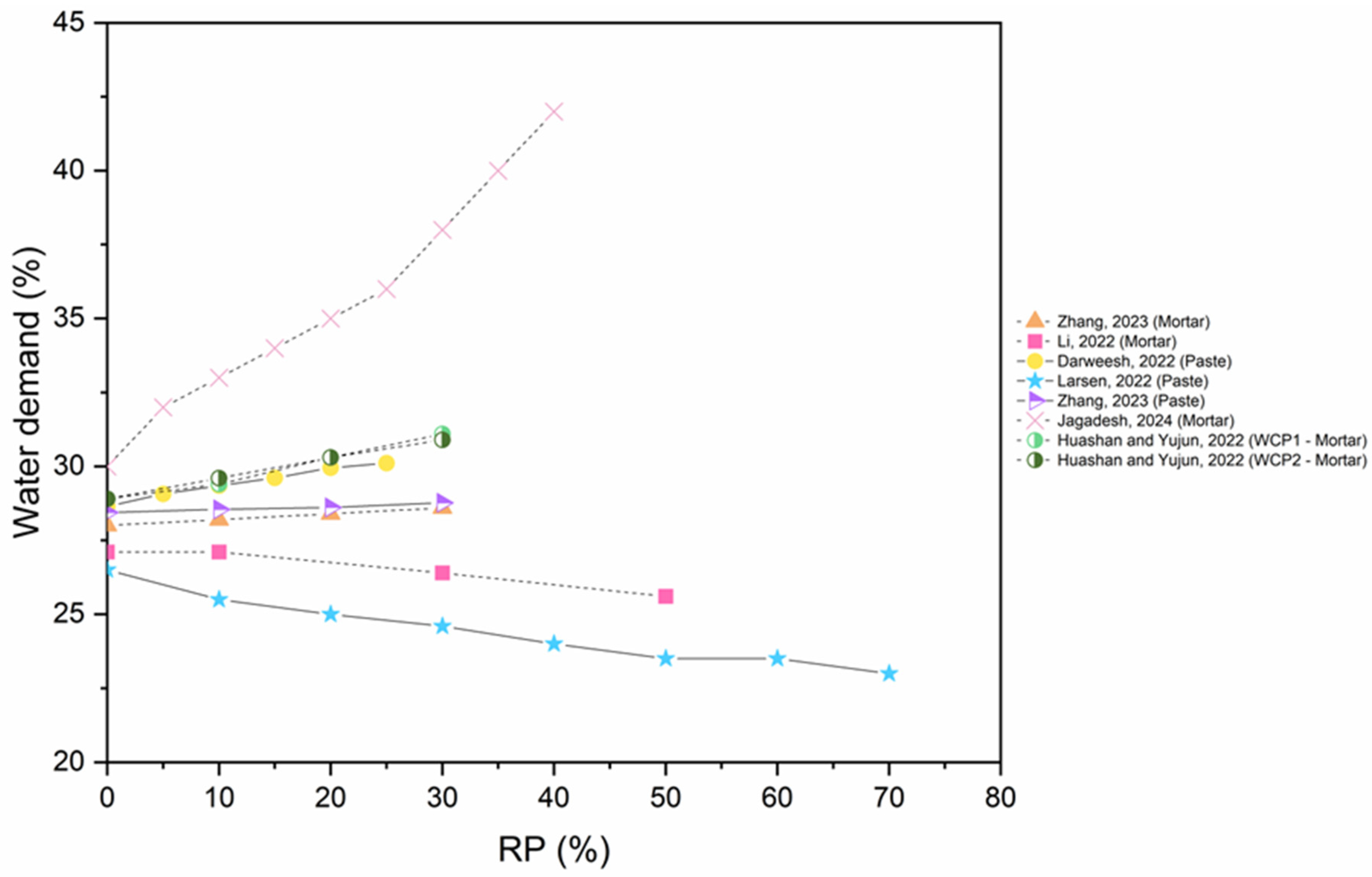
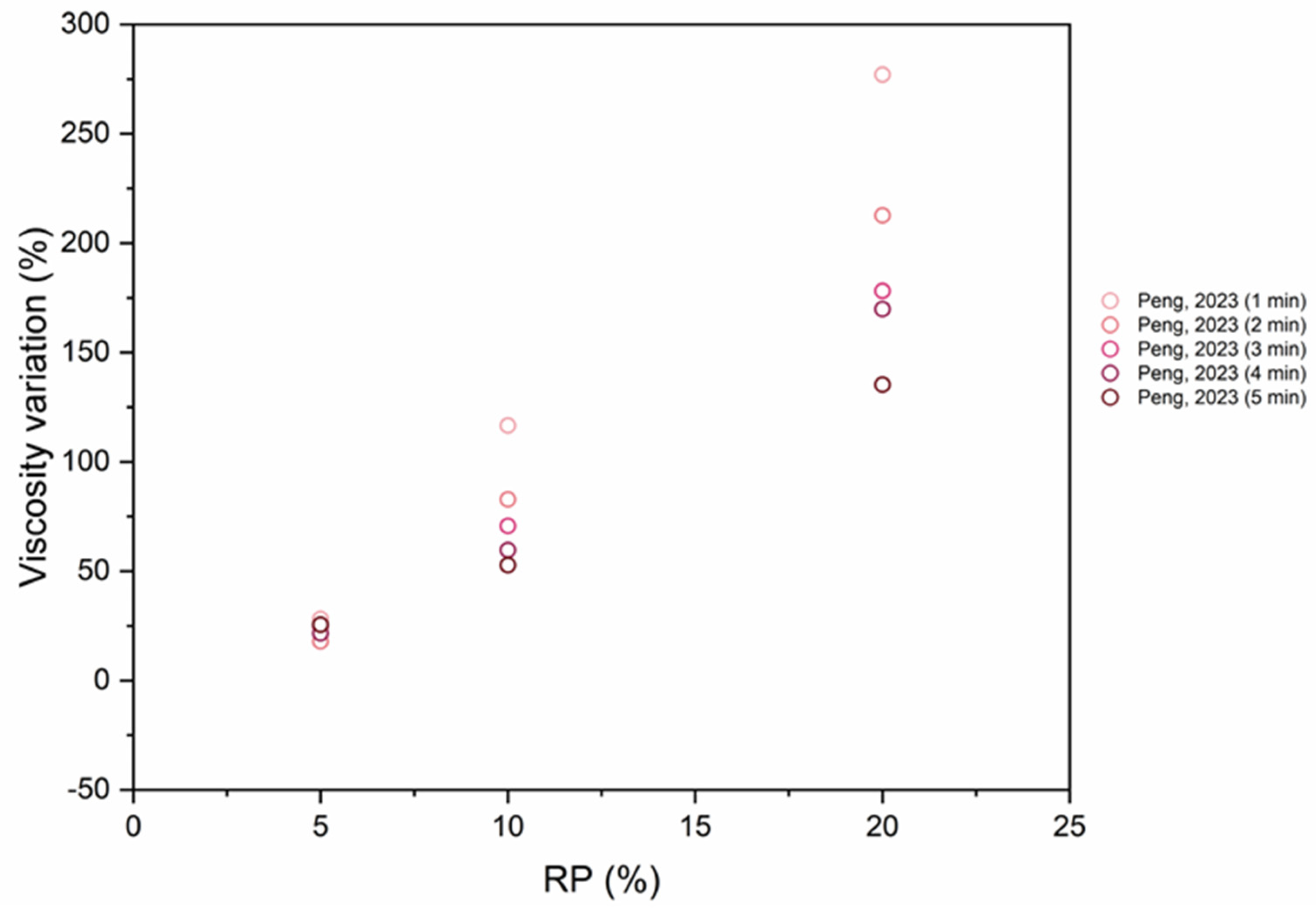
| Abbreviation | Designation |
|---|---|
| CFs | Concrete fines |
| CSFs | Concrete screening fines |
| CW | Concrete waste |
| CWFs | Concrete waste fines |
| CWP | Concrete waste powder |
| FRC | Fine recycled concrete |
| GCP | Ground concrete powder |
| GRC | Ground recycled concrete |
| GWCP | Ground waste concrete powder |
| HHCW | Humid hardened concrete waste |
| RP | Recycled powder |
| RCFs | Recycled concrete fines |
| RCCFs | Recycled crushed concrete fines |
| RCFP | Recycled concrete fine powder |
| RCP | Recycled concrete powder |
| RCWP | Recycled concrete waste powder |
| RFAP | Recycled fine aggregate powder |
| RFP | Recycled fine powder |
| RHCP | Recycled hardened concrete powder |
| RPC | Recycled powder concrete |
| RWC | Recycled waste concrete |
| RWCP | Recycled waste concrete powder |
| WC | Waste concrete |
| WCFs | Waste concrete fines |
| WCP | Waste concrete powder |
| WCRP | Waste concrete recycled powder |
| WP | Waste powder |
| WPC | Waste powder concrete |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaptan, K.; Cunha, S.; Aguiar, J. A Review of the Utilization of Recycled Powder from Concrete Waste as a Cement Partial Replacement in Cement-Based Materials: Fundamental Properties and Activation Methods. Appl. Sci. 2024, 14, 9775. https://doi.org/10.3390/app14219775
Kaptan K, Cunha S, Aguiar J. A Review of the Utilization of Recycled Powder from Concrete Waste as a Cement Partial Replacement in Cement-Based Materials: Fundamental Properties and Activation Methods. Applied Sciences. 2024; 14(21):9775. https://doi.org/10.3390/app14219775
Chicago/Turabian StyleKaptan, Kubilay, Sandra Cunha, and José Aguiar. 2024. "A Review of the Utilization of Recycled Powder from Concrete Waste as a Cement Partial Replacement in Cement-Based Materials: Fundamental Properties and Activation Methods" Applied Sciences 14, no. 21: 9775. https://doi.org/10.3390/app14219775
APA StyleKaptan, K., Cunha, S., & Aguiar, J. (2024). A Review of the Utilization of Recycled Powder from Concrete Waste as a Cement Partial Replacement in Cement-Based Materials: Fundamental Properties and Activation Methods. Applied Sciences, 14(21), 9775. https://doi.org/10.3390/app14219775